A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective
Abstract
1. Introduction
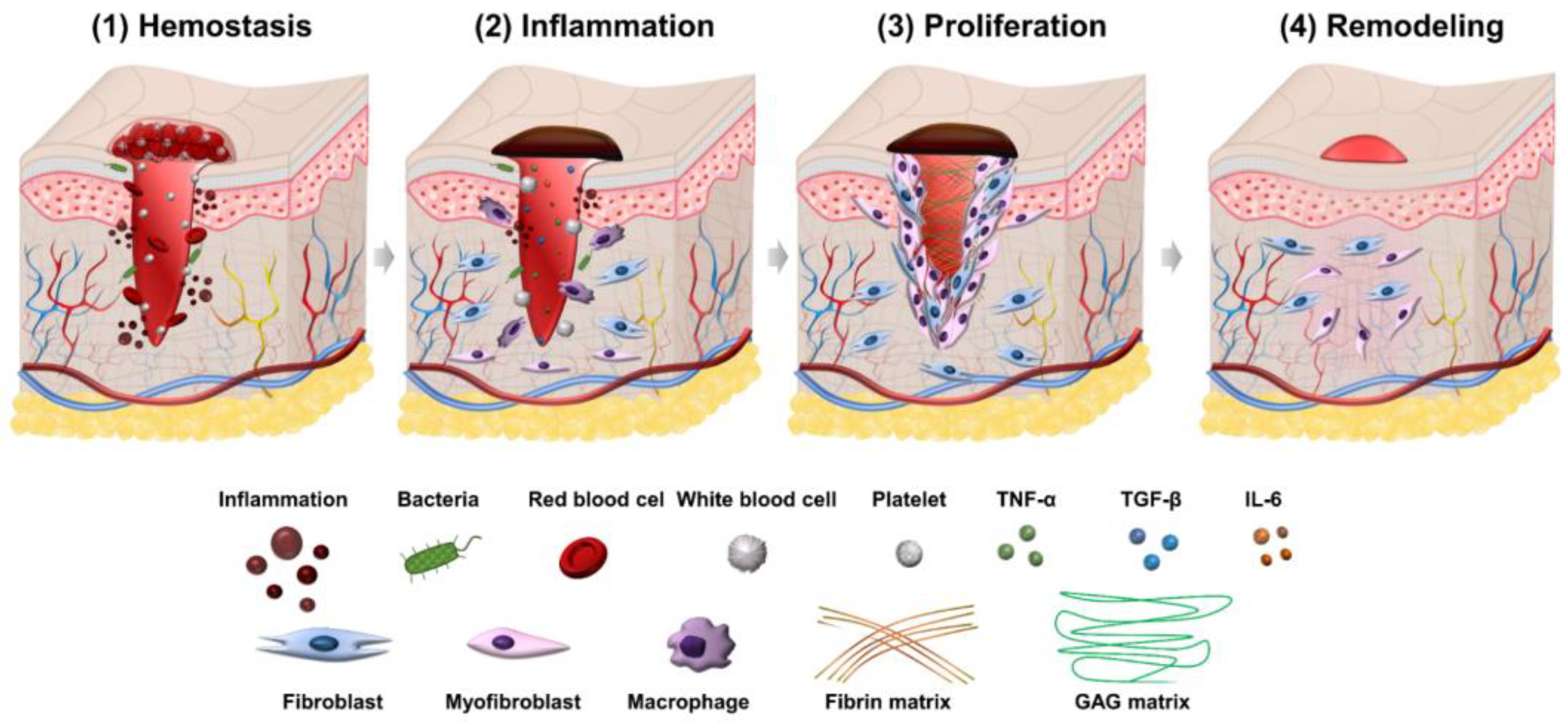
2. The Process of Wound Healing
2.1. Hemostasis Phase
2.2. Inflammation Phase
2.3. Proliferation Phase
2.4. Remodeling Phase
3. Classification of Natural Compounds for Wound Healing by Their Properties
3.1. Natural Compounds with Anti-Inflammation Properties
3.1.1. Myricetin
3.1.2. Calophyllolide (CP)
3.1.3. Steroidal Glycoside
3.1.4. Verbascoside (Acteoside)
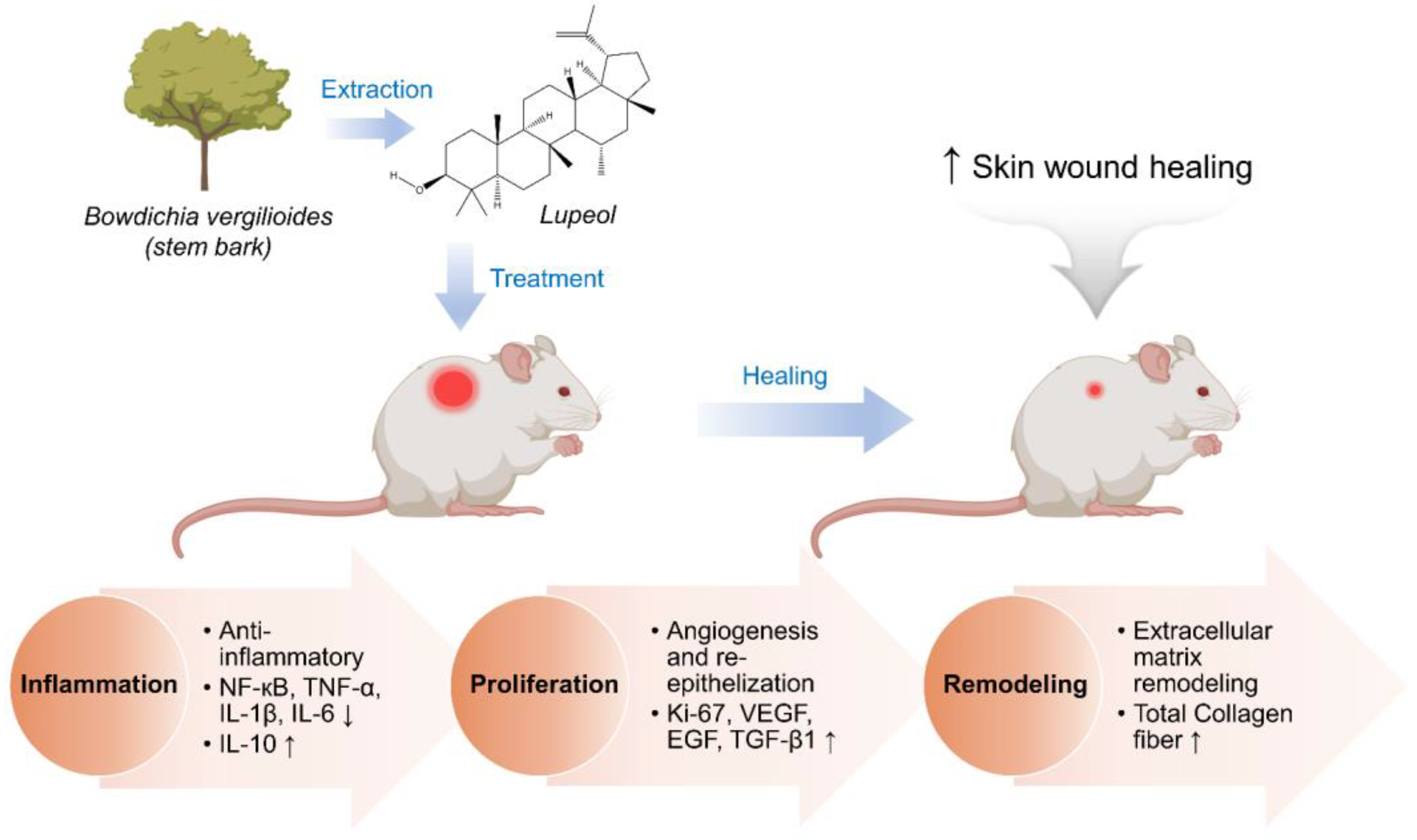
3.1.5. Lupeol
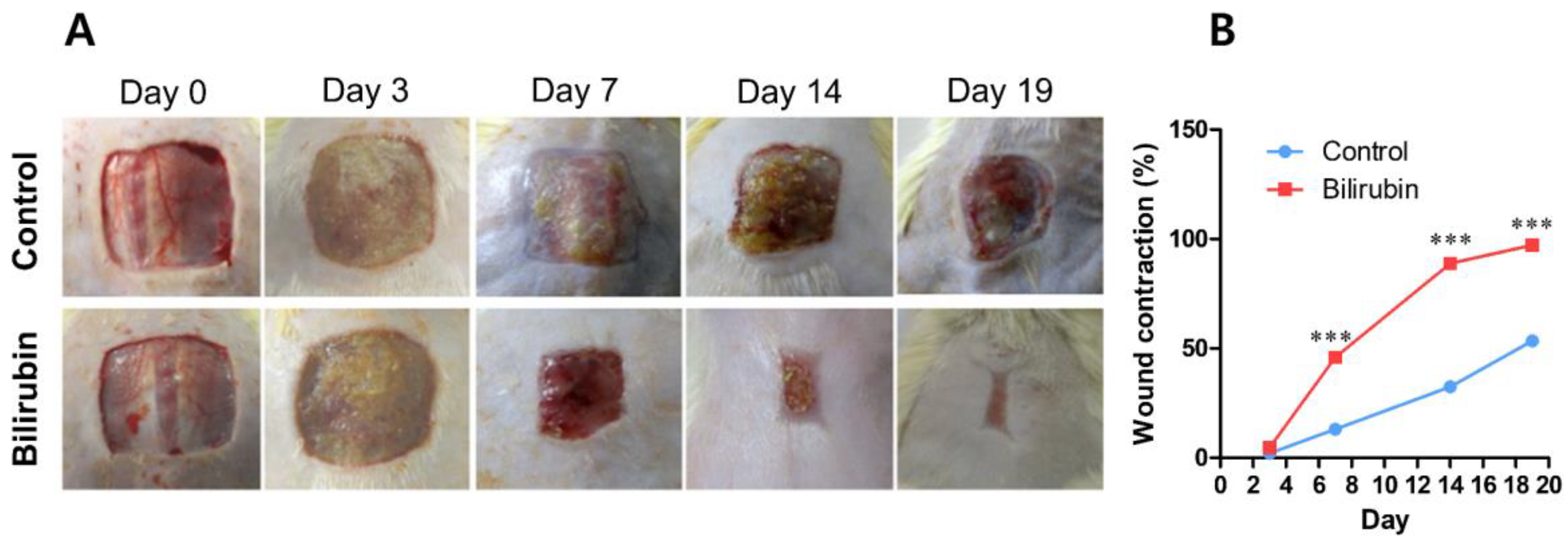
3.1.6. Bilirubin
3.1.7. Pinocembrin
3.2. Natural Compounds with Anti-Oxidant Properties
3.2.1. Curcumin
3.2.2. Quercetin
3.2.3. Catechin
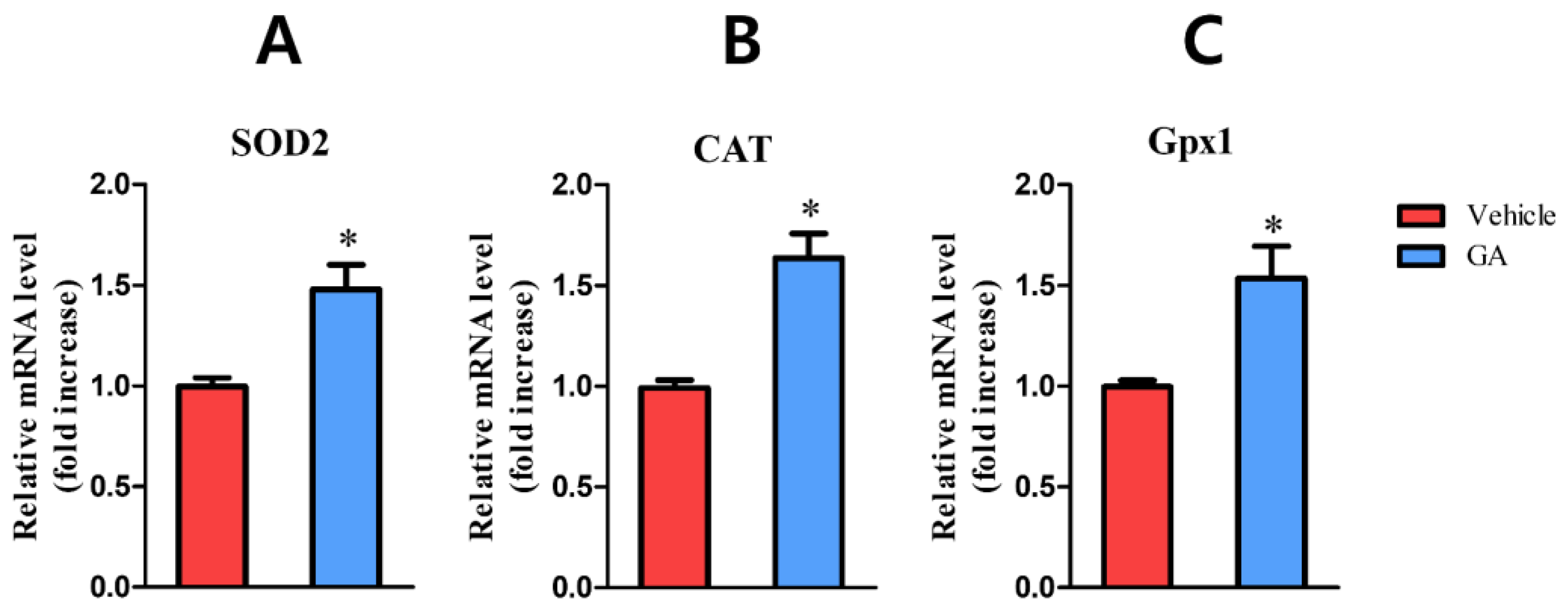
3.2.4. Galic Acid (GA)
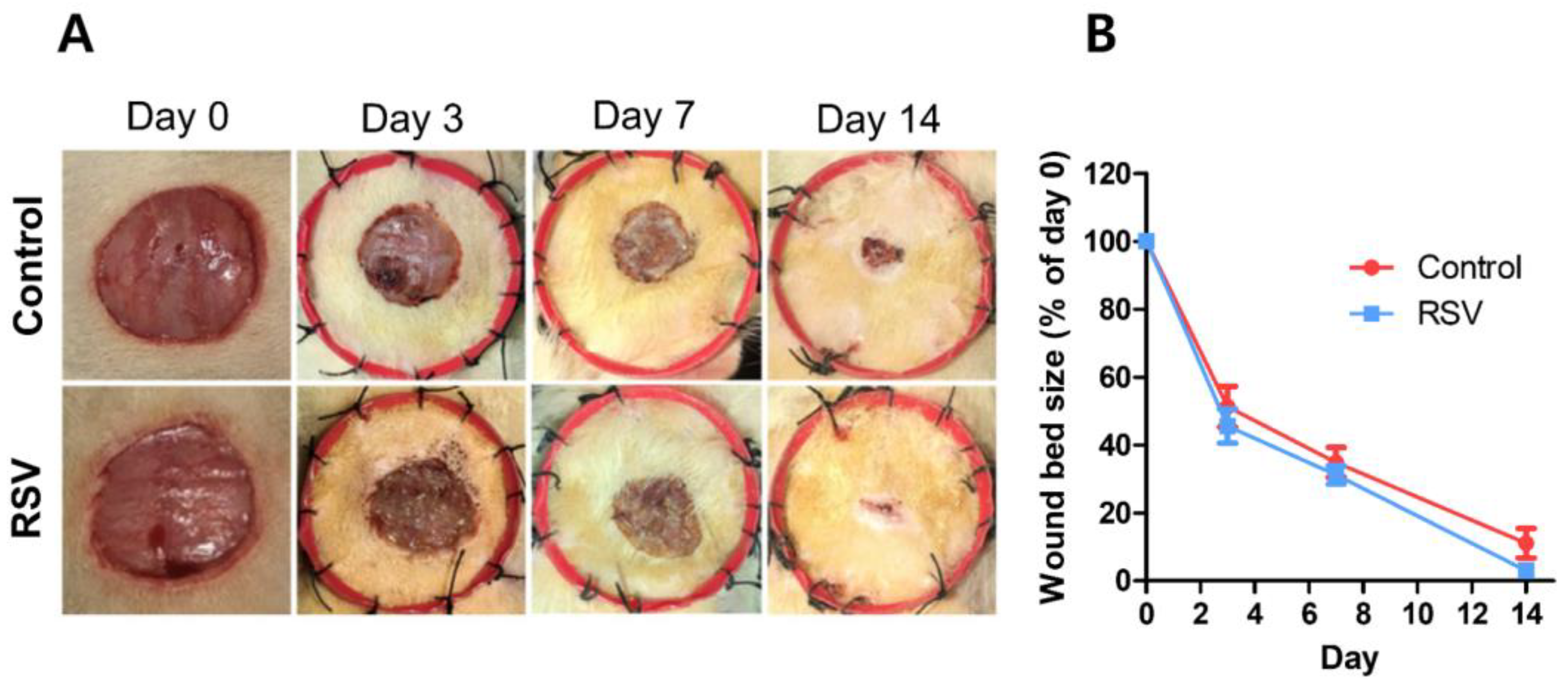
3.2.5. Resveratrol (RSV)
3.2.6. Naringenin
3.3. Natural Compounds with Antibacterial Properties
3.3.1. Chitosan and Chitin
3.3.2. Honey Bee
3.3.3. Propolis
3.3.4. Tannins
3.3.5. Allicin
3.3.6. Terpene Esters
3.4. Natural Compounds with Collagen Promotion Properties
3.4.1. Saponins
3.4.2. Cryptotanshinone
3.4.3. Artocarpin
3.4.4. β-Glucans
3.4.5. Amino Acids and Peptides
4. Current Trending Use of Natural Compounds in Wound Healing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Agyare, C.; Akindele, A.J.; Steenkamp, V. Natural Products and/or Isolated Compounds on Wound Healing. Evid.-Based Complement. Altern. Med. 2019, 2019, 4594965. [Google Scholar] [CrossRef] [PubMed]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Schreml, S.; Szeimies, R.-M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound Healing in the 21st Century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef] [PubMed]
- Ryall, C.; Duarah, S.; Chen, S.; Yu, H.; Wen, J. Advancements in Skin Delivery of Natural Bioactive Products for Wound Management: A Brief Review of Two Decades. Pharmaceutics 2022, 14, 1072. [Google Scholar] [CrossRef]
- Vitale, S.; Colanero, S.; Placidi, M.; Di Emidio, G.; Tatone, C.; Amicarelli, F.; D’Alessandro, A.M. Phytochemistry and Biological Activity of Medicinal Plants in Wound Healing: An Overview of Current Research. Molecules 2022, 27, 3566. [Google Scholar] [CrossRef]
- Ibrahim, N.; Wong, S.; Mohamed, I.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A. Wound Healing Properties of Selected Natural Products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef]
- Artem Ataide, J.; Caramori Cefali, L.; Machado Croisfelt, F.; Arruda Martins Shimojo, A.; Oliveira-Nascimento, L.; Gava Mazzola, P. Natural Actives for Wound Healing: A Review. Phyther. Res. 2018, 32, 1664–1674. [Google Scholar] [CrossRef]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell. Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J.A.A. Functional Properties of Honey, Propolis, and Royal Jelly. J. Food Sci. 2008, 73, 117–124. [Google Scholar] [CrossRef]
- Radha, M.H.; Laxmipriya, N.P. Evaluation of Biological Properties and Clinical Effectiveness of Aloe Vera: A Systematic Review. J. Tradit. Complement. Med. 2015, 5, 21–26. [Google Scholar] [CrossRef]
- Fana, S.E.; Ahmadpour, F.; Rasouli, H.R.; Tehrani, S.S.; Maniati, M. The Effects of Natural Compounds on Wound Healing in Iranian Traditional Medicine: A Comprehensive Review. Complement. Ther. Clin. Pract. 2021, 42, 101275. [Google Scholar] [CrossRef] [PubMed]
- Hajialyani, M.; Tewari, D.; Sobarzo-Sánchez, E.; Nabavi, S.M.; Farzaei, M.H.; Abdollahi, M. Natural Product-Based Nanomedicines for Wound Healing Purposes: Therapeutic Targets and Drug Delivery Systems. Int. J. Nanomed. 2018, 13, 5023–5043. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, C.D.; Neacsu, I.A.; Grumezescu, A.M.; Andronescu, E. Bee-Derived Products: Chemical Composition and Applications in Skin Tissue Engineering. Pharmaceutics 2022, 14, 750. [Google Scholar] [CrossRef] [PubMed]
- Strodtbeck, F. Physiology of Wound Healing. Newborn Infant Nurs. Rev. 2001, 1, 43–52. [Google Scholar] [CrossRef]
- Martin, P. Wound Healing--Aiming for Perfect Skin Regeneration. Science 1997, 276, 75–81. [Google Scholar] [CrossRef]
- Eming, S.A.; Krieg, T.; Davidson, J.M. Inflammation in Wound Repair: Molecular and Cellular Mechanisms. J. Investig. Dermatol. 2007, 127, 514–525. [Google Scholar] [CrossRef]
- Tziotzios, C.; Profyris, C.; Sterling, J. Cutaneous Scarring: Pathophysiology, Molecular Mechanisms, and Scar Reduction Therapeutics. J. Am. Acad. Dermatol. 2012, 66, 13–24. [Google Scholar] [CrossRef]
- Koh, T.J.; DiPietro, L.A. Inflammation and Wound Healing: The Role of the Macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Desmouliere, A.; Darby, I.A.; Laverdet, B.; Bonté, F. Fibroblasts and Myofibroblasts in Wound Healing. Clin. Cosmet. Investig. Dermatol. 2014, 7, 301. [Google Scholar] [CrossRef]
- Grey, J.E.; Enoch, S.; Harding, K.G. Wound Assessment. BMJ 2006, 332, 285–288. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-Wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E.; Wilgus, T.A. Vascular Endothelial Growth Factor and Angiogenesis in the Regulation of Cutaneous Wound Repair. Adv. Wound Care 2014, 3, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Miricescu, D.; Badoiu, S.C.; Stanescu-Spinu, I.-I.; Totan, A.R.; Stefani, C.; Greabu, M. Growth Factors, Reactive Oxygen Species, and Metformin—Promoters of the Wound Healing Process in Burns? Int. J. Mol. Sci. 2021, 22, 9512. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The Interplay of Fibroblasts, the Extracellular Matrix, and Inflammation in Scar Formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef]
- Chen, H.; Li, G.; Liu, Y.; Ji, S.; Li, Y.; Xiang, J.; Zhou, L.; Gao, H.; Zhang, W.; Sun, X.; et al. Pleiotropic Roles of CXCR4 in Wound Repair and Regeneration. Front. Immunol. 2021, 12, 668758. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Levin, M.; Udi, Y.; Solomonov, I.; Sagi, I. Next Generation Matrix Metalloproteinase Inhibitors—Novel Strategies Bring New Prospects. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 1927–1939. [Google Scholar] [CrossRef]
- Rajkumar, V.S.; Shiwen, X.; Bostrom, M.; Leoni, P.; Muddle, J.; Ivarsson, M.; Gerdin, B.; Denton, C.P.; Bou-Gharios, G.; Black, C.M.; et al. Platelet-Derived Growth Factor-β Receptor Activation Is Essential for Fibroblast and Pericyte Recruitment during Cutaneous Wound Healing. Am. J. Pathol. 2006, 169, 2254–2265. [Google Scholar] [CrossRef]
- Wang, C.; Brisson, B.K.; Terajima, M.; Li, Q.; Hoxha, K.; Han, B.; Goldberg, A.M.; Sherry Liu, X.; Marcolongo, M.S.; Enomoto-Iwamoto, M.; et al. Type III Collagen Is a Key Regulator of the Collagen Fibrillar Structure and Biomechanics of Articular Cartilage and Meniscus. Matrix Biol. 2020, 85–86, 47–67. [Google Scholar] [CrossRef]
- Zou, M.-L.; Teng, Y.-Y.; Wu, J.-J.; Liu, S.-Y.; Tang, X.-Y.; Jia, Y.; Chen, Z.-H.; Zhang, K.-W.; Sun, Z.-L.; Li, X.; et al. Fibroblasts: Heterogeneous Cells With Potential in Regenerative Therapy for Scarless Wound Healing. Front. Cell Dev. Biol. 2021, 9, 713605. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic Wounds: Treatment Consensus. Wound Repair Regen. 2022, 30, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Czubryt, M.P. Common Threads in Cardiac Fibrosis, Infarct Scar Formation, and Wound Healing. Fibrogenesis Tissue Repair 2012, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Ticona, L.A.; Sánchez, Á.R.; Sánchez-Corral, J.S.; Moreno, P.I.; Domenech, M.O. Anti-Inflammatory, pro-Proliferative and Antimicrobial Potential of the Compounds Isolated from Daemonorops Draco (Willd.) Blume. J. Ethnopharmacol. 2021, 268, 113668. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Lee, H.U.; Lee, Y.-C.C.; Kim, G.H.; Park, E.C.; Han, S.H.; Lee, J.J.G.; Choi, S.; Heo, N.S.; Kim, D.L.; et al. Wound Healing Potential of Antibacterial Microneedles Loaded with Green Tea Extracts. Mater. Sci. Eng. C 2014, 42, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Badiu, D.L.; Balu, A.M.; Barbes, L.; Luque, R.; Nita, R.; Radu, M.; Tanase, E.; Rosoiu, N. Physico-Chemical Characterisation of Lipids from Mytilus Galloprovincialis (L.) and Rapana Venosa and Their Healing Properties on Skin Burns. Lipids 2008, 43, 829–841. [Google Scholar] [CrossRef]
- Benedek, B.; Rothwangl-Wiltschnigg, K.; Rozema, E.; Gjoncaj, N.; Reznicek, G.; Jurenitsch, J.; Kopp, B.; Glasl, S. Yarrow (Achillea Millefolium L. s.l.): Pharmaceutical Quality of Commercial Samples. Pharmazie 2008, 63, 23–26. [Google Scholar]
- Bonte, F.; Dumas, M.; Chaudagne, C.; Meybeck, A. Influence of Asiatic Acid, Madecassic Acid, and Asiaticoside on Human Collagen I Synthesis. Planta Med. 1994, 60, 133–135. [Google Scholar] [CrossRef]
- Carvalho, F.A.; Uchina, H.S.; Borges, F.A.; Oyafuso, M.H.; Herculano, R.D.; Gremião, M.P.D.; Santos, A.G. Natural Membranes of Hevea Brasiliensis Latex as Delivery System for Casearia Sylvestris Leaf Components. Rev. Bras. Farmacogn. 2018, 28, 102–110. [Google Scholar] [CrossRef]
- Chen, W.C.; Liou, S.S.; Tzeng, T.F.; Lee, S.L.; Liu, I.M. Effect of Topical Application of Chlorogenic Acid on Excision Wound Healing in Rats. Planta Med. 2013, 79, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Cheng, H.L.; Kuan, Y.H.; Liang, T.J.; Chao, Y.Y.; Lin, H.C. Therapeutic Potential of Luteolin on Impaired Wound Healing in Streptozotocin-Induced Rats. Biomedicines 2021, 9, 761. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Lee, S.; Lee, M.J.; Lee, D.H.; Won, C.H.; Kim, S.M.; Chung, J.H. Dietary Aloe Vera Supplementation Improves Facial Wrinkles and Elasticity and It Increases the Type i Procollagen Gene Expression in Human Skin in Vivo. Ann. Dermatol. 2009, 21, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xie, J.; Xin, N.; Wang, Z. Panax Notoginseng Saponins Promote Wound Repair of Anterior Cruciate Ligament through Phosphorylation of PI3K, AKT and ERK. Int. J. Clin. Exp. Pathol. 2015, 8, 441–449. [Google Scholar]
- Alemzadeh, E.; Oryan, A. Effectiveness of a Crocus Sativus Extract on Burn Wounds in Rats. Planta Med. 2018, 84, 1191–1200. [Google Scholar] [CrossRef]
- Almasian, A.; Najafi, F.; Eftekhari, M.; Ardekani, M.R.S.; Sharifzadeh, M.; Khanavi, M. Polyurethane/Carboxymethylcellulose Nanofibers Containing Malva Sylvestris Extract for Healing Diabetic Wounds: Preparation, Characterization, in Vitro and in Vivo Studies. Mater. Sci. Eng. C 2020, 114, 111039. [Google Scholar] [CrossRef]
- Elshamy, A.I.; Ammar, N.M.; Hassan, H.A.; El-Kashak, W.A.; Al-Rejaie, S.S.; Abd-ElGawad, A.M.; Farrag, A.-R.R.H. Topical Wound Healing Activity of Myricetin Isolated from Tecomaria Capensis v. Aurea. Molecules 2020, 25, 4870. [Google Scholar] [CrossRef]
- Ambiga, S.; Narayanan, R.; Gowri, D.; Sukumar, D.; Madhavan, S. Evaluation of wound healing activity of flavonoids from ipomoea carnea Jacq. Anc. Sci. Life 2007, 26, 45–51. [Google Scholar]
- Song, M.; Chen, L.; Zhang, L.; Li, C.; Coffie, J.W.; Fang, Z.; Zhang, L.; Wang, S.; Gao, X.; Wang, H. Cryptotanshinone Enhances Wound Healing in Type 2 Diabetes with Modulatory Effects on Inflammation, Angiogenesis and Extracellular Matrix Remodelling. Pharm. Biol. 2020, 58, 845–853. [Google Scholar] [CrossRef]
- Yeh, C.-J.; Chen, C.-C.; Leu, Y.-L.; Lin, M.-W.; Chiu, M.-M.; Wang, S.-H. The Effects of Artocarpin on Wound Healing: In Vitro and in Vivo Studies. Sci. Rep. 2017, 7, 15599. [Google Scholar] [CrossRef]
- Majtan, J. Methylglyoxal—A Potential Risk Factor of Manuka Honey in Healing of Diabetic Ulcers. Evid.-Based Complement. Altern. Med. 2011, 2011, 295494. [Google Scholar] [CrossRef] [PubMed]
- Atrott, J.; Henle, T. Methylglyoxal in Manuka Honey—Correlation with Antibacterial Properties. Czech J. Food Sci. 2009, 27, S163–S165. [Google Scholar] [CrossRef]
- Wang, X.; Xu, P.; Yao, Z.; Fang, Q.; Feng, L.; Guo, R.; Cheng, B. Preparation of Antimicrobial Hyaluronic Acid/Quaternized Chitosan Hydrogels for the Promotion of Seawater-Immersion Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 360. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Jangir, B.L.; Nigam, A.; Kumar, V.; Sharma, S. Dose Regulated Cutaneous Wound Healing Potential of Quercetin in Male Rats. Wound Med. 2017, 19, 82–87. [Google Scholar] [CrossRef]
- Nguyen, V.-L.; Truong, C.-T.; Nguyen, B.C.Q.; Van Vo, T.-N.; Dao, T.-T.; Nguyen, V.-D.; Trinh, D.-T.T.; Huynh, H.K.; Bui, C.-B. Anti-Inflammatory and Wound Healing Activities of Calophyllolide Isolated from Calophyllum Inophyllum Linn. PLoS ONE 2017, 12, e0185674. [Google Scholar] [CrossRef] [PubMed]
- Di, R.; Murray, A.F.; Xiong, J.; Esposito, D.; Komarnytsky, S.; Gianfagna, T.J.; Munafo, J.P. Lily Steroidal Glycoalkaloid Promotes Early Inflammatory Resolution in Wounded Human Fibroblasts. J. Ethnopharmacol. 2020, 258, 112766. [Google Scholar] [CrossRef]
- Mohanty, C.; Das, M.; Sahoo, S.K. Sustained Wound Healing Activity of Curcumin Loaded Oleic Acid Based Polymeric Bandage in a Rat Model. Mol. Pharm. 2012, 9, 2801–2811. [Google Scholar] [CrossRef]
- Beserra, F.P.; Gushiken, L.F.S.; Vieira, A.J.; Bérgamo, D.A.; Bérgamo, P.L.; de Souza, M.O.; Hussni, C.A.; Takahira, R.K.; Nóbrega, R.H.; Martinez, E.R.M.; et al. From Inflammation to Cutaneous Repair: Topical Application of Lupeol Improves Skin Wound Healing in Rats by Modulating the Cytokine Levels, NF-ΚB, Ki-67, Growth Factor Expression, and Distribution of Collagen Fibers. Int. J. Mol. Sci. 2020, 21, 4952. [Google Scholar] [CrossRef]
- Sklenářová, R.; Svrčková, M.; Hodek, P.; Ulrichová, J.; Franková, J. Effect of the Natural Flavonoids Myricetin and Dihydromyricetin on the Wound Healing Process in Vitro. J. Appl. Biomed. 2021, 19, 149–158. [Google Scholar] [CrossRef]
- Phan, T.-T.; See, P.; Lee, S.-T.; Chan, S.-Y. Protective Effects of Curcumin against Oxidative Damage on Skin Cells In Vitro: Its Implication for Wound Healing. J. Trauma Inj. Infect. Crit. Care 2001, 51, 927–931. [Google Scholar] [CrossRef]
- Yang, D.; Moh, S.; Son, D.; You, S.; Kinyua, A.; Ko, C.; Song, M.; Yeo, J.; Choi, Y.-H.; Kim, K. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef] [PubMed]
- Genc, Y.; Harput, U.S.; Saracoglu, I. Active Compounds Isolated from Plantago Subulata L. via Wound Healing and Antiinflammatory Activity Guided Studies. J. Ethnopharmacol. 2019, 241, 112030. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Zhong, L.; Lu, S.; Hu, P.; Pan, Y.; Ma, X.; Yan, B.; Wei, Z.; Yang, G. Quercetin Promotes Cutaneous Wound Healing in Mice through Wnt/β-Catenin Signaling Pathway. J. Ethnopharmacol. 2022, 290, 115066. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Jangir, B.L.; Kumar, V.; Nigam, A.; Sharma, V. Quercetin Accelerated Cutaneous Wound Healing in Rats by Modulation of Different Cytokines and Growth Factors. Growth Factors 2020, 38, 105–119. [Google Scholar] [CrossRef]
- Zhao, X.; Pei, D.; Yang, Y.; Xu, K.; Yu, J.; Zhang, Y.; Zhang, Q.; He, G.; Zhang, Y.; Li, A.; et al. Green Tea Derivative Driven Smart Hydrogels with Desired Functions for Chronic Diabetic Wound Treatment. Adv. Funct. Mater. 2021, 31, 2009442. [Google Scholar] [CrossRef]
- Dube, A.; Ng, K.; Nicolazzo, J.A.; Larson, I. Effective Use of Reducing Agents and Nanoparticle Encapsulation in Stabilizing Catechins in Alkaline Solution. Food Chem. 2010, 122, 662–667. [Google Scholar] [CrossRef]
- Frydman, G.H.; Olaleye, D.; Annamalai, D.; Layne, K.; Yang, I.; Kaafarani, H.M.A.; Fox, J.G. Manuka Honey Microneedles for Enhanced Wound Healing and the Prevention and/or Treatment of Methicillin-Resistant Staphylococcus Aureus (MRSA) Surgical Site Infection. Sci. Rep. 2020, 10, 13229. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, H.; Yu, B.; Li, W.; Gao, F.; Zhang, K.; Zhang, H.; Shen, Y.; Cong, H. Chitosan Composite Hydrogels Cross-linked by Multifunctional Diazo Resin as Antibacterial Dressings for Improved Wound Healing. J. Biomed. Mater. Res. Part A 2020, 108, 1890–1898. [Google Scholar] [CrossRef]
- Dinda, M.; Mazumdar, S.; Das, S.; Ganguly, D.; Dasgupta, U.B.; Dutta, A.; Jana, K.; Karmakar, P. The Water Fraction of Calendula officinalis Hydroethanol Extract Stimulates In Vitro and In Vivo Proliferation of Dermal Fibroblasts in Wound Healing. Phyther. Res. 2016, 1707, 1696–1707. [Google Scholar] [CrossRef]
- Badiu, D.L.; Luque, R.; Dumitrescu, E.; Craciun, A.; Dinca, D. Amino Acids from Mytilus Galloprovincialis (L.) and Rapana Venosa Molluscs Accelerate Skin Wounds Healing via Enhancement of Dermal and Epidermal Neoformation. Protein. J. 2010, 29, 81–92. [Google Scholar] [CrossRef]
- Ulagesan, S.; Sankaranarayanan, K.; Kuppusamy, A. Functional Characterisation of Bioactive Peptide Derived from Terrestrial Snail Cryptozona Bistrialis and Its Wound-Healing Property in Normal and Diabetic-Induced Wistar Albino Rats. Int. Wound J. 2018, 15, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Akrawi, S.H.; Gorain, B.; Nair, A.B.; Choudhury, H.; Pandey, M.; Shah, J.N.; Venugopala, K.N. Development and Optimization of Naringenin-Loaded Chitosan-Coated Nanoemulsion for Topical Therapy in Wound Healing. Pharmaceutics 2020, 12, 893. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Yao, P.; Wu, H.; Zha, Z. Acceleration of Wound Healing in Traumatic Ulcers by Absorbable Collagen Sponge Containing Recombinant Basic Fibroblast Growth Factor. Biomed. Mater. 2006, 1, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Başer, K.H.; Demirci, B.; Demirci, F.; Koçak, S.; Akıncı, Ç.; Malyer, H.; Güleryüz, G. Composition and Antimicrobial Activity of the Essential Oil of Achillea Multifida. Planta Med. 2002, 68, 941–943. [Google Scholar] [CrossRef] [PubMed]
- Güzel, S.; Özay, Y.; Kumaş, M.; Uzun, C.; Özkorkmaz, E.G.; Yıldırım, Z.; Ülger, M.; Güler, G.; Çelik, A.; Çamlıca, Y.; et al. Wound healing properties, antimicrobial and antioxidant activities of Salvia kronenburgii and Salvia euphratica var. euphratica on excision and incision wound models in diabetic rats. Biomed. Pharmacother. 2019, 111, 1260–1276. [Google Scholar] [CrossRef]
- Tadić, V.; Arsić, I.; Zvezdanović, J.; Zugić, A.; Cvetković, D.; Pavkov, S. The Estimation of the Traditionally Used Yarrow (Achillea Millefolium L. Asteraceae) Oil Extracts with Anti-Inflamatory Potential in Topical Application. J. Ethnopharmacol. 2017, 199, 138–148. [Google Scholar] [CrossRef]
- Dorjsembe, B.; Lee, H.J.; Kim, M.; Dulamjav, B.; Jigjid, T.; Nho, C.W. Achillea Asiatica Extract and Its Active Compounds Induce Cutaneous Wound Healing. J. Ethnopharmacol. 2017, 206, 306–314. [Google Scholar] [CrossRef]
- Farahpour, M.R.; Pirkhezr, E.; Ashrafian, A.; Sonboli, A. Accelerated Healing by Topical Administration of Salvia Officinalis Essential Oil on Pseudomonas Aeruginosa and Staphylococcus Aureus Infected Wound Model. Biomed. Pharmacother. 2020, 128, 110120. [Google Scholar] [CrossRef]
- Fetse, J.; Kyekyeku, J.; Dueve, E.; Mensah, K. Wound Healing Activity of Total Alkaloidal Extract of the Root Bark of Alstonia Boonei (Apocynacea). Br. J. Pharm. Res. 2014, 4, 2642–2652. [Google Scholar] [CrossRef]
- Fronza, M.; Heinzmann, B.; Hamburger, M.; Laufer, S.; Merfort, I. Determination of the Wound Healing Effect of Calendula Extracts Using the Scratch Assay with 3T3 Fibroblasts. J. Ethnopharmacol. 2009, 126, 463–467. [Google Scholar] [CrossRef]
- Fu, J.; Huang, J.; Lin, M.; Xie, T.; You, T. Quercetin Promotes Diabetic Wound Healing via Switching Macrophages From M1 to M2 Polarization. J. Surg. Res. 2020, 246, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Gadekar, R.; Saurabh, M.K.; Thakur, G.S.; Saurabh, A. Study of Formulation, Characterisation and Wound Healing Potential of Transdermal Patches of Curcumin. Asian J. Pharm. Clin. Res. 2012, 5, 225–230. [Google Scholar]
- De Campos, E.P.; Trombini, L.N.; Rodrigues, R.; Portella, D.L.; Werner, A.C.; Ferraz, M.C.; De Oliveira, R.V.M.H.; Cogo, J.C.; Oshima-Franco, Y.; Aranha, N.; et al. Healing Activity of Casearia Sylvestris Sw. in Second-Degree Scald Burns in Rodents. BMC Res. Notes 2015, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, M.M.; Kshirsagar, S.B.; Sahane, R.S. Evaluation of Wound Healing Activity of Ferulic Acid in Diabetic Rats. Int. Wound J. 2014, 11, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Ghanadian, M.; Soltani, R.; Homayouni, A.; Khorvash, F.; Jouabadi, S.M.; Abdollahzadeh, M. The Effect of Plantago Major Hydroalcoholic Extract on the Healing of Diabetic Foot and Pressure Ulcers: A Randomized Open-Label Controlled Clinical Trial. Int. J. Low. Extrem. Wounds 2022. [Google Scholar] [CrossRef]
- Gourishetti, K.; Keni, R.; Nayak, P.G.; Jitta, S.R.; Bhaskaran, N.A.; Kumar, L.; Kumar, N.; Krishnadas, N.; Shenoy, R.R. Sesamol-Loaded Plga Nanosuspension for Accelerating Wound Healing in Diabetic Foot Ulcer in Rats. Int. J. Nanomedicine 2020, 15, 9265–9282. [Google Scholar] [CrossRef]
- Han, X.; Tao, Y.; Deng, Y.; Yu, J.; Sun, Y.; Jiang, G. Metformin Accelerates Wound Healing in Type 2 Diabetic Db/Db Mice. Mol. Med. Rep. 2017, 16, 8691–8698. [Google Scholar] [CrossRef]
- Hanafi, N.; Talebpour Amiri, F.; Shahani, S.; Enayatifard, R.; Ghasemi, M.; Karimpour, A.A. Licorice Cream Promotes Full-Thickness Wound Healing in Guinea Pigs. Marmara Pharm. J. 2018, 22, 411–421. [Google Scholar] [CrossRef]
- Hou, Q.; Li, M.; Lu, Y.H.; Liu, D.H.; Li, C.C. Burn Wound Healing Properties of Asiaticoside and Madecassoside. Exp. Ther. Med. 2016, 12, 1269–1274. [Google Scholar] [CrossRef]
- Hsiao, C.Y.; Hung, C.Y.; Tsai, T.H.; Chak, K.F. A Study of the Wound Healing Mechanism of a Traditional Chinese Medicine, Angelica Sinensis, Using a Proteomic Approach. Evid.-Based Complement. Altern. Med. 2012, 2012, 467531. [Google Scholar] [CrossRef]
- Hu, W.; Wang, A.M.; Wu, S.Y.; Zhang, B.; Liu, S.; Gou, Y.B.; Wang, J.M. Debriding Effect of Bromelain on Firearm Wounds in Pigs. J. Trauma. Inj. Infect. Crit. Care 2011, 71, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, A.D.; Alam, J.; Patil, M.V.K.; Sinha, A.; Bodhankar, S.L. Wound Healing Potential of Naringin Ointment Formulation via Regulating the Expression of Inflammatory, Apoptotic and Growth Mediators in Experimental Rats. Pharm. Biol. 2016, 54, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.W.; Han, Y.E.; Kim, J.; Oh, J.H.; Cho, Y.H.; Lee, E.J. 4-Hydroxybenzaldehyde Accelerates Acute Wound Healing through Activation of Focal Adhesion Signalling in Keratinocytes. Sci. Rep. 2017, 7, 14192. [Google Scholar] [CrossRef] [PubMed]
- Dinda, M.; Dasgupta, U.; Singh, N.; Bhattacharyya, D.; Karmakar, P. PI3K-Mediated Proliferation of Fibroblasts by Calendula officinalis Tincture: Implication in Wound Healing. Phyther. Res. 2015, 29, 607–616. [Google Scholar] [CrossRef]
- Karakaş, F.P.; Karakaş, A.; Boran, Ç.; Türker, A.U.; Yalçin, F.N.; Bilensoy, E. The Evaluation of Topical Administration of Bellis Perennis Fraction on Circular Excision Wound Healing in Wistar Albino Rats. Pharm. Biol. 2012, 50, 1031–1037. [Google Scholar] [CrossRef]
- Karatas, O.; Gevrek, F. Gallic Acid Liposome and Powder Gels Improved Wound Healing in Wistar Rats. Ann. Med. Res. 2019, 26, 2720. [Google Scholar] [CrossRef]
- Karimzadeh, S.; Farahpour, M.R. Topical Application of Salvia Officinalis Hydroethanolic Leaf Extract Improves Wound Healing Process. Indian J. Exp. Biol. 2017, 55, 98–106. [Google Scholar]
- Kharat, Z.; Amiri Goushki, M.; Sarvian, N.; Asad, S.; Dehghan, M.M.; Kabiri, M. Chitosan/PEO Nanofibers Containing Calendula officinalis Extract: Preparation, Characterization, in Vitro and in Vivo Evaluation for Wound Healing Applications. Int. J. Pharm. 2021, 609, 121132. [Google Scholar] [CrossRef]
- Khorasani, G.; Hosseinimehr, S.J.; Zamani, P.; Ghasemi, M.; Ahmadi, A. The Effect of Saffron (Crocus Sativus) Extract for Healing of Second-Degree Burn Wounds in Rats. Keio J. Med. 2008, 57, 190–195. [Google Scholar] [CrossRef]
- Kumar, S.; Ghosh, D.; Biswas, T.K.; Dutta, U.; Das, P.; Kundu, S. Spermatheca Gland Extract of Snail (Telescopium Telescopium) Has Wound Healing Potential: An Experimental Study in Rabbits. Int. J. Low. Extrem. Wounds 2008, 7, 204–209. [Google Scholar] [CrossRef]
- Labib, R.M.; Ayoub, I.M.; Michel, H.E.; Mehanny, M.; Kamil, V.; Hany, M.; Magdy, M.; Moataz, A.; Maged, B.; Mohamed, A. Appraisal on the Wound Healing Potential of Melaleuca Alternifolia and Rosmarinus officinalis L. Essential Oil-Loaded Chitosan Topical Preparations. PLoS ONE 2019, 14, e0219561. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kandhare, A.D.; Mukherjee, A.A.; Bodhankar, S.L. Hesperidin, a Plant Flavonoid Accelerated the Cutaneous Wound Healing in Streptozotocin-Induced Diabetic Rats: Role of TGF-B/SMADS and ANG-1/TIE-2 Signaling Pathways. EXCLI J. 2018, 17, 399–419. [Google Scholar] [PubMed]
- Li, X.; Zhai, Y.; Xi, B.; Ma, W.; Zhang, J.; Ma, X.; Miao, Y.; Zhao, Y.; Ning, W.; Zhou, H.; et al. Pinocembrin Ameliorates Skin Fibrosis via Inhibiting Tgf-β1 Signaling Pathway. Biomolecules 2021, 11, 1240. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Wu, J.Q.; Fan, R.Y.; He, Z.H.; Li, C.Y.; He, M.F. Isoliquiritin Promote Angiogenesis by Recruiting Macrophages to Improve the Healing of Zebrafish Wounds. Fish Shellfish Immunol. 2020, 100, 238–245. [Google Scholar] [CrossRef]
- Su, X.; Liu, X.; Wang, S.; Li, B.; Pan, T.; Liu, D.; Wang, F.; Diao, Y.; Li, K. Wound-Healing Promoting Effect of Total Tannins from Entada phaseoloides (L.) Merr. in Rats. Burns 2017, 43, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, S.; Singhai, A.K. Wound Healing Effect of Flavonoid Rich Fraction and Luteolin Isolated from Martynia Annua Linn. on Streptozotocin Induced Diabetic Rats. Asian Pac. J. Trop. Med. 2013, 6, 253–259. [Google Scholar] [CrossRef]
- Luo, Y.; Diao, H.; Xia, S.; Dong, L.; Chen, J.; Zhang, J. A Physiologically Active Polysaccharide Hydrogel Promotes Wound Healing. J. Biomed. Mater. Res. Part A 2010, 94, 193–204. [Google Scholar] [CrossRef]
- Luo, X.; Huang, P.; Yuan, B.; Liu, T.; Lan, F.; Lu, X.; Dai, L.; Liu, Y.; Yin, H. Astragaloside IV Enhances Diabetic Wound Healing Involving Upregulation of Alternatively Activated Macrophages. Int. Immunopharmacol. 2016, 35, 22–28. [Google Scholar] [CrossRef]
- Ma, X.; Lin, Y.; Liu, Y.; Li, W.; He, J.; Fang, M.; Lin, D. Effects of Apigenin Treatment on Random Skin Flap Survival in Rats. Front. Pharmacol. 2021, 12, 625733. [Google Scholar] [CrossRef]
- Manconi, M.; Manca, M.L.; Caddeo, C.; Cencetti, C.; di Meo, C.; Zoratto, N.; Nacher, A.; Fadda, A.M.; Matricardi, P. Preparation of Gellan-Cholesterol Nanohydrogels Embedding Baicalin and Evaluation of Their Wound Healing Activity. Eur. J. Pharm. Biopharm. 2018, 127, 244–249. [Google Scholar] [CrossRef]
- Mandawgade, S.D.; Patil, K.S. Wound Healing Potential of Some Active Principles of Lawsonia Alba Lam. Leaves. Indian J. Pharm. Sci. 2003, 65, 390–394. [Google Scholar]
- Marquele-Oliveira, F.; da Silva Barud, H.; Torres, E.C.; Machado, R.T.A.; Caetano, G.F.; Leite, M.N.; Frade, M.A.C.; Ribeiro, S.J.L.; Berretta, A.A. Development, Characterization and Pre-Clinical Trials of an Innovative Wound Healing Dressing Based on Propolis (EPP-AF®)-Containing Self-Microemulsifying Formulation Incorporated in Biocellulose Membranes. Int. J. Biol. Macromol. 2019, 136, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, S.; Governa, P.; Borgonetti, V.; Marcolongo, P.; Nanni, C.; Gamberucci, A.; Manetti, F.; Pessina, F.; Carullo, G.; Brizzi, A.; et al. Pinocembrin and Its Linolenoyl Ester Derivative Induce Wound Healing Activity in HaCaT Cell Line Potentially Involving a GPR120/FFA4 Mediated Pathway. Bioorg. Chem. 2021, 108, 104657. [Google Scholar] [CrossRef]
- Mellin, T.N.; Cashen, D.E.; Ronan, J.; Murphy, B.S.; DiSalvo, J.; Thomas, K.A. Acidic Fibroblast Growth Factor Accelerates Dermal Wound Healing in Diabetic Mice. J. Investig. Dermatol. 1995, 104, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Mittraphab, Y.; Nagata, M.; Matsumoto, M.; Shimizu, K. Antioxidant and Protective Effect of Acetone Extract of Entada phaseoloides Leaves on UVB-Irradiated Human Epidermal Keratinocytes (HaCaT Cells) by Inhibiting COX-2, INOS, and Caspase-3 Activation. Nat. Prod. Commun. 2022, 17, 1934578X221078627. [Google Scholar] [CrossRef]
- García-Salinas, S.; Evangelopoulos, M.; Gámez-Herrera, E.; Arruebo, M.; Irusta, S.; Taraballi, F.; Mendoza, G.; Tasciotti, E. Electrospun Anti-Inflammatory Patch Loaded with Essential Oils for Wound Healing. Int. J. Pharm. 2020, 577, 119067. [Google Scholar] [CrossRef]
- Mo, J.; Panichayupakaranant, P.; Kaewnopparat, N.; Nitiruangjaras, A.; Reanmongkol, W. Wound Healing Activities of Standardized Pomegranate Rind Extract and Its Major Antioxidant Ellagic Acid in Rat Dermal Wounds. J. Nat. Med. 2014, 68, 377–386. [Google Scholar] [CrossRef]
- Mochizuki, S.; Takano, M.; Sugano, N.; Ohtsu, M.; Tsunoda, K.; Koshi, R.; Yoshinuma, N. The Effect of B Vitamin Supplementation on Wound Healing in Type 2 Diabetic Mice. J. Clin. Biochem. Nutr. 2016, 58, 64–68. [Google Scholar]
- Mohammed, B.M.; Fisher, B.J.; Kraskauskas, D.; Ward, S.; Wayne, J.S.; Brophy, D.F.; Fowler, A.A.; Yager, D.R.; Natarajan, R. Vitamin C Promotes Wound Healing through Novel Pleiotropic Mechanisms. Int. Wound J. 2016, 13, 572–584. [Google Scholar] [CrossRef]
- Moura-Letts, G.; Villegas, L.F.; Marçalo, A.; Vaisberg, A.J.; Hammond, G.B. In Vivo Wound-Healing Activity of Oleanolic Acid Derived from the Acid Hydrolysis of Anredera Diffusa. J. Nat. Prod. 2006, 69, 978–979. [Google Scholar] [CrossRef]
- Nair, A.V.; Raman, M.; Doble, M. Cyclic β-(1→3) (1→6) Glucan/Carrageenan Hydrogels for Wound Healing Applications. RSC Adv. 2016, 6, 98545–98553. [Google Scholar] [CrossRef]
- Do Nascimento, J.E.T.; Rodrigues, A.L.M.; De Lisboa, D.S.; Liberato, H.R.; Falcão, M.J.C.; Da Silva, C.R.; Nobre Júnior, H.V.; Braz Filho, R.; De Paula Junior, V.F.; Alves, D.R.; et al. Chemical Composition and Antifungal in Vitro and in Silico, Antioxidant, and Anticholinesterase Activities of Extracts and Constituents of Ouratea Fieldingiana (DC.) Baill. Evid.-Based Complement. Altern. Med. 2018, 2018, 1748487. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, E.; Hosseinimehr, S.J.; Azadbakht, M.; Akbari, J.; Enayati-Fard, R.; Azizi, S. Effect of Malva Sylvestris Cream on Burn Injury and Wounds in Rats. Avicenna J. Phytomedicine 2015, 5, 341–354. [Google Scholar]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In Vitro Studies to Evaluate the Wound Healing Properties of Calendula officinalis Extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Nikita, G.; Vivek, P.; Chhaya, G. Wound-Healing Activity of an Oligomer of Alkannin/Shikonin, Isolated from Root Bark of Onosma Echioides. Nat. Prod. Res. 2015, 29, 1584–1588. [Google Scholar] [CrossRef]
- Oloumi, M.M.; Derakhshanfar, A.; Nikpoor, A. Healing Potential of Liquorice Root Extract on Dermal Wounds in Rats. J. Vetinary Res. 2007, 62, 147–154. [Google Scholar]
- Novianty, R.A.; Chrismawaty, B.E.; Subagyo, G. Effect of Allicin for Re-Epithelialization During Healing in Oral Ulcer Model. Indones. J. Dent. Res. 2015, 1, 87. [Google Scholar] [CrossRef][Green Version]
- Ozdemir, O.; Ozkan, K.; Hatipoglu, F.; Uyaroglu, A.; Arican, M. Effect of Asiaticoside, Collagenase, and Alpha-Chymotrypsin on Wound Healing in Rabbits. Wounds 2016, 28, 279–286. [Google Scholar]
- Parente, L.M.L.; Lino Júnior, R.D.S.; Tresvenzol, L.M.F.; Vinaud, M.C.; De Paula, J.R.; Paulo, N.M. Wound Healing and Anti-Inflammatory Effect in Animal Models of Calendula officinalis L. Growing in Brazil. Evid.-Based Complement. Altern. Med. 2012, 2012, 375671. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Shahrzad, A.; Abed, K.; Hamedi, B. Wound Healing Activity of Malva Sylvestris and Punica Granatum in Alloxan-Induced Diabetic Rats. Acta Pol. Pharm. Drug Res. 2010, 67, 511–516. [Google Scholar]
- Primarizky, H.; Yuniarti, W.M.; Lukiswanto, B.S. Ellagic Acid Activity in Healing Process of Incision Wound on Male Albino Rats (Rattus Norvegicus). KnE Life Sci. 2017, 3, 224. [Google Scholar] [CrossRef]
- Rashed, A.N.; Afifi, F.U.; Disi, A.M. Simple Evaluation of the Wound Healing Activity of a Crude Extract of Portulaca Oleracea L. (Growing in Jordan) in Mus Musculus JVI-1. J. Ethnopharmacol. 2003, 88, 131–136. [Google Scholar] [CrossRef]
- Ren, J.; Yang, M.; Xu, F.; Chen, J.; Ma, S. Acceleration of Wound Healing Activity with Syringic Acid in Streptozotocin Induced Diabetic Rats. Life Sci. 2019, 233, 116728. [Google Scholar] [CrossRef] [PubMed]
- Scrima, M.; Melito, C.; Merola, F.; Iorio, A.; Vito, N.; Giori, A.M.; Ferravante, A. Evaluation of Wound Healing Activity of Salvia Haenkei Hydroalcoholic Aerial Part Extract on in Vitro and in Vivo Experimental Models. Clin. Cosmet. Investig. Dermatol. 2020, 13, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Sh Ahmed, A.; Taher, M.; Mandal, U.K.; Jaffri, J.M.; Susanti, D.; Mahmood, S.; Zakaria, Z.A. Pharmacological Properties of Centella Asiatica Hydrogel in Accelerating Wound Healing in Rabbits. BMC Complement. Altern. Med. 2019, 19, 1–7. [Google Scholar] [CrossRef]
- Shafeie, N.; Naini, A.T.; Jahromi, H.K. Comparison of Different Concentrations of Calendula officinalis Gel on Cutaneous Wound Healing. Biomed. Pharmacol. J. 2015, 8, 979–992. [Google Scholar] [CrossRef]
- Shukla, A.; Rasik, A.M.; Patnaik, G.K. Depletion of Reduced Glutathione, Ascorbic Acid, Vitamin E and Antioxidant Defence Enzymes in a Healing Cutaneous Wound. Free Radic. Res. 1997, 26, 93–101. [Google Scholar] [CrossRef]
- Ram, M.; Singh, V.; Kumawat, S.; Kant, V.; Tandan, S.K.; Kumar, D. Bilirubin Modulated Cytokines, Growth Factors and Angiogenesis to Improve Cutaneous Wound Healing Process in Diabetic Rats. Int. Immunopharmacol. 2016, 30, 137–149. [Google Scholar] [CrossRef]
- Singh, S.D.J.; Krishna, V.; Mankani, K.L.; Manjunatha, B.K.; Vidya, S.M.; Manohara, Y.N. Wound Healing Activity of the Leaf Extracts and Deoxyelephantopin Isolated from Elephantopus Scaber Linn. Indian J. Pharmacol. 2005, 37, 238–242. [Google Scholar]
- Siriwattanasatorn, M.; Itharat, A.; Thongdeeying, P.; Ooraikul, B. In Vitro Wound Healing Activities of Three Most Commonly Used Thai Medicinal Plants and Their Three Markers. Evid.-Based Complement. Altern. Med. 2020, 2020, 6795383. [Google Scholar] [CrossRef]
- Somboonwong, J.; Kankaisre, M.; Tantisira, B.; Tantisira, M.H. Wound Healing Activities of Different Extracts of Centella Asiatica in Incision and Burn Wound Models: An Experimental Animal Study. BMC Complement. Altern. Med. 2012, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Wang, F.; Weng, Z.; Song, H.; Fang, Y.; Tang, X.; Shen, X. A Wheat Germ-Derived Peptide YDWPGGRN Facilitates Skin Wound-Healing Processes. Biochem. Biophys. Res. Commun. 2020, 524, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; Küpeli Akkol, E.; Keles, H.; Yesilada, E.; Sarker, S.D. Exploration of the Wound Healing Potential of Helichrysum Graveolens (Bieb.) Sweet: Isolation of Apigenin as an Active Component. J. Ethnopharmacol. 2013, 149, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of Antioxidant Activity of Vanillin by Using Multiple Antioxidant Assays. Biochim. Biophys. Acta Gen. Subj. 2011, 1810, 170–177. [Google Scholar] [CrossRef]
- Tang, T.; Yin, L.; Yang, J.; Shan, G. Emodin, an Anthraquinone Derivative from Rheum Officinale Baill, Enhances Cutaneous Wound Healing in Rats. Eur. J. Pharmacol. 2007, 567, 177–185. [Google Scholar] [CrossRef]
- Thaloor, D.; Miller, K.J.; Gephart, J.; Mitchell, P.O.; Pavlath, G.K. Systemic Administration of the NF-ΚB Inhibitor Curcumin Stimulates Muscle Regeneration after Traumatic Injury. Am. J. Physiol. Cell Physiol. 1999, 277, C320–C329. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Sharad, S.; Maheshwari, R.K. Phytochemicals in Wound Healing. Adv. Wound Care 2016, 5, 230–241. [Google Scholar] [CrossRef]
- Tsoutsos, D.; Kakagia, D.; Tamparopoulos, K. The Efficacy of Helix Aspersa Müller Extract in the Healing of Partial Thickness Burns: A Novel Treatment for Open Burn Management Protocols. J. Dermatolog. Treat. 2009, 20, 219–222. [Google Scholar] [CrossRef]
- Zhou, X.; Ruan, Q.; Ye, Z.; Chu, Z.; Xi, M.; Li, M.; Hu, W.; Guo, X.; Yao, P.; Xie, W. Resveratrol Accelerates Wound Healing by Attenuating Oxidative Stress-Induced Impairment of Cell Proliferation and Migration. Burns 2021, 47, 133–139. [Google Scholar] [CrossRef]
- Umasankar, K.; Nambikkairaj, B.; Manley Backyavathy, D. Effect of Topical Treatment of Rosmarinus officinalis Essential Oil on Wound Healing in Streptozotocin Induced Diabetic Rats. Nat. Environ. Pollut. Technol. 2012, 11, 607–611. [Google Scholar]
- Vidya, S.M.; Krishna, V.; Manjunatha, B.K.; Bharath, B.R.; Rajesh, K.P.; Manjunatha, H.; Mankani, K.L. Wound Healing Phytoconstituents from Seed Kernel of Entada Pursaetha DC. and Their Molecular Docking Studies with Glycogen Synthase Kinase 3-β. Med. Chem. Res. 2012, 21, 3195–3203. [Google Scholar] [CrossRef]
- Wahedi, H.M.; Park, Y.U.; Moon, E.Y.; Kim, S.Y. Juglone Ameliorates Skin Wound Healing by Promoting Skin Cell Migration through Rac1/Cdc42/PAK Pathway. Wound Repair Regen. 2016, 24, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Meng, H. Cytotoxic, Anti-Inflammatory and Hemostatic Spirostane-Steroidal Saponins from the Ethanol Extract of the Roots of Bletilla Striata. Fitoterapia 2015, 101, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, T.; Fu, A.; Mao, Z.; Yi, L.; Tang, S.; Yang, J. Hesperidin Enhances Angiogenesis via Modulating Expression of Growth and Inflammatory Factor in Diabetic Foot Ulcer in Rats. Eur. J. Inflamm. 2018, 16, 2058739218775255. [Google Scholar] [CrossRef]
- Yang, W.T.; Ke, C.Y.; Wu, W.T.; Tseng, Y.H.; Lee, R.P. Antimicrobial and Anti-Inflammatory Potential of Angelica Dahurica and Rheum Officinale Extract Accelerates Wound Healing in Staphylococcus Aureus-Infected Wounds. Sci. Rep. 2020, 10, 5596. [Google Scholar] [CrossRef]
- Yen, J.H.; Chio, W.T.; Chuang, C.J.; Yang, H.L.; Huang, S.T. Improved Wound Healing by Naringin Associated with MMP and the VEGF Pathway. Molecules 2022, 27, 1695. [Google Scholar] [CrossRef]
- Yu, M.H.; Choi, J.H.; Chae, I.G.; Im, H.G.; Yang, S.A.; More, K.; Lee, I.S.; Lee, J. Suppression of LPS-Induced Inflammatory Activities by Rosmarinus officinalis L. Food Chem. 2013, 136, 1047–1054. [Google Scholar] [CrossRef]
- Zangeneh, A.; Pooyanmehr, M.; Zangeneh, M.M.; Moradi, R.; Rasad, R.; Kazemi, N. Therapeutic Effects of Glycyrrhiza Glabra Aqueous Extract Ointment on Cutaneous Wound Healing in Sprague Dawley Male Rats. Comp. Clin. Path. 2019, 28, 1507–1514. [Google Scholar] [CrossRef]
- Zeka, K.; Ruparelia, K.C.; Sansone, C.; Macchiarelli, G.; Continenza, M.A.; Arroo, R.R.J. New Hydrogels Enriched with Antioxidants from Saffron Crocus Can Find Applications in Wound Treatment and/or Beautification. Skin Pharmacol. Physiol. 2018, 31, 95–98. [Google Scholar] [CrossRef]
- Orhan, I.E.; Akkol, E.K.; Suntar, I.; Yesilada, E. Assessment of Anticholinesterase and Antioxidant Properties of the Extracts and (+)-Catechin Obtained from Arceuthobium oxycedri (D.C.) M. Bieb (Dwarf Mistletoe). South African J. Bot. 2019, 120, 309–312. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhu, B.H. Arnebin-1 Promotes the Angiogenesis of Human Umbilical Vein Endothelial Cells and Accelerates the Wound Healing Process in Diabetic Rats. J. Ethnopharmacol. 2014, 154, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Huang, W.D.; Gao, Q.; Su, M.L.; Yang, Y.F.; Liu, Z.C.; Zhu, B.H. Arnebin-1 Promotes Angiogenesis by Inducing ENOS, VEGF and HIF-1α Expression through the PI3K-Dependent Pathway. Int. J. Mol. Med. 2015, 36, 685–697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, X.; Han, W.; Cheng, J.; Qin, Y. Wound Healing Effect of an Astragalus Membranaceus Polysaccharide and Its Mechanism. Mol. Med. Rep. 2017, 15, 4077–4083. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, Q.; Yan, S.; Zhou, J.; Huang, L.; Zhu, H.; Ye, F.; Zhang, Y.; Chen, L.; Chen, L.; et al. Bletilla Striata Polysaccharide Promotes Diabetic Wound Healing Through Inhibition of the NLRP3 Inflammasome. Front. Pharmacol. 2021, 12, 659215. [Google Scholar] [CrossRef]
- Zhi, Y.; Wang, H.; Huang, B.; Yan, G.; Yan, L.Z.; Zhang, W.; Zhang, J. Panax Notoginseng Saponins Suppresses TRPM7 via the PI3K/AKT Pathway to Inhibit Hypertrophic Scar Formation in Vitro. Burns 2021, 47, 894–905. [Google Scholar] [CrossRef]
- Zhou, G.; Ruhan, A.; Ge, H.; Wang, L.; Liu, M.; Wang, B.; Su, H.; Yan, M.; Xi, Y.; Fan, Y. Research on a Novel Poly (Vinyl Alcohol)/Lysine/Vanillin Wound Dressing: Biocompatibility, Bioactivity and Antimicrobial Activity. Burns 2014, 40, 1668–1678. [Google Scholar] [CrossRef]
- Abu-Al-Basal, M.A. Healing Potential of Rosmarinus officinalis L. on Full-Thickness Excision Cutaneous Wounds in Alloxan-Induced-Diabetic BALB/c Mice. J. Ethnopharmacol. 2010, 131, 443–450. [Google Scholar] [CrossRef]
- Afshar, M.; Ravarian, B.; Zardast, M.; Moallem, S.A.; Fard, M.H.; Valavi, M. Evaluation of Cutaneous Wound Healing Activity of Malva Sylvestris Aqueous Extract in BALB/c Mice. Iran. J. Basic Med. Sci. 2015, 18, 616–622. [Google Scholar]
- Deng, Z.H.; Yin, J.; Luo, W.; Kotian, R.N.; Gao, S.; Yi, Z.Q.; Xiao, W.F.; Li, W.P.; Li, Y.S. The Effect of Earthworm Extract on Promoting Skin Wound Healing. Biosci. Rep. 2018, 38, 1–11. [Google Scholar] [CrossRef]
- Diao, H.; Li, X.; Chen, J.; Luo, Y.; Chen, X.; Dong, L.; Wang, C.; Zhang, C.; Zhang, J. Bletilla Striata Polysaccharide Stimulates Inducible Nitric Oxide Synthase and Proinflammatory Cytokine Expression in Macrophages. J. Biosci. Bioeng. 2008, 105, 85–89. [Google Scholar] [CrossRef]
- Reinke, J.M.; Sorg, H. Wound Repair and Regeneration. Eur. Surg. Res. 2012, 49, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from Inflammation to Proliferation: A Critical Step during Wound Healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Cañedo-Dorantes, L.; Cañedo-Ayala, M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflam. 2019, 2019, 3706315. [Google Scholar] [CrossRef]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. A Systematic Review of the Effect of Centella Asiatica on Wound Healing. Int. J. Environ. Res. Public Health 2022, 19, 3266. [Google Scholar] [CrossRef] [PubMed]
- Babu, K.A.; Sivakrishnan, S.; Jasemine, S. Evaluation of in Vivo Wound Healing Activity of Ursolic Acid Rich Chloroform Extract of Hedyotis Herbacea Linn Ointment. J. Pharm. Res. Int. 2021, 33, 246–255. [Google Scholar] [CrossRef]
- Beserra, F.P.; Vieira, A.J.; Gushiken, L.F.S.; de Souza, E.O.; Hussni, M.F.; Hussni, C.A.; Nóbrega, R.H.; Martinez, E.R.M.; Jackson, C.J.; de Azevedo Maia, G.L.; et al. Lupeol, a Dietary Triterpene, Enhances Wound Healing in Streptozotocin-Induced Hyperglycemic Rats with Modulatory Effects on Inflammation, Oxidative Stress, and Angiogenesis. Oxid. Med. Cell. Longev. 2019, 2019, 3182627. [Google Scholar] [CrossRef]
- de Moura Sperotto, N.D.; Steffens, L.; Veríssimo, R.M.; Henn, J.G.; Péres, V.F.; Vianna, P.; Chies, J.A.B.; Roehe, A.; Saffi, J.; Moura, D.J. Wound Healing and Anti-Inflammatory Activities Induced by a Plantago Australis Hydroethanolic Extract Standardized in Verbascoside. J. Ethnopharmacol. 2018, 225, 178–188. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Bi, K. Bioactive Flavonoids in Medicinal Plants: Structure, Activity and Biological Fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Wang, S.-J.; Tong, Y.; Lu, S.; Yang, R.; Liao, X.; Xu, Y.-F.; Li, X. Anti-Inflammatory Activity of Myricetin Isolated from Myrica Rubra Sieb. et Zucc. Leaves. Planta Med. 2010, 76, 1492–1496. [Google Scholar] [CrossRef]
- Rehman, M.U.; Rather, I.A. Myricetin Abrogates Cisplatin-Induced Oxidative Stress, Inflammatory Response, and Goblet Cell Disintegration in Colon of Wistar Rats. Plants 2019, 9, 28. [Google Scholar] [CrossRef]
- Semwal, D.K.; Semwal, R.B.; Combrinck, S.; Viljoen, A. Myricetin: A Dietary Molecule with Diverse Biological Activities. Nutrients 2016, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Xie, Y.; Yao, Y.; Li, G.; Yuan, X.; Shen, H. A Novel Strategy for Pharmaceutical Cocrystal Generation without Knowledge of Stoichiometric Ratio: Myricetin Cocrystals and a Ternary Phase Diagram. Pharm. Res. 2015, 32, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Lin, G.; Xie, Y.; Duan, J.; Ma, P.; Li, G.; Ji, G. Quantitative Determination of Myricetin in Rat Plasma by Ultra Performance Liquid Chromatography Tandem Mass Spectrometry and Its Absolute Bioavailability. Drug Res. (Stuttg). 2013, 64, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, T.N.; Saxena, R.C.; Nigam, S.K.; Misra, G.; Bhargava, K.P. Calophyllolide—A New Non-Steroidal Anti-Inflammatory Agent. Indian J. Med. Res. 1980, 72, 762–765. [Google Scholar] [PubMed]
- Yimdjo, M.C.; Azebaze, A.G.; Nkengfack, A.E.; Meyer, A.M.; Bodo, B.; Fomum, Z.T. Antimicrobial and Cytotoxic Agents from Calophyllum Inophyllum. Phytochemistry 2004, 65, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.B.; Mathur, C.N.; Seth, S.D.S. Calophyllolide, a Complex Coumarin Anticoagulant from Calophyllum Inophyllum Linn. J. Pharm. Pharmacol. 2011, 14, 534–535. [Google Scholar] [CrossRef]
- Schapoval, E.E.; Winter de Vargas, M.R.; Chaves, C.G.; Bridi, R.; Zuanazzi, J.A.; Henriques, A.T. Antiinflammatory and Antinociceptive Activities of Extracts and Isolated Compounds from Stachytarpheta Cayennensis. J. Ethnopharmacol. 1998, 60, 53–59. [Google Scholar] [CrossRef]
- Lee, K.-W.; Kim, H.J.; Lee, Y.S.; Park, H.-J.; Choi, J.-W.; Ha, J.; Lee, K.-T. Acteoside Inhibits Human Promyelocytic HL-60 Leukemia Cell Proliferation via Inducing Cell Cycle Arrest at G0/G1 Phase and Differentiation into Monocyte. Carcinogenesis 2007, 28, 1928–1936. [Google Scholar] [CrossRef]
- Hausmann, M.; Obermeier, F.; Paper, D.H.; Balan, K.; Dunger, N.; Menzel, K.; Falk, W.; Schoelmerich, J.; Herfarth, H.; Rogler, G. In Vivo Treatment with the Herbal Phenylethanoid Acteoside Ameliorates Intestinal Inflammation in Dextran Sulphate Sodium-Induced Colitis. Clin. Exp. Immunol. 2007, 148, 373–381. [Google Scholar] [CrossRef]
- Sudhahar, V.; Kumar, S.A.; Varalakshmi, P. Role of Lupeol and Lupeol Linoleate on Lipemic–Oxidative Stress in Experimental Hypercholesterolemia. Life Sci. 2006, 78, 1329–1335. [Google Scholar] [CrossRef]
- Alqahtani, A.; Hamid, K.; Kam, A.; Wong, K.H.; Abdelhak, Z.; Razmovski-Naumovski, V.; Chan, K.; Li, K.M.; Groundwater, P.W.; Li, G.Q. The Pentacyclic Triterpenoids in Herbal Medicines and Their Pharmacological Activities in Diabetes and Diabetic Complications. Curr. Med. Chem. 2013, 20, 908–931. [Google Scholar] [PubMed]
- Badshah, H.; Ali, T.; Rehman, S.; Amin, F.; Ullah, F.; Kim, T.H.; Kim, M.O. Protective Effect of Lupeol Against Lipopolysaccharide-Induced Neuroinflammation via the P38/c-Jun N-Terminal Kinase Pathway in the Adult Mouse Brain. J. Neuroimmune Pharmacol. 2016, 11, 48–60. [Google Scholar] [CrossRef]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin Is an Antioxidant of Possible Physiological Importance. Science 1987, 235, 1043–1046. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Millimouno, F.M.; Ali Eltayb, W.; Ali, M.; Li, J.; Li, X. Pinocembrin: A Novel Natural Compound with Versatile Pharmacological and Biological Activities. BioMed Res. Int. 2013, 2013, 379850. [Google Scholar] [CrossRef]
- Drewes, S.E.; van Vuuren, S.F. Antimicrobial Acylphloroglucinols and Dibenzyloxy Flavonoids from Flowers of Helichrysum Gymnocomum. Phytochemistry 2008, 69, 1745–1749. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Recio, M.C.; Schinella, G.R.; Máñez, S.; Giner, R.M.; Cerdá-Nicolás, M.; Ríos, J.L. Assessment of the Anti-Inflammatory Activity and Free Radical Scavenger Activity of Tiliroside. Eur. J. Pharmacol. 2003, 461, 53–61. [Google Scholar] [CrossRef]
- Amri, B.; Martino, E.; Vitulo, F.; Corana, F.; Kaâb, L.B.-B.; Rui, M.; Rossi, D.; Mori, M.; Rossi, S.; Collina, S. Marrubium Vulgare L. Leave Extract: Phytochemical Composition, Antioxidant and Wound Healing Properties. Molecules 2017, 22, 1851. [Google Scholar] [CrossRef]
- Schwentker, A.; Vodovotz, Y.; Weller, R.; Billiar, T.R. Nitric Oxide and Wound Repair: Role of Cytokines? Nitric Oxide 2002, 7, 1–10. [Google Scholar] [CrossRef]
- EDWARDS, J. In Vitro Inhibition of Human Neutrophil Elastase by Oleic Acid Albumin Formulations from Derivatized Cotton Wound Dressings. Int. J. Pharm. 2004, 284, 1–12. [Google Scholar] [CrossRef]
- Baek, S.; Park, H.; Kim, M.; Lee, D. Preparation of PCL/(+)-Catechin/Gelatin Film for Wound Healing Using Air-Jet Spinning. Appl. Surf. Sci. 2020, 509, 145033. [Google Scholar] [CrossRef]
- Al-Roujayee, A.S. Naringenin Improves the Healing Process of Thermally-Induced Skin Damage in Rats. J. Int. Med. Res. 2017, 45, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Bonté, F.; Noel-Hudson, M.-S.; Wepierre, J.; Meybeck, A. Protective Effect of Curcuminoids on Epidermal Skin Cells under Free Oxygen Radical Stress. Planta Med. 1997, 63, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Chainani-Wu, N. Safety and Anti-Inflammatory Activity of Curcumin: A Component of Tumeric (Curcuma longa). J. Altern. Complement. Med. 2003, 9, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Joe, B.; Vijaykumar, M.; LOKESH, B.R. Biological Properties of Curcumin-Cellular and Molecular Mechanisms of Action. Crit. Rev. Food Sci. Nutr. 2004, 44, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Aceituno-Medina, M.; Mendoza, S.; Rodríguez, B.A.; Lagaron, J.M.; López-Rubio, A. Improved Antioxidant Capacity of Quercetin and Ferulic Acid during In-Vitro Digestion through Encapsulation within Food-Grade Electrospun Fibers. J. Funct. Foods 2015, 12, 332–341. [Google Scholar] [CrossRef]
- Bains, R.; Bains, V. The Antioxidant Master Glutathione and Periodontal Health. Dent. Res. J. 2015, 12, 389. [Google Scholar] [CrossRef]
- Gugler, R.; Leschik, M.; Dengler, H.J. Disposition of Quercetin in Man after Single Oral and Intravenous Doses. Eur. J. Clin. Pharmacol. 1975, 9, 229–234. [Google Scholar] [CrossRef]
- Caro, A.A.; Davis, A.; Fobare, S.; Horan, N.; Ryan, C.; Schwab, C. Antioxidant and Pro-Oxidant Mechanisms of (+) Catechin in Microsomal CYP2E1-Dependent Oxidative Stress. Toxicol. Vitr. 2019, 54, 1–9. [Google Scholar] [CrossRef]
- Ye, J.-H.H.; Augustin, M.A. Nano- and Micro-Particles for Delivery of Catechins: Physical and Biological Performance. Crit. Rev. Food Sci. Nutr. 2019, 59, 1563–1579. [Google Scholar] [CrossRef]
- Ng, T.B.; He, J.S.; Niu, S.M.; Pi, Z.F.; Shao, W.; Liu, F.; Zhao, L. A Gallic Acid Derivative and Polysaccharides with Antioxidative Activity from Rose (Rosa Rugosa ) Flowers. J. Pharm. Pharmacol. 2010, 56, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Luo, X.-D.; Protiva, P.; Yang, H.; Ma, C.; Basile, M.J.; Weinstein, I.B.; Kennelly, E.J. Bioactive Novel Polyphenols from the Fruit of Manilkara Zapota (Sapodilla). J. Nat. Prod. 2003, 66, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Soltani, M.; Naghizadeh, B.; Farbood, Y.; Mashak, A.; Sarkaki, A. A Possible Mechanism for the Anxiolytic-like Effect of Gallic Acid in the Rat Elevated plus Maze. Pharmacol. Biochem. Behav. 2014, 117, 40–46. [Google Scholar] [CrossRef]
- Burns, J.; Yokota, T.; Ashihara, H.; Lean, M.E.J.; Crozier, A. Plant Foods and Herbal Sources of Resveratrol. J. Agric. Food Chem. 2002, 50, 3337–3340. [Google Scholar] [CrossRef]
- Marques, F.Z.; Markus, M.A.; Morris, B.J. Resveratrol: Cellular Actions of a Potent Natural Chemical That Confers a Diversity of Health Benefits. Int. J. Biochem. Cell Biol. 2009, 41, 2125–2128. [Google Scholar] [CrossRef]
- Malhotra, A.; Bath, S.; Elbarbry, F. An Organ System Approach to Explore the Antioxidative, Anti-Inflammatory, and Cytoprotective Actions of Resveratrol. Oxid. Med. Cell. Longev. 2015, 2015, 803971. [Google Scholar] [CrossRef] [PubMed]
- Means, J.; Gerdes, B.; Koulen, P. Distinct Mechanisms Underlying Resveratrol-Mediated Protection from Types of Cellular Stress in C6 Glioma Cells. Int. J. Mol. Sci. 2017, 18, 1521. [Google Scholar] [CrossRef]
- Bilgic, T. Partial Effect of Resveratrol on Wound Healing: Study on Wistar Albino Rats. Eurasian J. Med. Investig. 2022, 5, 460–468. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef]
- Patel, K.; Singh, G.K.; Patel, D.K. A Review on Pharmacological and Analytical Aspects of Naringenin. Chin. J. Integr. Med. 2018, 24, 551–560. [Google Scholar]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Fazly Bazzaz, B.S. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Yanagibayashi, S.; Kishimoto, S.; Ishihara, M.; Murakami, K.; Aoki, H.; Takikawa, M.; Fujita, M.; Sekido, M.; Kiyosawa, T. Novel Hydrocolloid-Sheet as Wound Dressing to Stimulate Healing-Impaired Wound Healing in Diabetic Db/Db Mice. Biomed. Mater. Eng. 2012, 22, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Laforenza, U.; Patrone, M.; Moccia, F.; Ranzato, E. Honey-Mediated Wound Healing: H2O2 Entry through AQP3 Determines Extracellular Ca2+ Influx. Int. J. Mol. Sci. 2019, 20, 764. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical Formulations and Wound Healing Applications of Chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-Based Hydrogels for Controlled, Localized Drug Delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Chen, C.-Y. Antibacterial Characteristics and Activity of Acid-Soluble Chitosan. Bioresour. Technol. 2008, 99, 2806–2814. [Google Scholar] [CrossRef]
- Song, E.-H.; Shang, J.; Ratner, D.M. Polysaccharides. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 137–155. [Google Scholar]
- LogithKumar, R.; KeshavNarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A Review of Chitosan and Its Derivatives in Bone Tissue Engineering. Carbohydr. Polym. 2016, 151, 172–188. [Google Scholar] [CrossRef]
- Khoshfetrat, A.B.; Khanmohammadi, M.; Sakai, S.; Taya, M. Enzymatically-Gellable Galactosylated Chitosan: Hydrogel Characteristics and Hepatic Cell Behavior. Int. J. Biol. Macromol. 2016, 92, 892–899. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Elella, M.H.A.A.; Sabaa, M.W. Cytotoxicity and Metal Ions Removal Using Antibacterial Biodegradable Hydrogels Based on N-Quaternized Chitosan/Poly(Acrylic Acid). Int. J. Biol. Macromol. 2017, 98, 302–313. [Google Scholar] [CrossRef]
- Bagher, Z.; Ehterami, A.; Safdel, M.H.; Khastar, H.; Semiari, H.; Asefnejad, A.; Davachi, S.M.; Mirzaii, M.; Salehi, M. Wound Healing with Alginate/Chitosan Hydrogel Containing Hesperidin in Rat Model. J. Drug Deliv. Sci. Technol. 2020, 55, 101379. [Google Scholar] [CrossRef]
- Verma, J.; Kanoujia, J.; Parashar, P.; Tripathi, C.B.; Saraf, S.A. Wound Healing Applications of Sericin/Chitosan-Capped Silver Nanoparticles Incorporated Hydrogel. Drug Deliv. Transl. Res. 2017, 7, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Caetano, G.F.; Frade, M.A.C.; Andrade, T.A.M.; Leite, M.N.; Bueno, C.Z.; Moraes, Â.M.; Ribeiro-Paes, J.T. Chitosan-Alginate Membranes Accelerate Wound Healing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1013–1022. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Sandri, G.; Dellera, E.; Rossi, S.; Ferrari, F.; Mori, M.; Caramella, C. Ionic Polymeric Micelles Based on Chitosan and Fatty Acids and Intended for Wound Healing. Comparison of Linoleic and Oleic Acid. Eur. J. Pharm. Biopharm. 2014, 87, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Zubair, R.; Aziz, N. As Smooth as Honey—The Historical Use of Honey as Topical Medication. JAMA Dermatology 2015, 151, 1102. [Google Scholar] [CrossRef]
- Kumar, P.; Kothari, V. (Eds.) Wound Healing Research; Springer: Singapore, 2021; ISBN 978-981-16-2676-0. [Google Scholar]
- Christy, E.M.-L.; Clarke, A.M.; Ndip, R.N. An Overview of Honey: Therapeutic Properties and Contribution in Nutrition and Human Health. African J. Microbiol. Res. 2011, 5, 844–852. [Google Scholar]
- Mandal, M.D.; Mandal, S. Honey: Its Medicinal Property and Antibacterial Activity. Asian Pac. J. Trop. Biomed. 2011, 1, 154–160. [Google Scholar] [CrossRef]
- Bosio, K.; Avanzini, C.; D’avolio, A.; Ozino, O.; Savoia, D. In Vitro Activity of Propolis against Streptococcus Pyogenes. Lett. Appl. Microbiol. 2000, 31, 174–177. [Google Scholar] [CrossRef]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical Aspects of Propolis Research in Modern Times. Evid.-Based Complement. Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef]
- Afrouzan, H.; Tahghighi, A.; Zakeri, S.; Es-haghi, A. Chemical Composition and Antimicrobial Activities of Iranian Propolis. Iran. Biomed. J. 2018, 22, 50–65. [Google Scholar] [PubMed]
- Huang, S.; Zhang, C.-P.; Wang, K.; Li, G.; Hu, F.-L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [PubMed]
- Orhan, H.; Marol, S.; Hepşen, İ.F.; Şahin, G. Effects of Some Probable Antioxidants on Selenite-Induced Cataract Formation and Oxidative Stress-Related Parameters in Rats. Toxicology 1999, 139, 219–232. [Google Scholar] [CrossRef]
- Toreti, V.C.; Sato, H.H.; Pastore, G.M.; Park, Y.K. Recent Progress of Propolis for Its Biological and Chemical Compositions and Its Botanical Origin. Evid.-Based Complement. Altern. Med. 2013, 2013, 697390. [Google Scholar] [CrossRef] [PubMed]
- Uzel, A.; Sorkun, K.K.; Önçaǧ, Ö.; Çoǧulu, D.; Gençay, Ö.; Salih, B. Chemical Compositions and Antimicrobial Activities of Four Different Anatolian Propolis Samples. Microbiol. Res. 2005, 160, 189–195. [Google Scholar] [CrossRef]
- Czyżewska, U.; Konończuk, J.; Teul, J.; Drągowski, P.; Pawlak-Morka, R.; Surażyński, A.; Miltyk, W. Verification of Chemical Composition of Commercially Available Propolis Extracts by Gas Chromatography–Mass Spectrometry Analysis. J. Med. Food 2015, 18, 584–591. [Google Scholar] [CrossRef]
- Fujisawa, H.; Watanabe, K.; SUMA, K.; Origuchi, K.; Matsufuji, H.; SEKI, T.; ARIGA, T. Antibacterial Potential of Garlic-Derived Allicin and Its Cancellation by Sulfhydryl Compounds. Biosci. Biotechnol. Biochem. 2009, 73, 1948–1955. [Google Scholar] [CrossRef]
- Leng, B.-F.; Qiu, J.-Z.; Dai, X.-H.; Dong, J.; Wang, J.-F.; Luo, M.-J.; Li, H.-E.; Niu, X.-D.; Zhang, Y.; Ai, Y.-X.; et al. Allicin Reduces the Production of α-Toxin by Staphylococcus Aureus. Molecules 2011, 16, 7958–7968. [Google Scholar] [CrossRef]
- Arzanlou, M.; Bohlooli, S. Inhibition of Streptolysin O by Allicin—An Active Component of Garlic. J. Med. Microbiol. 2010, 59, 1044–1049. [Google Scholar] [CrossRef]
- Alhashim, M.; Lombardo, J. Mechanism of Action of Topical Garlic on Wound Healing. Dermatologic Surg. 2018, 44, 630–634. [Google Scholar] [CrossRef]
- Trusheva, B.; Todorov, I.; Ninova, M.; Najdenski, H.; Daneshmand, A.; Bankova, V. Antibacterial mono- and sesquiterpene esters of benzoic acids from Iranian propolis. Chem. Cent. J. 2010, 4, 8. [Google Scholar] [CrossRef]
- SUGUNA, L.; CHANDRAKASAN, G.; JOSEPH, K.T. Influence of Honey on Collagen Metabolism during Wound Healing in Rats. J. Clin. Biochem. Nutr. 1992, 13, 7–12. [Google Scholar] [CrossRef]
- Hormozi, M.; Assaei, R.; Boroujeni, M.B. The Effect of Aloe Vera on the Expression of Wound Healing Factors (TGFβ1 and BFGF) in Mouse Embryonic Fibroblast Cell: In Vitro Study. Biomed. Pharmacother. 2017, 88, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Seo, G.; Hyun, C.; Choi, S.; Kim, Y.M.; Cho, M. The Wound Healing Effect of Four Types of Beta-Glucan. Appl. Biol. Chem. 2019, 62, 20. [Google Scholar] [CrossRef]
- Mahibalan, S.; Stephen, M.; Nethran, R.T.; Khan, R.; Begum, S. Dermal Wound Healing Potency of Single Alkaloid (Betaine) versus Standardized Crude Alkaloid Enriched-Ointment of Evolvulus Alsinoides. Pharm. Biol. 2016, 54, 2851–2856. [Google Scholar] [CrossRef] [PubMed]
- Agra, L.C.; Ferro, J.N.S.; Barbosa, F.T.; Barreto, E. Triterpenes with Healing Activity: A Systematic Review. J. Dermatolog. Treat. 2015, 26, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Güçlü-Üstündağ, Ö.; Mazza, G.; Guclu-Ustundag, Ö.; Mazza, G. Saponins: Properties, Applications and Processing. Crit. Rev. Food Sci. Nutr. 2007, 47, 231–258. [Google Scholar] [CrossRef] [PubMed]
- Baumann, E.; Stoya, G.; Völkner, A.; Richter, W.; Lemke, C.; Linss, W. Hemolysis of Human Erythrocytes with Saponin Affects the Membrane Structure. Acta Histochem. 2000, 102, 21–35. [Google Scholar] [CrossRef]
- Killeen, G.F.; Madigan, C.A.; Connolly, C.R.; Walsh, G.A.; Clark, C.; Hynes, M.J.; Timmins, B.F.; James, P.; Headon, D.R.; Power, R.F. Antimicrobial Saponins of Yucca Schidigera and the Implications of Their in Vitro Properties for Their in Vivo Impact. J. Agric. Food Chem. 1998, 46, 3178–3186. [Google Scholar] [CrossRef]
- Konishi, M.; Hano, Y.; Takayama, M.; Nomura, T.; Hamzah, A.S.; Ahmad, R.B.; Jasmani, H. Triterpenoid Saponins from Hedyotis Nudicaulis. Phytochemistry 1998, 48, 525–528. [Google Scholar] [CrossRef]
- Simões, C.M.O.; Amoros, M.; Girre, L. Mechanism of Antiviral Activity of Triterpenoid Saponins. Phyther. Res. 1999, 13, 323–328. [Google Scholar] [CrossRef]
- Yogeeswari, P.; Sriram, D. Betulinic Acid and Its Derivatives: A Review on Their Biological Properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Just, M.; Recio, M.; Giner, R.; Cuéllar, M.; Máñez, S.; Bilia, A.; Ríos, J.-L. Anti-Inflammatory Activity of Unusual Lupane Saponins from Bupleurum Fruticescens. Planta Med. 1998, 64, 404–407. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Giner, R.M.; Recio, M.C.; Máñez, S.; Cerdá-Nicolás, M.; Ríos, J.-L. In Vivo Anti-Inflammatory Activity of Saponins from Bupleurum Rotundifolium. Life Sci. 2001, 68, 1199–1206. [Google Scholar] [CrossRef]
- Lee, C.-W.; Ko, H.-H.; Lin, C.-C.; Chai, C.-Y.; Chen, W.-T.; Yen, F.-L. Artocarpin Attenuates Ultraviolet B-Induced Skin Damage in Hairless Mice by Antioxidant and Anti-Inflammatory Effect. Food Chem. Toxicol. 2013, 60, 123–129. [Google Scholar] [CrossRef]
- Han, A.-R.R.; Kang, Y.-J.J.; Windono, T.; Lee, S.K.; Seo, E.-K.K. Prenylated Flavonoids from the Heartwood of Artocarpus Communis with Inhibitory Activity on Lipopolysaccharide-Induced Nitric Oxide Production. J. Nat. Prod. 2006, 69, 719–721. [Google Scholar] [CrossRef]
- Yao, X.; Wu, D.; Dong, N.; Ouyang, P.; Pu, J.; Hu, Q.; Wang, J.; Lu, W.; Huang, J. Moracin C, A Phenolic Compound Isolated from Artocarpus Heterophyllus, Suppresses Lipopolysaccharide-Activated Inflammatory Responses in Murine Raw264.7 Macrophages. Int. J. Mol. Sci. 2016, 17, 1199. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, C.-W.; Tzeng, W.-S.; Lin, L.-T.; Lee, C.-W.; Yen, F.-L.; Lin, C.-C. Enhanced Autophagic Activity of Artocarpin in Human Hepatocellular Carcinoma Cells through Improving Its Solubility by a Nanoparticle System. Phytomedicine 2016, 23, 528–540. [Google Scholar] [CrossRef]
- Arung, E.T.; Wicaksono, B.D.; Handoko, Y.A.; Kusuma, I.W.; Shimizu, K.; Yulia, D.; Sandra, F. Cytotoxic Effect of Artocarpin on T47D Cells. J. Nat. Med. 2010, 64, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Bohn, J.A.; BeMiller, J.N. (1→3)-β-d-Glucans as Biological Response Modifiers: A Review of Structure-Functional Activity Relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Browder, W.; Williams, D.; Pretus, H.; Olivero, G.; Enrichens, F.; Mao, P.; Franchello, A. Beneficial Effect of Enhanced Macrophage Function in the Trauma Patient. Ann. Surg. 1990, 211, 605–612, discussion 612–613. [Google Scholar]
- Sherwood, E.R.; Williams, D.L.; McNamee, R.B.; Jones, E.L.; Browder, I.W.; Di Luzio, N.R. In Vitro Tumoricidal Activity of Resting and Glucan-Activated Kupffer Cells. J. Leukoc. Biol. 1987, 42, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Pretus, H.A.; Ensley, H.E.; McNamee, R.B.; Jones, E.L.; Browder, I.W.; Williams, D.L. Isolation, Physicochemical Characterization and Preclinical Efficacy Evaluation of Soluble Scleroglucan. J. Pharmacol. Exp. Ther. 1991, 257, 500–510. [Google Scholar]
- Majtan, J.; Jesenak, M. β-Glucans: Multi-Functional Modulator of Wound Healing. Molecules 2018, 23, 806. [Google Scholar] [CrossRef]
- Mellin, T.N.; Mennie, R.J.; Cashen, D.E.; Ronan, J.J.; Capparella, J.; James, M.L.; Disalvo, J.; Frank, J.; Linemeyer, D.; Gimenez-Gallego, G.; et al. Acidic Fibroblast Growth Factor Accelerates Dermal Wound Healing. J Investig. Dermatol. 1992, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shearer, J.D.; Richards, J.R.; Mills, C.D.; Caldwell, M.D. Differential Regulation of Macrophage Arginine Metabolism: A Proposed Role in Wound Healing. Am. J. Physiol. Metab. 1997, 272, E181–E190. [Google Scholar] [CrossRef]
- Tsao, N. Advanced Wound Care Technologies 2020–2030. IDTechEx. 2022. Available online: https://www.idtechex.com/en/research-report/advanced-wound-care-technologies-2020-2030/682 (accessed on 19 August 2022).
- Buwalda, S.J.; Vermonden, T.; Hennink, W.E. Hydrogels for Therapeutic Delivery: Current Developments and Future Directions. Biomacromolecules 2017, 18, 316–330. [Google Scholar] [CrossRef] [PubMed]



| Compound | Origin | Using Part | Other Bioactivities | Targeting Phase | ExperimentalModel | Type of Wound | Ref. |
|---|---|---|---|---|---|---|---|
| Asiatic acid | Plant (Centella asiatica) | Leaves | Anti-microbial Anti-oxidant Pro-collagen | Inflammation Proliferation Remodeling | Human | Diabetic Burn | [174] |
| Pinocembrin | Plant | N/A | N/A | Inflammation | HaCaT cell | N/A | [113] |
| Ursolic acid | Plant (Hedyotis herbacea) | N/A | Anti-microbial | Inflammation | Rat | Incision Excision | [175] |
| Myricetin | Plant (Tecomaria capensis v. aurea) | N/A | Anti-oxidant Anti-allergic Analgesic | Inflammation | Rat | Excision | [47] |
| Myricetin | Plant | N/A | Anti-oxidant | N/A | In vitro | N/A | [59] |
| Apigenin | Plant | Fruits Beans Tea leaves | Anti-oxidant Pro-angiogenic | Inflammation Proliferation | Rat | Random skin flaps | [109] |
| Lupeol | Plant (Bowdichia virgilioides Kunth) | Stem bark | Anti-oxidant | Inflammation Proliferation Remodeling | Rat | Excision | [58] |
| Lupeol | Plant (Bowdichia virgilioides Kunth) | Stem bark | Anti-oxidant | Inflammation Proliferation | Rat | Excision | [176] |
| Steroidal glycoside | Plant | N/A | Dermal fibroblast migration activity | Inflammation Proliferation Remodeling | Human dermal fibroblast cells | Human wound | [56] |
| Verbascoside | Plant (Plantago subulata) | Aerial parts | Anti-oxidant Anti-fugal Anti-bacterial Anti-viral | Inflammation Proliferation | L929 fibroblasts RAW 264.7 cells | N/A | [62] |
| Verbascoside | Plant (Plantago australis) | Leaves | Anti-oxidant Healing | Inflammation Proliferation | HaCaT cells Rat | Excision | [177] |
| Hesperetin | Plant | Citrus species | Anti-microbial Anti-oxidant | Inflammation Proliferation Remodeling | Rat | Excision diabetic foot ulcer | [102] |
| Hesperetin | Plant | Citrus species | Anti-oxidant Pro-collagen | Inflammation | Rat | Diabetic foot ulcer | [154] |
| Carophylolide | Plant (Calophyllum inophyllum Linn) | Seed | Anti-microbial Anti-coagulant | Inflammation | Mice | Incision | [55] |
| Artocarpin | Plant (A.communis.) | Heartwood | Anti-oxidative, Anti-microbial | Inflammation Proliferation | Mice HUVECs cells | Excision | [50] |
| Bilirubin | Mammals | Product of heme catabolism | Anti-oxidant | Inflammation Proliferation Remodeling | Rat | Excision | [138] |
| Compound | Origin | Using Part | Other Bioactivities | Target Phase | Experimental Model | Type of Wound | Ref. |
|---|---|---|---|---|---|---|---|
| Quercetin | Plant (Oxytropis falcata Bunge) | Fruits | Anti-inflammatory Anti-infection | Inflammation Proliferation Remodeling | Mice | Excision | [63] |
| Resveratrol | Plant | N/A | Anti-inflammatory Anti-bacterial | Inflammation Proliferation | HUVE cells Rat | Burn injury | [149] |
| Catechin | Plant (Green tea) | N/A | Anti-bacterial Anti-inflammatory Pro-angiogenic | Inflammation | Mice | Chronic diabetic wound | [65] |
| Catechin | N/A | N/A | N/A | N/A | Mouse NIH/3T3 fibroblast cell | N/A | [200] |
| Luteolin | Plant | N/A | Anti-inflammatory Anti-allergenic | Inflammation Proliferation | Rat | Excision | [42] |
| Syringic acid | Plant | Fruits | Anti-inflammatory Anti-microbial Anti-adipogenic | Inflammation Proliferation Remodeling | Rat | Incision diabetic wound | [133] |
| Metformin | N/A | N/A | Anti-hypoglycemic | Inflammation Proliferation | Mice | Diabetic wounds | [87] |
| Naringenin | Plant | Citrus fruits | Anti-inflammatory | Proliferation Inflammation | Rat | Thermally-induced skin damage | [201] |
| Galic acid | Plant | Fruits Leaves Flower | Anti-inflammatory Analgesic | Inflammation Proliferation | HaCaT MEF HF21 cells | Hyperglucidic conditions | [61] |
| Ferulic acid | Plant (vegetables, cereals, coffee) | Seed Fruits | Anti-inflammatory Antimicrobial | Inflammation Proliferation | Rat | Excision diabetic wounds | [84] |
| Curcumin | Plant | Turmeric | Anti-inflammatory | Inflammation Proliferation | Rat | Excision | [82] |
| Curcumin | Plant (Curcuma longa) | Turmeric | Anti-inflammatory Anti-infective | Inflammation | Rat | Excision | [57] |
| Curcumin | Plant | Turmeric | Anti-inflammatory | Inflammation | Human keratinocytes and fibroblasts | H2O2 condition | [60] |
| Curcumin | Plant (Curcuma longa) | Turmeric | N/A | Inflammation | Human keratinocytes | Hypoxanthine/xanthine oxidase injury | [202] |
| Compound | Origin | Using Part | Other Bioactivities | Target Phase | Experimental Model | Type of Wound | Ref. |
|---|---|---|---|---|---|---|---|
| Chitosan | Animal (Crab) | Shells | Anti-microbial Anti-inflammation | Inflammation | Diabetic db/db mice | Excision wound | [222] |
| Pinocembrin | Animal (Bee) | PropolisHoney | Anti-oxidation Anti-inflammatory Anti-apoptosis | Proliferation | Human Keloid fibroblast Mice | keloid xenograft | [103] |
| Lupeol | Plant (Bowdichia virgilioides Kunth) | Stem bark | Anti-oxidant Antidiabetic | Inflammation Proliferation Remodeling | Rat | Excision | [58] |
| Hydrogen peroxide | Animal (Bee) | Honey | N/A | Inflammation | HaCaT cells | N/A | [223] |
| Methylglyoxal | Animal (Bee) | Honey (Manuka) | N/A | N/A | N/A | N/A | [52] |
| Tannins | Plant (E. phaseoloides (L.) Merr) | N/A | Anti-oxidant Anti-inflammatory | Inflammation Proliferation Remodeling | Rat | Excision | [105] |
| Arnebin-1 | Plant (Arnebianobilis) | Root | Anti-fungal | Proliferation | Rat | Excision | [147] |
| Hydroalcoholic extract | Plant (Caseariasylvestris Sw.) | Leaves | Anti-inflammatory Antiseptic | Proliferation | Rodent | Scald burns | [83] |
| Dichloromethane andhexanoic fractions | Plant (Calendula officinalis L.) | Flower | Anti-inflammatory Anti-septic | Inflammation Proliferation | Rat | Excision | [129] |
| Lawsone | Plant (Lawsonia Alba Lam.) | Leaves | Anti-fungal Anti-parasitic Anti-viral | N/A | Rat | Excision Incision | [111] |
| Compound | Origin | Using Part | Other Bioactivities | Target Phase | Experimental Model | Type of Wound | Ref. |
|---|---|---|---|---|---|---|---|
| Honey | Animal (Bee) | Honey | Anti-bacterial | Proliferation | Rat | Excision | [253] |
| Calendula officinalis extract | Plant (Calendula officinalis) | Flower | Anti-bacterial | Proliferation | Rat | Excision | [98] |
| Saponins | Plant (Panax Notoginseng) | Root Rhizome | Anti-inflammation Anti-oxidant Anti-apoptosis Anti-coagulation | Remodeling | Hypertrophic scar fibroblast | N/A | [165] |
| Cryptotanshinone | Plant (Salvia miltiorrhiza Bge.) | N/A | Anti-inflammatory Anti-oxidative Anti-bacterial | Remodeling | Diabetic mice | Excision | [49] |
| Bexarotene, Taspine, and 2-hydroxy-1-naphthaldehyde Isonicotinoylhydrazone | Plant (Daemonorops draco) | N/A | Anti-bacterial Anti-inflammation | Inflammation Proliferation | THP-1, HaCaT, NIH-3T3 cells | N/A | [35] |
| Sesamol | Plant | Sesame oil | Anti-inflammatory Anti-oxidant | Inflammation Proliferation | Rat | Diabetic foot ulcer | [86] |
| Astragaloside IV | Plant (Astragali Radix) | N/A | Anti-inflammatory Anti-oxidative | Inflammation Proliferation | Mice | Excision | [108] |
| Polysaccharide APS2-1 | Plant (Astragalus membranaceus) | Roots | Anti-inflammatory | Inflammation Proliferation | Mice | Excision | [163] |
| Aloe vera gel | Plant (Aloe vera) | Leaves | Anti-inflammatory Anti-bacterial Anti-viral Anti-fugal | Proliferation | Mouse embryonic fibroblasts | N/A | [254] |
| Asiaticoside | Plant (Centella asiatica) | Aerial parts | Anti-oxidant | Proliferation | Rabbit | Incision | [135] |
| Gallic acid and quercetin | Plant (Glycyrrhiza glabra L.) | Roots | Anti-inflammatory Anti-bacterial Anti-microbial Antioxidant | Inflammation Proliferation | Pig | Excision | [88] |
| Asiatic acid | Plant (Centella asiatica) | Aerial parts | Anti-oxidative | Proliferation | Rat | Wound burn | [141] |
| β-Glucans | Fungi | N/A | Anti-biotic | Proliferation | Human dermal fibroblasts | N/A | [255] |
| Alkaloids | Plant (Evolvulus alsinoides) | Aerial parts | Anti-bacterial Anti-fugal Anti-oxidant | Proliferation | Rat | Incision | [256] |
| Asiaticoside and madecassoside | Plant (Centella asiatica) | N/A | Anti-oxidant | Proliferation | Rat | Burn injury | [89] |
| Triterpenes | Plant (Buddleia scordioides) | Leaves | N/A | Proliferation | Diabetic rat | Incision Excision | [257] |
| Deoxyelephantopin | Plant (Elephantopus scaber) | Leaves | Anti-inflammatory | Inflammation Proliferation | Rat | Incision | [139] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trinh, X.-T.; Long, N.-V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.-Y.; Heo, C.-Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. https://doi.org/10.3390/ijms23179573
Trinh X-T, Long N-V, Van Anh LT, Nga PT, Giang NN, Chien PN, Nam S-Y, Heo C-Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. International Journal of Molecular Sciences. 2022; 23(17):9573. https://doi.org/10.3390/ijms23179573
Chicago/Turabian StyleTrinh, Xuan-Tung, Nguyen-Van Long, Le Thi Van Anh, Pham Thi Nga, Nguyen Ngan Giang, Pham Ngoc Chien, Sun-Young Nam, and Chan-Yeong Heo. 2022. "A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective" International Journal of Molecular Sciences 23, no. 17: 9573. https://doi.org/10.3390/ijms23179573
APA StyleTrinh, X.-T., Long, N.-V., Van Anh, L. T., Nga, P. T., Giang, N. N., Chien, P. N., Nam, S.-Y., & Heo, C.-Y. (2022). A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. International Journal of Molecular Sciences, 23(17), 9573. https://doi.org/10.3390/ijms23179573






