Impact of Comorbidities of Patients with Psoriasis on Phototherapy Responses
Abstract
:1. Introduction
2. Results and Discussion
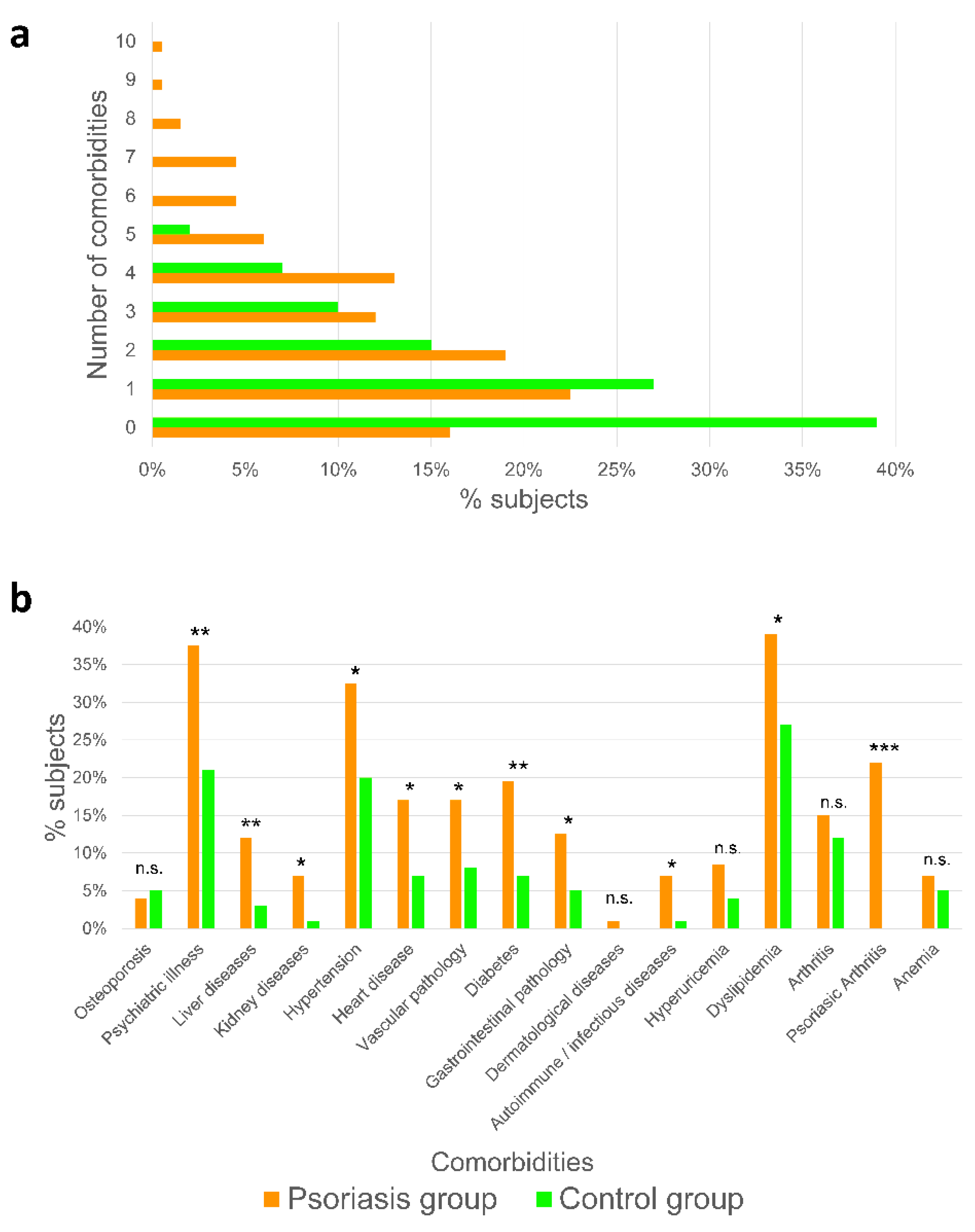
2.1. Psoriatic Patients Present More Associated Comorbidities Than Healthy Subjects
2.2. Men Show More Comorbidities Associated to Psoriasis Than Women
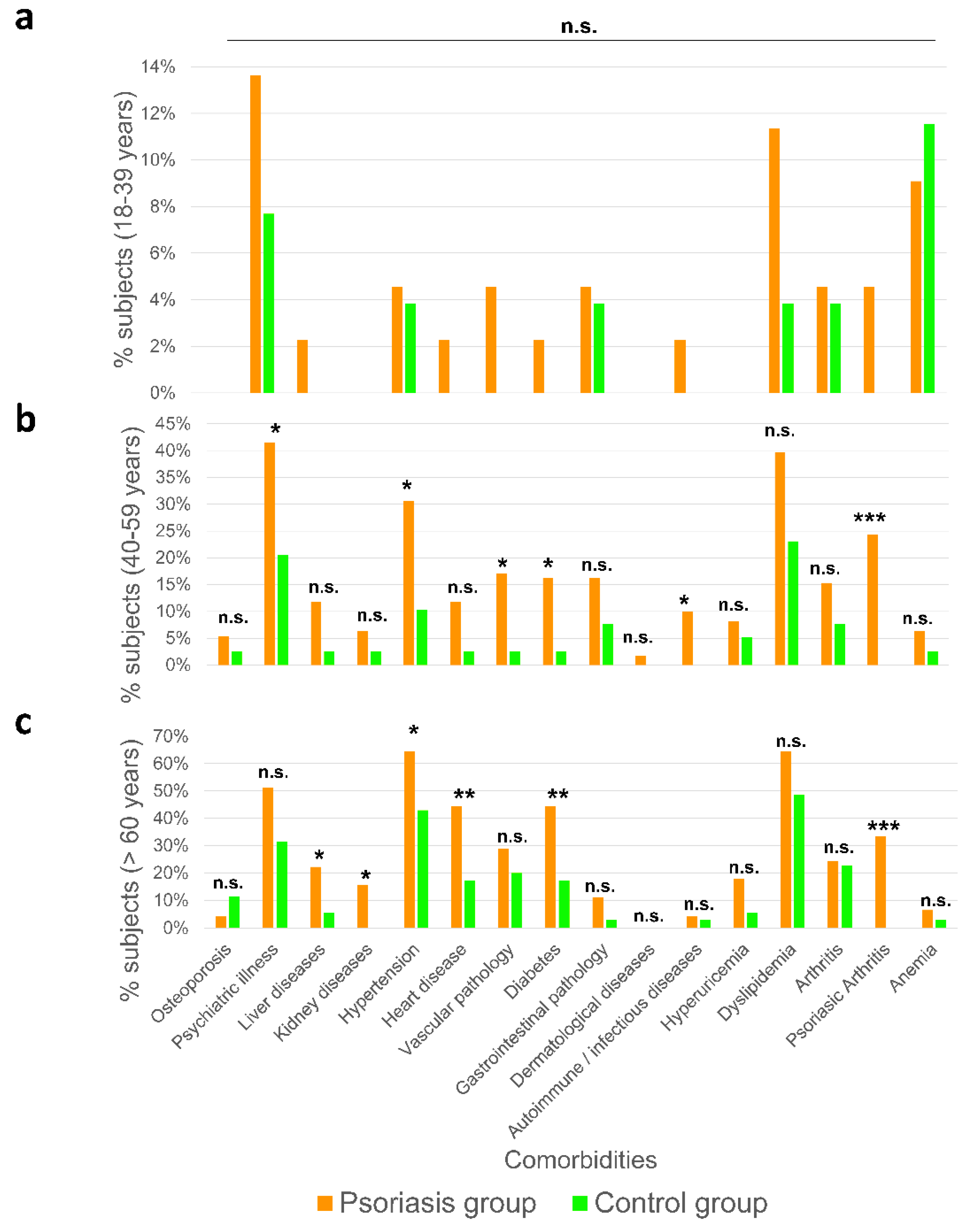
2.3. Premature Aging Is Associated with Comorbidities in Psoriatic Patients
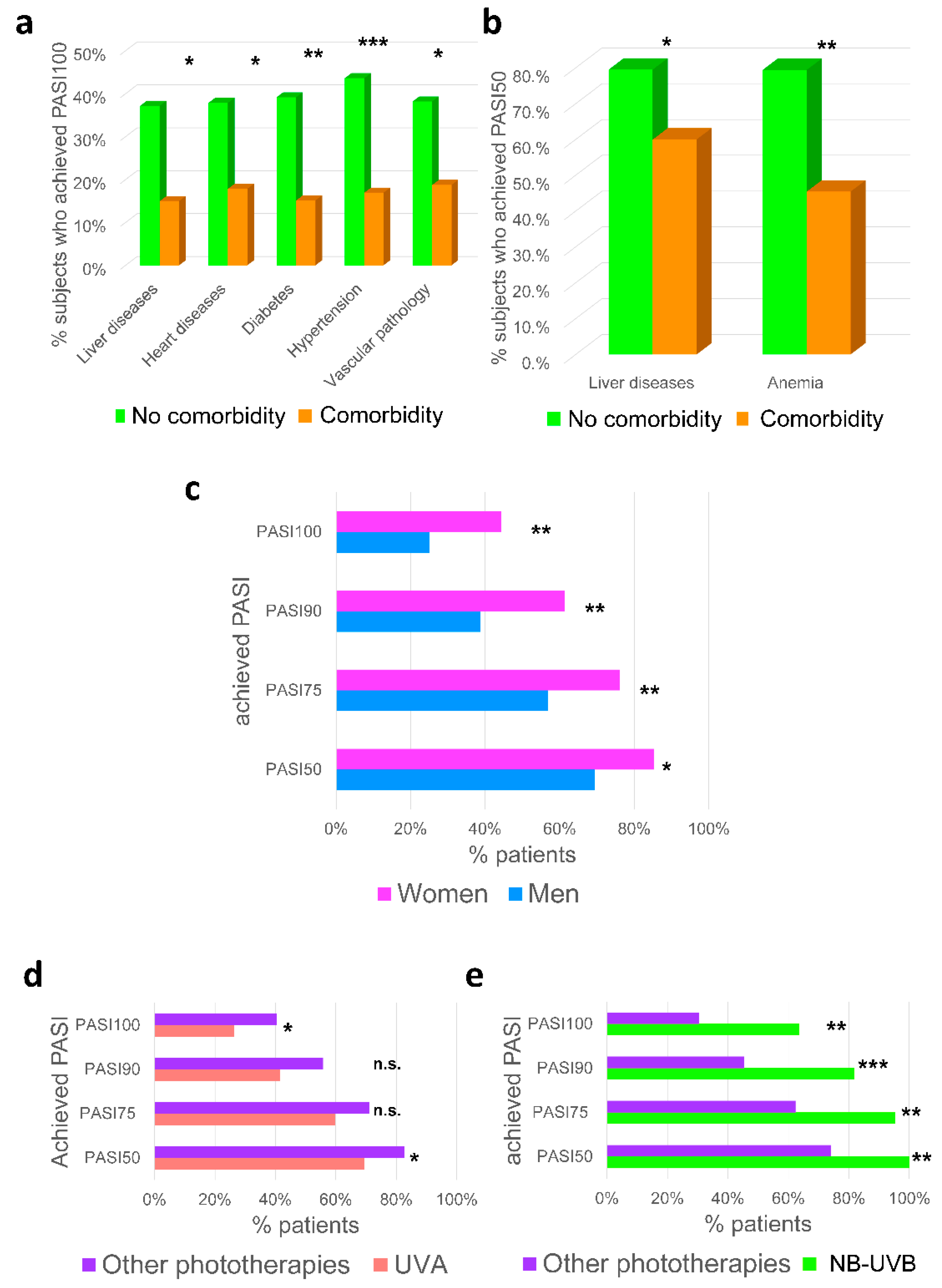
2.4. Impact of Comorbidities, Gender and Type of Phototherapy
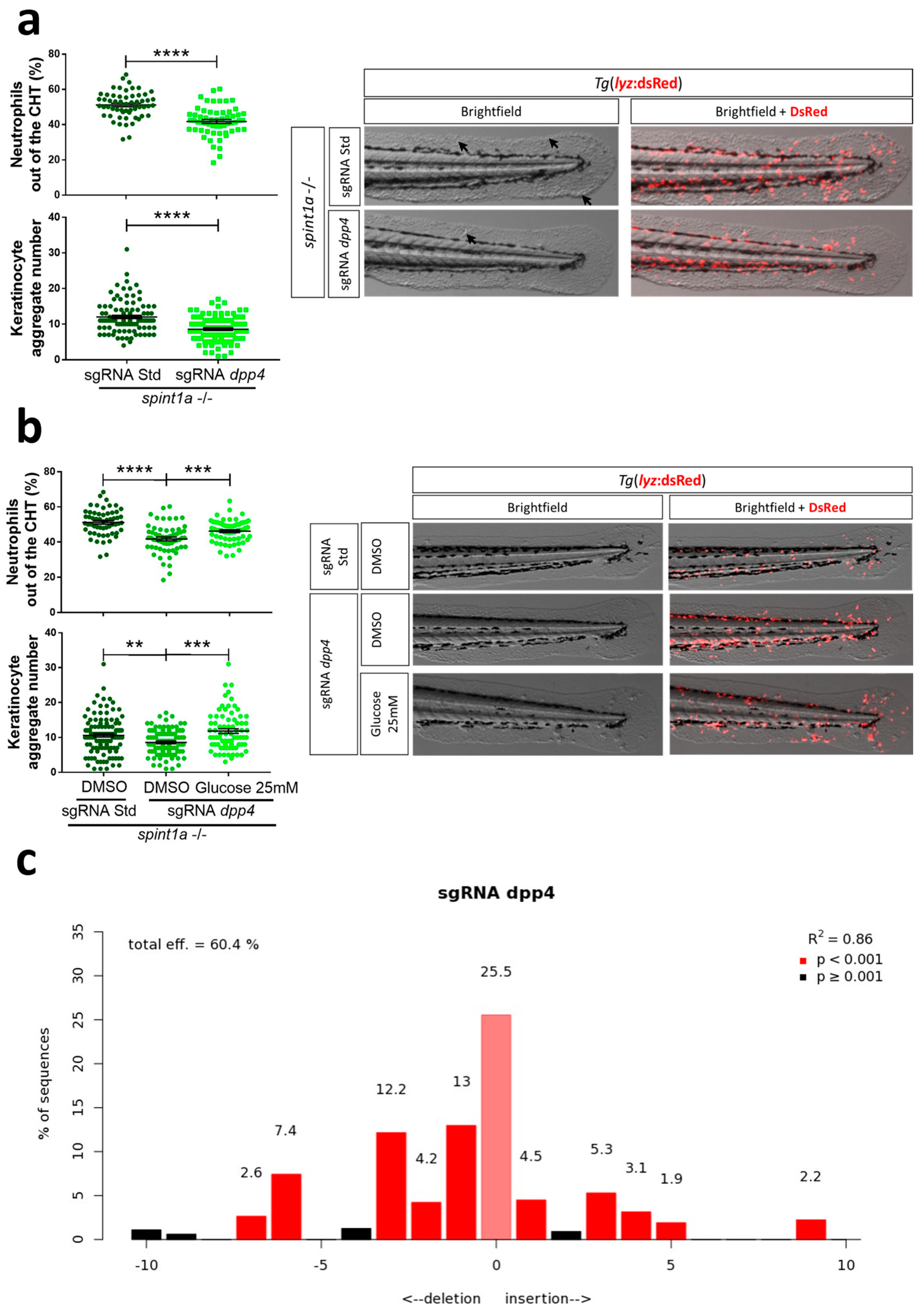
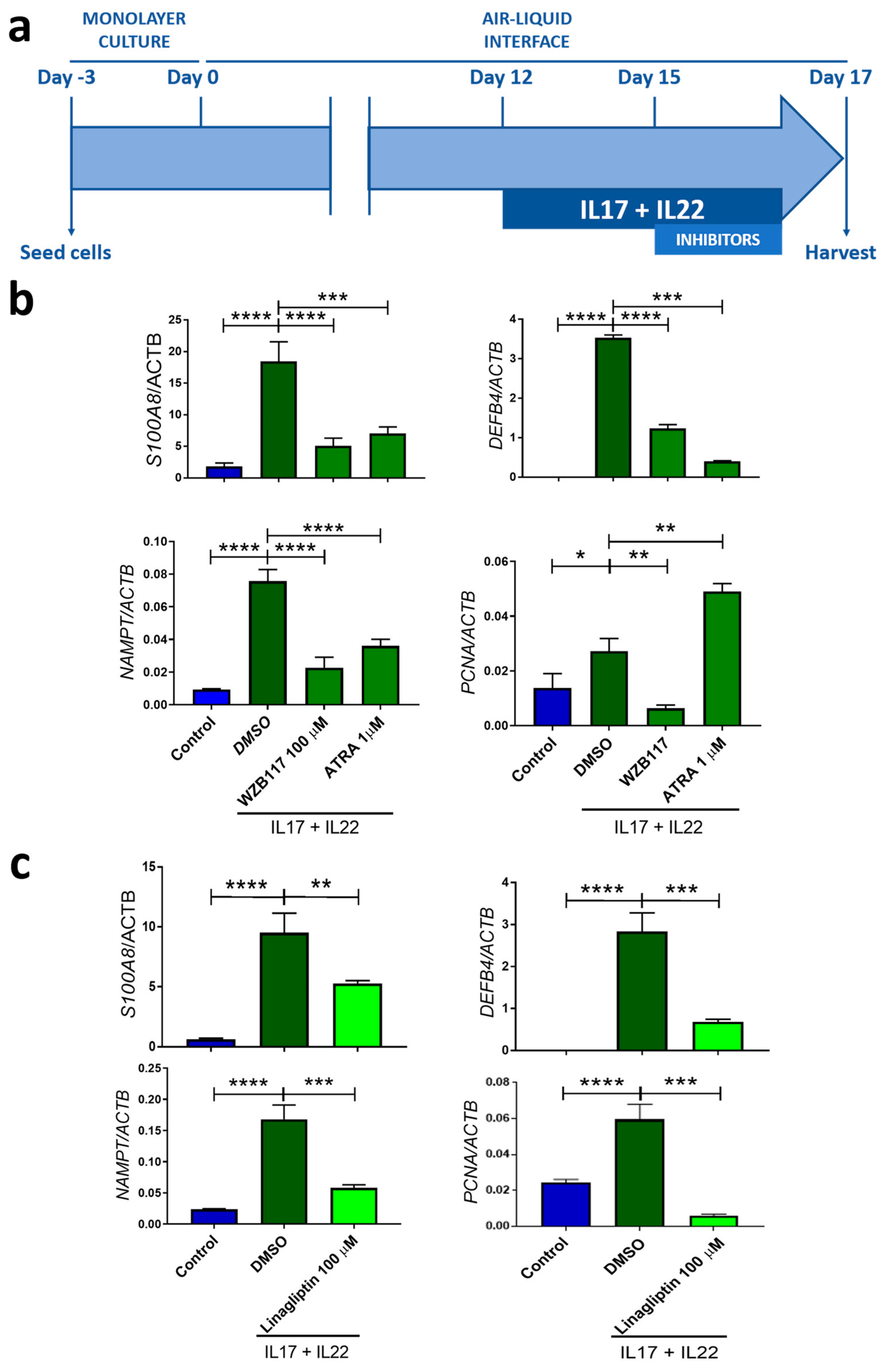
2.5. Zebrafish Larvae and Human 3D Psoriatic Skin Organoids Are Valid Models to Study Comorbidities Associated with Psoriasis
3. Materials and Methods
3.1. Study Design and Patient Cohort
3.2. Analysis of Comorbidities
3.3. Analysis of Phototherapy Treatments
3.4. Animals
3.5. Genetic Inhibition in Zebrafish
3.6. Chemical Treatments in Zebrafish
3.7. Imaging of Zebrafish Larvae
3.8. Human Organotypic 3D Models
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kvist-Hansen, A.; Kaiser, H.; Skov, L.; Hansen, P.R. Comorbidity in connection with psoriasis is more than psoriatic arthritis. Ugeskr. Laeger 2018, 180, 2. [Google Scholar]
- Blegvad, C.; Nybo Andersen, A.M.; Adam, A.; Zachariae, C.; Skov, L. Psoriasis as a Predictor of Cardiometabolic Comorbidity in Women: A Study Based on the Danish National Birth Cohort. Acta Dermatol. Venereol. 2019, 99, 274–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, A.; Bhuiyan, S.I.; Mahmud, M.M.; Siddique, R.U.; Shawkat, S.M.; Nandi, A.K. Comorbidities in Patients with Psoriasis. Mymensingh Med. J. 2019, 28, 894–899. [Google Scholar] [PubMed]
- Cingöz, K.; Gündüz, K.; İnanır, I. Patients’ knowledge about psoriasis and comorbidities; their participation in treatment decisions. J. Dermatol. Treat. 2021, 32, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Mde, F.; Rocha Bde, O.; Duarte, G.V. Psoriasis: Classical and emerging comorbidities. An. Bras. Dermatol. 2015, 90, 9–20. [Google Scholar] [CrossRef] [Green Version]
- Takeshita, J.; Grewal, S.; Langan, S.M.; Mehta, N.N.; Ogdie, A.; Van Voorhees, A.S.; Gelfand, J.M. Psoriasis and comorbid diseases: Epidemiology. J. Am. Acad. Dermatol. 2017, 76, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Dhana, A.; Yen, H.; Yen, H.; Cho, E. All-cause and cause-specific mortality in psoriasis: A systematic review and meta-analysis. J. Am. Acad. Dermatol. 2019, 80, 1332–1343. [Google Scholar] [CrossRef]
- Feldman, S.R.; Srivastava, B.; Abell, J.; Hoops, T.; Fakharzadeh, S.; Chakravarty, S.; Muser, E.; Dungee, D.; Quinn, S.; Leone-Perkins, M.; et al. Gastrointestinal Signs and Symptoms Related to Inflammatory Bowel Disease in Patients With Moderate-to-Severe Psoriasis. J. Drugs Dermatol. 2018, 17, 1298–1308. [Google Scholar]
- Alariny, A.F.; Farid, C.I.; Elweshahi, H.M.; Abbood, S.S. Psychological and Sexual Consequences of Psoriasis Vulgaris on Patients and Their Partners. J. Sex. Med. 2019, 16, 1900–1911. [Google Scholar] [CrossRef]
- Tribó, M.J.; Turroja, M.; Castaño-Vinyals, G.; Bulbena, A.; Ros, E.; García-Martínez, P.; Tausk, F.; Sagristà, M.; Pujol, R.M.; Ferran, M.; et al. Patients with Moderate to Severe Psoriasis Associate with Higher Risk of Depression and Anxiety Symptoms: Results of a Multivariate Study of 300 Spanish Individuals with Psoriasis. Acta Dermatol. Venereol. 2019, 99, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Kromer, C.; Mohr, J.; Celis, D.; Poortinga, S.; Gerdes, S.; Mössner, R.; Wilsmann-Theis, D. Screening for depression in psoriasis patients during a dermatological consultation: A first step towards treatment. J. Dtsch. Dermatol. Ges. 2021, 19, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Aleem, D.; Tohid, H. Pro-inflammatory Cytokines, Biomarkers, Genetics and the Immune System: A Mechanistic Approach of Depression and Psoriasis. Rev. Colomb. Psiquiatr. Engl. Ed. 2018, 47, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Marangell, L.B.; Nakamura, M.; Armstrong, A.; Jeon, C.; Bhutani, T.; Wu, J.J. Depression and suicidality in psoriasis: Review of the literature including the cytokine theory of depression. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Awosika, O.; Eleryan, M.G.; Rengifo-Pardo, M.; Doherty, L.; Martin, L.W.; Ehrlich, A. A Case-control Study to Evaluate the Prevalence of Nonalcoholic Fatty Liver Disease Among Patients with Moderate-to-severe Psoriasis. J. Clin. Aesthet. Dermatol. 2018, 11, 33–37. [Google Scholar]
- Ortolan, A.; Lorenzin, M.; Tadiotto, G.; Russo, F.P.; Oliviero, F.; Felicetti, M.; D’Incà, R.; Favero, M.; Piaserico, S.; Doria, A.; et al. Metabolic syndrome, non-alcoholic fatty liver disease and liver stiffness in psoriatic arthritis and psoriasis patients. Clin. Rheumatol. 2019, 38, 2843–2850. [Google Scholar] [CrossRef]
- Queiro, R.; Lorenzo, A.; Tejón, P.; Pardo, E.; Coto, P. Hypertension is associated with increased age at the onset of psoriasis and a higher body mass index in psoriatic disease. Clin. Rheumatol. 2019, 38, 2063–2068. [Google Scholar] [CrossRef]
- Hjuler, K.F.; Gormsen, L.C.; Vendelbo, M.H.; Egeberg, A.; Nielsen, J.; Iversen, L. Systemic Inflammation and Evidence of a Cardio-splenic Axis in Patients with Psoriasis. Acta Derm. Venereol. 2018, 98, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Shiba, M.; Kato, T.; Izumi, T.; Miyamoto, S.; Nakane, E.; Haruna, T.; Inoko, M. Risk of myocardial infarction in patients with psoriasis: A cross-sectional patient-population study in a Japanese hospital. J. Cardiol. 2019, 73, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Salihbegovic, E.M.; Hadzigrahic, N.; Suljagic, E.; Kurtalic, N.; Hadzic, J.; Zejcirovic, A.; Bijedic, M.; Handanagic, A. Psoriasis and dyslipidemia. Mater. Sociomed. 2015, 27, 15–17. [Google Scholar] [CrossRef] [Green Version]
- Feldman, S.R.; Hur, P.; Zhao, Y.; Tian, H.; Wei, Z.; Wang, X.; Herrera, V. Incidence rates of comorbidities among patients with psoriasis in the United States. Dermatol. Online J. 2018, 24, 10. [Google Scholar] [CrossRef]
- Gisondi, P.; Fostini, A.C.; Fossà, I.; Girolomoni, G.; Targher, G. Psoriasis and the metabolic syndrome. Clin. Dermatol. 2018, 36, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.G.; Thomsen, S.F. Type 2 diabetes and psoriasis: Links and risks. Psoriasis (Auckl) 2019, 17, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Borsky, P.; Chmelarova, M.; Fiala, Z.; Hamakova, K.; Palicka, V.; Krejsek, J.; Andrys, C.; Kremlacek, J.; Rehacek, V.; Beranek, M.; et al. Aging in psoriasis vulgaris: Female patients are epigenetically older than healthy controls. Immun. Ageing 2021, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wu, M.X. A clinical review of phototherapy for psoriasis. Lasers Med. Sci. 2018, 33, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, N.M.; Sbroglio, L.L.; Buffara, M.O.; Baka, J.L.C.E.; Pessoa, A.S.; Azulay-Abulafia, L. Phototherapy. An. Bras. Dermatol. 2021, 96, 397–407. [Google Scholar] [CrossRef]
- Florek, A.G.; Wang, C.J.; Armstrong, A.W. Treatment preferences and treatment satisfaction among psoriasis patients: A systematic review. Arch. Dermatol. Res. 2018, 310, 271–319. [Google Scholar] [CrossRef]
- Cuesta-Montero, L. Fototerapia en la Población de Alicante: Características Epidemiológicas y Clínicas, Análisis de Respuesta, Efectos Secundarios Y Riesgos de Fotocarcinogénesis; Miguel Hernández University: Elche, Spain, 2015. [Google Scholar]
- Fernández-Guarino, M.; Aboin-Gonzalez, S.; Barchino, L.; Velazquez, D.; Arsuaga, C.; Lázaro, P. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol. Ther. 2016, 29, 19–23. [Google Scholar] [CrossRef]
- Zhang, Z.; Zi, Z.; Lee, E.E.; Zhao, J.; Contreras, D.C.; South, A.P.; Abel, E.D.; Chong, B.F.; Vandergriff, T.; Hosler, G.A.; et al. Differential glucose requirement in skin homeostasis and injury identifies a therapeutic target for psoriasis. Nat. Med. 2018, 24, 617–627. [Google Scholar] [CrossRef]
- Martinez-Morcillo, F.J.; Canton-Sandoval, J.; Martinez-Menchon, T.; Corbalan-Velez, R.; Mesa-Del-Castillo, P.; Perez-Oliva, A.B.; Garcia-Moreno, D.; Mulero, V. Non-canonical roles of NAMPT and PARP in inflammation. Dev. Comp. Immunol. 2021, 115, 103881. [Google Scholar] [CrossRef]
- Martinez-Navarro, F.J.; Martinez-Morcillo, F.J.; Lopez-Munoz, A.; Pardo-Sanchez, I.; Martinez-Menchon, T.; Corbalan-Velez, R.; Cayuela, M.L.; Perez-Oliva, A.B.; Garcia-Moreno, D.; Mulero, V. The vitamin B6-regulated enzymes PYGL and G6PD fuel NADPH oxidases to promote skin inflammation. Dev. Comp. Immunol. 2020, 108, 103666. [Google Scholar] [CrossRef]
- Lynch, M.; Malara, A.; Timoney, I.; Ahern, T.; Awdeh, F.; Sweeney, C.; Galligan, M.; Venckens, S.; Kelly, G.; Hughes, R.; et al. Dipeptidyl Peptidase-4 Inhibition in Psoriasis Patients with Diabetes: A Double-Blind Randomized Controlled Trial. Dermatology 2021, 237, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lin, L.; Chen, P.; Yu, Y.; Chen, S.; Chen, X.; Shao, Z. Treatment with liraglutide, a glucagon-like peptide-1 analogue, improves effectively the skin lesions of psoriasis patients with type 2 diabetes: A prospective cohort study. Diabetes Res. Clin. Pract. 2019, 150, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Ahern, T.B.; Timoney, I.; Sweeney, C.; Kelly, G.; Hughes, R.; Tobin, A.M.; O’Shea, D.; Kirby, B. Dipeptidyl peptidase-4 inhibition and narrow-band ultraviolet-B light in psoriasis (DINUP): Study protocol for a randomised controlled trial. Trials 2016, 17, 29. [Google Scholar] [CrossRef] [Green Version]
- Bao, L.; Li, J.; Perez White, B.E.; Patel, P.M.; Amber, K.T. Inhibition of dipeptidyl-peptidase 4 induces upregulation of the late cornified envelope cluster in keratinocytes. Arch. Dermatol. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, T.; Shinohara, M.; Tanimoto, N.; Kumagai, C.; Hashimoto, K. Sitagliptin, a dipeptidyl peptidase-IV inhibitor, improves psoriasis. Dermatology 2012, 224, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.; Malara, A.; Timoney, I.; Vencken, S.; Ahern, T.; Awdeh, F.; Sweeney, C.; Galligan, M.; Kelly, G.; Hughes, R.; et al. Sitagliptin and Narrow-Band Ultraviolet-B for Moderate Psoriasis (DINUP): A Randomised Controlled Clinical Trial. Dermatology 2022, 238, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish Danio* (Brachydanio) Rerio; University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Hall, C.; Flores, M.V.; Storm, T.; Crosier, K.; Crosier, P. The zebrafish lysozyme C promoter drives myeloid-specific expression in transgenic fish. BMC Dev. Biol. 2007, 7, 42. [Google Scholar] [CrossRef] [Green Version]
- Amsterdam, A.; Burgess, S.; Golling, G.; Chen, W.; Sun, Z.; Townsend, K.; Farrington, S.; Haldi, M.; Hopkins, N. A large-scale insertional mutagenesis screen in zebrafish. Genes Dev. 1999, 13, 2713–2724. [Google Scholar] [CrossRef] [Green Version]
- Brinkman, E.K.; Chen, T.; Amendola, M.; van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Res. 2014, 42, e168. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatás-Lalana, B.; Cantón-Sandoval, J.; Rodríguez-Ruiz, L.; Corbalán-Vélez, R.; Martínez-Menchón, T.; Pérez-Oliva, A.B.; Mulero, V. Impact of Comorbidities of Patients with Psoriasis on Phototherapy Responses. Int. J. Mol. Sci. 2022, 23, 9508. https://doi.org/10.3390/ijms23179508
Fatás-Lalana B, Cantón-Sandoval J, Rodríguez-Ruiz L, Corbalán-Vélez R, Martínez-Menchón T, Pérez-Oliva AB, Mulero V. Impact of Comorbidities of Patients with Psoriasis on Phototherapy Responses. International Journal of Molecular Sciences. 2022; 23(17):9508. https://doi.org/10.3390/ijms23179508
Chicago/Turabian StyleFatás-Lalana, Belén, Joaquín Cantón-Sandoval, Lola Rodríguez-Ruiz, Raúl Corbalán-Vélez, Teresa Martínez-Menchón, Ana B. Pérez-Oliva, and Victoriano Mulero. 2022. "Impact of Comorbidities of Patients with Psoriasis on Phototherapy Responses" International Journal of Molecular Sciences 23, no. 17: 9508. https://doi.org/10.3390/ijms23179508
APA StyleFatás-Lalana, B., Cantón-Sandoval, J., Rodríguez-Ruiz, L., Corbalán-Vélez, R., Martínez-Menchón, T., Pérez-Oliva, A. B., & Mulero, V. (2022). Impact of Comorbidities of Patients with Psoriasis on Phototherapy Responses. International Journal of Molecular Sciences, 23(17), 9508. https://doi.org/10.3390/ijms23179508






