Phospholipase A1 Member A Deficiency Alleviates Mannan-Induced Psoriatic Arthritis in Mice Model
Abstract
:1. Introduction
2. Results
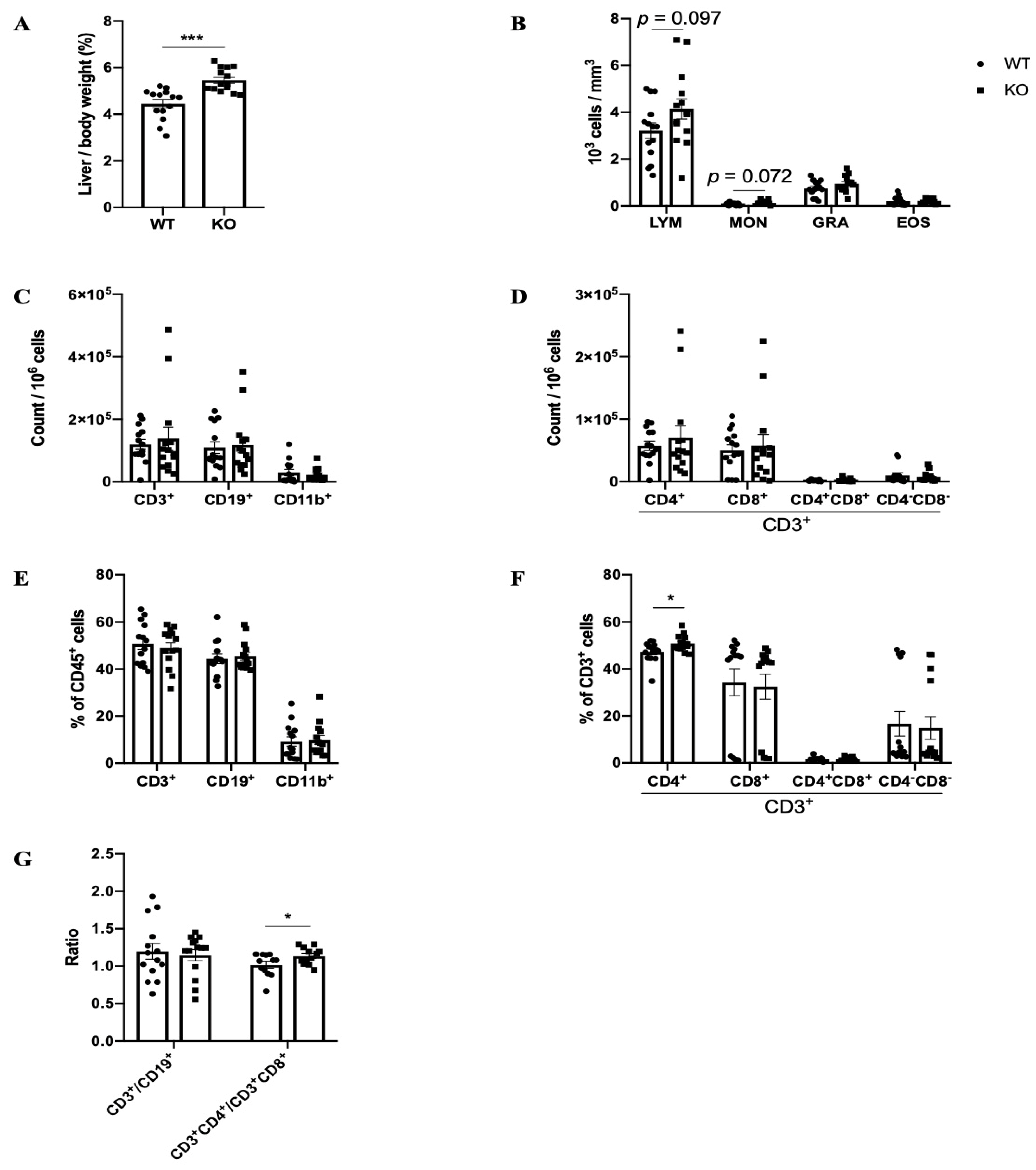
2.1. Phenotypes of Pla1a−/− Mice
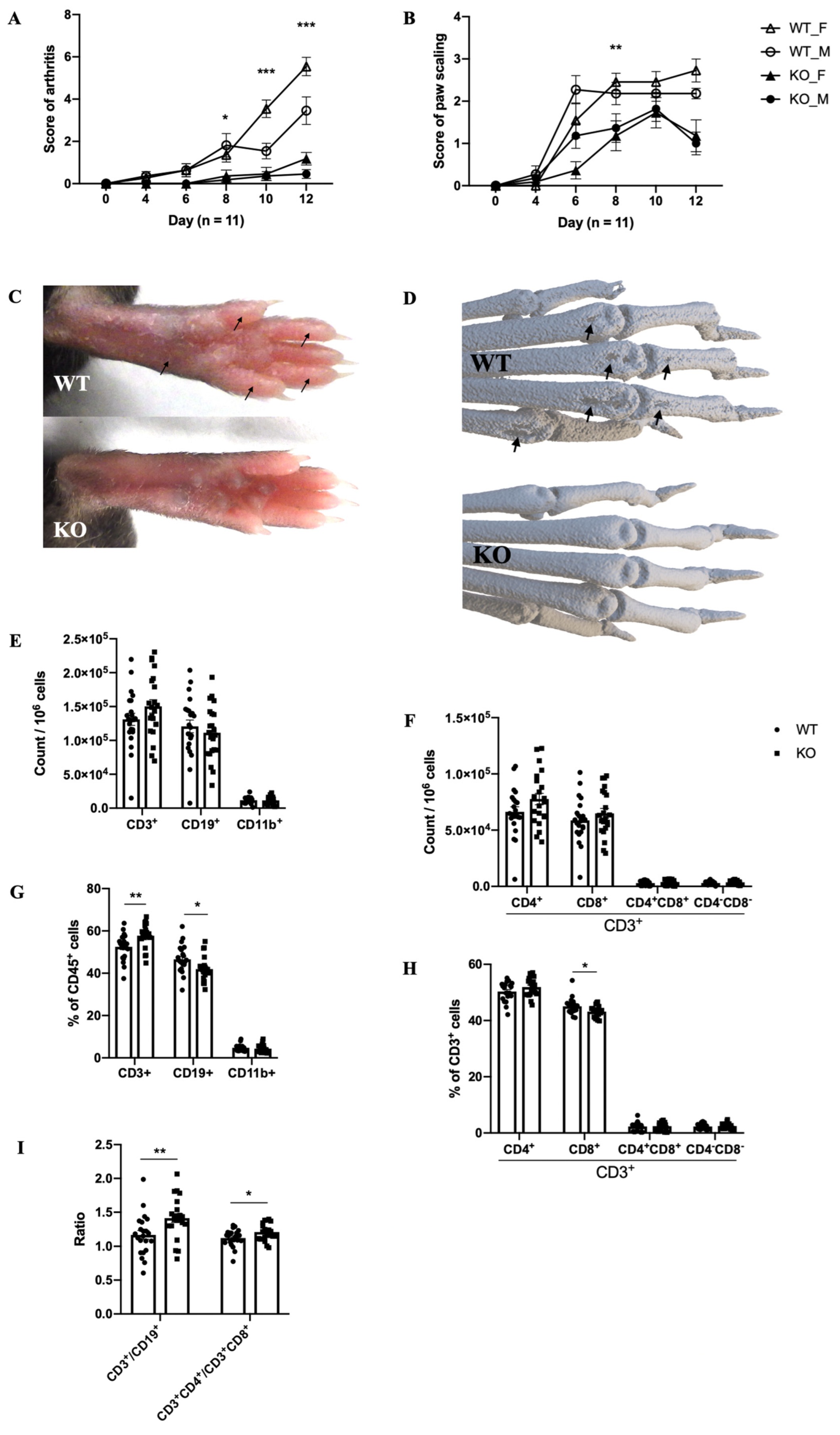
2.2. The Mouse Model of MIP
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Model of MIP
4.3. Immune Cell Sorting
4.4. Luminex
4.5. Micro-Computed Tomography (μCT) Image Acquisition
4.6. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.; Hasse, S.; Bourgoin, S.G. Phosphatidylserine-specific phospholipase A1: A friend or the devil in disguise. Prog. Lipid Res. 2021, 83, 101112. [Google Scholar] [CrossRef] [PubMed]
- Sawada, T.; Kurano, M.; Shirai, H.; Iwasaki, Y.; Tahara, K.; Hayashi, H.; Igarashi, K.; Fujio, K.; Aoki, J.; Yatomi, Y. Serum phosphatidylserine-specific phospholipase A(1) as a novel biomarker for monitoring systemic lupus erythematosus disease activity. Int. J. Rheum. Dis. 2019, 22, 2059–2066. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hasse, S.; Vaillancourt, M.; Zhao, C.; Davis, L.; Boilard, E.; Fortin, P.; Di Battista, J.; Poubelle, P.E.; Bourgoin, S.G. Phospholipase A1 Member A Activates Fibroblast-like Synoviocytes through the Autotaxin-Lysophosphatidic Acid Receptor Axis. Int. J. Mol. Sci. 2021, 22, 12685. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.; Stafford, L.; Bresnihan, B.; FitzGerald, O. A prospective, clinical and radiological study of early psoriatic arthritis: An early synovitis clinic experience. Rheumatology 2003, 42, 1460–1468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, S.; Hayami, Y.; Naniwa, T.; Ueda, R. The Th17/IL-23 Axis and Natural Immunity in Psoriatic Arthritis. Int. J. Rheumatol. 2012, 2012, 539683. [Google Scholar] [CrossRef] [PubMed]
- Hueber, A.J.; McInnes, I.B. Immune regulation in psoriasis and psoriatic arthritis--recent developments. Immunol. Lett. 2007, 114, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Khmaladze, I.; Kelkka, T.; Guerard, S.; Wing, K.; Pizzolla, A.; Saxena, A.; Lundqvist, K.; Holmdahl, M.; Nandakumar, K.S.; Holmdahl, R. Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice. Proc. Natl. Acad. Sci. USA 2014, 111, E3669–E3678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagert, C.; Sareila, O.; Kelkka, T.; Jalkanen, S.; Holmdahl, R. The Macrophage Mannose Receptor Regulate Mannan-Induced Psoriasis, Psoriatic Arthritis, and Rheumatoid Arthritis-Like Disease Models. Front. Immunol. 2018, 9, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, K.K.; Lindgaard, L.M.; Wogensen, L.; Dagnæs-Hansen, F.; Thomsen, J.S.; Sakaguchi, S.; Stengaard-Pedersen, K.; Hauge, E.M. SKG arthritis as a model for evaluating therapies in rheumatoid arthritis with special focus on bone changes. Rheumatol. Int. 2013, 33, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Scholz, T.; Yau, A.C.Y.; Guerard, S.; Hüffmeier, U.; Burkhardt, H.; Holmdahl, R. Mannan-induced Nos2 in macrophages enhances IL-17-driven psoriatic arthritis by innate lymphocytes. Sci. Adv. 2018, 4, eaas9864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skon-Hegg, C.; Zhang, J.; Wu, X.; Sagolla, M.; Ota, N.; Wuster, A.; Tom, J.; Doran, E.; Ramamoorthi, N.; Caplazi, P.; et al. LACC1 Regulates TNF and IL-17 in Mouse Models of Arthritis and Inflammation. J. Immunol. 2019, 202, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Li, Q.; Holmdahl, R. Natural Loss-of-Function Mutations in Qa2 and NCF1 Cause the Spread of Mannan-Induced Psoriasis. J. Investig. Dermatol. 2021, 141, 1765–1771.e1764. [Google Scholar] [CrossRef] [PubMed]
- Colucci, S.; Brunetti, G.; Cantatore, F.P.; Oranger, A.; Mori, G.; Quarta, L.; Cirulli, N.; Mancini, L.; Corrado, A.; Grassi, F.R.; et al. Lymphocytes and synovial fluid fibroblasts support osteoclastogenesis through RANKL, TNFalpha, and IL-7 in an in vitro model derived from human psoriatic arthritis. J. Pathol. 2007, 212, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, P.; Augustín, J.J.; Tamayo, E.; Iglesias, M.; Acinas, O.; Mendiguren, M.A.; Vázquez, J.A.; Genre, F.; San Segundo, D.; Merino, J.; et al. Therapeutic Effects of Anti-Bone Morphogenetic Protein and Activin Membrane-Bound Inhibitor Treatment in Psoriasis and Arthritis. Arthritis Rheumatol. 2020, 72, 1547–1558. [Google Scholar] [CrossRef] [PubMed]
- Ritchlin, C.T.; Haas-Smith, S.A.; Li, P.; Hicks, D.G.; Schwarz, E.M. Mechanisms of TNF-α- and RANKL-mediated osteoclastogenesis and bone resorption in psoriatic arthritis. J. Clin. Investig. 2003, 111, 821–831. [Google Scholar] [CrossRef] [PubMed]
| Paw Lysate (pg/mL, n = 5) | Paw Lysate (pg/mL, n = 5) | Plasma (pg/mL, n = 5) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control Mice | MIP | MIP | |||||||
| WT | KO | p Value | WT | KO | p Value | WT | KO | p Value | |
| Eotaxin | 72.97 ± 8.85 | 106.22 ± 64.79 | 0.339 | 176.10 ± 27.21 | 132.03 ± 11.59 | 0.018 | 238.52 ± 39.65 | 288.77 ± 26.23 | 0.067 |
| G-CSF | 6.60 ± 0.56 | 9.78 ± 3.19 | 0.085 | 152.47 ± 62.87 | 73.30 ± 28.62 | 0.051 | 355.06 ± 66.26 | 295.83 ± 64.08 | 0.235 |
| GM-CSF | 11.60 ± 0.88 | 12.13 ± 1.75 | 0.602 | 21.74 ± 1.94 | 26.24 ± 8.30 | 0.322 | 32.60 ± 3.45 | 33.89 ± 2.69 | 0.573 |
| IFNγ | 4.11 ± 0.93 | 3.43 ± 0.90 | 0.321 | 2.36 ± 0.31 | 2.81 ± 0.44 | 0.140 | 11.57 ± 4.20 | 15.63 ± 8.90 | 0.433 |
| IL-1α | 1298.44 ± 206.51 | 1745.13 ± 567.59 | 0.177 | 1586.86 ± 302.61 | 2259.35 ± 546.18 | 0.063 | 128.78 ± 22.24 | 146.74 ± 26.17 | 0.326 |
| IL-1β | 9.66 ± 0.90 | 9.02 ± 1.21 | 0.417 | 23.04 ± 5.52 | 21.38 ± 7.29 | 0.725 | 12.78 ± 0.54 | 16.82 ± 10.01 | 0.443 |
| IL-2 | 7.52 ± 2.80 | 5.35 ± 1.97 | 0.241 | 3.55 ± 0.23 | 3.39 ± 0.48 | 0.582 | 29.44 ± 8.83 | 29.93 ± 16.02 | 0.959 |
| IL-3 | 1.85 ± 0.11 | 1.79 ± 0.14 | 0.552 | 8.69 ± 11.07 | 3.34 ± 2.41 | 0.372 | 3.11 ± 0.43 | 3.80 ± 1.62 | 0.439 |
| IL-4 | 0.54 ± 0.13 | 0.50 ± 0.09 | 0.650 | 0.83 ± 0.58 | 0.64 ± 0.15 | 0.541 | 0.82 ± 0.11 | 1.09 ± 0.35 | 0.179 |
| IL-5 | 3.43 ± 1.90 | 3.74 ± 2.06 | 0.828 | 2.13 ± 0.14 | 2.41 ± 0.79 | 0.500 | 7.04 ± 1.53 | 5.97 ± 1.27 | 0.311 |
| IL-6 | 6.05 ± 0.36 | 5.58 ± 1.83 | 0.623 | 20.88 ± 5.07 | 14.67 ± 10.82 | 0.329 | 5.35 ± 2.48 | 5.66 ± 2.29 | 0.856 |
| IL-7 | 7.92 ± 0.75 | 6.62 ± 0.62 | 0.028 | 7.00 ± 0.75 | 5.61 ± 0.50 | 0.015 | 20.22 ± 11.64 | 9.37 ± 1.32 | 0.101 |
| IL-9 | 24.23 ± 1.19 | 22.58 ± 2.01 | 0.196 | 21.11 ± 1.02 | 19.35 ± 1.09 | 0.046 | 83.84 ± 30.47 | 69.96 ± 7.32 | 0.402 |
| IL-10 | 26.15 ± 3.70 | 20.18 ± 7.17 | 0.177 | 19.86 ± 8.83 | 13.93 ± 4.41 | 0.264 | 25.12 ± 2.64 | 25.44 ± 6.10 | 0.925 |
| IL-12p40 | 44.37 ± 4.56 | 34.16 ± 7.92 | 0.054 | 31.02 ± 6.35 | 29.32 ± 1.87 | 0.620 | 24.97 ± 2.85 | 27.10 ± 2.25 | 0.274 |
| IL-12p70 | 16.47 ± 1.41 | 15.87 ± 1.19 | 0.532 | 26.65 ± 13.31 | 19.73 ± 4.60 | 0.354 | 36.88 ± 7.88 | 51.29 ± 9.54 | 0.048 |
| IL-13 | 3.49 ± 1.49 | 3.18 ± 0.85 | 0.725 | 2.98 ± 0.81 | 2.11 ± 0.42 | 0.095 | 204.48 ± 37.72 | 217.22 ± 59.07 | 0.725 |
| IL-15 | 40.84 ± 6.33 | 46.98 ± 3.24 | 0.123 | 41.18 ± 3.37 | 36.88 ± 3.62 | 0.120 | 228.26 ± 132.86 | 135.21 ± 13.93 | 0.201 |
| IL-17 | 1.12 ± 0.17 | 1.12 ± 0.54 | 0.989 | 84.83 ± 33.40 | 26.87 ± 15.76 | 0.014 | 3.83 ± 1.04 | 3.70 ± 1.19 | 0.875 |
| IP-10 | 2.41 ± 0.44 | 2.75 ± 0.75 | 0.456 | 7.62 ± 1.20 | 4.33 ± 1.04 | 0.003 | 70.23 ± 25.89 | 51.20 ± 10.47 | 0.210 |
| KC | 29.40 ± 4.74 | 35.14 ± 13.30 | 0.440 | 136.06 ± 20.35 | 82.05 ± 29.22 | 0.016 | 25.48 ± 6.42 | 23.39 ± 11.65 | 0.761 |
| LIF | 9.62 ± 1.32 | 11.01 ± 0.61 | 0.093 | 25.66 ± 5.20 | 14.76 ± 1.98 | 0.004 | 8.50 ± 10.72 | 2.49 ± 0.80 | 0.296 |
| MCP-1 | 78.25 ± 16.37 | 113.02 ± 49.95 | 0.222 | 229.29 ± 129.40 | 139.90 ± 76.34 | 0.268 | 45.41 ± 5.00 | 62.20 ± 41.74 | 0.448 |
| M-CSF | 21.42 ± 4.50 | 20.41 ± 3.21 | 0.724 | 16.24 ± 2.10 | 15.91 ± 3.43 | 0.872 | 38.51 ± 2.67 | 36.84 ± 2.66 | 0.403 |
| MIG | 5.99 ± 3.50 | 6.83 ± 3.54 | 0.745 | 19.63 ± 8.05 | 12.96 ± 3.61 | 0.169 | 259.55 ± 35.17 | 607.34 ± 371.66 | 0.099 |
| MIP-1α | 27.90 ± 1.47 | 28.19 ± 1.88 | 0.810 | 70.12 ± 10.76 | 58.56 ± 12.08 | 0.191 | 136.01 ± 17.20 | 130.96 ± 27.26 | 0.762 |
| MIP-1β | 18.18 ± 4.50 | 17.24 ± 3.04 | 0.739 | 44.31 ± 6.53 | 32.14 ± 7.34 | 0.038 | 95.25 ± 10.97 | 101.15 ± 27.23 | 0.698 |
| MIP-2 | 180.89 ± 11.94 | 167.39 ± 26.58 | 0.381 | 1049.48 ± 301.44 | 859.24 ± 304.88 | 0.401 | 733.26 ± 3.52 | 735.67 ± 2.42 | 0.292 |
| RANTES | 1.14 ± 0.15 | 1.22 ± 0.22 | 0.576 | 2.97 ± 1.24 | 2.21 ± 0.64 | 0.308 | 21.92 ± 3.13 | 26.81 ± 7.59 | 0.268 |
| TNF-α | 3.41 ± 0.30 | 3.40 ± 0.60 | 0.991 | 10.90 ± 2.52 | 9.16 ± 2.77 | 0.380 | 18.17 ± 1.28 | 19.83 ± 3.82 | 0.433 |
| VEGF | 21.07 ± 1.71 | 24.68 ± 5.73 | 0.261 | 75.54 ± 7.03 | 54.11 ± 13.11 | 0.020 | 1.48 ± 0.41 | 1.37 ± 0.20 | 0.658 |
| LIX | 110.40 ± 52.50 | 85.28 ± 65.33 | 0.565 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Aoudjit, F.; Bourgoin, S.G. Phospholipase A1 Member A Deficiency Alleviates Mannan-Induced Psoriatic Arthritis in Mice Model. Int. J. Mol. Sci. 2022, 23, 8559. https://doi.org/10.3390/ijms23158559
Zhao Y, Aoudjit F, Bourgoin SG. Phospholipase A1 Member A Deficiency Alleviates Mannan-Induced Psoriatic Arthritis in Mice Model. International Journal of Molecular Sciences. 2022; 23(15):8559. https://doi.org/10.3390/ijms23158559
Chicago/Turabian StyleZhao, Yang, Fawzi Aoudjit, and Sylvain G. Bourgoin. 2022. "Phospholipase A1 Member A Deficiency Alleviates Mannan-Induced Psoriatic Arthritis in Mice Model" International Journal of Molecular Sciences 23, no. 15: 8559. https://doi.org/10.3390/ijms23158559
APA StyleZhao, Y., Aoudjit, F., & Bourgoin, S. G. (2022). Phospholipase A1 Member A Deficiency Alleviates Mannan-Induced Psoriatic Arthritis in Mice Model. International Journal of Molecular Sciences, 23(15), 8559. https://doi.org/10.3390/ijms23158559








