Photofunctions in Hybrid Systems of Schiff Base Metal Complexes and Metal or Semiconductor (Nano)Materials
Abstract
1. Introduction
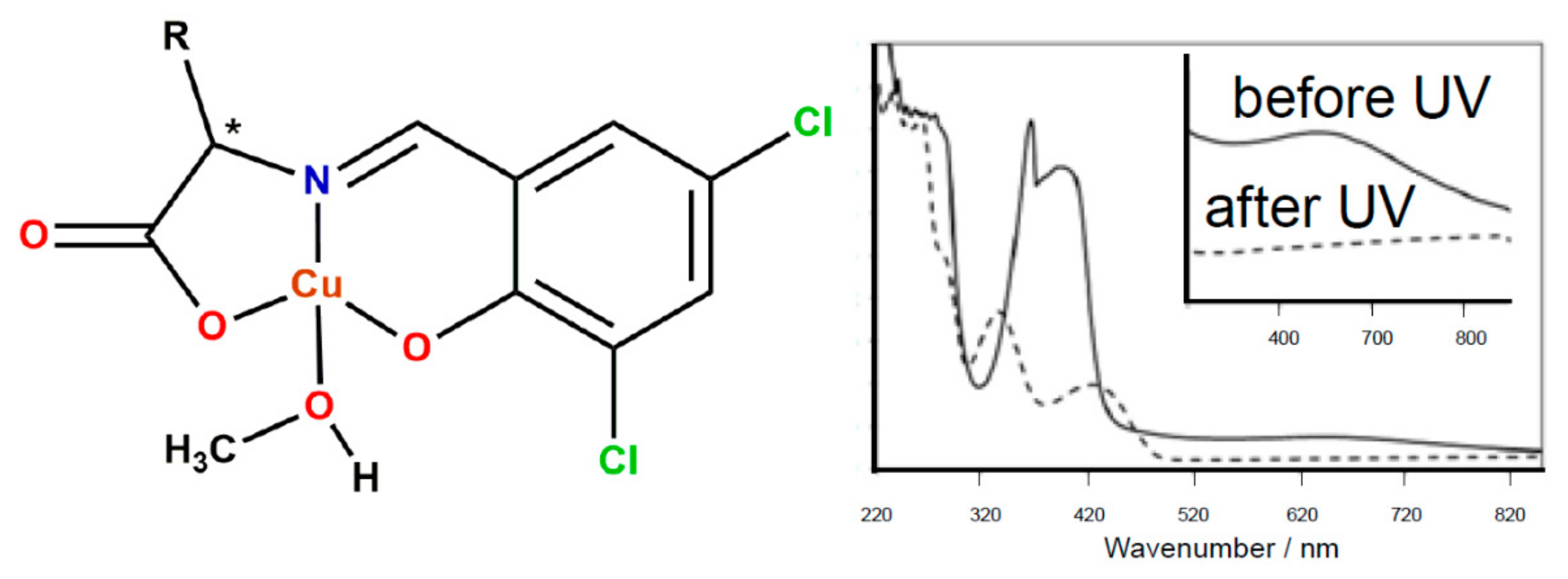
2. Photocatalysis—UV-Induced Reduction of Cu(II) Complexes by TiO2
3. Sensitization in Solar Cells (DSSC)
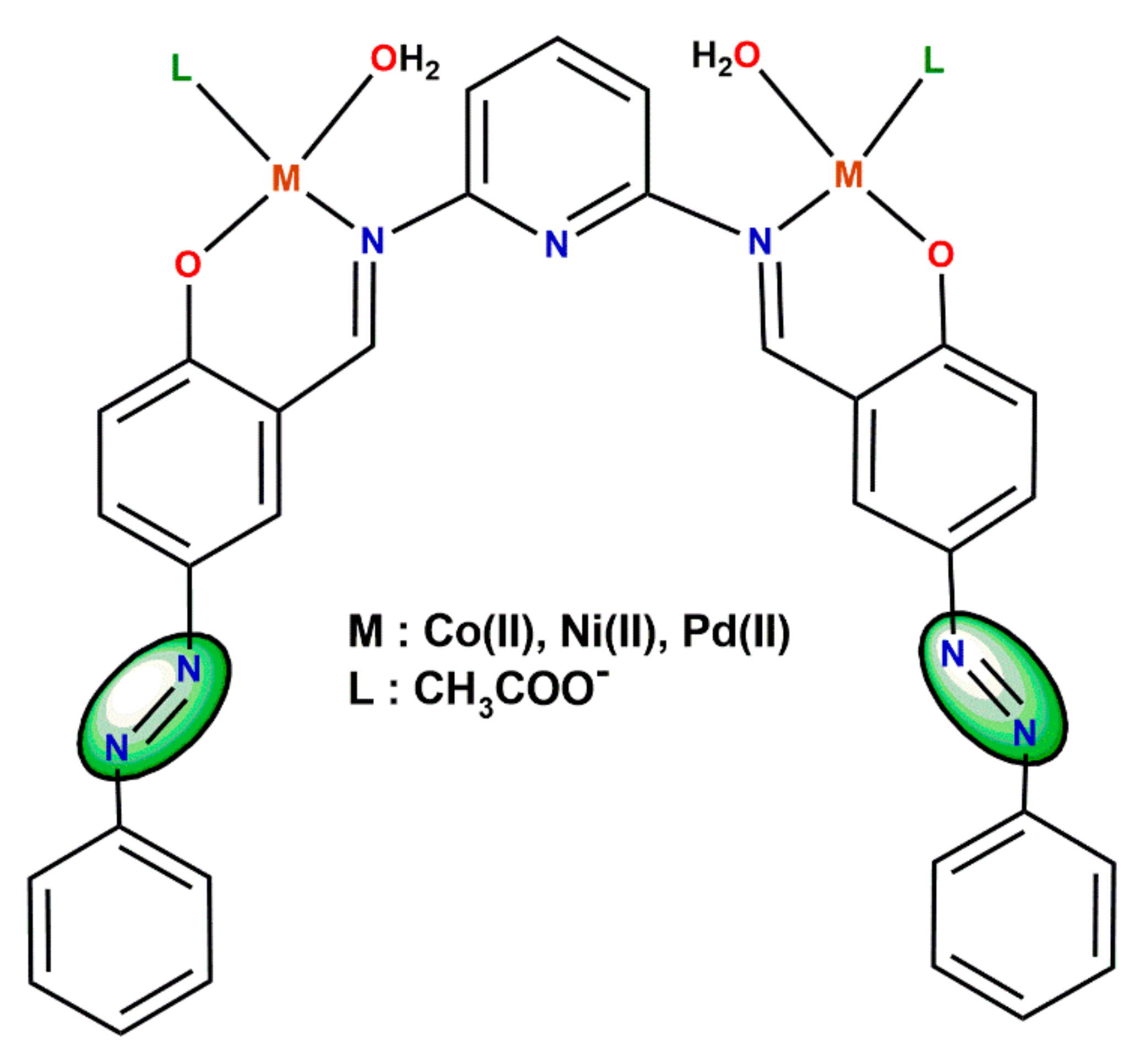
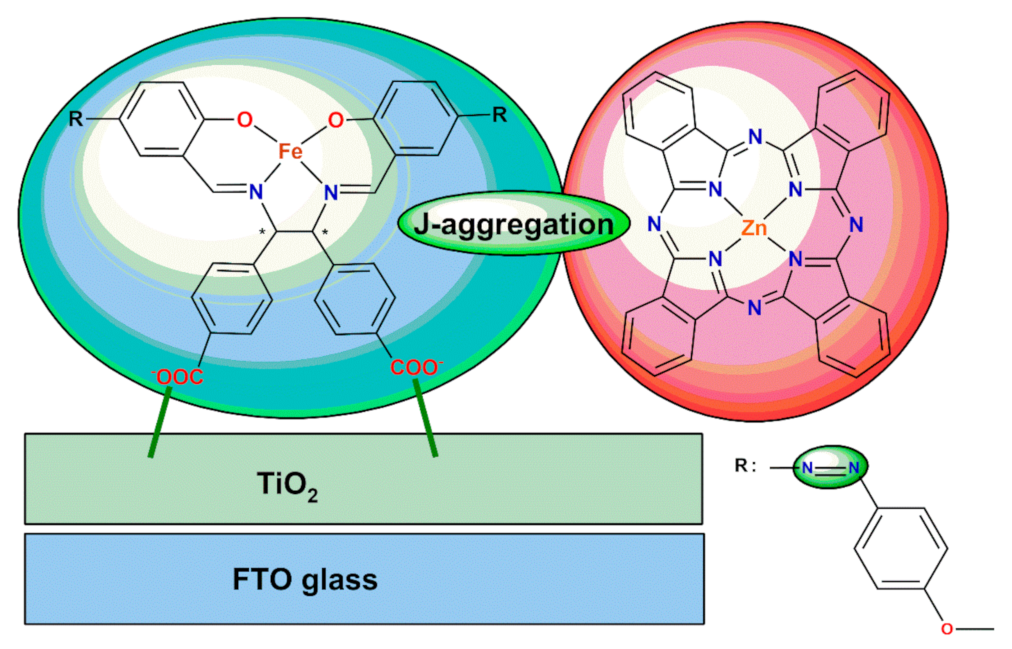
4. Co-Sensitization Using the Schiff Base Metal Complexes
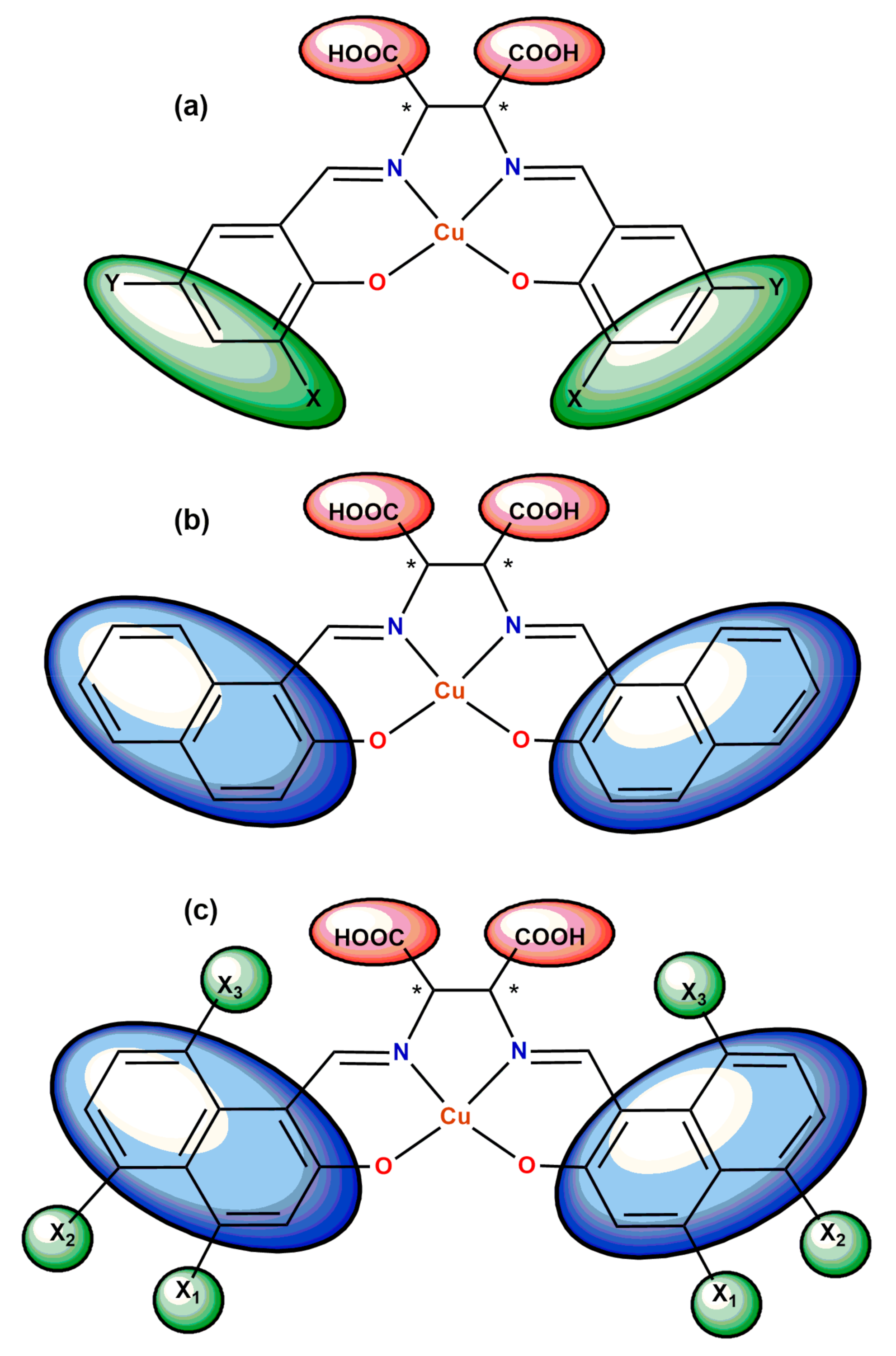
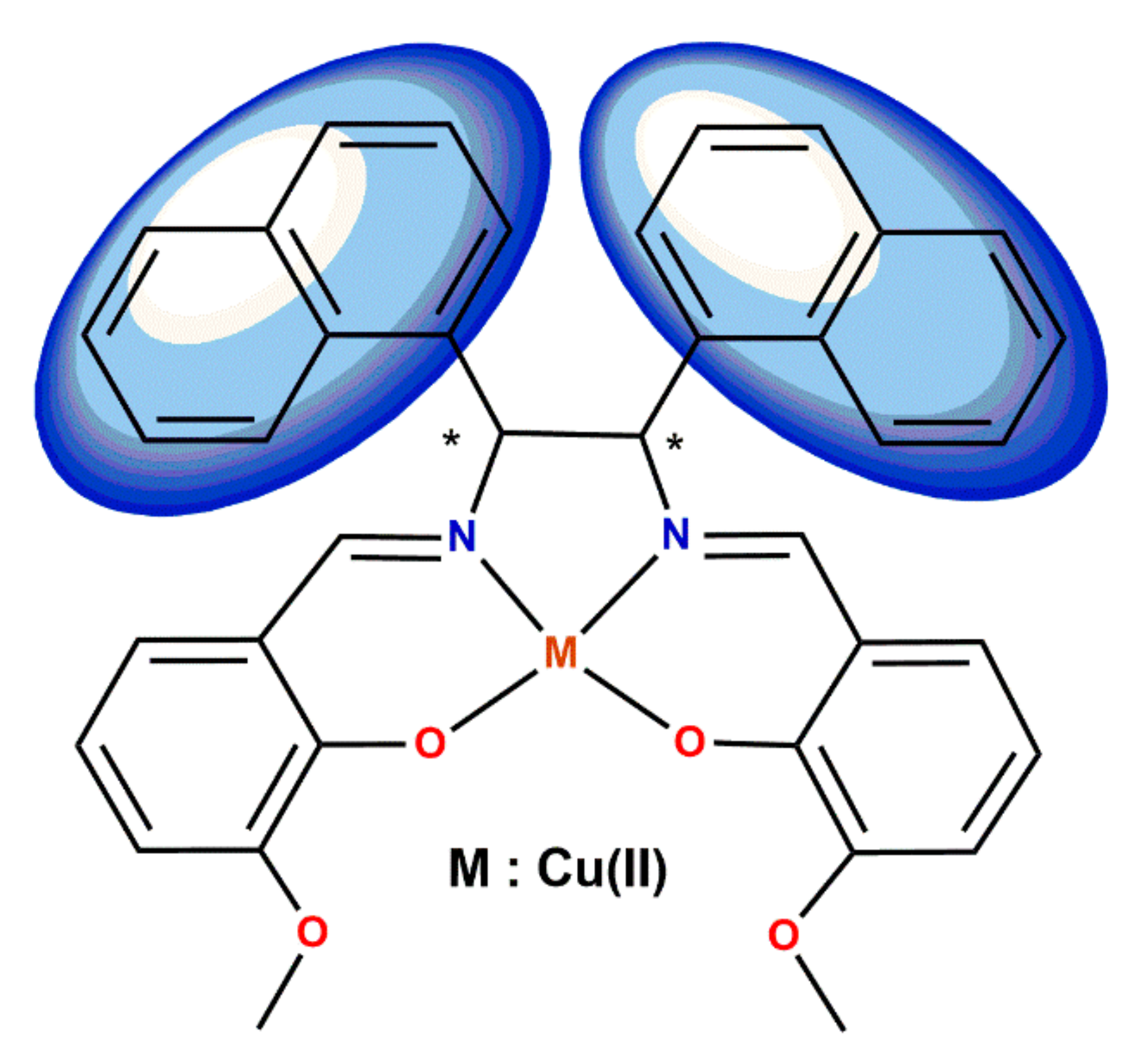
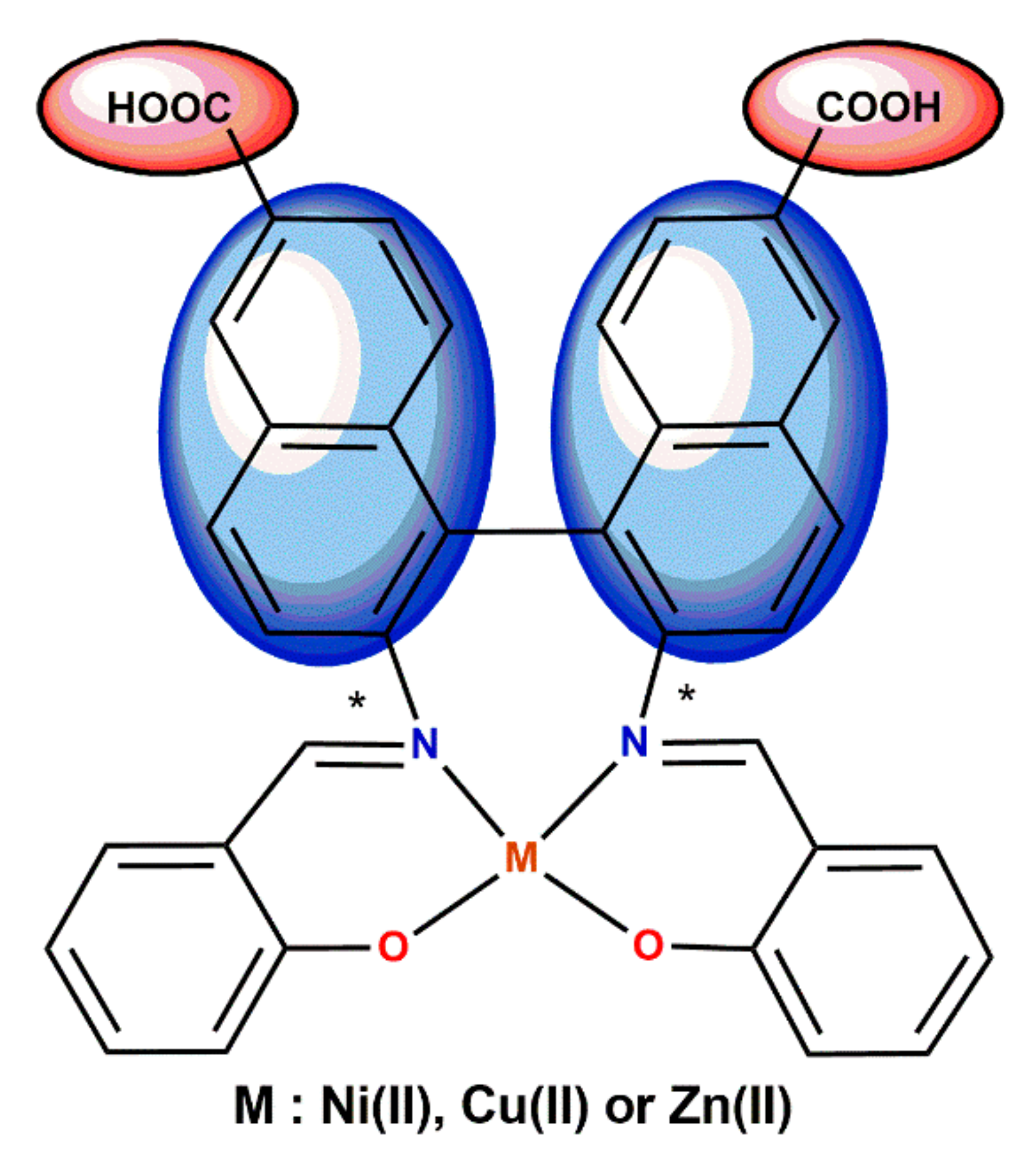
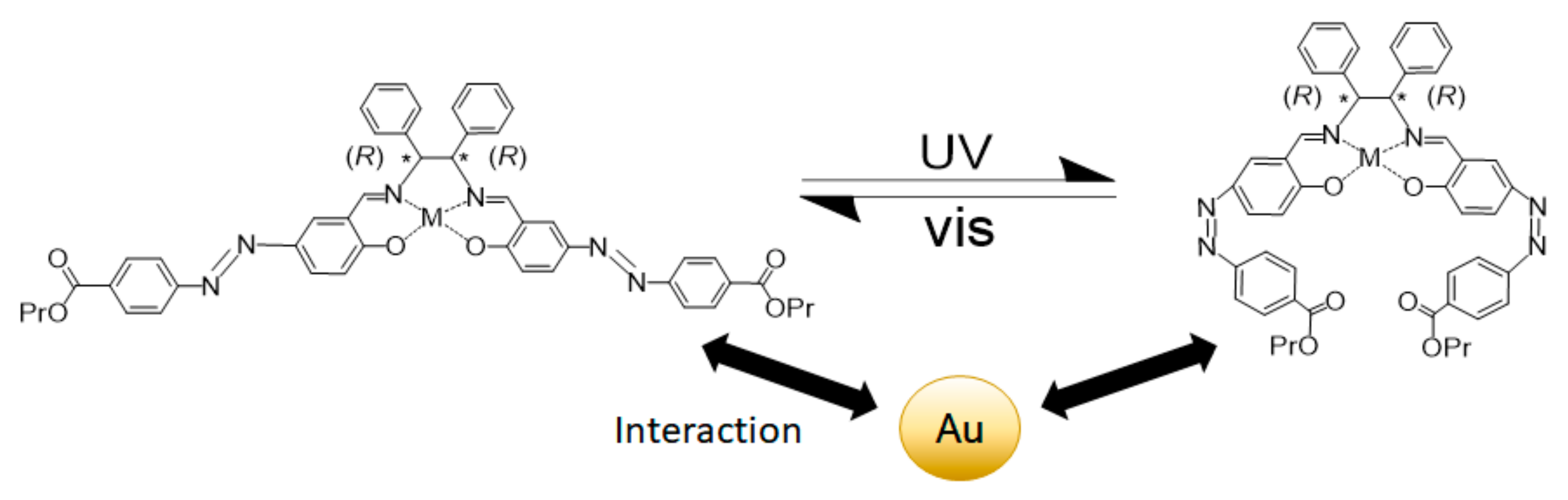
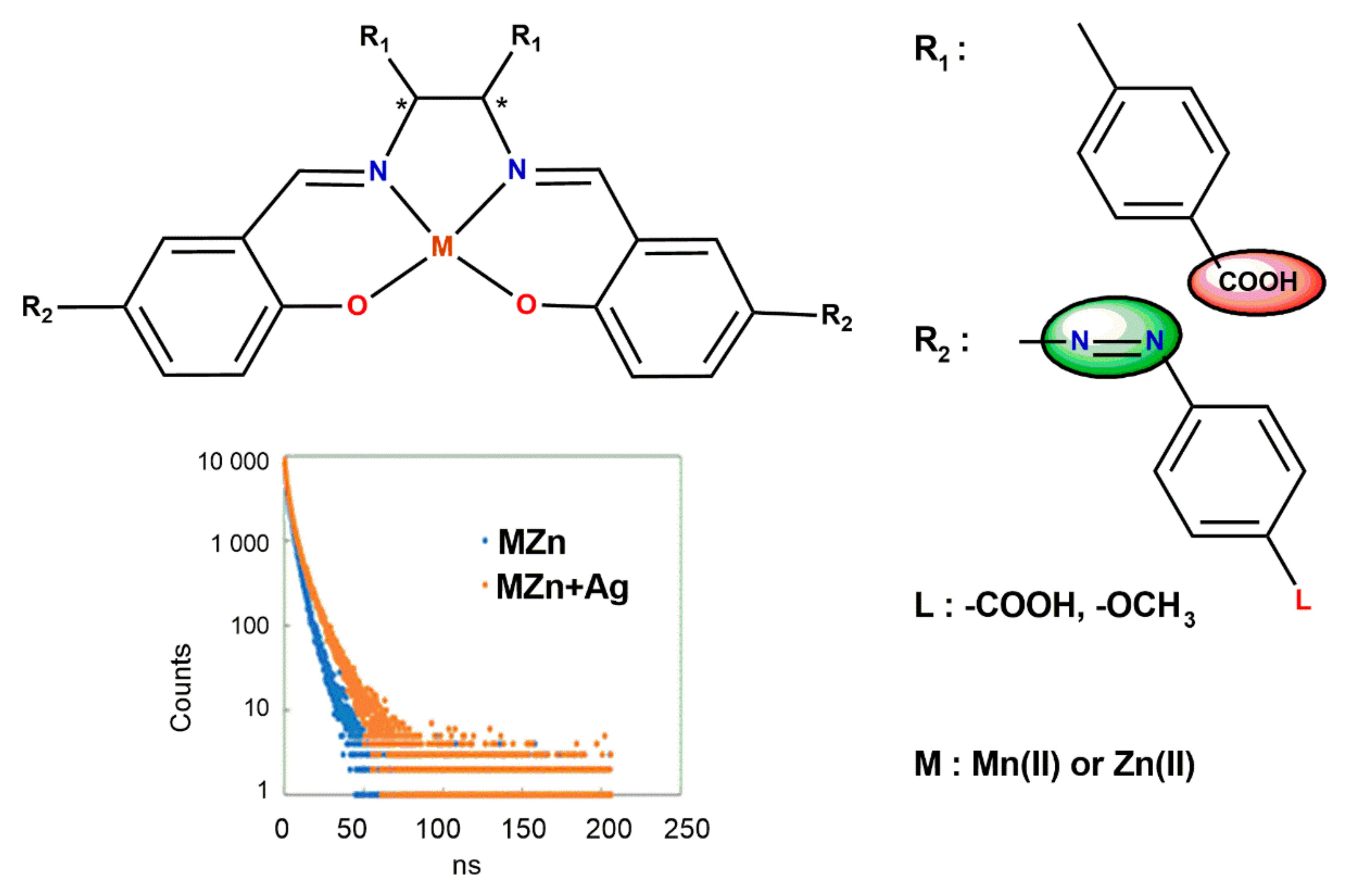
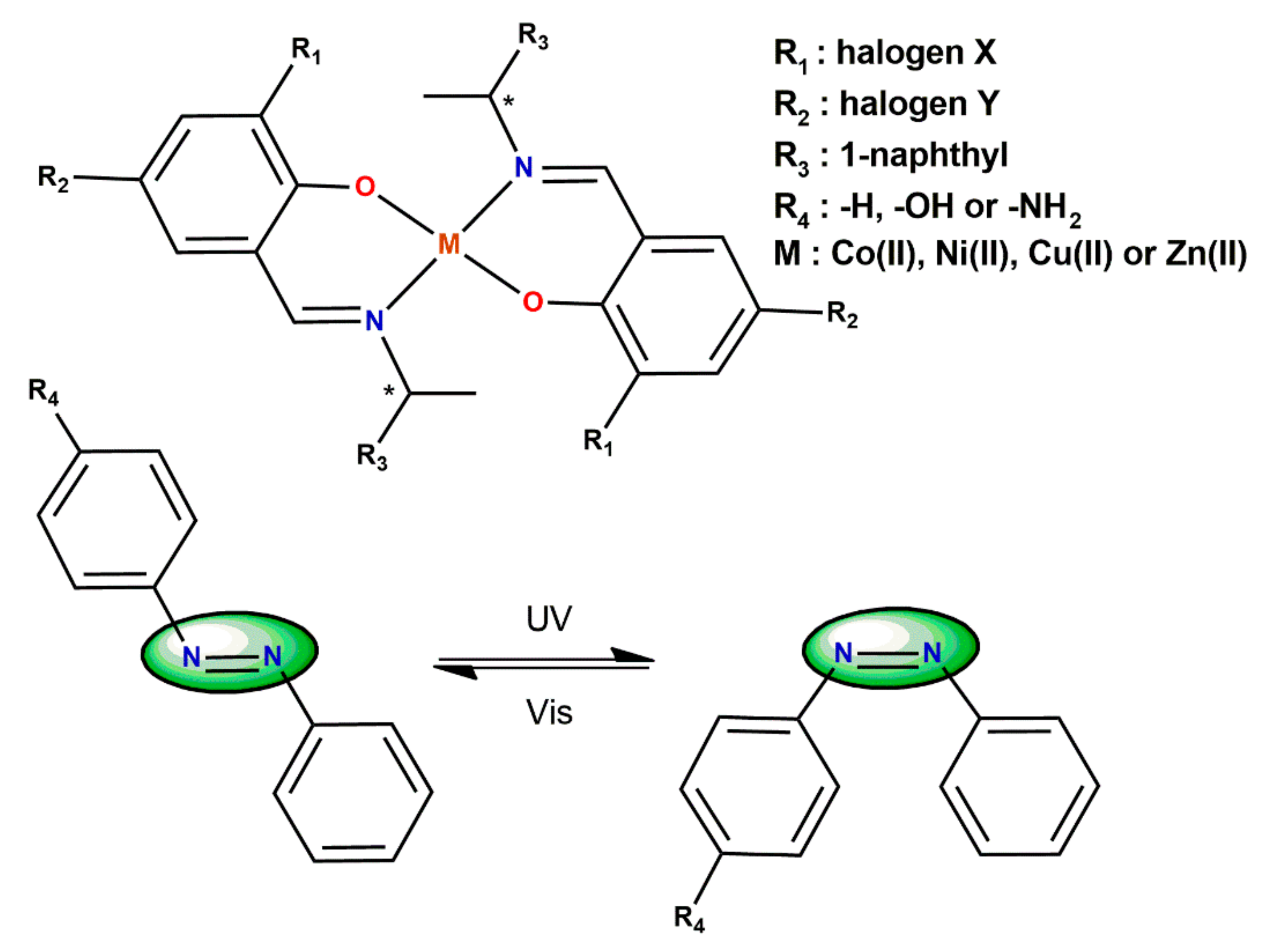
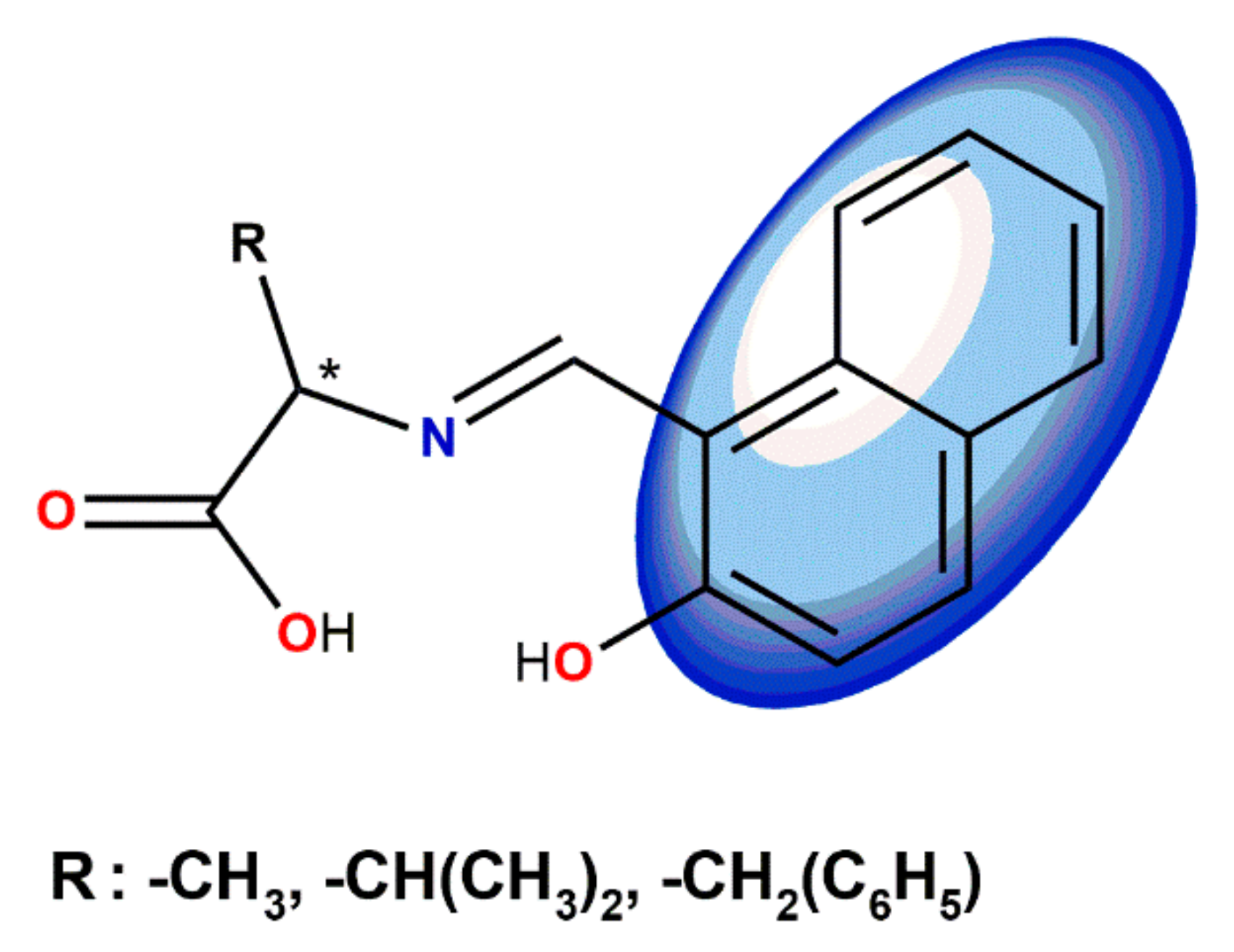
5. Ligand-Induced Chirality in Chiral Schiff Base Complex @ Nanoparticles Composites
6. Chiral Schiff Base Complexes @ Polymer Matrix
7. Chiral Schiff Base Complexes @ Metal-Dendrimer Matrices
8. Applications in the Environmental Protection—The Reduction of Cr(VI) to Cr(III) by TiO2 Photocatalyst in the Presence of Schiff Base Transition Metal Complexes
9. Applications in Cosmetology—Schiff Base Complexes as Sunscreens
10. Real-Time Visualization of Cellular Processes—QDs Doped with Schiff Base Complex for Real-Time Visualization of Cell Membrane Damages
11. Schiff Base Complexes @ CdTe QDs as Cytocompatible Fluorophores for Bio-Labeling
12. Light Activated Prodrug Applications
13. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CD | Circular Dichroism |
| IR | Infrared |
| IR-FEL | Infrared Free Electron Laser |
| PAMAM | poly(amido-amine) (PAMAM) dendrimer |
| PET | Photoinduced Electron Transfer |
| QDs | quantum dots |
References
- Yang, X.; Wang, D. Photocatalysis: From Fundamental Principles to Materials and Applications. ACS Appl. Energy Mater. 2018, 1, 6657–6693. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Updates on the Roadmap for Photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Quan, Q.; Zhang, N.; Xu, Y.-J. Multifarious roles of carbon quantum dots in heterogeneous photocatalysis. J. Energy Chem. 2016, 25, 927–935. [Google Scholar] [CrossRef]
- Sharma, S.; Umar, A.; Sood, S.; Mehta, S.K.; Kansal, S.K. Photoluminescent C-dots: An overview on the recent development in the synthesis, physiochemical properties and potential applications. J. Alloys Compd. 2018, 748, 818–853. [Google Scholar] [CrossRef]
- Pal, A.; Sk, M.P.; Chattopadhyay, A. Recent advances in crystalline carbon dots for superior application potential. Mater. Adv. 2020, 1, 525–553. [Google Scholar] [CrossRef]
- Hazaraimi, M.H.; Goh, P.S.; Lau, W.J.; Ismail, A.F.; Wu, Z.; Subramaniam, M.N.; Lim, J.W.; Kanakaraju, D. The state-of-the-art development of photocatalysts for the degradation of persistent herbicides in wastewater. Sci. Total Environ. 2022, 843, 156975. [Google Scholar] [CrossRef]
- Liu, X.; Hamon, J.-R. Recent developments in penta-, hexa- and heptadentate Schiff base ligands and their metal complexes. Coord. Chem. Rev. 2019, 389, 94–118. [Google Scholar] [CrossRef]
- Mehrabian, M.; Dalir, S.; Mahmoudi, G.; Miroslaw, B.; Babashkina, M.G.; Dektereva, A.V.; Safin, D.A. A Highly Stable All-Inorganic CsPbBr3 Perovskite Solar Cell. Eur. J. Inorg. Chem. 2019, 2019, 3699–3703. [Google Scholar] [CrossRef]
- Hu, H.; Wang, L.; Wang, L.; Li, L.; Feng, S. Imine-functionalized polysiloxanes for supramolecular elastomers with tunable mechanical properties. Polym. Chem. 2020, 11, 7721–7728. [Google Scholar] [CrossRef]
- González, D.M.; Hernández, L.A.; Oyarce, J.; Alfaro, A.; Novoa, N.; Cisterna, J.; Brito, I.; Carrillo, D.; Manzur, C. A new and efficient high-performance electrochemical glucose sensor based on a metallopolymer derived from a cobaltate (III) Schiff base complex. Synth. Met. 2021, 271, 116633. [Google Scholar] [CrossRef]
- Li, N.; Kang, G.; Liu, H.; Li, M.; Qiu, W.; Wang, Q.; Liu, L.; Yu, J.; Li, B.; Li, F.; et al. Hybrid nanoparticles based on novel Schiff Base for durable flame retardant and antibacterial properties. Compos. Part B Eng. 2022, 238, 109905. [Google Scholar] [CrossRef]
- Deng, B.B.; Cheng, T.T.; Hu, Y.T.; Cheng, S.P.; Huang, C.R.; Yu, H.; Wang, Z.X. The first salicylaldehyde Schiff base organic-inorganic hybrid lead iodide perovskite ferroelectric. Chem. Commun. 2022, 58, 2192–2195. [Google Scholar] [CrossRef]
- Afrin Dalia, S.; Farhana Afsan, B.; Md Saddam Hossain, B.; Nuruzzaman Khan, M.; Md Kudrat-E-Zahan, B.; Md Mahasin Ali, B.; Md Kudrat-E-Zahan, C.; Afsan, F.; Saddam Hossain, M.; Zakaria, C.; et al. A short review on chemistry of schiff base metal complexes and their catalytic application. Int. J. Chem. Stud. 2018, 6, 2859–2866. [Google Scholar]
- Radecka-Paryzek, W. Self-assembly in schiff base lanthanide complexes—From supramolecular dimers to coordination polymers. Can. J. Chem. 2009, 87, 1–7. [Google Scholar] [CrossRef]
- Liu, X.; Manzur, C.; Novoa, N.; Celedón, S.; Carrillo, D.; Hamon, J.-R.R. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Tsantis, S.T.; Tzimopoulos, D.I.; Holynska, M.; Perlepes, S.P. Oligonuclear Actinoid Complexes with Schiff Bases as Ligands—Older Achievements and Recent Progress. Int. J. Mol. Sci. 2020, 21, 555. [Google Scholar] [CrossRef]
- Miroslaw, B. Homo-and hetero-oligonuclear complexes of platinum group metals (PGM) coordinated by imine Schiff base ligands. Int. J. Mol. Sci. 2020, 21, 3493. [Google Scholar] [CrossRef]
- Sztanke, K.; Maziarka, A.; Osinka, A.; Sztanke, M. An insight into synthetic Schiff bases revealing antiproliferative activities in vitro. Bioorganic Med. Chem. 2013, 21, 3648–3666. [Google Scholar] [CrossRef]
- Raczuk, E.; Dmochowska, B.; Samaszko-Fiertek, J.; Madaj, J. Different Schiff Bases—Structure, Importance and Classification. Molecules 2022, 27, 787. [Google Scholar] [CrossRef]
- Catalano, A.; Sinicropi, M.S.; Iacopetta, D.; Ceramella, J.; Mariconda, A.; Rosano, C.; Scali, E.; Saturnino, C.; Longo, P. A review on the advancements in the field of metal complexes with schiff bases as antiproliferative agents. Appl. Sci. 2021, 11, 6027. [Google Scholar] [CrossRef]
- Takeshita, Y.; Takakura, K.; Akitsu, T. Multifunctional composites of chiral valine derivative Schiff base Cu(II) complexes and TiO2. Int. J. Mol. Sci. 2015, 16, 3955–3969. [Google Scholar] [CrossRef]
- Yamamoto, S.; Akitsu, T. Fluorescence detection by photochromic dye of photoinduced electron transfer reactions between lysine and methionine derivative Schiff base copper(II) complexes and titanium oxide. Asian Chem. Lett. 2011, 15, 203–209. [Google Scholar]
- Kurata, M.; Yoshida, N.; Fukunaga, S.; Akitsu, T. Proposed Mechanism of Photo-Induced Reactions of Chiral Threonine Schiff Base Cu(II) Complexes with Imidazole by TiO 2. Contemp. Eng. Sci. 2013, 6, 255–260. [Google Scholar] [CrossRef]
- Takeshita, Y.; Nogami, A.; Akitsu, T. UV light-induced reaction of chiral arginine derivative-Schiff base Cu(II) complexes and TiO2. World Sci. Echo 2014, 1, 20–23. [Google Scholar]
- Takeshita, Y.; Akitsu, T. Multi-Functional Composites and Substituent Effects of Chiral Asparagine-Schiff Base Cu(II) Complexes and Titanium(IV) Oxide. Pure Appl. Chem. Sci. 2015, 3, 11–17. [Google Scholar] [CrossRef]
- Nishizuru, H.; Kimura, N.; Akitsu, T. Photo-induced reduction of hybrid systems of phenylalanine and other derivatives of Schiff base Cu(II) complexes and titanium(IV) oxide. Asian Chem. Lett. 2012, 16, 33–42. [Google Scholar]
- Nakayama, T.; Minemoto, M.; Nishizuru, H.; Akitsu, T. Spectroelectrochemistry of photoinduced electron transfer reactions between leucine and serine derivative Schiff base copper(II) complexes and titanium Oxide. Asian Chem. Lett. 2011, 15, 215–219. [Google Scholar]
- Yoshida, N.; Akitsu, T. Reaction of Hybrid Systems Composed of Cu(II) Complexes Having Chiral Schiff Base Amino-Acid Ester Derivatives and TiO2. In Integrating Approach to Photofunctional Hybrid Materials for Energy and the Environment; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 111–124. ISBN 978-1624176388. [Google Scholar]
- Gong, J.; Sumathy, K.; Qiao, Q.; Zhou, Z. Review on dye-sensitized solar cells (DSSCs): Advanced techniques and research trends. Renew. Sustain. Energy Rev. 2017, 68, 234–246. [Google Scholar] [CrossRef]
- Francis, O.I.; Ikenna, A.; Francis, O.I.; Ikenna, A. Review of Dye-Sensitized Solar Cell (DSSCs) Development. Nat. Sci. 2021, 13, 496–509. [Google Scholar] [CrossRef]
- Baby, R.; Nixon, P.D.; Kumar, N.M.; Subathra, M.S.P.; Ananthi, N. A comprehensive review of dye-sensitized solar cell optimal fabrication conditions, natural dye selection, and application-based future perspectives. Environ. Sci. Pollut. Res. Int. 2022, 29, 371–404. [Google Scholar] [CrossRef]
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced research trends in dye-sensitized solar cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Tsunoda, Y.; Tanaka, S.; Haraguchi, T.; Sugiyama, M.; Noor, S.; Akitsu, T. Orbital and molecular design of new naphthyl-salen type transition metal complexes toward DSSC dyes. J. Indian Chem. Soc. 2017, 94, 761–772. [Google Scholar]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Mahadevi, P.; Sumathi, S. Mini review on the performance of Schiff base and their metal complexes as photosensitizers in dye-sensitized solar cells. Synth. Commun. 2020, 50, 2237–2249. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Takahashi, K.; Akitsu, T. Molecular design through TD-DFT calculation of chiral salen Cu II complexes toward NIR absorption for DSSC. J. Indian Chem. Soc 2016, 93, 921–927. [Google Scholar]
- Cappel, U.B.; Karlsson, M.H.; Pschirer, N.G.; Eickemeyer, F.; Schöneboom, J.; Erk, P.; Boschloo, G.; Hagfeldt, A. A broadly absorbing perylene dye for solid-state dye-sensitized solar cells. J. Phys. Chem. C 2009, 113, 14595–14597. [Google Scholar] [CrossRef]
- Milan, R.; Selopal, G.S.; Cavazzini, M.; Orlandi, S.; Boaretto, R.; Caramori, S.; Concina, I.; Pozzi, G. Dye-sensitized solar cells based on a push-pull zinc phthalocyanine bearing diphenylamine donor groups: Computational predictions face experimental reality. Sci. Rep. 2017, 7, 15675. [Google Scholar] [CrossRef]
- Ragoussi, M.E.; Ince, M.; Torres, T. Recent Advances in Phthalocyanine-Based Sensitizers for Dye-Sensitized Solar Cells. European J. Org. Chem. 2013, 2013, 6475–6489. [Google Scholar] [CrossRef]
- Yamane, S.; Hiyoshi, Y.; Tanaka, S.; Ikenomoto, S.; Numata, T.; Takakura, K.; Haraguchi, T.; Palafox, M.A.; Hara, M.; Sugiyama, M.; et al. Substituent Effect of Chiraldiphenyl Salen Metal (M = Fe(II), Co(II), Ni(II), Cu(II), Zn(II)) Complexes for New Conceptual DSSC Dyes. J. Chem. Chem. Eng. 2018, 11, 135–151. [Google Scholar]
- Takahashi, K.; Tanaka, S.; Yamaguchi, M.; Tsunoda, Y.; Akitsu, T.; Sugiyama, M.; Soni, R.K.; Moon, D. Dual purpose Br-containing Schiff base Cu(II) complexes for DSSC dyes and polymer flame retardants. J. Korean Chem. Soc. 2017, 61, 129–131. [Google Scholar]
- Matsuno, M.; Noor, S.; Numata, T.; Haraguchi, T.; Akitsu, T.; Hara, M. Synthesis and structural characterization of new [CuII-TiO2] composites from CuII-salen as precursors. J. Indian Chem. Soc. 2017, 94, 1089–1098. [Google Scholar]
- Tsaturyan, A.; Machida, Y.; Akitsu, T.; Gozhikova, I.; Shcherbakov, I. Binaphthyl-containing Schiff base complexes with carboxyl groups for dye sensitized solar cell: An experimental and theoretical study. J. Mol. Struct. 2018, 1162, 54–62. [Google Scholar] [CrossRef]
- Tanaka, S.; Sato, H.; Ishida, Y.; Deng, Y.; Haraguchi, T.; Akitsu, T.; Sugiyama, M.; Hara, M.; Moon, D. Photo-Control of Adsorption of Dye Metal Complexes Incorporating Chiral Schiff Base Ligands Containing Azo-Groups on TiO2. J. Korean Chem. Soc. 2018, 62, 328–332. [Google Scholar]
- Sato, H.; Beppu, I.; Haraguchi, T.; Akitsu, T.; Parida, R.; Giri, S.; Roymahapatra, G.; Hubert Joe, I. Optical properties of chiral Schiff base MnII, CoII, NiII complexes having azobenzene. J. Indian Chem. Soc. 2018, 95, 1487–1495. [Google Scholar]
- Gencer Imer, A.; Syan, R.H.B.; Gülcan, M.; Ocak, Y.S.; Tombak, A. The novel pyridine based symmetrical Schiff base ligand and its transition metal complexes: Synthesis, spectral definitions and application in dye sensitized solar cells (DSSCs). J. Mater. Sci. Mater. Electron. 2018, 29, 898–905. [Google Scholar] [CrossRef]
- Akitsu, T.; Iwama, J. Salen-type metal complexes based on structural database of X-ray crystallography. In Computational and Data-Driven Chemistry Using Artificial Intelligence; Elsevier: Amsterdam, The Netherlands, 2022; pp. 69–109. ISBN 9780128222492. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Fan, R.; Wang, P.; Li, L. Enhance the performances of dye-sensitized solar cell by a new type of sensitizer to co-sensitize zinc oxide photoelectrode with ruthenium complex. Dye. Pigment. 2012, 92, 1314–1319. [Google Scholar] [CrossRef]
- Dong, Y.W.; Fan, R.Q.; Wang, P.; Wei, L.G.; Wang, X.M.; Zhang, H.J.; Gao, S.; Yang, Y.L.; Wang, Y.L. Synthesis and characterization of substituted Schiff-base ligands and their d10 metal complexes: Structure-induced luminescence tuning behaviors and applications in co-sensitized solar cells. Dalt. Trans. 2015, 44, 5306–5322. [Google Scholar] [CrossRef]
- Kuznetsova, V.; Gromova, Y.; Martinez-Carmona, M.; Purcell-Milton, F.; Ushakova, E.; Cherevkov, S.; Maslov, V.; Gun’Ko, Y.K. Ligand-induced chirality and optical activity in semiconductor nanocrystals: Theory and applications. Nanophotonics 2020, 10, 797–824. [Google Scholar] [CrossRef]
- Allenmark, S. Induced circular dichroism by chiral molecular interaction. Chirality 2003, 15, 409–422. [Google Scholar] [CrossRef]
- Gawroński, J.; Grajewski, J. The significance of induced circular dichroism. Org. Lett. 2003, 5, 3301–3303. [Google Scholar] [CrossRef]
- Akitsu, T.; Kim, S.; Nakane, D. Induced CD on metal nanoclusters or other materials by chiral Schiff base metal complexes. Trends Photochem. Photobiol. 2021, 20, 27–36. [Google Scholar]
- Tsutsumi, Y.; Sunaga, N.; Haraguchi, T.; Akitsu, T. Induced CD from chiral Schiff base metal complexes involving azo-dye groups to gold nanoparticles. J. Indian Chem. Soc. 2017, 94, 1163–1172. [Google Scholar]
- Sunaga, N.; Haraguchi, T.; Akitsu, T. Orientation of Chiral Schiff Base Metal Complexes Involving Azo-Groups for Induced CD on Gold Nanoparticles by Polarized UV Light Irradiation. Symmetry 2019, 11, 1094. [Google Scholar] [CrossRef]
- Oshima, M.; Matsuno, M.; Yuki, T.; Nobumitsu, S.; Haraguchi, T.; Akitsu, T.; Oshima, M.; Matsuno, M.; Yuki, T.; Nobumitsu, S.; et al. Synthesis of Chiral Schiff Base Metal Complex Inducing CD and Elucidation of Structure of Adsorption on Surface of Gold Nanoparticles. Int. J. Org. Chem. 2017, 7, 153–170. [Google Scholar] [CrossRef][Green Version]
- Kimura, N.; Nishizuru, H.; Aritake, Y.; Akitsu, T. Observation of reciprocal induced CD between colloidal gold nanoparticles and chiral Schiff base Zn(II) complexes with parallel dipole moments. J. Chem. Chem. Eng. 2013, 7, 390–394. [Google Scholar]
- Aritake, Y.; Nakayama, T.; Nishizuru, H.; Akitsu, T. Observation of induced CD on CdSe nanoparticles from chiral Schiff base Ni(II), Cu(II), Zn(II) complexes. Inorg. Chem. Commun. 2011, 14, 423–425. [Google Scholar] [CrossRef]
- Bazadze, M.A.; Ebralidze, N.A.; Ebralidze, T.D. Weigert’s effect mechanism observed in dyes. Appl. Opt. 2002, 41, 78–79. [Google Scholar]
- Petrova, S.S.; Chichinadze, N.M.; Shaverdova, V.G. Kinetics of the Weigert effect in azo dyes embedded in polymeric matrices with different activities. Tech. Phys. 2005, 50, 227–231. [Google Scholar] [CrossRef]
- Iftime, G.; Labarthet, F.L.; Natansohn, A.; Rochon, P. Control of Chirality of an Azobenzene Liquid Crystalline Polymer with Circularly Polarized Light. J. Am. Chem. Soc. 2000, 122, 12646–12650. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, S.; Hu, J.; Fujiki, M.; Zou, G. The chirality induction and modulation of polymers by circularly polarized light. Symmetry (Basel). 2019, 11, 474. [Google Scholar] [CrossRef]
- Saiga, K.; Haraguchi, T.; Kitahama, Y.; Hosokai, T.; Matsuzaki, H.; Moon, D.; Sugiyama, M.; Hara, M.; Akitsu, T.; Saiga, K.; et al. Optical Properties of Chiral Azo-Schiff Base Mn(II) and Zn(II) Complexes with Silver Nanoparticles. J. Mater. Sci. Chem. Eng. 2021, 9, 1–10. [Google Scholar] [CrossRef]
- Albeladi, H.K.; Al-Romaizan, A.N.; Hussein, M.A. Role of cross-linking process on the performance of PMMA. Int. J. Biosens. Bioelectron. 2017, 3, 279–284. [Google Scholar]
- Zafar, M.S. Prosthodontic applications of polymethyl methacrylate (PMMA): An update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Rahman, F.; Carbaugh, D.J.; Wright, J.T.; Rajan, P.; Pandya, S.G.; Kaya, S. A review of polymethyl methacrylate (PMMA) as a versatile lithographic resist—With emphasis on UV exposure. Microelectron. Eng. 2020, 224, 111238. [Google Scholar] [CrossRef]
- Akitsu, T. Photofunctional supramolecular solution systems of chiral Schiff base nickel(II), copper(II), and zinc(II) complexes and photochromic azobenzenes. Polyhedron 2007, 26, 2527–2535. [Google Scholar] [CrossRef]
- Akitsu, T.; Itoh, T. Polarized spectroscopy of hybrid materials of chiral Schiff base cobalt(II), nickel(II), copper(II), and zinc(II) complexes and photochromic azobenzenes in PMMA films. Polyhedron 2010, 29, 477–487. [Google Scholar] [CrossRef]
- Akitsu, T.; Einaga, Y. Synthesis, crystal structures and electronic properties of Schiff base nickel (II) complexes: Towards solvatochromism induced by a photochromic solute. Polyhedron 2005, 24, 1869–1877. [Google Scholar] [CrossRef]
- Balogh, L.; Laverdure, K.S.; Gido, S.P.; Mott, A.G.; Miller, M.J.; Ketchel, B.P.; Tomalia, D.A. Dendrimer-Metal Nanocomposites. MRS Online Proc. Libr. 1999, 576, 69–75. [Google Scholar] [CrossRef]
- Akitsu, T.; Yamaguchi, J.; Uchida, N.; Aritake, Y. The studies of conditions for inducing chirality to Cu(II) complexes by chiral Zn(II) and Ni(II) complexes with Schiff base. Res. Lett. Mater. Sci. 2009, 2009, 484172. [Google Scholar] [CrossRef]
- Yoshida, N.; Tsaturyan, A.; Akitsu, T.; Tsunoda, Y.; Shcherbakov, I. Wavelengths dependence of photo-induced reactions of hybrid systems of Schiff base Cu(II) complexes and TiO2 for Cr(VI) reduction. Russ. Chem. Bull. 2017, 11, 2057–2065. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Tsatsuryan, A.; Haraguchi, T.; Shcherbakov, I.; Akitsu, T. Photochemical reduction of Cr(VI) compounds by amino acid Schiff base copper complexes with a hydroxyl group and titanium oxide composites in aqueous solutions. New J. Chem. 2020, 44, 16665–16674. [Google Scholar] [CrossRef]
- Nakagame, R.; Tsaturyan, A.; Haraguchi, T.; Pimonova, Y.; Lastovina, T.; Akitsu, T.; Shcherbakov, I. Photochemical reaction of amino acid Schiff base derived Cu complexes with extended π-system and their titanium oxide composites. Inorg. Chim. Acta 2019, 486, 221–231. [Google Scholar] [CrossRef]
- Onami, Y.; Koya, R.; Kawasaki, T.; Aizawa, H.; Nakagame, R.; Miyagawa, Y.; Haraguchi, T.; Akitsu, T.; Tsukiyama, K.; Palafox, M.A. Investigation by DFT Methods of the Damage of Human Serum Albumin Including Amino Acid Derivative Schiff Base Zn(II) Complexes by IR-FEL Irradiation. Int. J. Mol. Sci. 2019, 20, 2846. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Q.; Ma, D.K.; Zhang, Y.G.; Chen, W.; Huang, S.M. N-doped carbon quantum dots for TiO2-based photocatalysts and dye-sensitized solar cells. Nano Energy 2013, 2, 545–552. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.K.; Holloway, P.H. Quantum dots and their multimodal applications: A review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Jain, K.K. Applications of nanobiotechnology in clinical diagnostics. Clin. Chem. 2007, 53, 2002–2009. [Google Scholar] [CrossRef]
- Pisanic, T.R.; Zhang, Y.; Wang, T.H. Quantum dots in diagnostics and detection: Principles and paradigms. Analyst 2014, 139, 2968–2981. [Google Scholar] [CrossRef]
- Moulick, A.; Heger, Z.; Milosavljevic, V.; Richtera, L.; Barroso-Flores, J.; Merlos Rodrigo, M.A.; Buchtelova, H.; Podgajny, R.; Hynek, D.; Kopel, P.; et al. Real-Time Visualization of Cell Membrane Damage Using Gadolinium-Schiff Base Complex-Doped Quantum Dots. ACS Appl. Mater. Interfaces 2018, 10, 35859–35868. [Google Scholar] [CrossRef]
- Buchtelova, H.; Strmiska, V.; Skubalova, Z.; Dostalova, S.; Michalek, P.; Krizkova, S.; Hynek, D.; Kalina, L.; Richtera, L.; Moulick, A.; et al. Improving cytocompatibility of CdTe quantum dots by Schiff-base-coordinated lanthanides surface doping. J. Nanobiotechnology 2018, 16, 43. [Google Scholar] [CrossRef]
- Pervaiz, M.; Munir, A.; Riaz, A.; Saeed, Z.; Younas, U.; Imran, M.; Ullah, S.; Bashir, R.; Rashid, A.; Adnan, A. Review article-Amalgamation, scrutinizing, and biological evaluation of the antimicrobial aptitude of thiosemicarbazide Schiff bases derivatives metal complexes. Inorg. Chem. Commun. 2022, 141, 109459. [Google Scholar] [CrossRef]
- De Fátima, Â.; Pereira C de, P.; Olímpio, C.R.S.D.G.; de Freitas Oliveira, B.G.; Franco, L.L.; da Silva, P.H.C. Schiff bases and their metal complexes as urease inhibitors—A brief review. J. Adv. Res. 2018, 13, 113–126. [Google Scholar] [CrossRef]
- Ritter, E.; Przybylski, P.; Brzezinski, B.; Bartl, F. Schiff Bases in Biological Systems. Curr. Org. Chem. 2009, 13, 241–249. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Mohamed, I.M.A.A. A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni-Suef Univ. J. Basic Appl. Sci. 2015, 4, 119–133. [Google Scholar] [CrossRef]
- Peterson, M.D.; Holbrook, R.J.; Meade, T.J.; Weiss, E.A. Photoinduced electron transfer from PbS quantum dots to cobalt(III) schiff base complexes: Light activation of a protein inhibitor. J. Am. Chem. Soc. 2013, 135, 13162–13167. [Google Scholar] [CrossRef][Green Version]
- Holbrook, R.J.; Weinberg, D.J.; Peterson, M.D.; Weiss, E.A.; Meade, T.J. Light-activated protein inhibition through photoinduced electron transfer of a ruthenium(II)-cobalt(III) bimetallic complex. J. Am. Chem. Soc. 2015, 137, 3379–3385. [Google Scholar] [CrossRef]
- Renfrew, A. Selective Activation of Transition-Metal Complexes in Medicine. Encycl. Inorg. Bioinorg. Chem. 2018, 1–11. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akitsu, T.; Miroslaw, B.; Sudarsan, S. Photofunctions in Hybrid Systems of Schiff Base Metal Complexes and Metal or Semiconductor (Nano)Materials. Int. J. Mol. Sci. 2022, 23, 10005. https://doi.org/10.3390/ijms231710005
Akitsu T, Miroslaw B, Sudarsan S. Photofunctions in Hybrid Systems of Schiff Base Metal Complexes and Metal or Semiconductor (Nano)Materials. International Journal of Molecular Sciences. 2022; 23(17):10005. https://doi.org/10.3390/ijms231710005
Chicago/Turabian StyleAkitsu, Takashiro, Barbara Miroslaw, and Shanmugavel Sudarsan. 2022. "Photofunctions in Hybrid Systems of Schiff Base Metal Complexes and Metal or Semiconductor (Nano)Materials" International Journal of Molecular Sciences 23, no. 17: 10005. https://doi.org/10.3390/ijms231710005
APA StyleAkitsu, T., Miroslaw, B., & Sudarsan, S. (2022). Photofunctions in Hybrid Systems of Schiff Base Metal Complexes and Metal or Semiconductor (Nano)Materials. International Journal of Molecular Sciences, 23(17), 10005. https://doi.org/10.3390/ijms231710005







