Blockade of Platelet Glycoprotein Ibα Augments Neuroprotection in Orai2-Deficient Mice during Middle Cerebral Artery Occlusion
Abstract
:1. Introduction
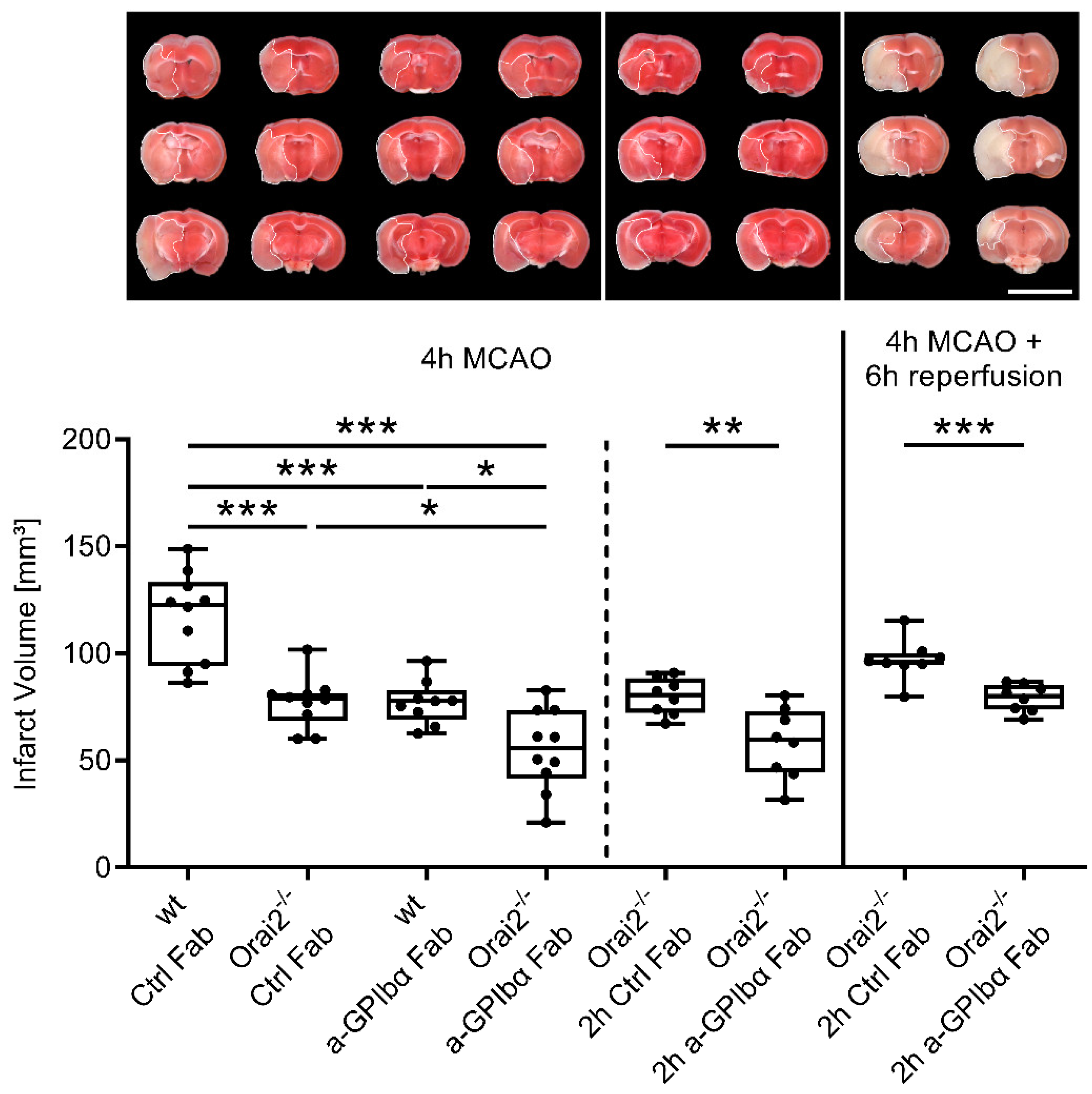
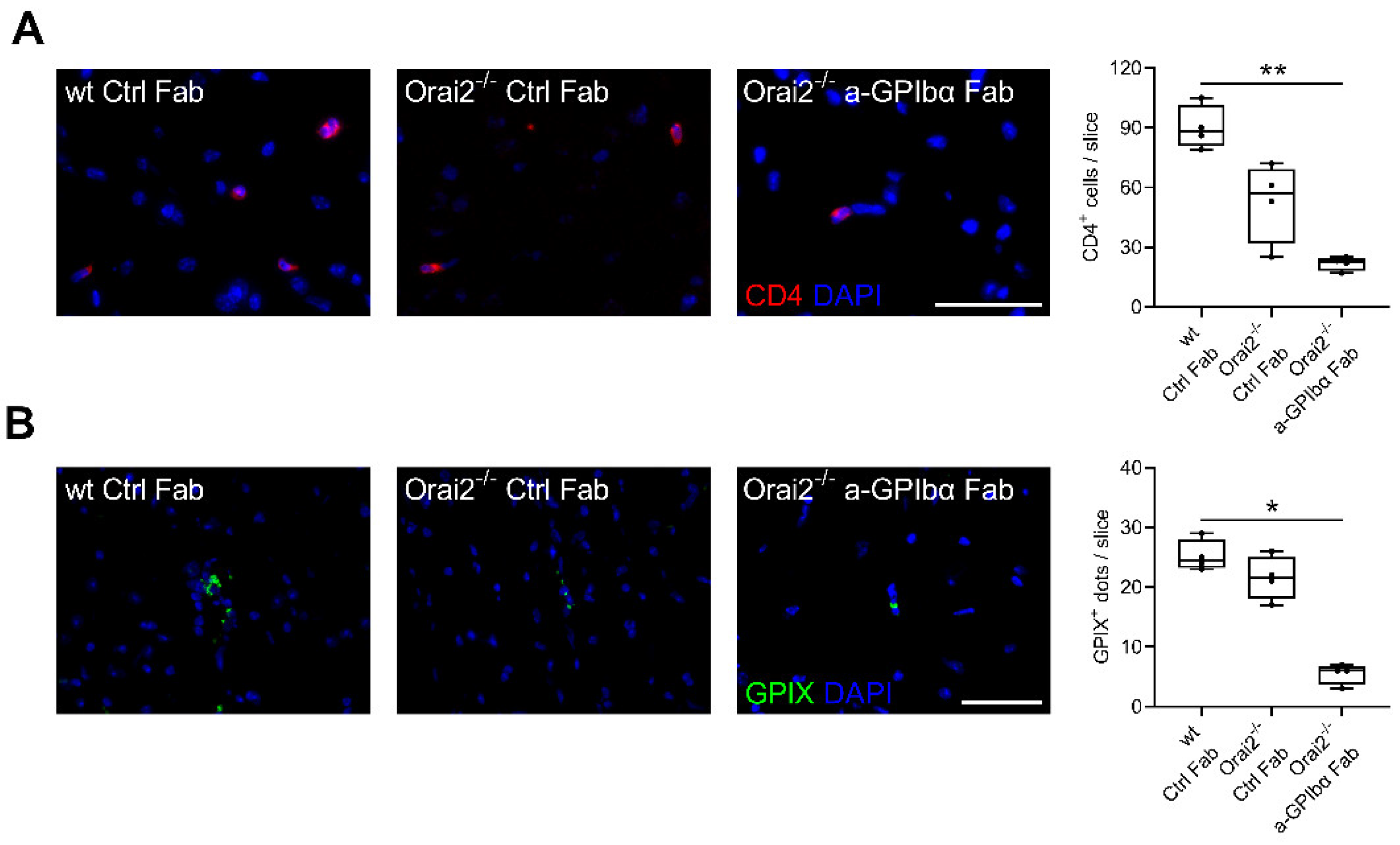
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Ischemia Model
4.3. Triphenyltetrazolium Chloride (TTC) Staining
4.4. Animal Treatment
4.5. Immunohistochemistry
4.6. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shuaib, A.; Butcher, K.; Mohammad, A.A.; Saqqur, M.; Liebeskind, D.S. Collateral blood vessels in acute ischaemic stroke: A potential therapeutic target. Lancet Neurol. 2011, 10, 909–921. [Google Scholar] [CrossRef]
- Hill, M.D.; Goyal, M.; Menon, B.K.; Nogueira, R.G.; McTaggart, R.A.; Demchuk, A.M.; Poppe, A.Y.; Buck, B.H.; Field, T.S.; Dowlatshahi, D.; et al. Efficacy and safety of nerinetide for the treatment of acute ischaemic stroke (ESCAPE-NA1): A multicentre, double-blind, randomised controlled trial. Lancet 2020, 395, 878–887. [Google Scholar] [CrossRef]
- Stoll, G.; Nieswandt, B. Thrombo-inflammation in acute ischaemic stroke—Implications for treatment. Nat. Rev. Neurol. 2019, 15, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Schuhmann, M.K.; Bieber, M.; Franke, M.; Kollikowski, A.M.; Stegner, D.; Heinze, K.G.; Nieswandt, B.; Pham, M.; Stoll, G. Platelets and lymphocytes drive progressive penumbral tissue loss during middle cerebral artery occlusion in mice. J. Neuroinflamm. 2021, 18, 46. [Google Scholar] [CrossRef] [PubMed]
- Kleinschnitz, C.; Pozgajova, M.; Pham, M.; Bendszus, M.; Nieswandt, B.; Stoll, G. Targeting platelets in acute experimental stroke: Impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation 2007, 115, 2323–2330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuhmann, M.K.; Guthmann, J.; Stoll, G.; Nieswandt, B.; Kraft, P.; Kleinschnitz, C. Blocking of platelet glycoprotein receptor Ib reduces “thrombo-inflammation” in mice with acute ischemic stroke. J. Neuroinflamm. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stegner, D.; Hofmann, S.; Schuhmann, M.K.; Kraft, P.; Herrmann, A.M.; Popp, S.; Hohn, M.; Popp, M.; Klaus, V.; Post, A.; et al. Loss of Orai2-Mediated Capacitative Ca(2+) Entry Is Neuroprotective in Acute Ischemic Stroke. Stroke 2019, 50, 3238–3245. [Google Scholar] [CrossRef] [PubMed]
- Kollikowski, A.M.; Cattus, F.; Haag, J.; Feick, J.; Marz, A.G.; Weidner, F.; Schuhmann, M.K.; Mullges, W.; Stoll, G.; Pham, M.; et al. Progression of cerebral infarction before and after thrombectomy is modified by prehospital pathways. J. Neurointerv. Surg. 2022, 14, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Stoll, G.; Pham, M. Beyond recanalization—A call for action in acute stroke. Nat. Rev. Neurol. 2020, 16, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Gilgen, M.; Slotboom, J.; El-Koussy, M.; Zubler, C.; Kiefer, C.; Luedi, R.; Mono, M.L.; Heldner, M.R.; Weck, A.; et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain 2013, 136, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Essig, F.; Kollikowski, A.M.; Mullges, W.; Stoll, G.; Haeusler, K.G.; Schuhmann, M.K.; Pham, M. Local Cerebral Recombinant Tissue Plasminogen Activator Concentrations During Acute Stroke. JAMA Neurol. 2021, 78, 615–617. [Google Scholar] [CrossRef] [PubMed]
- Ahnstedt, H.; McCullough, L.D.; Cipolla, M.J. The Importance of Considering Sex Differences in Translational Stroke Research. Transl. Stroke Res. 2016, 7, 261–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Davalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Seifert, H.A.; Benedek, G.; Liang, J.; Nguyen, H.; Kent, G.; Vandenbark, A.A.; Saugstad, J.A.; Offner, H. Sex differences in regulatory cells in experimental stroke. Cell Immunol. 2017, 318, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Bieber, M.; Schuhmann, M.K.; Kollikowski, A.M.; Stegner, D.; Nieswandt, B.; Pham, M.; Stoll, G. Targeting platelet glycoprotein VI attenuates progressive ischemic brain damage before recanalization during middle cerebral artery occlusion in mice. Exp. Neurol. 2021, 344, 113804. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bieber, M.; Schuhmann, M.K.; Bellut, M.; Stegner, D.; Heinze, K.G.; Pham, M.; Nieswandt, B.; Stoll, G. Blockade of Platelet Glycoprotein Ibα Augments Neuroprotection in Orai2-Deficient Mice during Middle Cerebral Artery Occlusion. Int. J. Mol. Sci. 2022, 23, 9496. https://doi.org/10.3390/ijms23169496
Bieber M, Schuhmann MK, Bellut M, Stegner D, Heinze KG, Pham M, Nieswandt B, Stoll G. Blockade of Platelet Glycoprotein Ibα Augments Neuroprotection in Orai2-Deficient Mice during Middle Cerebral Artery Occlusion. International Journal of Molecular Sciences. 2022; 23(16):9496. https://doi.org/10.3390/ijms23169496
Chicago/Turabian StyleBieber, Michael, Michael K. Schuhmann, Maximilian Bellut, David Stegner, Katrin G. Heinze, Mirko Pham, Bernhard Nieswandt, and Guido Stoll. 2022. "Blockade of Platelet Glycoprotein Ibα Augments Neuroprotection in Orai2-Deficient Mice during Middle Cerebral Artery Occlusion" International Journal of Molecular Sciences 23, no. 16: 9496. https://doi.org/10.3390/ijms23169496
APA StyleBieber, M., Schuhmann, M. K., Bellut, M., Stegner, D., Heinze, K. G., Pham, M., Nieswandt, B., & Stoll, G. (2022). Blockade of Platelet Glycoprotein Ibα Augments Neuroprotection in Orai2-Deficient Mice during Middle Cerebral Artery Occlusion. International Journal of Molecular Sciences, 23(16), 9496. https://doi.org/10.3390/ijms23169496





