Resistance Training Improves Beta Cell Glucose Sensing and Survival in Diabetic Models
Abstract
1. Introduction
2. Results
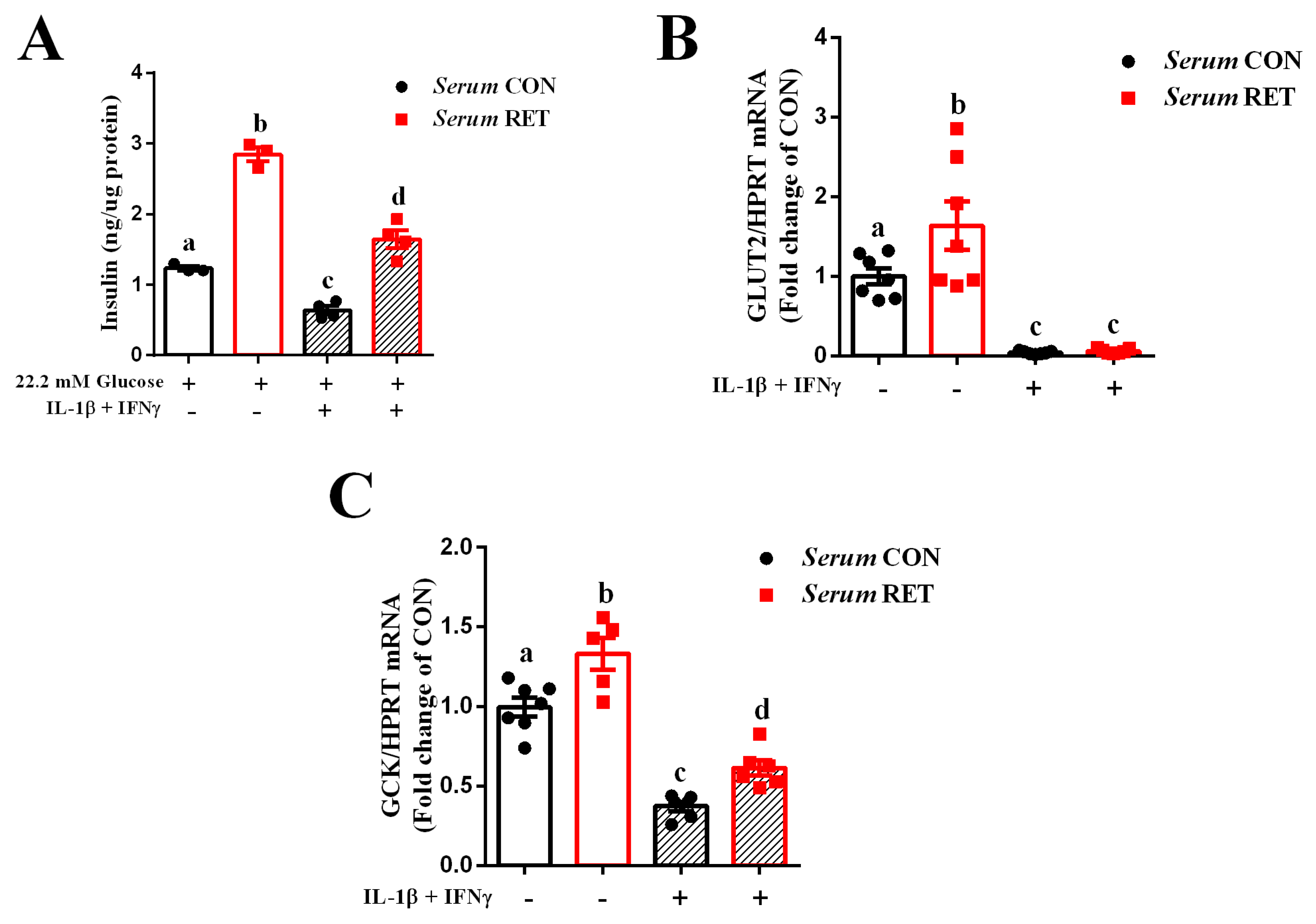
2.1. Serum from Resistance-Trained Mice Improves Glucose Sensing and Preserves Insulin Secretion in INS-1E Cells Exposed to Pro-Inflammatory Cytokines
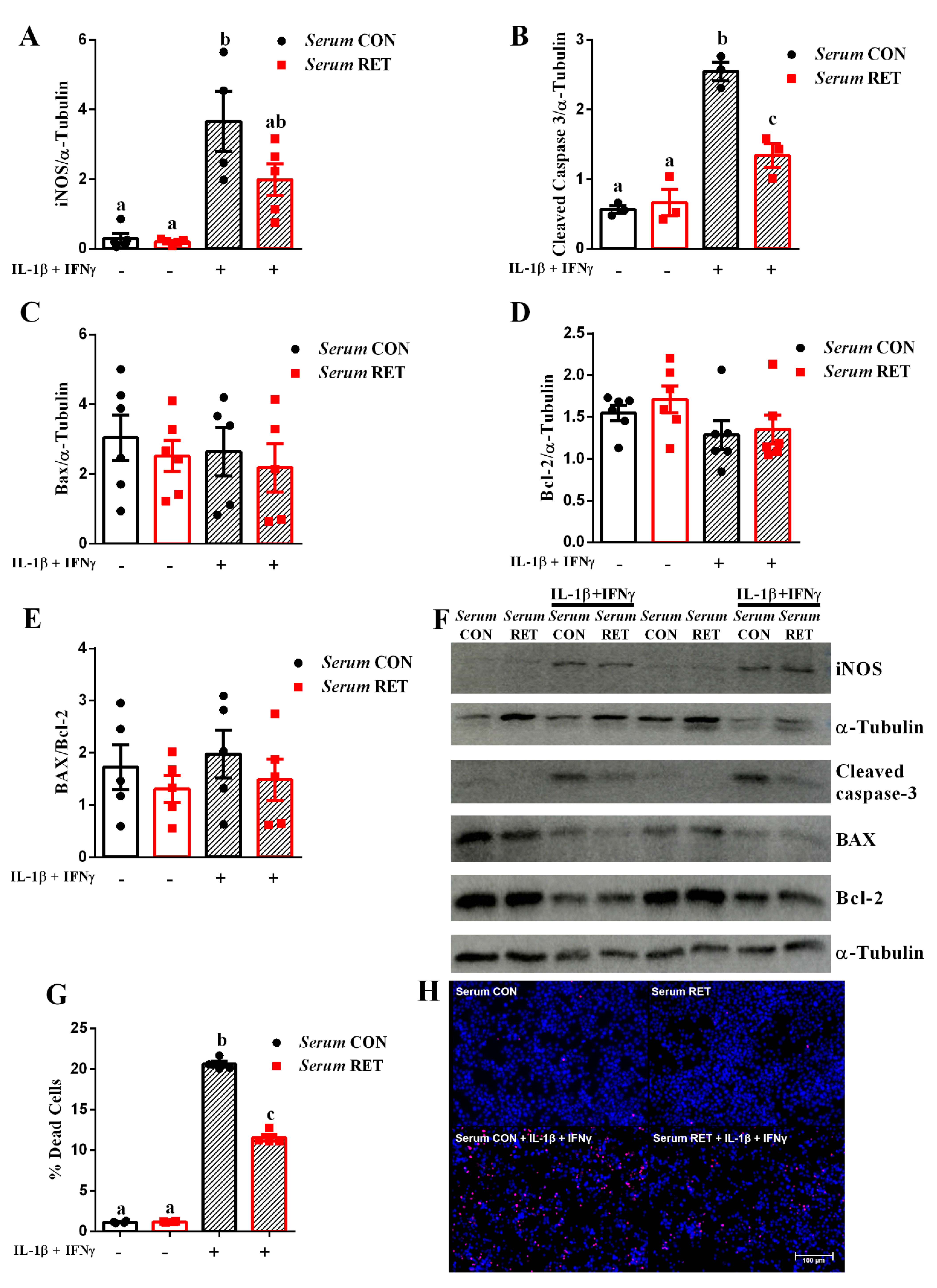
2.2. Serum from Resistance-Trained Mice Protects INS-1E Cells from Cytokine-Induced Apoptosis
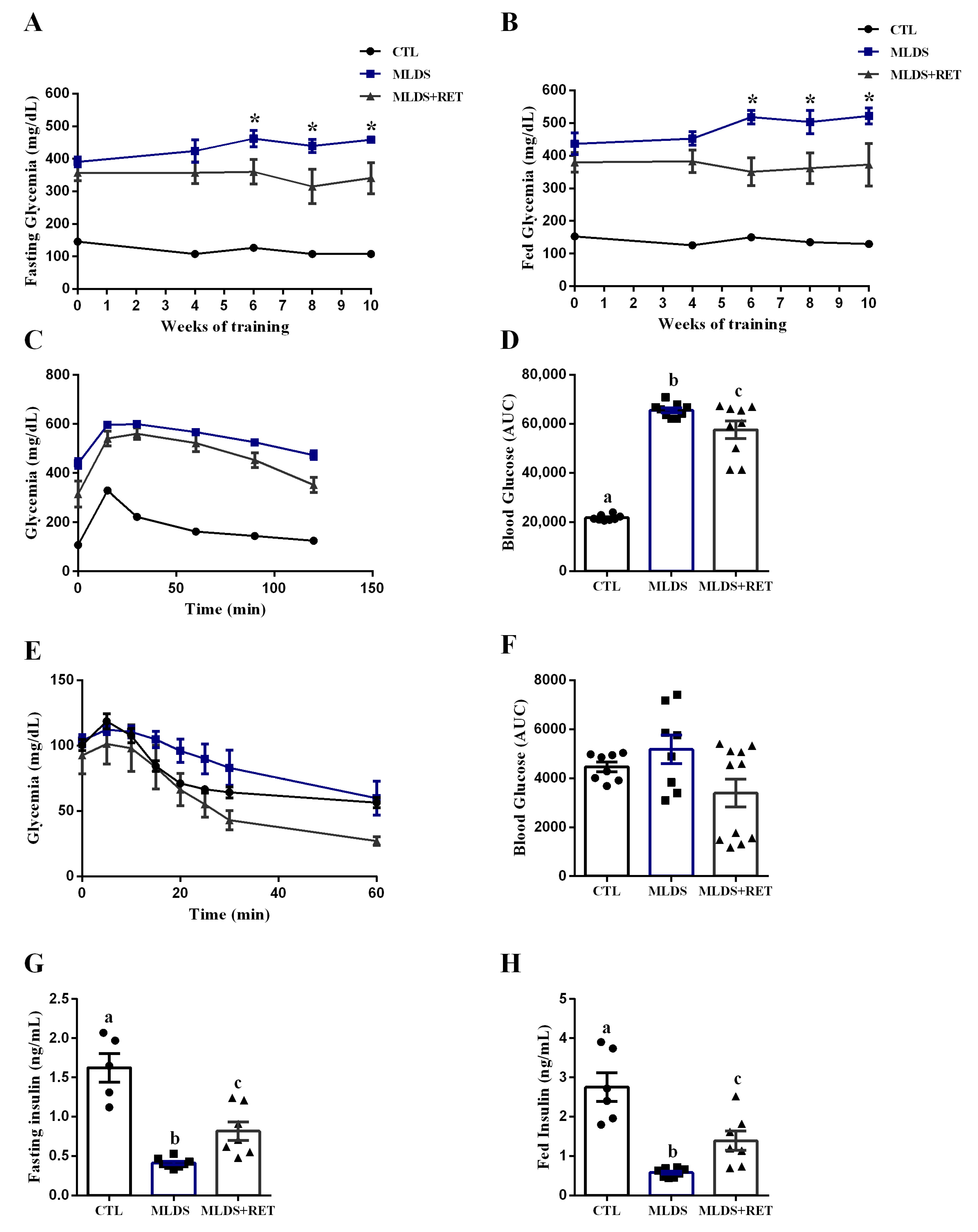
2.3. Resistance Training Induces Adaptation and Alterations in Body Composition in Type 1 Diabetic Mice
2.4. Resistance Exercise Training Modulates Glucose Metabolism in a Mice Model of Type 1 Diabetes
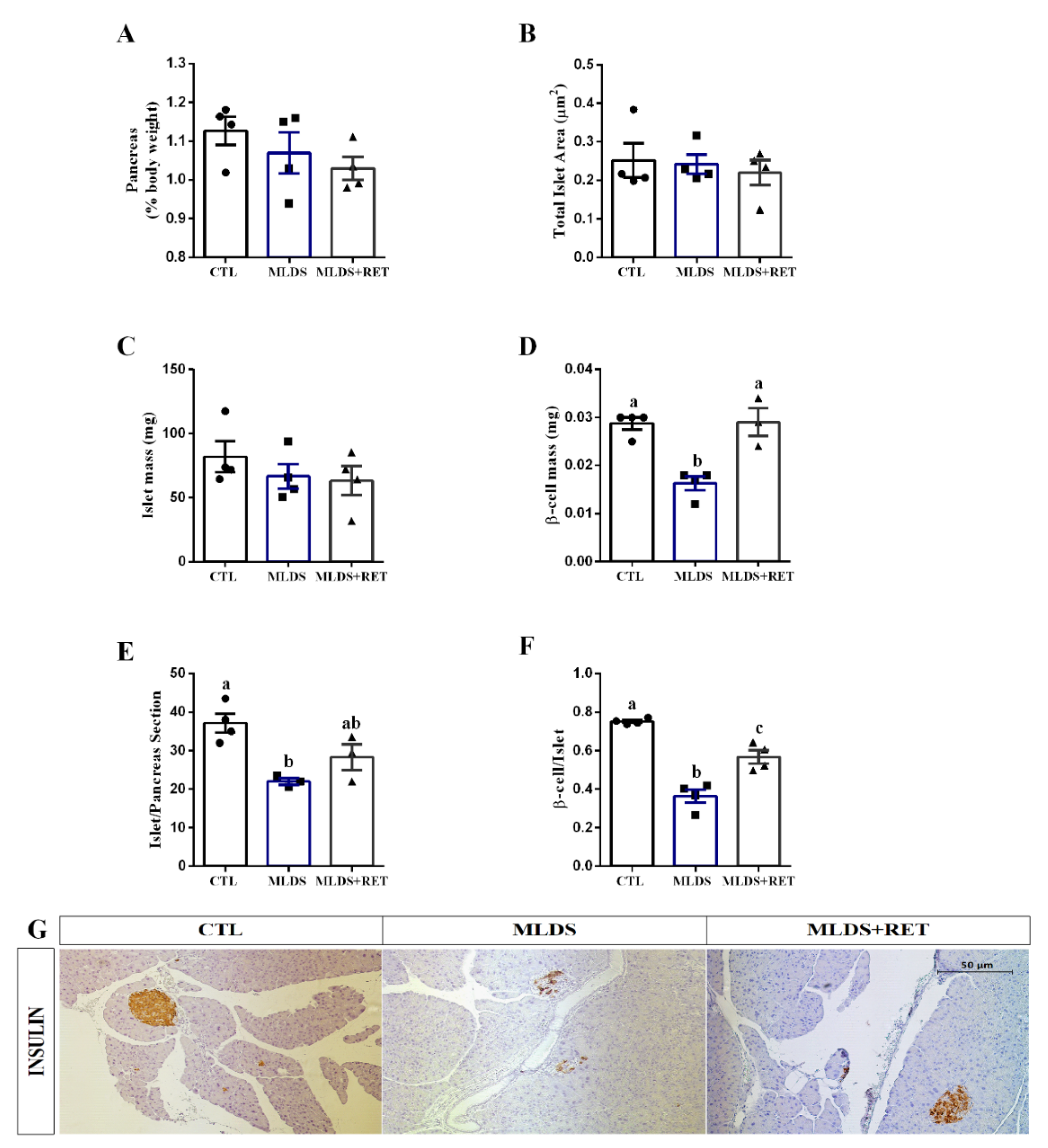
2.5. Resistance Training Increases Beta Cell Mass in Type 1 Diabetic Mice
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Mice
4.3. Resistance Exercise Training Protocol
4.4. INS-1E Cell Culture and Treatment
4.5. Insulin Secretion in INS-1E Cells
4.6. mRNA Isolation and Real-Time Quantitative PCR
4.7. Western Blot
4.8. HO-PI Fluorescence Quantification
4.9. Intraperitoneal Glucose (ipGTT) and Insulin (ipITT) Tolerance Tests
4.10. Plasma Insulin Measurement
4.11. Pancreas Morphometry and Immunohistochemistry
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apaolaza, P.S.; Balcacean, D.; Zapardiel-Gonzalo, J.; Nelson, G.; Lenchik, N.; Akhbari, P.; Gerling, I.; Richardson, S.J.; Rodriguez-Calvo, T.; nPOD-Virus Group. Islet expression of type I interferon response sensors is associated with immune infiltration and viral infection in type 1 diabetes. Sci. Adv. 2021, 7, eabd6527. [Google Scholar] [CrossRef] [PubMed]
- Bathina, S.; Das, U.N. Resolvin D1 Decreases Severity of Streptozotocin-Induced Type 1 Diabetes Mellitus by Enhancing BDNF Levels, Reducing Oxidative Stress, and Suppressing Inflammation. Int. J. Mol. Sci. 2021, 22, 1516. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Galgani, M.; Bruzzaniti, S.; Matarese, G. Immunometabolism and autoimmunity. Curr. Opin. Immunol. 2020, 67, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Metsios, G.S.; Moe, R.H.; Kitas, G.D. Exercise and inflammation. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101504. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, R.; Monedero-Carrasco, S.; Bizzozero-Peroni, B.; Garrido-Miguel, M.; Mesas, A.E.; Martínez-Vizcaíno, V. Effectiveness of Resistance Exercise on Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Systematic Review with Meta-Analysis. Diabetes Metab. J. 2022, 1–17. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Jacobs, P.G.; Castle, J.R.; Wilson, L.M.; Kuehl, K.; Branigan, D.; Gabo, V.; Guillot, F.; Riddell, M.C.; Haidar, A.; et al. Separating insulin-mediated and non-insulin-mediated glucose uptake during and after aerobic exercise in type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E425–E437. [Google Scholar] [CrossRef]
- Paula, F.M.; Leite, N.C.; Vanzela, E.C.; Kurauti, M.A.; Freitas-Dias, R.; Carneiro, E.M.; Boschero, A.C.; Zoppi, C.C. Exercise increases pancreatic β-cell viability in a model of type 1 diabetes through IL-6 signaling. FASEB J. 2015, 29, 1805–1816. [Google Scholar] [CrossRef]
- Paula, F.M.M.; Leite, N.C.; Borck, P.C.; Freitas-Dias, R.; Cnop, M.; Chacon-Mikahil, M.P.T.; Cavaglieri, C.R.; Marchetti, P.; Boschero, A.C.; Zoppi, C.C.; et al. Exercise training protects human and rodent β cells against endoplasmic reticulum stress and apoptosis. FASEB J. 2018, 32, 1524–1536. [Google Scholar] [CrossRef]
- Bacchi, E.; Negri, C.; Zanolin, M.E.; Milanese, C.; Faccioli, N.; Trombetta, M.; Zoppini, G.; Cevese, A.; Bonadonna, R.C.; Schena, F.; et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: A randomized controlled trial (the RAED2 study). Diabetes Care 2012, 35, 676–682. [Google Scholar] [CrossRef]
- Choi, S.B.; Jang, J.S.; Park, S. Estrogen and exercise may enhance beta-cell function and mass via insulin receptor substrate 2 induction in ovariectomized diabetic rats. Endocrinology 2005, 146, 4786–4794. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.B.; Jang, J.S.; Hong, S.M.; Jun, D.W.; Park, S. Exercise and dexamethasone oppositely modulate beta-cell function and survival via independent pathways in 90% pancreatectomized rats. J. Endocrinol. 2006, 190, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Codella, R.; Lanzoni, G.; Zoso, A.; Caumo, A.; Montesano, A.; Terruzzi, I.M.; Ricordi, C.; Luzi, L.; Inverardi, L. Moderate Intensity Training Impact on the Inflammatory Status and Glycemic Profiles in NOD Mice. J. Diabetes Res. 2015, 2015, 737586. [Google Scholar] [CrossRef] [PubMed]
- Coskun, O.; Ocakci, A.; Bayraktaroglu, T.; Kanter, M. Exercise training prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Tohoku J. Exp. Med. 2004, 203, 145–154. [Google Scholar] [CrossRef]
- Park, S.; Hong, S.M.; Lee, J.E.; Sung, S.R. Exercise improves glucose homeostasis that has been impaired by a high-fat diet by potentiating pancreatic beta-cell function and mass through IRS2 in diabetic rats. J. Appl. Physiol. 2007, 103, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Yardley, J.E.; Kenny, G.P.; Perkins, B.A.; Riddell, M.C.; Malcolm, J.; Boulay, P.; Khandwala, F.; Sigal, R.J. Effects of performing resistance exercise before versus after aerobic exercise on glycemia in type 1 diabetes. Diabetes Care 2012, 35, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Yardley, J.E.; Kenny, G.P.; Perkins, B.A.; Riddell, M.C.; Balaa, N.; Malcolm, J.; Boulay, P.; Khandwala, F.; Sigal, R.J. Resistance versus aerobic exercise: Acute effects on glycemia in type 1 diabetes. Diabetes Care 2013, 36, 537–542. [Google Scholar] [CrossRef]
- Álvarez, C.; Ramírez-Campillo, R.; Ramírez-Vélez, R.; Izquierdo, M. Effects and prevalence of nonresponders after 12 weeks of high-intensity interval or resistance training in women with insulin resistance: A randomized trial. J. Appl. Physiol. 2017, 122, 985–996. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, W.; Chen, Q.; Zhang, Y.; Kuo, C.H.; Korivi, M. Resistance Exercise Intensity is Correlated with Attenuation of HbA1c and Insulin in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2019, 16, 140. [Google Scholar] [CrossRef]
- Reddy, R.; Wittenberg, A.; Castle, J.R.; El Youssef, J.; Winters-Stone, K.; Gillingham, M.; Jacobs, P.G. Effect of Aerobic and Resistance Exercise on Glycemic Control in Adults with Type 1 Diabetes. Can. J. Diabetes 2019, 43, 406–414.e1. [Google Scholar] [CrossRef]
- Bronczek, G.A.; Soares, G.M.; de Barros, J.F.; Vettorazzi, J.F.; Kurauti, M.A.; Marconato-Júnior, E.; Zangerolamo, L.; Marmentini, C.; Boschero, A.C.; Costa-Júnior, J.M. Resistance exercise training improves glucose homeostasis by enhancing insulin secretion in C57BL/6 mice. Sci. Rep. 2021, 11, 8574. [Google Scholar] [CrossRef] [PubMed]
- Wolkowicz, K.L.; Aiello, E.M.; Vargas, E.; Teymourian, H.; Tehrani, F.; Wang, J.; Pinsker, J.E.; Doyle, F.J.; Patti, M.E.; Laffel, L.M.; et al. A review of biomarkers in the context of type 1 diabetes: Biological sensing for enhanced glucose control. Bioeng. Transl. Med. 2021, 6, e10201. [Google Scholar] [CrossRef] [PubMed]
- Berger, C.; Zdzieblo, D. Glucose transporters in pancreatic islets. Pflug. Arch. 2020, 472, 1249–1272. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.S.; Hossain, K.S.; Das, S.; Kundu, S.; Adegoke, E.O.; Rahman, M.A.; Hannan, M.A.; Uddin, M.J.; Pang, M.G. Role of Insulin in Health and Disease: An Update. Int. J. Mol. Sci. 2021, 22, 6403. [Google Scholar] [CrossRef]
- Shomali, N.; Mahmoudi, J.; Mahmoodpoor, A.; Zamiri, R.E.; Akbari, M.; Xu, H.; Shotorbani, S.S. Harmful effects of high amounts of glucose on the immune system: An updated review. Biotechnol. Appl. Biochem. 2021, 68, 404–410. [Google Scholar] [CrossRef]
- Ohneda, M.; Johnson, J.H.; Inman, L.R.; Chen, L.; Suzuki, K.; Goto, Y.; Alam, T.; Ravazzola, M.; Orci, L.; Unger, R.H. GLUT2 expression and function in beta-cells of GK rats with NIDDM. Dissociation between reductions in glucose transport and glucose-stimulated insulin secretion. Diabetes 1993, 42, 1065–1072. [Google Scholar] [CrossRef]
- Ostenson, C.G.; Efendic, S. Islet gene expression and function in type 2 diabetes; studies in the Goto-Kakizaki rat and humans. Diabetes Obes. Metab. 2007, 9 (Suppl. S2), 180–186. [Google Scholar] [CrossRef]
- Portha, B.; Giroix, M.H.; Serradas, P.; Gangnerau, M.N.; Movassat, J.; Rajas, F.; Bailbe, D.; Plachot, C.; Mithieux, G.; Marie, J.C. Beta-cell function and viability in the spontaneously diabetic GK rat: Information from the GK/Par colony. Diabetes 2001, 50 (Suppl. S1), S89–S93. [Google Scholar] [CrossRef]
- Orci, L.; Unger, R.H.; Ravazzola, M.; Ogawa, A.; Komiya, I.; Baetens, D.; Lodish, H.F.; Thorens, B. Reduced beta-cell glucose transporter in new onset diabetic BB rats. J. Clin. Invest 1990, 86, 1615–1622. [Google Scholar] [CrossRef]
- Thorens, B.; Weir, G.C.; Leahy, J.L.; Lodish, H.F.; Bonner-Weir, S. Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats. Proc. Natl. Acad. Sci. USA 1990, 87, 6492–6496. [Google Scholar] [CrossRef]
- Wang, Z.; Gleichmann, H. GLUT2 in pancreatic islets: Crucial target molecule in diabetes induced with multiple low doses of streptozotocin in mice. Diabetes 1998, 47, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Király, M.A.; Bates, H.E.; Kaniuk, N.A.; Yue, J.T.; Brumell, J.H.; Matthews, S.G.; Riddell, M.C.; Vranic, M. Swim training prevents hyperglycemia in ZDF rats: Mechanisms involved in the partial maintenance of beta-cell function. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E271–E283. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Hong, S.M.; Lee, J.E.; Sung, S.R.; Kim, S.H. Chlorpromazine attenuates pancreatic beta-cell function and mass through IRS2 degradation, while exercise partially reverses the attenuation. J. Psychopharmacol. 2008, 22, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Mandrup-Poulsen, T. A choice of death—The signal-transduction of immune-mediated beta-cell apoptosis. Diabetologia 2001, 44, 2115–2133. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, A.; Suarez-Pinzon, W.L. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem. Pharmacol. 1998, 55, 1139–1149. [Google Scholar] [CrossRef]
- Rojas, J.; Bermudez, V.; Palmar, J.; Martínez, M.S.; Olivar, L.C.; Nava, M.; Tomey, D.; Rojas, M.; Salazar, J.; Garicano, C.; et al. Pancreatic Beta Cell Death: Novel Potential Mechanisms in Diabetes Therapy. J. Diabetes Res. 2018, 2018, 9601801. [Google Scholar] [CrossRef]
- Calegari, V.C.; Zoppi, C.C.; Rezende, L.F.; Silveira, L.R.; Carneiro, E.M.; Boschero, A.C. Endurance training activates AMP-activated protein kinase, increases expression of uncoupling protein 2 and reduces insulin secretion from rat pancreatic islets. J. Endocrinol. 2011, 208, 257–264. [Google Scholar] [CrossRef]
- Su, S.H.; Jen, C.J.; Chen, H.I. NO signaling in exercise training-induced anti-apoptotic effects in human neutrophils. Biochem. Biophys. Res. Commun. 2011, 405, 58–63. [Google Scholar] [CrossRef]
- Szalai, Z.; Szász, A.; Nagy, I.; Puskás, L.G.; Kupai, K.; Király, A.; Berkó, A.M.; Pósa, A.; Strifler, G.; Baráth, Z.; et al. Anti-inflammatory effect of recreational exercise in TNBS-induced colitis in rats: Role of NOS/HO/MPO system. Oxid. Med. Cell. Longev. 2014, 2014, 925981. [Google Scholar] [CrossRef]
- Chavoshan, B.; Fournier, M.; Lewis, M.I.; Porszasz, J.; Storer, T.W.; Da, X.; Rambod, M.; Casaburi, R. Testosterone and resistance training effects on muscle nitric oxide synthase isoforms in COPD men. Respir. Med. 2012, 106, 269–275. [Google Scholar] [CrossRef][Green Version]
- Al-Jarrah, M.; Obaidat, H.; Bataineh, Z.; Walton, L.; Al-Khateeb, A. Endurance exercise training protects against the upregulation of nitric oxide in the striatum of MPTP/probenecid mouse model of Parkinson’s disease. NeuroRehabilitation 2013, 32, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Oharomari, L.K.; de Moraes, C.; Navarro, A.M. Exercise Training but not Curcumin Supplementation Decreases Immune Cell Infiltration in the Pancreatic Islets of a Genetically Susceptible Model of Type 1 Diabetes. Sports Med. Open 2017, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, T.; Li, Y.; Yuan, C.; Ma, H.; Seeram, N.P.; Liu, F.; Mu, Y.; Huang, X.; Li, L. Hypoglycemic and hypolipidemic effects of triterpenoid-enriched Jamun (Eugenia jambolana Lam.) fruit extract in streptozotocin-induced type 1 diabetic mice. Food Funct. 2018, 9, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Du, X.; Zhang, Z.; Zhou, J. Trigonelline inhibits caspase 3 to protect β cells apoptosis in streptozotocin-induced type 1 diabetic mice. Eur. J. Pharmacol. 2018, 836, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.P.; Ye, R.J.; Yang, J.M.; Liu, N.; Zhang, W.J.; Ma, L.; Sun, T.; Niu, J.G.; Zheng, P.; Yu, J.Q. Protective effects of Salidroside on spermatogenesis in streptozotocin induced type-1 diabetic male mice by inhibiting oxidative stress mediated blood-testis barrier damage. Chem. Biol. Interact. 2020, 315, 108869. [Google Scholar] [CrossRef]
- Carvalho, A.L.; DeMambro, V.E.; Guntur, A.R.; Le, P.; Nagano, K.; Baron, R.; de Paula, F.J.A.; Motyl, K.J. High fat diet attenuates hyperglycemia, body composition changes, and bone loss in male streptozotocin-induced type 1 diabetic mice. J. Cell. Physiol. 2018, 233, 1585–1600. [Google Scholar] [CrossRef]
- Kurauti, M.A.; Soares, G.M.; Marmentini, C.; Bronczek, G.A.; Branco, R.C.S.; Boschero, A.C. Insulin and aging. Vitam. Horm. 2021, 115, 185–219. [Google Scholar] [CrossRef]
- Chetan, M.R.; Thrower, S.L.; Narendran, P. What is type 1 diabetes? Medicine 2019, 47, 5–9. [Google Scholar] [CrossRef]
- Sala, D.; Zorzano, A. Differential control of muscle mass in type 1 and type 2 diabetes mellitus. Cell. Mol. Life Sci. 2015, 72, 3803–3817. [Google Scholar] [CrossRef]
- Gunawardana, S.C.; Piston, D.W. Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 2012, 61, 674–682. [Google Scholar] [CrossRef]
- Croymans, D.M.; Paparisto, E.; Lee, M.M.; Brandt, N.; Le, B.K.; Lohan, D.; Lee, C.C.; Roberts, C.K. Resistance training improves indices of muscle insulin sensitivity and β-cell function in overweight/obese, sedentary young men. J. Appl. Physiol. 2013, 115, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, L.; Mahani, M.N. Effects of resistance training on insulin resistance and pancreatic beta-cells function in male patients with type 2 diabetes. J. Phys. Act. Horm. 2019, 3, 19–34. [Google Scholar]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Barlow, J.P.; Solomon, T.P. Do skeletal muscle-secreted factors influence the function of pancreatic β-cells? Am. J. Physiol. Endocrinol. Metab. 2018, 314, E297–E307. [Google Scholar] [CrossRef]
- Natalicchio, A.; Marrano, N.; Biondi, G.; Spagnuolo, R.; Labarbuta, R.; Porreca, I.; Cignarelli, A.; Bugliani, M.; Marchetti, P.; Perrini, S.; et al. The Myokine Irisin Is Released in Response to Saturated Fatty Acids and Promotes Pancreatic β-Cell Survival and Insulin Secretion. Diabetes 2017, 66, 2849–2856. [Google Scholar] [CrossRef] [PubMed]
- Fulgenzi, G.; Hong, Z.; Tomassoni-Ardori, F.; Barella, L.F.; Becker, J.; Barrick, C.; Swing, D.; Yanpallewar, S.; Croix, B.S.; Wess, J.; et al. Novel metabolic role for BDNF in pancreatic β-cell insulin secretion. Nat. Commun. 2020, 11, 1950. [Google Scholar] [CrossRef]
- Nakayasu, E.S.; Syed, F.; Tersey, S.A.; Gritsenko, M.A.; Mitchell, H.D.; Chan, C.Y.; Dirice, E.; Turatsinze, J.V.; Cui, Y.; Kulkarni, R.N.; et al. Comprehensive Proteomics Analysis of Stressed Human Islets Identifies GDF15 as a Target for Type 1 Diabetes Intervention. Cell Metab. 2020, 31, 363–374.e6. [Google Scholar] [CrossRef]
- Zhao, C.; Qiao, C.; Tang, R.H.; Jiang, J.; Li, J.; Martin, C.B.; Bulaklak, K.; Wang, D.W.; Xiao, X. Overcoming Insulin Insufficiency by Forced Follistatin Expression in β-cells of db/db Mice. Mol. Ther. 2015, 23, 866–874. [Google Scholar] [CrossRef]
- Rutti, S.; Dusaulcy, R.; Hansen, J.S.; Howald, C.; Dermitzakis, E.T.; Pedersen, B.K.; Pinget, M.; Plomgaard, P.; Bouzakri, K. Angiogenin and Osteoprotegerin are type II muscle specific myokines protecting pancreatic beta-cells against proinflammatory cytokines. Sci. Rep. 2018, 8, 10072. [Google Scholar] [CrossRef]
- Kurauti, M.A.; Freitas-Dias, R.; Ferreira, S.M.; Vettorazzi, J.F.; Nardelli, T.R.; Araujo, H.N.; Santos, G.J.; Carneiro, E.M.; Boschero, A.C.; Rezende, L.F.; et al. Acute Exercise Improves Insulin Clearance and Increases the Expression of Insulin-Degrading Enzyme in the Liver and Skeletal Muscle of Swiss Mice. PLoS ONE 2016, 11, e0160239. [Google Scholar] [CrossRef]
- Kurauti, M.A.; Costa-Júnior, J.M.; Ferreira, S.M.; Dos Santos, G.J.; Protzek, A.O.; Nardelli, T.R.; de Rezende, L.F.; Boschero, A.C. Acute exercise restores insulin clearance in diet-induced obese mice. J. Endocrinol. 2016, 229, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, S.; Handschin, C. Skeletal muscle as an endocrine organ: PGC-1α, myokines and exercise. Bone 2015, 80, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef] [PubMed]
- Biolo, G.; Maggi, S.P.; Williams, B.D.; Tipton, K.D.; Wolfe, R.R. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am. J. Physiol. 1995, 268, E514–E520. [Google Scholar] [CrossRef] [PubMed]
- Chesley, A.; MacDougall, J.D.; Tarnopolsky, M.A.; Atkinson, S.A.; Smith, K. Changes in human muscle protein synthesis after resistance exercise. J. Appl. Physiol. 1992, 73, 1383–1388. [Google Scholar] [CrossRef]
- MacDougall, J.D.; Tarnopolsky, M.A.; Chesley, A.; Atkinson, S.A. Changes in muscle protein synthesis following heavy resistance exercise in humans: A pilot study. Acta Physiol. Scand. 1992, 146, 403–404. [Google Scholar] [CrossRef]
- Curran, M.; Drayson, M.T.; Andrews, R.C.; Zoppi, C.; Barlow, J.P.; Solomon, T.P.J.; Narendran, P. The benefits of physical exercise for the health of the pancreatic β-cell: A review of the evidence. Exp. Physiol. 2020, 105, 579–589. [Google Scholar] [CrossRef]
- Király, M.A.; Bates, H.E.; Yue, J.T.; Goche-Montes, D.; Fediuc, S.; Park, E.; Matthews, S.G.; Vranic, M.; Riddell, M.C. Attenuation of type 2 diabetes mellitus in the male Zucker diabetic fatty rat: The effects of stress and non-volitional exercise. Metabolism 2007, 56, 732–744. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, J.; Tian, H.; Pei, Y.; Lu, Y.; Han, X.; Liu, Y.; Zhong, W.; Sun, B.; Fang, F.; et al. The DPP-4 inhibitor MK0626 and exercise protect islet function in early pre-diabetic kkay mice. Peptides 2013, 49, 91–99. [Google Scholar] [CrossRef]
- Bronczek, G.A.; Vettorazzi, J.F.; Soares, G.M.; Kurauti, M.A.; Santos, C.; Bonfim, M.F.; Carneiro, E.M.; Balbo, S.L.; Boschero, A.C.; Costa Júnior, J.M. The Bile Acid TUDCA Improves Beta-Cell Mass and Reduces Insulin Degradation in Mice with Early-Stage of Type-1 Diabetes. Front. Physiol. 2019, 10, 561. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. Pharmacol. 2015, 70, 5.47.1–5.47.20. [Google Scholar] [CrossRef] [PubMed]
- Hornberger, T.A.; Farrar, R.P. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Can. J. Appl. Physiol. 2004, 29, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.M.; Rodrigues, K.C.D.C.; Anaruma, C.P.; Sant’Ana, M.R.; de Campos, T.D.P.; Gaspar, R.S.; Canciglieri, R.D.S.; de Melo, D.G.; Mekary, R.A.; da Silva, A.S.R.; et al. Short-term strength training reduces gluconeogenesis and NAFLD in obese mice. J. Endocrinol. 2019, 241, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Lima, Y.C.; Kurauti, M.A.; da Fonseca Alves, G.; Ferezini, J.; Piovan, S.; Malta, A.; de Almeida, F.L.A.; Gomes, R.M.; de Freitas Mathias, P.C.; Milani, P.G.; et al. Whey protein sweetened with. Nutr. Metab. 2019, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Soares, G.M.; Zangerolamo, L.; Costa-Júnior, J.M.; Vettorazzi, J.F.; Carneiro, E.M.; Saad, S.T.; Boschero, A.C.; Barbosa-Sampaio, H.C. Whole-Body ARHGAP21-Deficiency Improves Energetic Homeostasis in Lean and Obese Mice. Front. Endocrinol. 2019, 10, 338. [Google Scholar] [CrossRef]
- Mateus Gonçalves, L.; Vettorazzi, J.F.; Vanzela, E.C.; Figueiredo, M.S.; Batista, T.M.; Zoppi, C.C.; Boschero, A.C.; Carneiro, E.M. Amino acid restriction increases β-cell death under challenging conditions. J. Cell. Physiol. 2019, 234, 16679–16684. [Google Scholar] [CrossRef]
- Dos Santos, C.; Rafacho, A.; Ferreira, S.M.; Vettorazzi, J.F.; Dos Reis Araújo, T.; Mateus Gonçalves, L.; Ruhrmann, S.; Bacos, K.; Ling, C.; Boschero, A.C.; et al. Excess of glucocorticoids during late gestation impairs the recovery of offspring’s β-cell function after a postnatal injury. FASEB J. 2021, 35, e21828. [Google Scholar] [CrossRef]
- Chung, C.H.; Hao, E.; Piran, R.; Keinan, E.; Levine, F. Pancreatic β-cell neogenesis by direct conversion from mature α-cells. Stem Cells 2010, 28, 1630–1638. [Google Scholar] [CrossRef]
- Lubaczeuski, C.; Balbo, S.L.; Ribeiro, R.A.; Vettorazzi, J.F.; Santos-Silva, J.C.; Carneiro, E.M.; Bonfleur, M.L. Vagotomy ameliorates islet morphofunction and body metabolic homeostasis in MSG-obese rats. Braz. J. Med. Biol. Res. 2015, 48, 447–457. [Google Scholar] [CrossRef]

| CTL | MLDS | MLDS + RET | |
|---|---|---|---|
| Retroperitoneal fat pad (% body weight) | 0.515 ± 0.08 a | 0.098 ± 0.02 b | 0.184 ± 0.03 b |
| Perigonadal fat pad (% body weight) | 1.414 ± 0.19 a | 0.210 ± 0.07 b | 0.595 ± 0.09 c |
| Gastrocnemius (% body weight) | 1.025 ± 0.03 a | 0.836 ± 0.03 b | 1.087 ± 0.01 a |
| Soleus (% body weight) | 0.041 ± 0.003 a | 0.028 ± 0.001 b | 0.054 ± 0.002 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bronczek, G.A.; Soares, G.M.; Marmentini, C.; Boschero, A.C.; Costa-Júnior, J.M. Resistance Training Improves Beta Cell Glucose Sensing and Survival in Diabetic Models. Int. J. Mol. Sci. 2022, 23, 9427. https://doi.org/10.3390/ijms23169427
Bronczek GA, Soares GM, Marmentini C, Boschero AC, Costa-Júnior JM. Resistance Training Improves Beta Cell Glucose Sensing and Survival in Diabetic Models. International Journal of Molecular Sciences. 2022; 23(16):9427. https://doi.org/10.3390/ijms23169427
Chicago/Turabian StyleBronczek, Gabriela Alves, Gabriela Moreira Soares, Carine Marmentini, Antonio Carlos Boschero, and José Maria Costa-Júnior. 2022. "Resistance Training Improves Beta Cell Glucose Sensing and Survival in Diabetic Models" International Journal of Molecular Sciences 23, no. 16: 9427. https://doi.org/10.3390/ijms23169427
APA StyleBronczek, G. A., Soares, G. M., Marmentini, C., Boschero, A. C., & Costa-Júnior, J. M. (2022). Resistance Training Improves Beta Cell Glucose Sensing and Survival in Diabetic Models. International Journal of Molecular Sciences, 23(16), 9427. https://doi.org/10.3390/ijms23169427






