Localized Heat Therapy Improves Mitochondrial Respiratory Capacity but Not Fatty Acid Oxidation
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics
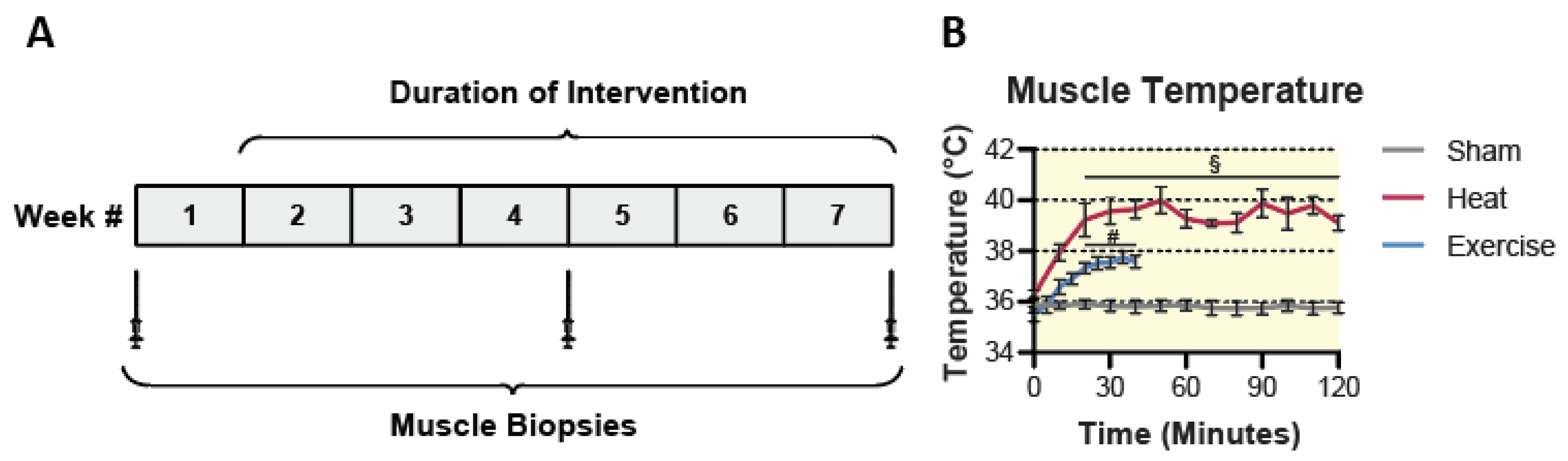
2.2. Sham, HT, and EX Interventions
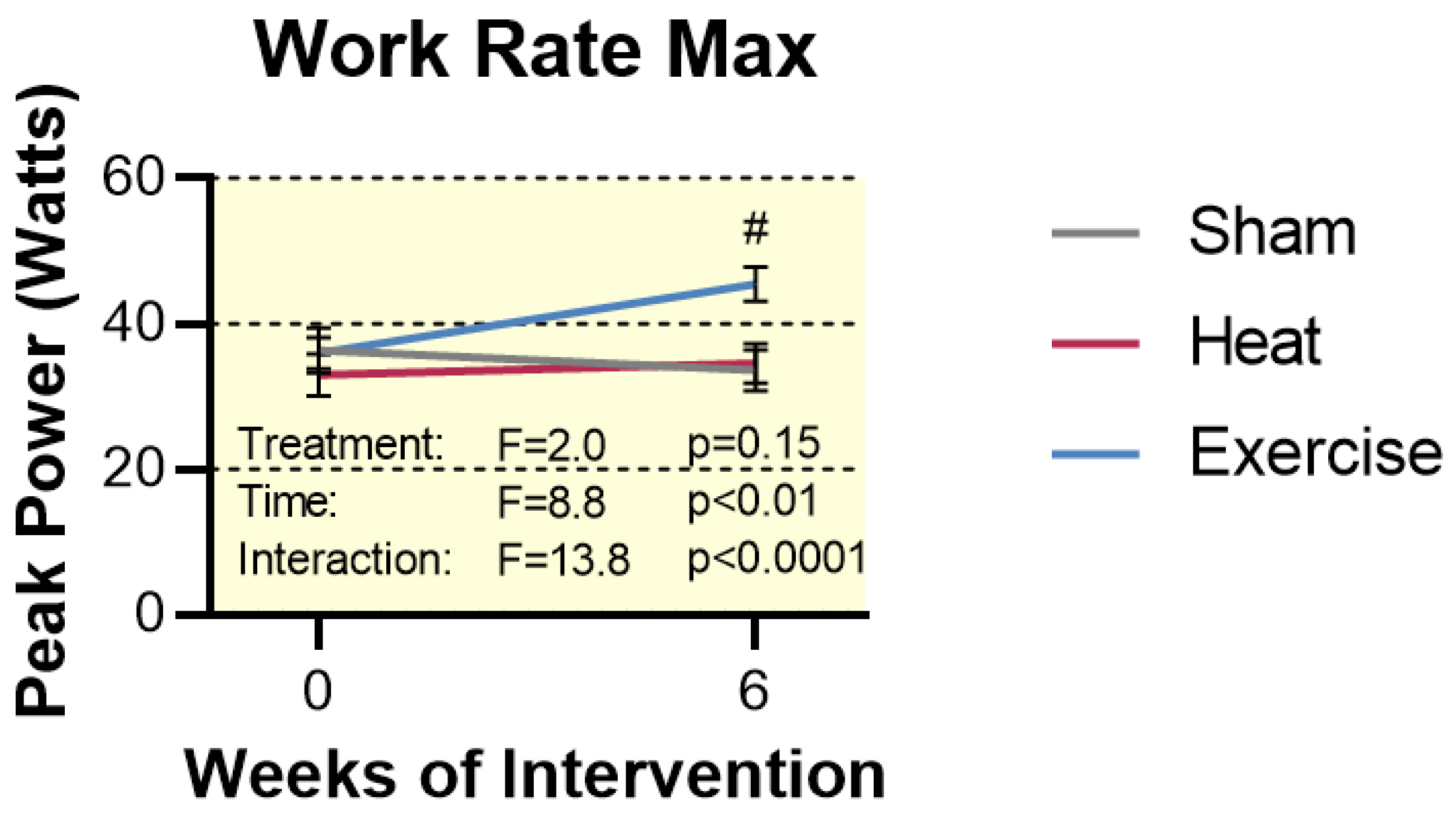
2.3. Work Rate Max
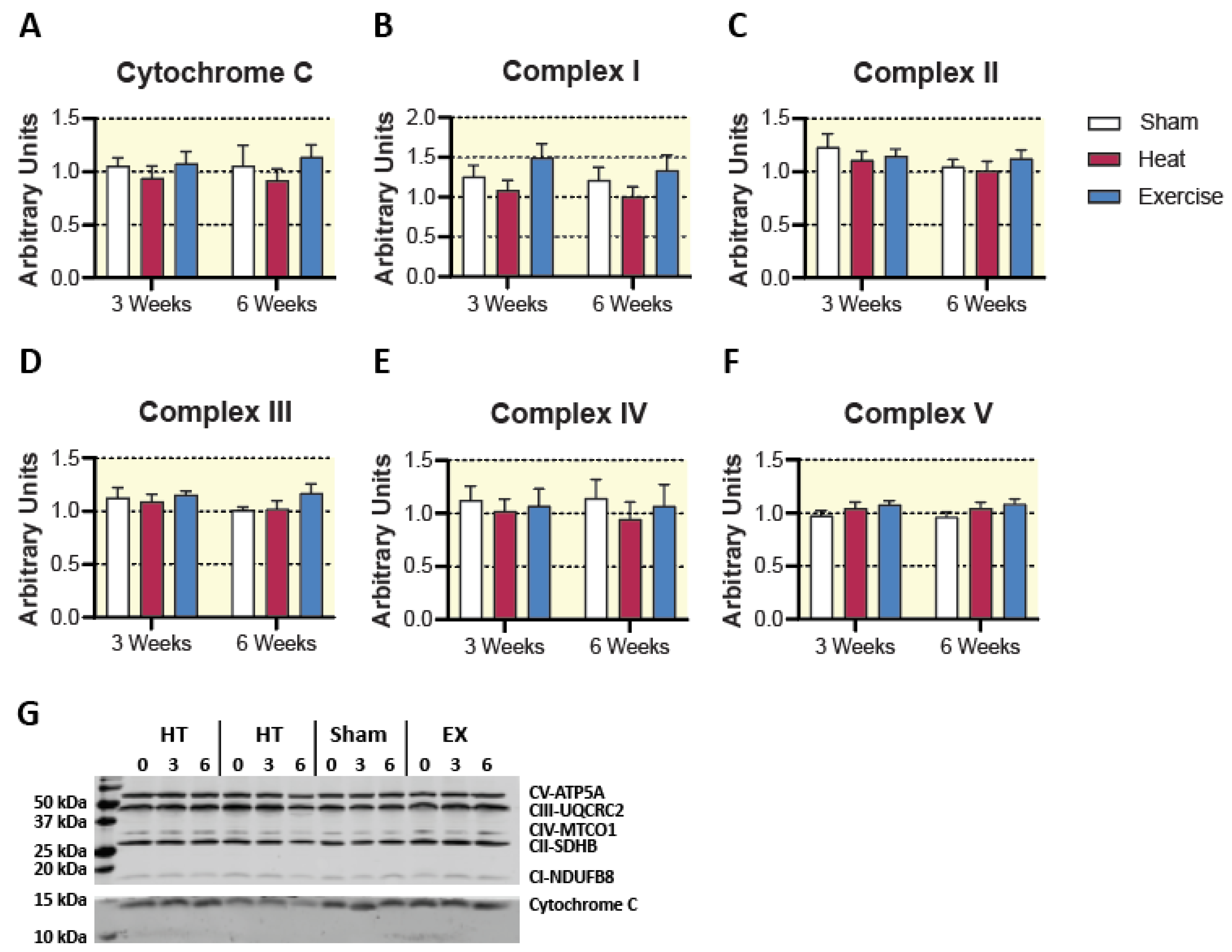
2.4. Mitochondrial Content
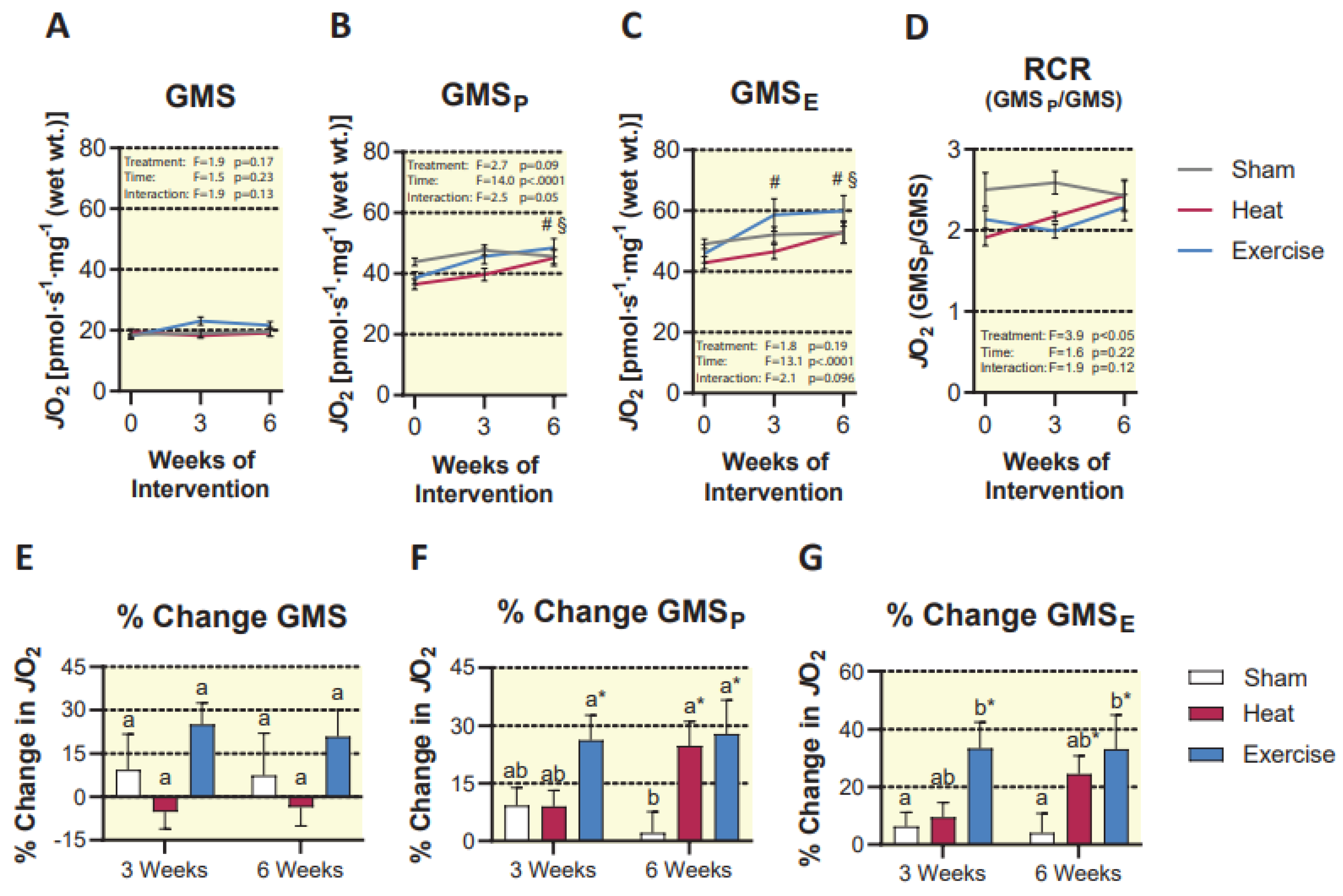
2.5. Mitochondrial Respiration
3. Discussion
4. Experimental Considerations
5. Materials and Methods
5.1. Subject Characteristics
5.2. Study Description
5.3. Exercise Protocol
5.4. Localized Heat Therapy
5.5. Sham Therapy
5.6. Muscle Temperature
5.7. Muscle Biopsies
5.8. Mitochondrial Respiration
5.9. Tissue Homogenization
5.10. Citrate Synthase and HAD Activity
5.11. Western Blotting
5.12. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benton, C.R.; Nickerson, J.G.; Lally, J.; Han, X.X.; Holloway, G.P.; Glatz, J.F.; Luiken, J.J.; Graham, T.E.; Heikkila, J.J.; Bonen, A. Modest PGC-1alpha overexpression in muscle in vivo is sufficient to increase insulin sensitivity and palmitate oxidation in subsarcolemmal, not intermyofibrillar, mitochondria. J. Biol. Chem. 2008, 283, 4228–4240. [Google Scholar] [CrossRef] [Green Version]
- Dodds, R.M.; Davies, K.; Granic, A.; Hollingsworth, K.G.; Warren, C.; Gorman, G.; Turnbull, D.M.; Sayer, A.A. Mitochondrial respiratory chain function and content are preserved in the skeletal muscle of active very old men and women. Exp. Gerontol. 2018, 113, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; Booth, F.W.; Winder, W.W.; Holloszy, J.O. Skeletal muscle respiratory capacity, endurance, and glycogen utilization. Am. J. Physiol. 1975, 228, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buso, A.; Comelli, M.; Picco, R.; Isola, M.; Magnesa, B.; Pisot, R.; Rittweger, J.; Salvadego, D.; Simunic, B.; Grassi, B.; et al. Mitochondrial Adaptations in Elderly and Young Men Skeletal Muscle Following 2 Weeks of Bed Rest and Rehabilitation. Front. Physiol. 2019, 10, 474. [Google Scholar] [CrossRef] [PubMed]
- Morton, A.B.; Mor Huertas, A.; Hinkley, J.M.; Ichinoseki-Sekine, N.; Christou, D.D.; Smuder, A.J. Mitochondrial accumulation of doxorubicin in cardiac and diaphragm muscle following exercise preconditioning. Mitochondrion 2019, 45, 52–62. [Google Scholar] [CrossRef]
- Pinti, M.V.; Fink, G.K.; Hathaway, Q.A.; Durr, A.J.; Kunovac, A.; Hollander, J.M. Mitochondrial dysfunction in type 2 diabetes mellitus: An organ-based analysis. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E268–E285. [Google Scholar] [CrossRef]
- Remchak, M.E.; Piersol, K.L.; Bhatti, S.; Spaeth, A.M.; Buckman, J.F.; Malin, S.K. Considerations for Maximizing the Exercise “Drug” to Combat Insulin Resistance: Role of Nutrition, Sleep, and Alcohol. Nutrients 2021, 13, 1708. [Google Scholar] [CrossRef]
- Sangwung, P.; Petersen, K.F.; Shulman, G.I.; Knowles, J.W. Mitochondrial Dysfunction, Insulin Resistance, and Potential Genetic Implications. Endocrinology 2020, 161. [Google Scholar] [CrossRef]
- Brown, J.L.; Rosa-Caldwell, M.E.; Lee, D.E.; Blackwell, T.A.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Hardee, J.P.; Carson, J.A.; Wiggs, M.P.; et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2017, 8, 926–938. [Google Scholar] [CrossRef]
- Hesselink, M.K.; Schrauwen-Hinderling, V.; Schrauwen, P. Skeletal muscle mitochondria as a target to prevent or treat type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 633–645. [Google Scholar] [CrossRef]
- Sirago, G.; Conte, E.; Fracasso, F.; Cormio, A.; Fehrentz, J.A.; Martinez, J.; Musicco, C.; Camerino, G.M.; Fonzino, A.; Rizzi, L.; et al. Growth hormone secretagogues hexarelin and JMV2894 protect skeletal muscle from mitochondrial damages in a rat model of cisplatin-induced cachexia. Sci. Rep. 2017, 7, 13017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holloszy, J.O. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967, 242, 2278–2282. [Google Scholar] [CrossRef]
- Oliveira, A.N.; Richards, B.J.; Slavin, M.; Hood, D.A. Exercise Is Muscle Mitochondrial Medicine. Exerc. Sport Sci. Rev. 2021, 49, 67–76. [Google Scholar] [CrossRef]
- Hood, D.A. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl. Physiol. Nutr. Metab. 2009, 34, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Irrcher, I.; Ljubicic, V.; Hood, D.A. Interactions between ROS and AMP kinase activity in the regulation of PGC-1alpha transcription in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2009, 296, C116–C123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorenza, M.; Gunnarsson, T.P.; Hostrup, M.; Iaia, F.M.; Schena, F.; Pilegaard, H.; Bangsbo, J. Metabolic stress-dependent regulation of the mitochondrial biogenic molecular response to high-intensity exercise in human skeletal muscle. J. Physiol. 2018, 596, 2823–2840. [Google Scholar] [CrossRef]
- Irrcher, I.; Ljubicic, V.; Kirwan, A.F.; Hood, D.A. AMP-activated protein kinase-regulated activation of the PGC-1alpha promoter in skeletal muscle cells. PLoS ONE 2008, 3, e3614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.T.; Brooks, G.A. Mild heat stress induces mitochondrial biogenesis in C2C12 myotubes. J. Appl. Physiol. 2012, 112, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Matsunaga, Y.; Masuda, H.; Takahashi, Y.; Takahashi, Y.; Terada, S.; Hoshino, D.; Hatta, H. Postexercise whole body heat stress additively enhances endurance training-induced mitochondrial adaptations in mouse skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R931–R943. [Google Scholar] [CrossRef] [Green Version]
- Ihsan, M.; Deldicque, L.; Molphy, J.; Britto, F.; Cherif, A.; Racinais, S. Skeletal Muscle Signaling Following Whole-Body and Localized Heat Exposure in Humans. Front. Physiol. 2020, 11, 839. [Google Scholar] [CrossRef] [PubMed]
- Gupte, A.A.; Bomhoff, G.L.; Touchberry, C.D.; Geiger, P.C. Acute heat treatment improves insulin-stimulated glucose uptake in aged skeletal muscle. J. Appl. Physiol. 2011, 110, 451–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebok, J.; Edel, Z.; Vancsa, S.; Farkas, N.; Kiss, S.; Eross, B.; Torok, Z.; Balogh, G.; Balogi, Z.; Nagy, R.; et al. Heat therapy shows benefit in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Int. J. Hyperth. 2021, 38, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Archer, A.E.; Von Schulze, A.T.; Geiger, P.C. Exercise, heat shock proteins and insulin resistance. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20160529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafen, P.S.; Preece, C.N.; Sorensen, J.R.; Hancock, C.R.; Hyldahl, R.D. Repeated exposure to heat stress induces mitochondrial adaptation in human skeletal muscle. J. Appl. Physiol. 2018, 125, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Hafen, P.S.; Abbott, K.; Bowden, J.; Lopiano, R.; Hancock, C.R.; Hyldahl, R.D. Daily heat treatment maintains mitochondrial function and attenuates atrophy in human skeletal muscle subjected to immobilization. J. Appl. Physiol. 2019, 127, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Hyldahl, R.D.; Hafen, P.S.; Nelson, W.B.; Ahmadi, M.; Pfeifer, B.; Mehling, J.; Gifford, J.R. Passive muscle heating attenuates the decline in vascular function caused by limb disuse. J. Physiol. 2021, 599, 4581–4596. [Google Scholar] [CrossRef]
- Richardson, R.S.; Poole, D.C.; Knight, D.R.; Kurdak, S.S.; Hogan, M.C.; Grassi, B.; Johnson, E.C.; Kendrick, K.F.; Erickson, B.K.; Wagner, P.D. High muscle blood flow in man: Is maximal O2 extraction compromised? J. Appl. Physiol. 1993, 75, 1911–1916. [Google Scholar] [CrossRef]
- Andersen, P.; Adams, R.P.; Sjogaard, G.; Thorboe, A.; Saltin, B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J. Appl. Physiol. 1985, 59, 1647–1653. [Google Scholar] [CrossRef]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef]
- Groennebaek, T.; Nielsen, J.; Jespersen, N.R.; Botker, H.E.; de Paoli, F.V.; Miller, B.F.; Vissing, K. Utilization of biomarkers as predictors of skeletal muscle mitochondrial content after physiological intervention and in clinical settings. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E886–E889. [Google Scholar] [CrossRef]
- Gollnick, P.D.; Saltin, B. Significance of skeletal muscle oxidative enzyme enhancement with endurance training. Clin. Physiol. 1982, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Strijcker, D.; Lapauw, B.; Ouwens, D.M.; Van de Velde, D.; Hansen, D.; Petrovic, M.; Cuvelier, C.; Tonoli, C.; Calders, P. High intensity interval training is associated with greater impact on physical fitness, insulin sensitivity and muscle mitochondrial content in males with overweight/obesity, as opposed to continuous endurance training: A randomized controlled trial. J. Musculoskelet. Neuronal Interact. 2018, 18, 215–226. [Google Scholar]
- MacInnis, M.J.; Zacharewicz, E.; Martin, B.J.; Haikalis, M.E.; Skelly, L.E.; Tarnopolsky, M.A.; Murphy, R.M.; Gibala, M.J. Superior mitochondrial adaptations in human skeletal muscle after interval compared to continuous single-leg cycling matched for total work. J. Physiol. 2017, 595, 2955–2968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Memme, J.M.; Erlich, A.T.; Phukan, G.; Hood, D.A. Exercise and mitochondrial health. J. Physiol. 2021, 599, 803–817. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Borras, C.; Pallardo, F.V.; Sastre, J.; Ji, L.L.; Vina, J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J. Physiol. 2005, 567 Pt 1, 113–120. [Google Scholar] [CrossRef]
- Wadley, G.D.; Nicolas, M.A.; Hiam, D.S.; McConell, G.K. Xanthine oxidase inhibition attenuates skeletal muscle signaling following acute exercise but does not impair mitochondrial adaptations to endurance training. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E853–E862. [Google Scholar] [CrossRef] [Green Version]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Bluher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winder, W.W. Energy-sensing and signaling by AMP-activated protein kinase in skeletal muscle. J. Appl. Physiol. 2001, 91, 1017–1028. [Google Scholar] [CrossRef]
- Lee, W.J.; Kim, M.; Park, H.S.; Kim, H.S.; Jeon, M.J.; Oh, K.S.; Koh, E.H.; Won, J.C.; Kim, M.S.; Oh, G.T.; et al. AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPARalpha and PGC-1. Biochem. Biophys. Res. Commun. 2006, 340, 291–295. [Google Scholar] [CrossRef]
- Menzies, K.J.; Singh, K.; Saleem, A.; Hood, D.A. Sirtuin 1-mediated effects of exercise and resveratrol on mitochondrial biogenesis. J. Biol. Chem. 2013, 288, 6968–6979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Xu, L.; Alberobello, A.T.; Gavrilova, O.; Bagattin, A.; Skarulis, M.; Liu, J.; Finkel, T.; Mueller, E. Celastrol Protects against Obesity and Metabolic Dysfunction through Activation of a HSF1-PGC1alpha Transcriptional Axis. Cell Metab. 2015, 22, 695–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henstridge, D.C.; Bruce, C.R.; Drew, B.G.; Tory, K.; Kolonics, A.; Estevez, E.; Chung, J.; Watson, N.; Gardner, T.; Lee-Young, R.S.; et al. Activating HSP72 in rodent skeletal muscle increases mitochondrial number and oxidative capacity and decreases insulin resistance. Diabetes 2014, 63, 1881–1894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, R.S.; Morris, E.M.; Wheatley, J.L.; Archer, A.E.; McCoin, C.S.; White, K.S.; Wilson, D.R.; Meers, G.M.; Koch, L.G.; Britton, S.L.; et al. Deficiency in the Heat Stress Response Could Underlie Susceptibility to Metabolic Disease. Diabetes 2016, 65, 3341–3351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Q.; Cai, H.; Wei, L.; Lan, R. Chitosan oligosaccharides alleviate acute heat stress-induced oxidative damage by activating ERK1/2-mediated HO-1 and GSH-Px gene expression in breast muscle of broilers. Poult. Sci. 2022, 101, 101515. [Google Scholar] [CrossRef] [PubMed]
- Kikusato, M.; Yoshida, H.; Furukawa, K.; Toyomizu, M. Effect of heat stress-induced production of mitochondrial reactive oxygen species on NADPH oxidase and heme oxygenase-1 mRNA levels in avian muscle cells. J. Therm. Biol. 2015, 52, 8–13. [Google Scholar] [CrossRef]
- Montilla, S.I.; Johnson, T.P.; Pearce, S.C.; Gardan-Salmon, D.; Gabler, N.K.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Lonergan, S.M.; Selsby, J.T. Heat stress causes oxidative stress but not inflammatory signaling in porcine skeletal muscle. Temperature 2014, 1, 42–50. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, T.E.; Mayorga, E.J.; Roths, M.; Rhoads, R.P.; Baumgard, L.H.; Selsby, J.T. The effect of Mitoquinol (MitoQ) on heat stressed skeletal muscle from pigs, and a potential confounding effect of biological sex. J. Therm. Biol. 2021, 97, 102900. [Google Scholar] [CrossRef]
- Racinais, S.; Oksa, J. Temperature and neuromuscular function. Scand. J. Med. Sci. Sports 2010, 20 (Suppl. S3), 1–18. [Google Scholar] [CrossRef]
- Karunanithi, S.; Barclay, J.W.; Robertson, R.M.; Brown, I.R.; Atwood, H.L. Neuroprotection at Drosophila synapses conferred by prior heat shock. J. Neurosci. 1999, 19, 4360–4369. [Google Scholar] [CrossRef] [Green Version]
- Granata, C.; Jamnick, N.A.; Bishop, D.J. Training-Induced Changes in Mitochondrial Content and Respiratory Function in Human Skeletal Muscle. Sports Med. 2018, 48, 1809–1828. [Google Scholar] [CrossRef] [PubMed]
- Granata, C.; Caruana, N.J.; Botella, J.; Jamnick, N.A.; Huynh, K.; Kuang, J.; Janssen, H.A.; Reljic, B.; Mellett, N.A.; Laskowski, A.; et al. High-intensity training induces non-stoichiometric changes in the mitochondrial proteome of human skeletal muscle without reorganisation of respiratory chain content. Nat. Commun. 2021, 12, 7056. [Google Scholar] [CrossRef] [PubMed]
- Funai, K.; Summers, S.A.; Rutter, J. Reign in the membrane: How common lipids govern mitochondrial function. Curr. Opin. Cell Biol. 2020, 63, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Greggio, C.; Jha, P.; Kulkarni, S.S.; Lagarrigue, S.; Broskey, N.T.; Boutant, M.; Wang, X.; Conde Alonso, S.; Ofori, E.; Auwerx, J.; et al. Enhanced Respiratory Chain Supercomplex Formation in Response to Exercise in Human Skeletal Muscle. Cell Metab. 2017, 25, 301–311. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Shiba, S.; Horie-Inoue, K.; Shimokata, K.; Inoue, S. A stabilizing factor for mitochondrial respiratory supercomplex assembly regulates energy metabolism in muscle. Nat. Commun. 2013, 4, 2147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picard, M. Mitochondrial synapses: Intracellular communication and signal integration. Trends Neurosci. 2015, 38, 468–474. [Google Scholar] [CrossRef]
- Ehrenborg, E.; Krook, A. Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator-activated receptor delta. Pharmacol. Rev. 2009, 61, 373–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.A. Peroxisome proliferator-activated receptors and the regulation of mammalian lipid metabolism. Biochem. Soc. Trans. 2002, 30 Pt 6, 1086–1090. [Google Scholar] [CrossRef]
- Fritzen, A.M.; Lundsgaard, A.M.; Kiens, B. Tuning fatty acid oxidation in skeletal muscle with dietary fat and exercise. Nat. Rev. Endocrinol. 2020, 16, 683–696. [Google Scholar] [CrossRef]
- Eigendorf, J.; Maassen, M.; Apitius, D.; Maassen, N. Energy Metabolism in Continuous, High-Intensity, and Sprint Interval Training Protocols with Matched Mean Intensity. J. Strength Cond. Res. 2021, 35, 3104–3110. [Google Scholar] [CrossRef]
- Peake, J.M.; Tan, S.J.; Markworth, J.F.; Broadbent, J.A.; Skinner, T.L.; Cameron-Smith, D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E539–E552. [Google Scholar] [CrossRef] [Green Version]
- Hancock, C.R.; Han, D.H.; Chen, M.; Terada, S.; Yasuda, T.; Wright, D.C.; Holloszy, J.O. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc. Natl. Acad. Sci. USA 2008, 105, 7815–7820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyssel, K.; Alligier, M.; Meugnier, E.; Chanseaume, E.; Loizon, E.; Canto, C.; Disse, E.; Lambert-Porcheron, S.; Brozek, J.; Blond, E.; et al. Regulation of energy metabolism and mitochondrial function in skeletal muscle during lipid overfeeding in healthy men. J. Clin. Endocrinol. Metab. 2014, 99, E1254–E1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.B.; Kim, T.W. Passive heat loading links lipolysis and regulation of fibroblast growth factor-21 in humans. J. Therm. Biol. 2014, 45, 163–167. [Google Scholar] [CrossRef] [PubMed]
- O’Hearn, K.; Tingelstad, H.C.; Blondin, D.; Tang, V.; Filion, L.G.; Haman, F. Heat exposure increases circulating fatty acids but not lipid oxidation at rest and during exercise. J. Therm. Biol. 2016, 55, 39–46. [Google Scholar] [CrossRef]
- Hooper, P.L. Hot-tub therapy for type 2 diabetes mellitus. N. Engl. J. Med. 1999, 341, 924–925. [Google Scholar] [CrossRef] [Green Version]
- Kavanagh, K.; Davis, A.T.; Jenkins, K.A.; Flynn, D.M. Effects of heated hydrotherapy on muscle HSP70 and glucose metabolism in old and young vervet monkeys. Cell Stress Chaperones 2016, 21, 717–725. [Google Scholar] [CrossRef] [Green Version]
- Hesketh, K.; Shepherd, S.O.; Strauss, J.A.; Low, D.A.; Cooper, R.J.; Wagenmakers, A.J.M.; Cocks, M. Passive heat therapy in sedentary humans increases skeletal muscle capillarization and eNOS content but not mitochondrial density or GLUT4 content. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H114–H123. [Google Scholar] [CrossRef]
- Kim, K.; Reid, B.A.; Casey, C.A.; Bender, B.E.; Ro, B.; Song, Q.; Trewin, A.J.; Petersen, A.C.; Kuang, S.; Gavin, T.P.; et al. Effects of repeated local heat therapy on skeletal muscle structure and function in humans. J. Appl. Physiol. 2020, 128, 483–492. [Google Scholar] [CrossRef]
- Staron, R.S.; Hagerman, F.C.; Hikida, R.S.; Murray, T.F.; Hostler, D.P.; Crill, M.T.; Ragg, K.E.; Toma, K. Fiber type composition of the vastus lateralis muscle of young men and women. J. Histochem. Cytochem. 2000, 48, 623–629. [Google Scholar] [CrossRef] [Green Version]
- Gouzi, F.; Maury, J.; Molinari, N.; Pomies, P.; Mercier, J.; Prefaut, C.; Hayot, M. Reference values for vastus lateralis fiber size and type in healthy subjects over 40 years old: A systematic review and metaanalysis. J. Appl. Physiol. 2013, 115, 346–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bathgate, K.E.; Bagley, J.R.; Jo, E.; Talmadge, R.J.; Tobias, I.S.; Brown, L.E.; Coburn, J.W.; Arevalo, J.A.; Segal, N.L.; Galpin, A.J. Muscle health and performance in monozygotic twins with 30 years of discordant exercise habits. Eur. J. Appl. Physiol. 2018, 118, 2097–2110. [Google Scholar] [CrossRef] [PubMed]
- Serrano, N.; Colenso-Semple, L.M.; Lazauskus, K.K.; Siu, J.W.; Bagley, J.R.; Lockie, R.G.; Costa, P.B.; Galpin, A.J. Extraordinary fast-twitch fiber abundance in elite weightlifters. PLoS ONE 2019, 14, e0207975. [Google Scholar] [CrossRef] [Green Version]
- Gosker, H.R.; Zeegers, M.P.; Wouters, E.F.; Schols, A.M. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: A systematic review and meta-analysis. Thorax 2007, 62, 944–949. [Google Scholar] [CrossRef] [Green Version]
- Ortenblad, N.; Nielsen, J.; Boushel, R.; Soderlund, K.; Saltin, B.; Holmberg, H.C. The Muscle Fiber Profiles, Mitochondrial Content, and Enzyme Activities of the Exceptionally Well-Trained Arm and Leg Muscles of Elite Cross-Country Skiers. Front. Physiol. 2018, 9, 1031. [Google Scholar] [CrossRef] [PubMed]
- Howald, H.; Hoppeler, H.; Claassen, H.; Mathieu, O.; Straub, R. Influences of endurance training on the ultrastructural composition of the different muscle fiber types in humans. Pflug. Arch. 1985, 403, 369–376. [Google Scholar] [CrossRef]
- Shanely, R.A.; Zwetsloot, K.A.; Triplett, N.T.; Meaney, M.P.; Farris, G.E.; Nieman, D.C. Human skeletal muscle biopsy procedures using the modified Bergstrom technique. J. Vis. Exp. 2014, 10, 51812. [Google Scholar]
- Pesta, D.; Gnaiger, E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012, 810, 25–58. [Google Scholar]



| Age (y) | Height (cm) | Weight (kg) | BMI | Skinfold Thickness (mm) | Baseline WR Max (watts) | |
|---|---|---|---|---|---|---|
|
Sham (M = 6, F = 5) | 20.5 ± 0.5 | 172.3 ± 3.5 | 61.5 ± 3.4 | 20.6 ± 0.7 | 21.0 ± 3.2 | 35.0 ± 2.5 |
|
HT (M = 5, F = 7) | 21.8 ± 1.3 | 168.3 ± 1.8 | 62.3 ± 3.0 | 22.0 ± 1.0 | 22.2 ± 2.2 | 34.2 ± 2.4 |
|
EX (M = 4, F = 4) | 22.7 ± 1.7 | 170.3 ± 2.5 | 67.7 ± 4.8 | 23.1 ± 1.3 | 26.3 ± 3.2 | 36.8 ± 2.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchant, E.D.; Kaluhiokalani, J.P.; Wallace, T.E.; Ahmadi, M.; Dorff, A.; Linde, J.J.; Leach, O.K.; Hyldahl, R.D.; Gifford, J.R.; Hancock, C.R. Localized Heat Therapy Improves Mitochondrial Respiratory Capacity but Not Fatty Acid Oxidation. Int. J. Mol. Sci. 2022, 23, 8500. https://doi.org/10.3390/ijms23158500
Marchant ED, Kaluhiokalani JP, Wallace TE, Ahmadi M, Dorff A, Linde JJ, Leach OK, Hyldahl RD, Gifford JR, Hancock CR. Localized Heat Therapy Improves Mitochondrial Respiratory Capacity but Not Fatty Acid Oxidation. International Journal of Molecular Sciences. 2022; 23(15):8500. https://doi.org/10.3390/ijms23158500
Chicago/Turabian StyleMarchant, Erik D., Jamie P. Kaluhiokalani, Taysom E. Wallace, Mohadeseh Ahmadi, Abigail Dorff, Jessica J. Linde, Olivia K. Leach, Robert D. Hyldahl, Jayson R. Gifford, and Chad R. Hancock. 2022. "Localized Heat Therapy Improves Mitochondrial Respiratory Capacity but Not Fatty Acid Oxidation" International Journal of Molecular Sciences 23, no. 15: 8500. https://doi.org/10.3390/ijms23158500
APA StyleMarchant, E. D., Kaluhiokalani, J. P., Wallace, T. E., Ahmadi, M., Dorff, A., Linde, J. J., Leach, O. K., Hyldahl, R. D., Gifford, J. R., & Hancock, C. R. (2022). Localized Heat Therapy Improves Mitochondrial Respiratory Capacity but Not Fatty Acid Oxidation. International Journal of Molecular Sciences, 23(15), 8500. https://doi.org/10.3390/ijms23158500






