Burn-Induced Acute Kidney Injury–Two-Lane Road: From Molecular to Clinical Aspects
Abstract
1. Introduction
- AKI—duration of ≤7 days, presenting an increase of serum creatinine level more than 50% within 7 days or by ≥0.3 mg/dL within 2 days or oliguria more than 4 h; at this point, no structural changes are defined.
- AKD (acute kidney disease or disorders)—duration of ≤3 months, presenting AKI or a glomerular filtration rate (GFR) < 60 mL/min/1.73 m2 or a decrease of GFR more than 35% from the baseline value or increase of serum creatinine level of 50% higher than baseline; different structural anomalies can be noted, such as albuminuria, hematuria, pyuria, etc.
- After 3 months of renal impairment, according to the definition, chronic kidney disease (CKD) is considered (GFR < 60 mL/min/1.73 m2).
- Stage 1—an increase in serum creatinine level ≥ 0.3 mg/dL or 1.5–1.9 times higher than the baseline value.
- Stage 2—an increase in serum creatinine level more than 2–2.9 times higher than baseline.
- Stage 3—an increase in serum creatinine level ≥ 3 times higher or >4 mg/dL than baseline or the requirement of renal replacement therapy initiation.
- Risk (R)—an increase in serum creatinine level of 1.5–1.9 times higher than the baseline value or a decrease of GFR > 25% or a urine output < 0.5 mL/body weight/hour within 6–12 h.
- Injury (I)—an increase in serum creatinine level of 2–2.9 times higher than baseline or a decrease of GFR > 50% or a urine output < 0.5 mL/body weight/hour within 12 h.
- Failure (F)—an increase in serum creatinine level of 3 times higher or >4 mg/dL than baseline or a decrease of GFR > 75% or a urine output < 0.5 mL/body weight/hour within 24 h or anuria notice more than 24 h.
- Loss (L)—the loss of renal function > 4 weeks.
- End Stage (E)—the loss of renal function > 3 months.
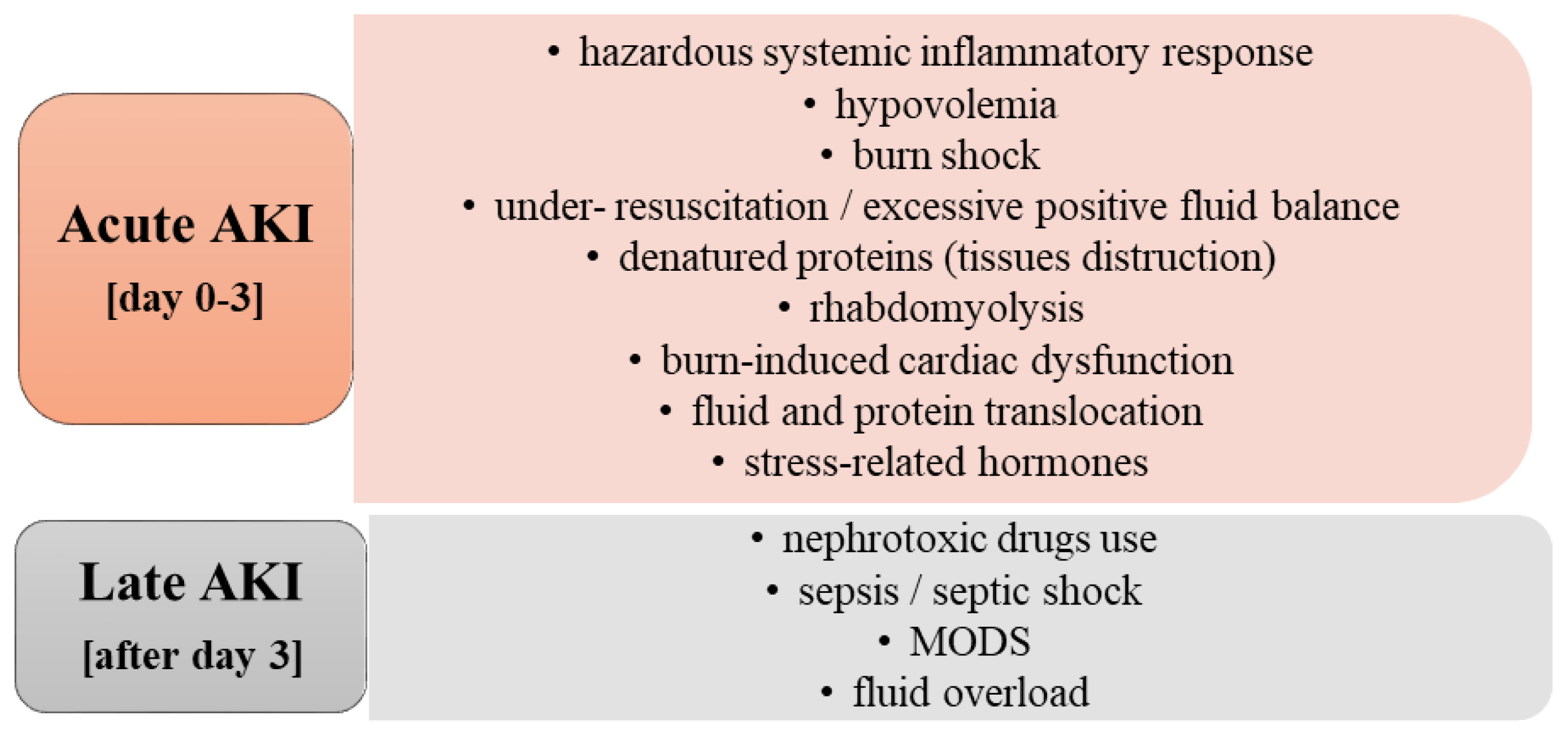
2. Etiology and Risk Factors of Acute Kidney Injury in Burn Patients
2.1. Acute AKI
- ▪
- Hemodynamic changes,
- Abnormal renal autoregulation—in cases of a systolic blood pressure value < 80 mmHg or due to an insult that interferes with prostaglandins activity,
- Intrarenal vasoconstriction and the injury of the endothelial cells—an endothelial impairment is noticed that contributes to the decrease of nitric oxide and prostaglandins, the increase of endothelin, and the swelling of endothelial cells, and also an important synthesis of adhesion molecules (such as intracellular adhesion molecule 1). These changes determine significant intrarenal vasoconstriction, abnormal autoregulation, hypoxia, increased synthesis of reactive oxygen species, with a direct impact on the drop fall of GFR,
- Tubuloglomerular feedback—it could present a beneficial role as there is a limitation in delivering sodium to the impaired tubules, once the GFR decreases.
- ▪
- Injury of the tubular epithelial cells
- Cell death—it occurs only in a few renal tubules, especially due to apoptosis, not necrosis,
- Disruption of actin cytoskeleton—it is responsible for the loss of polarity that, in the proximal convoluted tubule, contributes to impaired sodium reabsorption and, consequently, elevated distal sodium chloride, which represents the trigger for the onset of tubuloglomerular feedback. Furthermore, actin cytoskeleton disruption determines cell detachment and decreased cell-matrix adhesion leading to an accumulation of tubular cells in the tubules; consequently, cast formation and intratubular obstruction are emphasized,
- Backleak—its onset is linked to the loss of adhesion molecules and junction proteins, leading to the leak back of the filtrate into the renal interstitium, favoring also interstitial inflammation. This abnormality is especially noticed in severe forms of acute tubular necrosis.
- ▪
- Inflammatory state—due to the presence of ischemia, different pro-inflammatory immune mechanisms are activated
- High synthesis of toll-like receptors 2 and 4 that contribute to chemokines release,
- Activation of the alternative pathway of the complement that favors the synthesis of cytokines (such as interleukin 6, tumor necrosis factor-α, etc.) and chemokines, contributing to the increase of leukocytes and a direct vasoactive effect,
- Neutrophil activation along with the presence of reactive species of oxygen and proteases will lead to an increased renal injury. Similarly, the activation of monocytes will exacerbate the severity of the injury,
- Additionally, T and B cell upregulation can contribute to the extension of renal impairment.
- ▪
- Sympathetic overactivity simultaneous with an insufficient response of the adrenal gland,
- ▪
- Hypovolemia that leads to important myocardial ischemia,
- ▪
- Tumor necrosis factor-alpha (TNF-α) activation, which has a direct impact on myocardial suppression; in the presence of endotoxins or thermal injury, the myocytes synthesize TNF-α, which contributes to an impaired response to catecholamine, to a low ejection fraction, and even to the presence of biventricular dilatation.
2.2. Late AKI
- ▪
- Intraabdominal hypertension, defined as an increased intraabdominal pressure over 12 mm Hg, represents an important risk factor. In face of intraabdominal hypertension >20 mmHg (considered the value mostly associated with organ dysfunction onset) and abdominal syndrome development, the renal perfusion is decreased inducing reduced GFR, which along with inflammatory cytokines and denatured proteins, contributes to burn-induced AKI progression;
- ▪
- Interstitial edema leads to increased interstitial pressure, altered renal oxygenation, and impaired cellular junctions; the kidneys’ response to this new high pressure is inadequate due to the renal capsule limitation. All these disturbances produced by the presence of interstitial edema are contributing to the onset of renal congestion, decreased renal perfusion, and a significant lowered GFR, which finally determine the development of AKI;
- ▪
- Endothelial dysfunction produces glycocalyx impairment and capillary leakage that determine interstitial edema, and reduced systemic intravascular volume, leading to decreased renal perfusion and subsequently to AKI;
- ▪
- Atrial natriuretic peptide (ANP) is synthetized due to hypervolemia that leads to the stretching of atria and blood vessels. The presence of ANP contributes to the impairment of glycocalyx and further on the capillary leakage that, as already mentioned, are incriminated in the development of AKI;
- ▪
- Bowel wall edema favors the access of bacteria into the systemic vascular circulation, leading to sepsis that represents an important cause of AKI by altering the endothelial function.
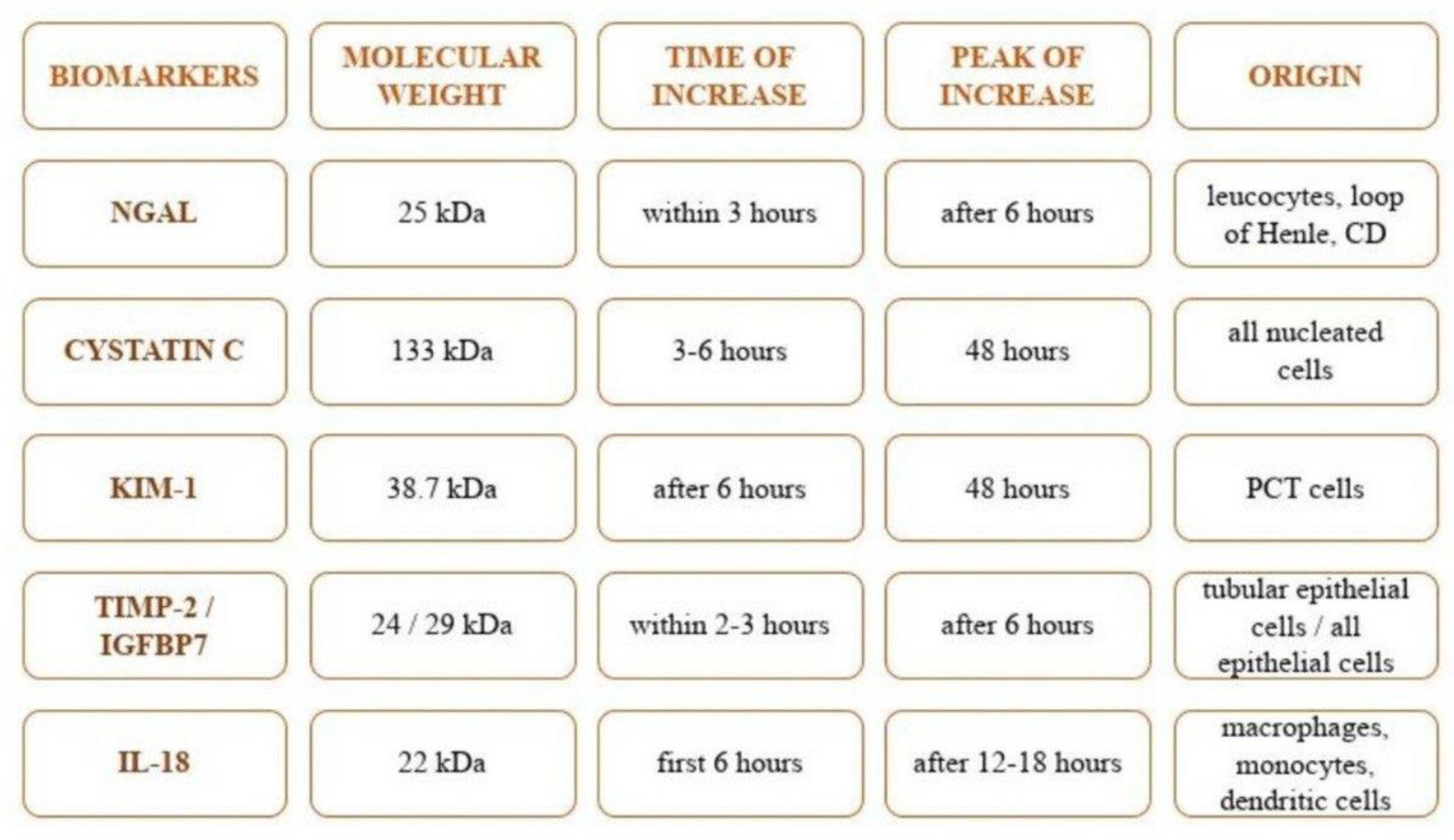
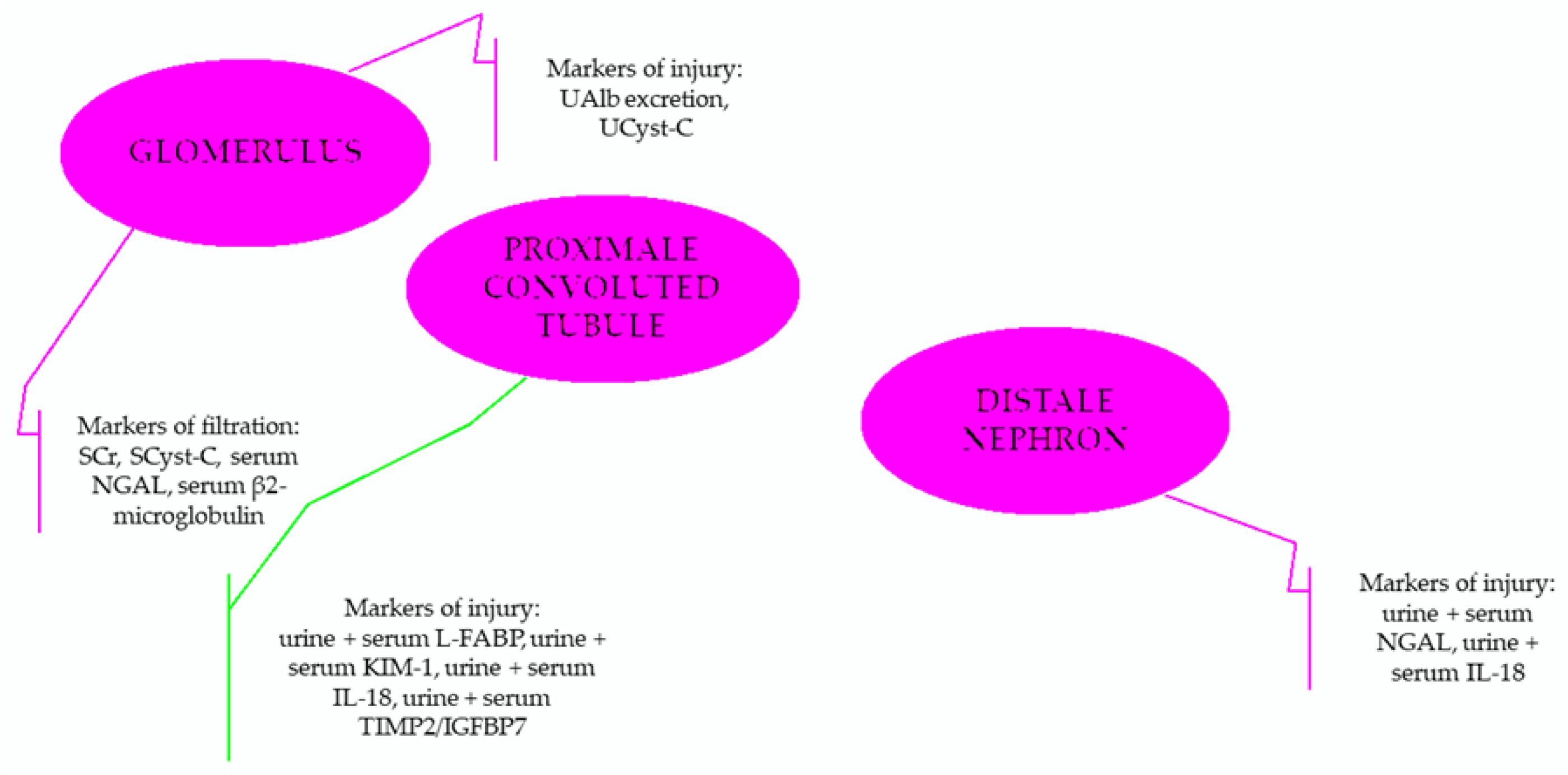
3. Particular Biomarkers of Renal Injury
- For the assessment of homeostasis (including the required volume repletion)—urine output,
- For the evaluation of glomerular filtration—serum creatinine,
- For the assessment of tubular injury—NGAL (neutrophil gelatinase-associated lipocalin),
- For determining the renal function reserve—furosemide stress test and measured renal functional reserve (using high oral protein intake to evaluate renal response to this significant protein load; GFR was measured before and after the intake),
- For monitoring the system stress—TIMP-2 (tissue inhibitor of metalloproteinase-2) and IGFBP7 (insulin-like growth factor-binding protein 7).
3.1. Neutrophil Gelatinase-Associated Lipocalin (NGAL)
3.2. Cystatin C
3.3. Kidney Injury Molecule-1 (KIM-1)
3.4. Tissue Inhibitor of Metalloproteinase-2 (TIMP-2) and Insulin-like Growth Factor-Binding Protein 7 (IGFBP7)
3.5. Interleukin-18 (IL-18)
- Serum creatinine + NGAL—can predict the risk of mortality, lengths of hospital stay, the need for intensive care management, and also renal replacement therapy requirement.
- Serum creatinine + NGAL + KIM-1—predict better the need of renal replacement therapy and the risk of mortality within 7 days form the onset of AKI comparing with only the evaluation of serum creatinine.
- NGAL + Cyst-C—highlight the risk of severe forms of AKI onset.
4. Clinical Use of Particular Biomarkers in Burn-Induced AKI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Putra, O.N.; Saputro, I.D.; Diana, D. Rifle Criteria for Acute Kidney Injury in Burn Patients: Prevalence and Risk Factors. Ann. Burns Fire Disasters 2021, 34, 252–258. [Google Scholar] [PubMed]
- Palmieri, T.; Lavrentieva, A.; Greenhalgh, D.G. Acute kidney injury in critically ill burn patients. Risk factors, progression and impact on mortality. Burns 2010, 36, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin. Pract. 2012, 120, 179–184. [Google Scholar] [CrossRef]
- Soni, S.S.; Ronco, C.; Katz, N.; Cruz, D.N. Early diagnosis of acute kidney injury: The promise of novel biomarkers. Blood Purif. 2009, 28, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S. Defining AKD: The Spectrum of AKI, AKD, and CKD. Nephron 2022, 146, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Meshram, S.V. Predictors of Mortality in Acute Kidney Injury Patients Admitted to Medicine Intensive Care Unit in a Rural Tertiary Care Hospital. Indian J. Crit. Care Med. 2018, 22, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Neyra, J.A.; Madni, T.; Imran, J.; Phelan, H.; Arnoldo, B.; Wolf, S.E. Acute kidney injury after burn. Burns 2017, 43, 898–908. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Yang, Z.; Zhang, Y.; Chen, Y.; Gong, Y.; Chen, Y.; Chen, J.; Yuan, L.; Luo, G.; Peng, Y.; et al. Late-Onset Acute Kidney Injury is a Poor Prognostic Sign for Severe Burn Patients. Front. Surg. 2022, 9, 842999. [Google Scholar] [CrossRef]
- Witkowski, W.; Kaweck, M.; Surowiecka-Pastewka, A.; Klimm, W.; Szamotulska, K.; Niemczyk, S. Early and Late Acute Kidney Injury in Severely Burned Patients. Med. Sci. Monit. 2016, 22, 3755–3763. [Google Scholar] [CrossRef]
- Coca, S.G.; Bauling, P.; Schifftner, T.; Howard, C.S.; Teitelbaum, I.; Parikh, C.R. Contribution of acute kidney injury toward morbidity and mortality in burns: A contemporary analysis. Am. J. Kidney Dis. 2007, 49, 517–523. [Google Scholar] [CrossRef]
- Dépret, F.; Boutin, L.; Jarkovský, J.; Chaussard, M.; Soussi, S.; Bataille, A.; Oueslati, H.; Moreno, N.; de Tymowski, C.; Parenica, J.; et al. Prediction of major adverse kidney events in critically ill burn patients. Burns 2018, 44, 1887–1894. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.T.; Li, X.; Kulangara, R.; Adams-Huet, B.; Huen, S.C.; Madni, T.D.; Imran, J.B.; Phelan, H.A.; Arnoldo, B.D.; Moe, O.W.; et al. Acute Kidney Injury After Burn: A Cohort Study from the Parkland Burn Intensive Care Unit. J. Burn. Care Res. 2019, 40, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Folkestad, T.; Brurberg, K.G.; Nordhuus, K.M.; Tveiten, C.K.; Guttormsen, A.B.; Os, I.; Beitland, S. Acute kidney injury in burn patients admitted to the intensive care unit: A systematic review and meta-analysis. Crit. Care 2020, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Brusselaers, N.; Monstrey, S.; Colpaert, K.; Decruyenaere, J.; Blot, S.I.; Hoste, E.A. Outcome of acute kidney injury in severe burns: A systematic review and meta-analysis. Intensive Care Med. 2010, 36, 915–925. [Google Scholar] [CrossRef]
- Ko, A.; Song, J.; Golovko, G.; El Ayadi, A.; Ozhathil, D.K.; Wermine, K.; Africa, R.E.; Gotewal, S.; Reynolds, S.; Wolf, S.E. Higher risk of acute kidney injury and death with rhabdomyolysis in severely burned patients. Surgery 2022, 171, 1412–1416. [Google Scholar] [CrossRef] [PubMed]
- Planas, M.; Wachtel, T.; Frank, H.; Henderson, L.W. Characterization of acute renal failure in the burned patient. Arch. Intern. Med. 1982, 142, 2087–2091. [Google Scholar] [CrossRef]
- Wu, G.; Xiao, Y.; Wang, C.; Hong, X.; Sun, Y.; Ma, B.; Wang, G.; Xia, Z. Risk Factors for Acute Kidney Injury in Patients with Burn Injury: A Meta-Analysis and Systematic Review. J. Burn Care Res. 2017, 38, 271–282. [Google Scholar] [CrossRef]
- Yang, H.T.; Yim, H.; Cho, Y.S.; Kym, D.; Hur, J.; Kim, J.H.; Chun, W.; Kim, H.S. Assessment of biochemical markers in the early post-burn period for predicting acute kidney injury and mortality in patients with major burn injury: Comparison of serum creatinine, serum cystatin-C, plasma and urine neutrophil gelatinase-associated lipocalin. Crit. Care 2014, 8, R151. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Barrow, R.E.; Wolf, S.E.; Herndon, D.N. Mortality in burned children with acute renal failure. Arch. Surg. 1998, 133, 752–756. [Google Scholar] [CrossRef]
- Schrier, R.W.; Wang, W. Acute renal failure and sepsis. N. Engl. J. Med. 2004, 351, 159–169. [Google Scholar] [CrossRef]
- Prowle, J.R.; Kirwan, C.J.; Bellomo, R. Fluid management for the prevention and attenuation of acute kidney injury. Nat. Rev. Nephrol. 2014, 10, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Wasung, M.E.; Chawla, L.S.; Madero, M. Biomarkers of renal function, which and when? Clin. Chim. Acta 2015, 438, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, O.; Golbetz, H.; Kriss, J.P.; Myers, B.D. Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int. 1985, 28, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Kym, D.; Cho, Y.S.; Yoon, J.; Yim, H.; Yang, H.T. Evaluation of diagnostic biomarkers for acute kidney injury in major burn patients. Ann. Surg. Treat. Res. 2015, 88, 281–288. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bellomo, R.; Ronco, C.; Mehta, R.L.; Asfar, P.; Boisramé-Helms, J.; Darmon, M.; Diehl, J.L.; Duranteau, J.; Hoste, E.A.J.; Olivier, J.B.; et al. Acute kidney injury in the ICU: From injury to recovery: Reports from the 5th Paris International Conference. Ann. Intensive Care 2017, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Wang, W.; Poole, B.; Mitra, A. Acute renal failure: Definitions, diagnosis, pathogenesis, and therapy. J. Clin. Investig. 2004, 114, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.E.; Sarhane, K.A.; Fagan, S.P.; Goverman, J. Renal dysfunction in burns: A review. Ann. Burns Fire Disasters 2013, 26, 16–25. [Google Scholar]
- Chun, W.; Kim, Y.; Yoon, J.; Lee, S.; Yim, H.; Cho, Y.S.; Kym, D.; Hur, J.; Yang, H.T. Assessment of Plasma Neutrophil Gelatinase-Associated Lipocalin for Early Detection of Acute Kidney Injury and Prediction of Mortality in Severely Burned Patients. J. Burn Care Res. 2018, 39, 387–393. [Google Scholar] [CrossRef]
- Yim, H.; Kym, D.; Seo, D.K.; Yoon, J.; Yang, H.T.; Lee, J.; Cho, Y.S.; Hur, J.; Chun, W.; Han, S.W. Serum cystatin C and microalbuminuria in burn patients with acute kidney injury. Eur. J. Clin. Investig. 2015, 45, 594–600. [Google Scholar] [CrossRef]
- Sakyi, S.A.; Ephraim, R.K.D.; Adoba, P.; Amoani, B.; Buckman, T.; Mantey, R.; Eghan, B.A. Tissue inhibitor metalloproteinase 2 (TIMP-2) and insulin-like growth factor binding protein 7 (IGFBP7) best predicts the development of acute kidney injury. Heliyon 2021, 7, e07960. [Google Scholar] [CrossRef]
- Kang, H.K.; Kim, D.K.; Lee, B.H.; Om, A.S.; Hong, J.H.; Koh, H.C.; Lee, C.H.; Shin, I.C.; Kang, J.S. Urinary N-acetyl-beta-D-glucosaminidase and malondialdehyde as a markers of renal damage in burned patients. J. Korean Med. Sci. 2001, 16, 598–602. [Google Scholar] [CrossRef] [PubMed]
- van Kuijk, J.P.; Flu, W.J.; Chonchol, M.; Hoeks, S.E.; Winkel, T.A.; Verhagen, H.J.; Bax, J.J.; Poldermans, D. Temporary perioperative decline of renal function is an independent predictor for chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.F.; Wu, V.C.; Huang, T.M.; Yeh, Y.C.; Wang, K.C.; Han, Y.Y.; Lin, Y.F.; Jhuang, Y.J.; Chao, C.T.; Shiao, C.C.; et al. Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit. Care 2012, 16, R123. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Lio, C.F.; Shih, S.C.; Lin, C.J.; Chen, Y.T.; Yu, C.M.; Sun, F.J.; Kuo, C.F.; Jia, X. The predisposing factors of AKI for prophylactic strategies in burn care. PeerJ 2020, 8, e9984. [Google Scholar] [CrossRef] [PubMed]
- Rakkolainen, I.; Lindbohm, J.V.; Vuola, J. Factors associated with acute kidney injury in the Helsinki Burn Centre in 2006–2015. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 105. [Google Scholar] [CrossRef] [PubMed]
- Holm, C.; Hörbrand, F.; von Donnersmarck, G.H.; Mühlbauer, W. Acute renal failure in severely burned patients. Burns 1999, 25, 171–178. [Google Scholar] [CrossRef]
- Mariano, F.; De Biase, C.; Hollo, Z.; Deambrosis, I.; Davit, A.; Mella, A.; Bergamo, D.; Maffei, S.; Rumbolo, F.; Papaleo, A.; et al. Long-Term Preservation of Renal Function in Septic Shock Burn Patients Requiring Renal Replacement Therapy for Acute Kidney Injury. J. Clin. Med. 2021, 10, 5760. [Google Scholar] [CrossRef]
- Ho, G.; Camacho, F.; Rogers, A.; Cartotto, R. Early Acute Kidney Injury Following Major Burns. J. Burn Care Res. 2021, 42, 126–134. [Google Scholar] [CrossRef]
- Shashaty, M.G.; Meyer, N.J.; Localio, A.R.; Gallop, R.; Bellamy, S.L.; Holena, D.N.; Lanken, P.N.; Kaplan, S.; Yarar, D.; Kawut, S.M.; et al. African American race, obesity, and blood product transfusion are risk factors for acute kidney injury in critically ill trauma patients. J. Crit. Care 2012, 27, 496–504. [Google Scholar] [CrossRef]
- Emara, S.S.; Alzaylai, A.A. Renal failure in burn patients: A review. Ann Burns Fire Disasters 2013, 26, 12–15. [Google Scholar]
- Sharfuddin, A.A.; Molitoris, B.A. Pathophysiology of ischemic acute kidney injury. Nat. Rev. Nephrol. 2011, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Gaut, J.P.; Liapis, H. Acute kidney injury pathology and pathophysiology: A retrospective review. Clin. Kidney J. 2020, 14, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers 2021, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Haseley, L.; Jefferson, J.A. Pathophysiology and etiology of acute kidney injury. In Comprehensive Clinical Nephrology, 6th ed.; Feehally, J., Floege, J., Tonelli, M., Johnson, R.J., Eds.; Elsevier: Philadelphia, PA, USA, 2019; pp. 786–801. [Google Scholar]
- Mosier, M.J.; Pham, T.N.; Klein, M.B.; Gibran, N.S.; Arnoldo, B.D.; Gamelli, R.L.; Tompkins, R.G.; Herndon, D.N. Early acute kidney injury predicts progressive renal dysfunction and higher mortality in severely burned adults. J. Burn Care Res. 2010, 31, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, D.M.; Gómez, B.I.; Dubick, M.A. Molecular mechanisms of trauma-induced acute kidney injury: Inflammatory and metabolic insights from animal models. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863 Pt B, 2661–2671. [Google Scholar] [CrossRef]
- Jeschke, M.G. Postburn Hypermetabolism: Past, Present, and Future. J. Burn Care Res. 2016, 37, 86–96. [Google Scholar] [CrossRef]
- Koonrangsesomboon, W.; Khwannimit, B. Impact of positive fluid balance on mortality and length of stay in septic shock patients. Indian J. Crit. Care Med. 2015, 19, 708–713. [Google Scholar] [CrossRef]
- Patil, V.P.; Salunke, B.G. Fluid overload and acute kidney injury. Indian J. Crit. Care Med. 2020, 24, S94–S97. [Google Scholar] [CrossRef]
- Chen, B.; Zhao, J.; Zhang, Z.; Li, G.; Jiang, H.; Huang, Y.; Li, X. Clinical characteristics and risk factors for severe burns complicated by early acute kidney injury. Burns 2020, 46, 1100–1106. [Google Scholar] [CrossRef]
- Thalji, S.Z.; Kothari, A.N.; Kuo, P.C.; Mosier, M.J. Acute Kidney Injury in Burn Patients: Clinically Significant Over the Initial Hospitalization and 1 Year After Injury: An Original Retrospective Cohort Study. Ann. Surg. 2017, 266, 376–382. [Google Scholar] [CrossRef]
- Tan, B.K.; Liew, Z.H.; Kaushik, M.; Cheah, A.K.W.; Tan, H.K. Early Initiation of Renal Replacement Therapy Among Burned Patients with Acute Kidney Injury. Ann. Plast. Surg. 2020, 84, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Su, C.L.; Chang, G.H.; Tsai, I.J.; Hsu, C.Y.; Wang, I.K.; Chang, C.C. Factors Impacting Survival in Patients with Major Burn-Induced Acute Kidney Injury Postrenal Replacement Therapy: A Nationwide Study With 15 Years Follow-Up in Taiwan. Ann. Plast. Surg. 2021, 86, S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, L.A.; Wilson, S.; Walker, R.G.; Singer, Y.; Cleland, H. Acute Kidney Injury: It’s not just the ‘big’ burns. Injury 2018, 49, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, H.H.; Sen, S.; Palmieri, T.L.; Blackmon, T.; Wajda, J.; Tran, N.K. Early Recognition of Burn- and Trauma-Related Acute Kidney Injury: A Pilot Comparison of Machine Learning Techniques. Sci. Rep. 2020, 10, 205. [Google Scholar] [CrossRef]
- Koyner, J.L.; Zarbock, A.; Basu, R.K.; Ronco, C. The impact of biomarkers of acute kidney injury on individual patient care. Nephrol. Dial. Transplant. 2020, 35, 1295–1305. [Google Scholar] [CrossRef]
- Zheng, C.M.; Liao, M.T.; Lin, M.Y.; Lo, L.; Wu, C.C.; Hsu, Y.H.; Lin, Y.F.; Lu, K.C. Biomarkers in acute kidney injury. Open J. Nephrol. 2013, 3, 51–60. [Google Scholar] [CrossRef][Green Version]
- Mishra, J.; Dent, C.; Tarabishi, R.; Mitsnefes, M.M.; Ma, Q.; Kelly, C.; Ruff, S.M.; Zahedi, K.; Shao, M.; Bean, J.; et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005, 365, 1231–1238. [Google Scholar] [CrossRef]
- Hong, D.Y.; Lee, J.H.; Park, S.O.; Baek, K.J.; Lee, K.R. Plasma neutrophil gelatinase-associated lipocalin as early biomarker for acute kidney injury in burn patients. J. Burn Care Res. 2013, 34, e326–e332. [Google Scholar] [CrossRef]
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Barasch, J.; Devarajan, P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543. [Google Scholar] [CrossRef]
- Supavekin, S.; Zhang, W.; Kucherlapati, R.; Kaskel, F.J.; Moore, L.C.; Devarajan, P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003, 63, 1714–1724. [Google Scholar] [CrossRef]
- Bolignano, D.; Donato, V.; Coppolino, G.; Campo, S.; Buemi, A.; Lacquaniti, A.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) as a marker of kidney damage. Am. J. Kidney Dis. 2008, 52, 595–605. [Google Scholar] [CrossRef] [PubMed]
- Howell, E.; Sen, S.; Palmieri, T.; Godwin, Z.; Bockhold, J.; Greenhalgh, D.; Tran, N.K. Point-of-care B-type natriuretic peptide and neutrophil gelatinase-associated lipocalin measurements for acute resuscitation: A pilot study. J. Burn Care Res. 2015, 36, e26–e33. [Google Scholar] [CrossRef] [PubMed]
- Rakkolainen, I.; Vuola, J. Plasma NGAL predicts early acute kidney injury no earlier than s-creatinine or cystatin C in severely burned patients. Burns 2016, 42, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P. Review: Neutrophil gelatinase-associated lipocalin: A troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010, 15, 419–428. [Google Scholar] [CrossRef]
- Axelsson, L.; Bergenfeldt, M.; Ohlsson, K. Studies of the release and turnover of a human neutrophil lipocalin. Scand. J. Clin. Lab. Investig. 1995, 55, 577–588. [Google Scholar] [CrossRef]
- Sen, S.; Godwin, Z.R.; Palmieri, T.; Greenhalgh, D.; Steele, A.N.; Tran, N.K. Whole blood neutrophil gelatinase-associated lipocalin predicts acute kidney injury in burn patients. J. Surg. Res. 2015, 196, 382–387. [Google Scholar] [CrossRef]
- Constantin, J.M.; Futier, E.; Perbet, S.; Roszyk, L.; Lautrette, A.; Gillart, T.; Guerin, R.; Jabaudon, M.; Souweine, B.; Bazin, J.E.; et al. Plasma neutrophil gelatinase-associated lipocalin is an early marker of acute kidney injury in adult critically ill patients: A prospective study. J. Crit. Care 2010, 25, e1–e6. [Google Scholar] [CrossRef]
- Desanti De Oliveira, B.; Xu, K.; Shen, T.H.; Callahan, M.; Kiryluk, K.; D’Agati, V.D.; Tatonetti, N.P.; Barasch, J.; Devarajan, P. Molecular nephrology: Types of acute tubular injury. Nat. Rev. Nephrol. 2019, 15, 599–612. [Google Scholar] [CrossRef]
- Herget-Rosenthal, S.; Marggraf, G.; Hüsing, J.; Göring, F.; Pietruck, F.; Janssen, O.; Philipp, T.; Kribben, A. Early detection of acute renal failure by serum cystatin C. Kidney Int. 2004, 66, 1115–1122. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, Y.S.; Kym, D.; Yoon, J.; Yim, H.; Hur, J.; Chun, W. Diagnostic performance of plasma and urine neutrophil gelatinase-associated lipocalin, cystatin C, and creatinine for acute kidney injury in burn patients: A prospective cohort study. PLoS ONE 2018, 13, e0199600. [Google Scholar] [CrossRef]
- Stevens, L.A.; Coresh, J.; Schmid, C.H.; Feldman, H.I.; Froissart, M.; Kusek, J.; Rossert, J.; Van Lente, F.; Bruce, R.D., 3rd; Zhang, Y.L.; et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3418 individuals with CKD. Am. J. Kidney Dis. 2008, 51, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Schmid, C.H.; Greene, T.; Li, L.; Beck, G.J.; Joffe, M.M.; Froissart, M.; Kusek, J.W.; Zhang, Y.L.; Coresh, J.; et al. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009, 75, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.I.; Tang, S.C.; Lai, K.N.; Leung, J.C. Kidney injury molecule-1: More than just an injury marker of tubular epithelial cells? J. Cell Physiol. 2013, 228, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Han, W.K.; Bailly, V.; Abichandani, R.; Thadhani, R.; Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A novel biomarker for human renal proximal tubule injury. Kidney Int. 2002, 62, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Cruz, D.N.; Goh, C.Y.; Haase-Fielitz, A.; Ronco, C.; Haase, M. Early biomarkers of renal injury. Congest. Heart Fail. 2010, 16 (Suppl. S1), S25–S31. [Google Scholar] [CrossRef]
- Arthur, J.M.; Hill, E.G.; Alge, J.L.; Lewis, E.C.; Neely, B.A.; Janech, M.G.; Tumlin, J.A.; Chawla, L.S.; Shaw, A.D.; SAKInet Investigators. Evaluation of 32 urine biomarkers to predict the progression of acute kidney injury after cardiac surgery. Kidney Int. 2014, 85, 431–438. [Google Scholar] [CrossRef]
- Alge, J.L.; Arthur, J.M. Biomarkers of AKI: A review of mechanistic relevance and potential therapeutic implications. Clin. J. Am. Soc. Nephrol. 2015, 10, 147–155. [Google Scholar] [CrossRef]
- Parikh, C.R.; Thiessen-Philbrook, H.; Garg, A.X.; Kadiyala, D.; Shlipak, M.G.; Koyner, J.L.; Edelstein, C.L.; Devarajan, P.; Patel, U.D.; Zappitelli, M.; et al. Performance of kidney injury molecule-1 and liver fatty acid-binding protein and combined biomarkers of AKI after cardiac surgery. Clin. J. Am. Soc. Nephrol. 2013, 8, 1079–1088. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Radu, S.; Costea, C.F.; Ciocoiu, M.; Carauleanu, A.; Lacatusu, C.M.; Maranduca, M.A.; Floria, M.; Rezus, C. The Predictive Role of the Biomarker Kidney Molecule-1 (KIM-1) in Acute Kidney Injury (AKI) Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2019, 20, 5238. [Google Scholar] [CrossRef]
- Price, P.M.; Safirstein, R.L.; Megyesi, J. The cell cycle and acute kidney injury. Kidney Int. 2009, 76, 604–613. [Google Scholar] [CrossRef]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; et al. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit. Care 2013, 17, R25. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C. Acute kidney injury: From clinical to molecular diagnosis. Crit. Care 2016, 20, 201. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Ankawi, G.; Zhang, J.; Digvijay, K.; Giavarina, D.; Yin, Y.; Ronco, C. Current understanding and future directions in the application of TIMP-2 and IGFBP7 in AKI clinical practice. Clin. Chem. Lab. Med. 2019, 57, 567–576. [Google Scholar] [CrossRef]
- Emlet, D.R.; Wen, X.; Kellum, J.A. Comments on the Review ‘Biomarkers in acute kidney injury—Pathophysiological basis and clinical performance’ Acta Physiol 2017, 219, 556–574: An update on kidney localization of IGFBP7 and TIMP2. Acta Physiol. (Oxf.) 2018, 222, e12934. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Gong, Z.; Wu, Y.; Tian, Y.; Liao, X. Diagnostic Value of Urine Tissue Inhibitor of Metalloproteinase-2 and Insulin-Like Growth Factor-Binding Protein 7 for Acute Kidney Injury: A Meta-Analysis. PLoS ONE 2017, 12, e0170214. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.Y.; Lim, S.Y.; Yang, J.H.; Oh, S.W.; Kim, M.G.; Jo, S.K. Urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor-binding protein 7 as biomarkers of patients with established acute kidney injury. Korean J. Intern. Med. 2020, 35, 662–671. [Google Scholar] [CrossRef]
- Oh, D.J. A long journey for acute kidney injury biomarkers. Ren. Fail. 2020, 42, 154–165. [Google Scholar] [CrossRef]
- Ren, H.; Zhou, X.; Dai, D.; Liu, X.; Wang, L.; Zhou, Y.; Luo, X.; Cai, Q. Assessment of urinary kidney injury molecule-1 and interleukin-18 in the early post-burn period to predict acute kidney injury for various degrees of burn injury. BMC Nephrol. 2015, 16, 142. [Google Scholar] [CrossRef]
- Melnikov, V.Y.; Ecder, T.; Fantuzzi, G.; Siegmund, B.; Lucia, M.S.; Dinarello, C.A.; Schrier, R.W.; Edelstein, C.L. Impaired IL-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. J. Clin. Investig. 2001, 107, 1145–1152. [Google Scholar] [CrossRef]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef]
- Tsigou, E.; Psallida, V.; Demponeras, C.; Boutzouka, E.; Baltopoulos, G. Role of new biomarkers: Functional and structural damage. Crit. Care Res. Pract. 2013, 2013, 361078. [Google Scholar] [CrossRef] [PubMed]
- Ling, W.; Zhaohui, N.; Ben, H.; Leyi, G.; Jianping, L.; Huili, D.; Jiaqi, Q. Urinary IL-18 and NGAL as early predictive biomarkers in contrast-induced nephropathy after coronary angiography. Nephron Clin. Pract. 2008, 108, c176–c181. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Abraham, E.; Ancukiewicz, M.; Edelstein, C.L. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J. Am. Soc. Nephrol. 2005, 16, 3046–3052. [Google Scholar] [CrossRef] [PubMed]
- Washburn, K.K.; Zappitelli, M.; Arikan, A.A.; Loftis, L.; Yalavarthy, R.; Parikh, C.R.; Edelstein, C.L.; Goldstein, S.L. Urinary interleukin-18 is an acute kidney injury biomarker in critically ill children. Nephrol. Dial. Transplant. 2008, 23, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Coca, S.G.; Thiessen-Philbrook, H.; Shlipak, M.G.; Koyner, J.L.; Wang, Z.; Edelstein, C.L.; Devarajan, P.; Patel, U.D.; Zappitelli, M.; et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J. Am. Soc. Nephrol. 2011, 22, 1748–1757. [Google Scholar] [CrossRef]
- Hirooka, Y.; Nozaki, Y. Interleukin-18 in Inflammatory Kidney Disease. Front. Med. (Lausanne) 2021, 8, 639103. [Google Scholar] [CrossRef]
- Ostermann, M.; Zarbock, A.; Goldstein, S.; Kashani, K.; Macedo, E.; Murugan, R.; Bell, M.; Forni, L.; Guzzi, L.; Joannidis, M.; et al. Recommendations on Acute Kidney Injury Biomarkers from the Acute Disease Quality Initiative Consensus Conference: A Consensus Statement. JAMA Netw. Open 2020, 3, e2019209. [Google Scholar] [CrossRef]
- Rizvi, M.S.; Kashani, K.B. Biomarkers for Early Detection of Acute Kidney Injury. J. Appl. Lab. Med. 2017, 2, 386–399. [Google Scholar] [CrossRef]
- Pajaro-Galvis, N.; Rico-Fontalvo, J.; Daza-Arnedo, R.; Cardona-Blanco, M.X.; Abuabara-Franco, E.; Leal-Martínez, V.; Cabrales-Juan, J.; CorreaGuerrero, J.; Bohórquez-Rivero, J.; Sáenz-López, J.; et al. Biomarkers in acute kidney injury. J. Clin. Nephrol. 2020, 4, 27–35. [Google Scholar] [CrossRef]
- Malyszko, J.; Lukaszyk, E.; Glowinska, I.; Durlik, M. Biomarkers of delayed graft function as a form of acute kidney injury in kidney transplantation. Sci. Rep. 2015, 5, 11684. [Google Scholar] [CrossRef]
- Coca, S.G.; Singanamala, S.; Parikh, C.R. Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int. 2012, 81, 442–448. [Google Scholar] [CrossRef] [PubMed]
- See, E.J.; Jayasinghe, K.; Glassford, N.; Bailey, M.; Johnson, D.W.; Polkinghorne, K.R.; Toussaint, N.D.; Bellomo, R. Long-term risk of adverse outcomes after acute kidney injury: A systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019, 95, 160–172. [Google Scholar] [CrossRef]
- Rakkolainen, I.; Mustonen, K.M.; Vuola, J. Long-Term Outcome After Renal Replacement Therapy in Severe Burns. J. Burn Care Res. 2020, 41, 866–870. [Google Scholar] [CrossRef]
- Honeycutt, A.A.; Segel, J.E.; Zhuo, X.; Hoerger, T.J.; Imai, K.; Williams, D. Medical costs of CKD in the Medicare population. J. Am. Soc. Nephrol. 2013, 24, 1478–1483. [Google Scholar] [CrossRef]
- Ronksley, P.E.; Tonelli, M.; Manns, B.J.; Weaver, R.G.; Thomas, C.M.; MacRae, J.M.; Ravani, P.; Quinn, R.R.; James, M.T.; Lewanczuk, R.; et al. Emergency Department Use among Patients with CKD: A Population-Based Analysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Barry, D.W. Clinical and laboratory diagnosis of acute renal failure. Best Pract. Res. Clin. Anaesthesiol. 2004, 18, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Haase, M.; Kellum, J.A.; Ronco, C. Subclinical AKI--an emerging syndrome with important consequences. Nat. Rev. Nephrol. 2012, 8, 735–739. [Google Scholar] [CrossRef]
- Lameire, N.; Hoste, E. Reflections on the definition, classification, and diagnostic evaluation of acute renal failure. Curr. Opin. Crit. Care 2004, 10, 468–475. [Google Scholar] [CrossRef]
- Rahman, M.; Shad, F.; Smith, M.C. Acute kidney injury: A guide to diagnosis and management. Am. Fam. Physician 2012, 86, 631–639. [Google Scholar]
- Shoaib, M.; Mahmud, S.N.; Safdar, M. Early Diagnosis of Acute Kidney Injury by Urinary Neutrophil Gelatinase Associated Lipocalin in Adult Critically Ill Patients. J. Ayub Med. Coll. Abbottabad 2019, 31, 12–15. [Google Scholar]
- Emami, A.; Javanmardi, F.; Rajaee, M.; Pirbonyeh, N.; Keshavarzi, A.; Fotouhi, M.; Hosseini, S.M. Predictive Biomarkers for Acute Kidney Injury in Burn Patients. J. Burn Care Res. 2019, 40, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Long, Z.; Lin, L.; Feng, Y.; Zhou, N.; Mai, Q. Serum cystatin C is an early biomarker for assessment of renal function in burn patients. Clin. Chem. Lab. Med. 2012, 50, 667–671. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Hollinger, A.; Vieillard-Baron, A.; Dépret, F.; Cariou, A.; Deye, N.; Fournier, M.C.; Jaber, S.; Damoisel, C.; Lu, Q.; et al. One-Year Prognosis of Kidney Injury at Discharge From the ICU: A Multicenter Observational Study. Crit. Care Med. 2019, 47, e953–e961. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.B.; Liu, G.L.; Yu, Z.Q.; Pan, P. Urinary KIM-1, IL-18 and Cys-c as early predictive biomarkers in gadolinium-based contrast-induced nephropathy in the elderly patients. Clin. Nephrol. 2013, 80, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Waskowski, J.; Pfortmueller, C.A.; Schenk, N.; Buehlmann, R.; Schmidli, J.; Erdoes, G.; Schefold, J.C. (TIMP2) × (IGFBP7) as early renal biomarker for the prediction of acute kidney injury in aortic surgery (TIGER). A single center observational study. PLoS ONE 2021, 16, e0244658. [Google Scholar] [CrossRef] [PubMed]
- Hatton, G.E.; Wang, Y.W.; Isbell, K.D.; Finkel, K.W.; Kao, L.S.; Wade, C.E. Urinary cell cycle arrest proteins urinary tissue inhibitor of metalloprotease 2 and insulin-like growth factor binding protein 7 predict acute kidney injury after severe trauma: A prospective observational study. J. Trauma Acute Care Surg. 2020, 89, 761–767. [Google Scholar] [CrossRef]
- Breglia, A.; Godi, I.; Virzì, G.M.; Guglielmetti, G.; Iannucci, G.; De Cal, M.; Brocca, A.; Carta, M.; Giavarina, D.; Ankawi, G.; et al. Subclinical Contrast-Induced Acute Kidney Injury in Patients Undergoing Cerebral Computed Tomography. Cardiorenal Med. 2020, 10, 125–136. [Google Scholar] [CrossRef]
- Bank, J.R.; Ruhaak, R.; Soonawala, D.; Mayboroda, O.; Romijn, F.P.; van Kooten, C.; Cobbaert, C.M.; de Fijter, J.W. Urinary TIMP-2 Predicts the Presence and Duration of Delayed Graft Function in Donation After Circulatory Death Kidney Transplant Recipients. Transplantation 2019, 103, 1014–1023. [Google Scholar] [CrossRef]
- Adler, C.; Heller, T.; Schregel, F.; Hagmann, H.; Hellmich, M.; Adler, J.; Reuter, H. TIMP-2/IGFBP7 predicts acute kidney injury in out-of-hospital cardiac arrest survivors. Crit. Care 2018, 22, 126. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculae, A.; Peride, I.; Tiglis, M.; Sharkov, E.; Neagu, T.P.; Lascar, I.; Checherita, I.A. Burn-Induced Acute Kidney Injury–Two-Lane Road: From Molecular to Clinical Aspects. Int. J. Mol. Sci. 2022, 23, 8712. https://doi.org/10.3390/ijms23158712
Niculae A, Peride I, Tiglis M, Sharkov E, Neagu TP, Lascar I, Checherita IA. Burn-Induced Acute Kidney Injury–Two-Lane Road: From Molecular to Clinical Aspects. International Journal of Molecular Sciences. 2022; 23(15):8712. https://doi.org/10.3390/ijms23158712
Chicago/Turabian StyleNiculae, Andrei, Ileana Peride, Mirela Tiglis, Evgeni Sharkov, Tiberiu Paul Neagu, Ioan Lascar, and Ionel Alexandru Checherita. 2022. "Burn-Induced Acute Kidney Injury–Two-Lane Road: From Molecular to Clinical Aspects" International Journal of Molecular Sciences 23, no. 15: 8712. https://doi.org/10.3390/ijms23158712
APA StyleNiculae, A., Peride, I., Tiglis, M., Sharkov, E., Neagu, T. P., Lascar, I., & Checherita, I. A. (2022). Burn-Induced Acute Kidney Injury–Two-Lane Road: From Molecular to Clinical Aspects. International Journal of Molecular Sciences, 23(15), 8712. https://doi.org/10.3390/ijms23158712





