Evaluation of Orbital Lymphoproliferative and Inflammatory Disorders by Gene Expression Analysis
Abstract
:1. Introduction
2. Results
2.1. Study Population
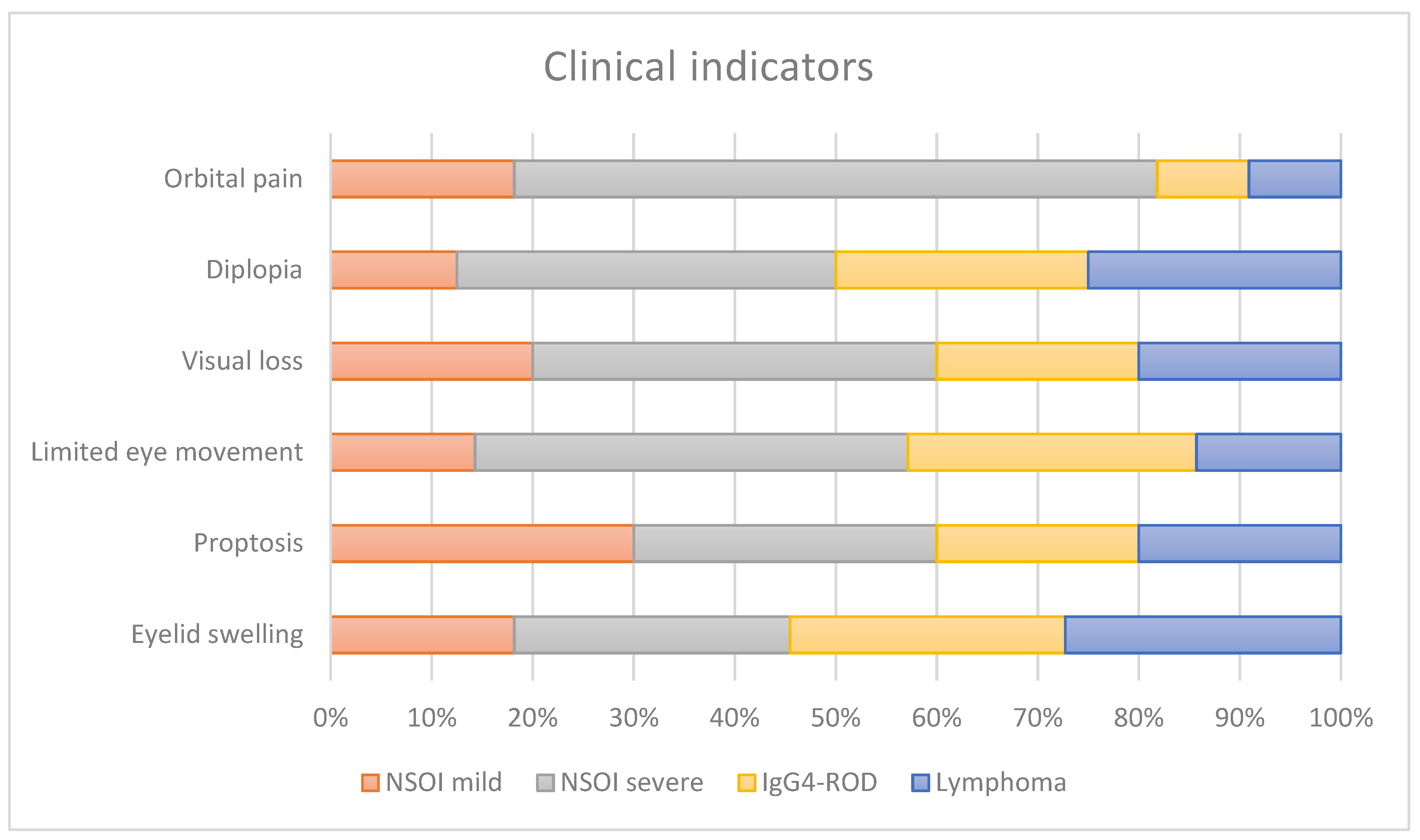
2.2. Clinical Examination
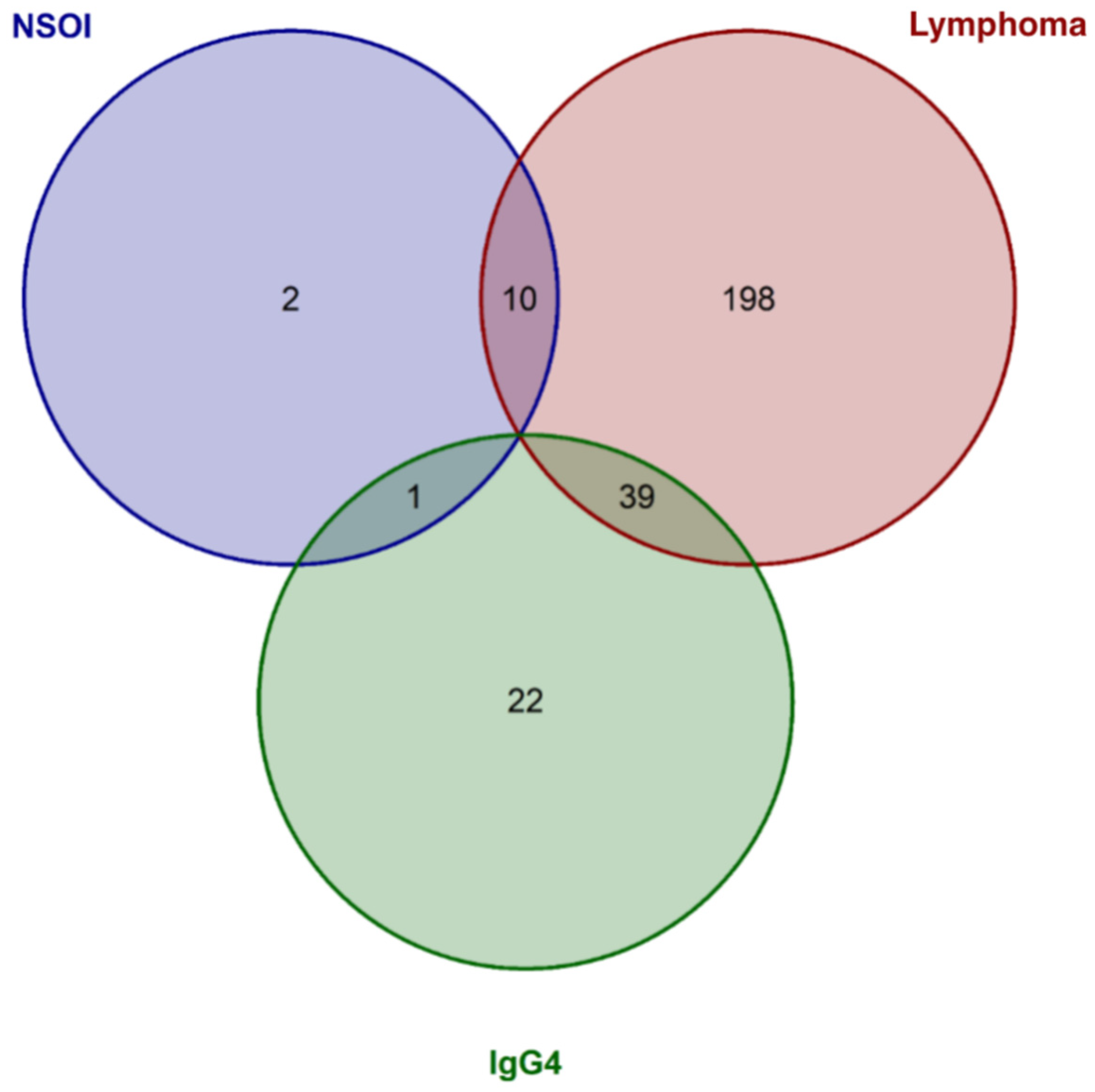
2.3. Expression Analysis
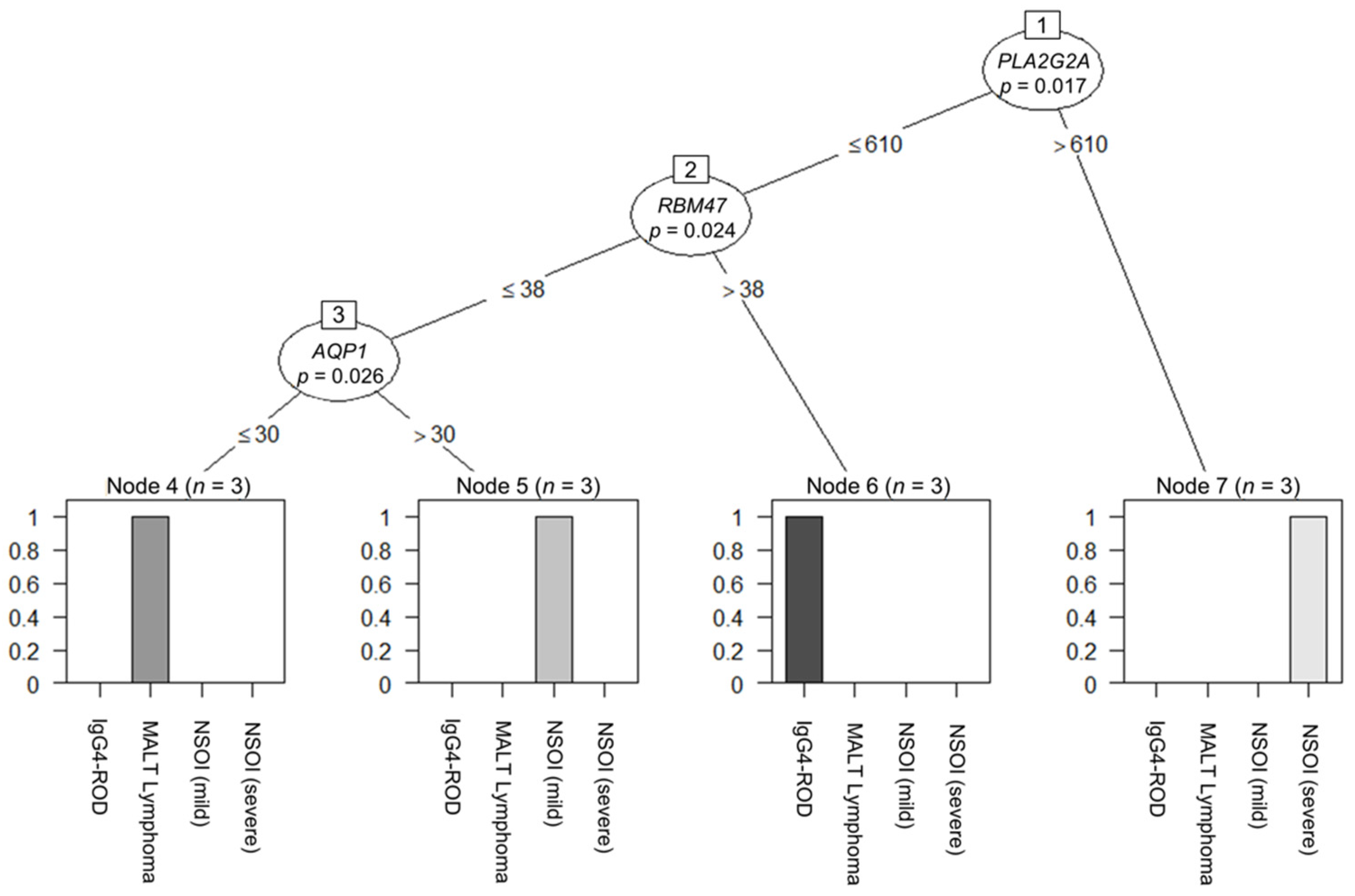
2.4. Decision-Tree-Based Analysis of Differences between Groups
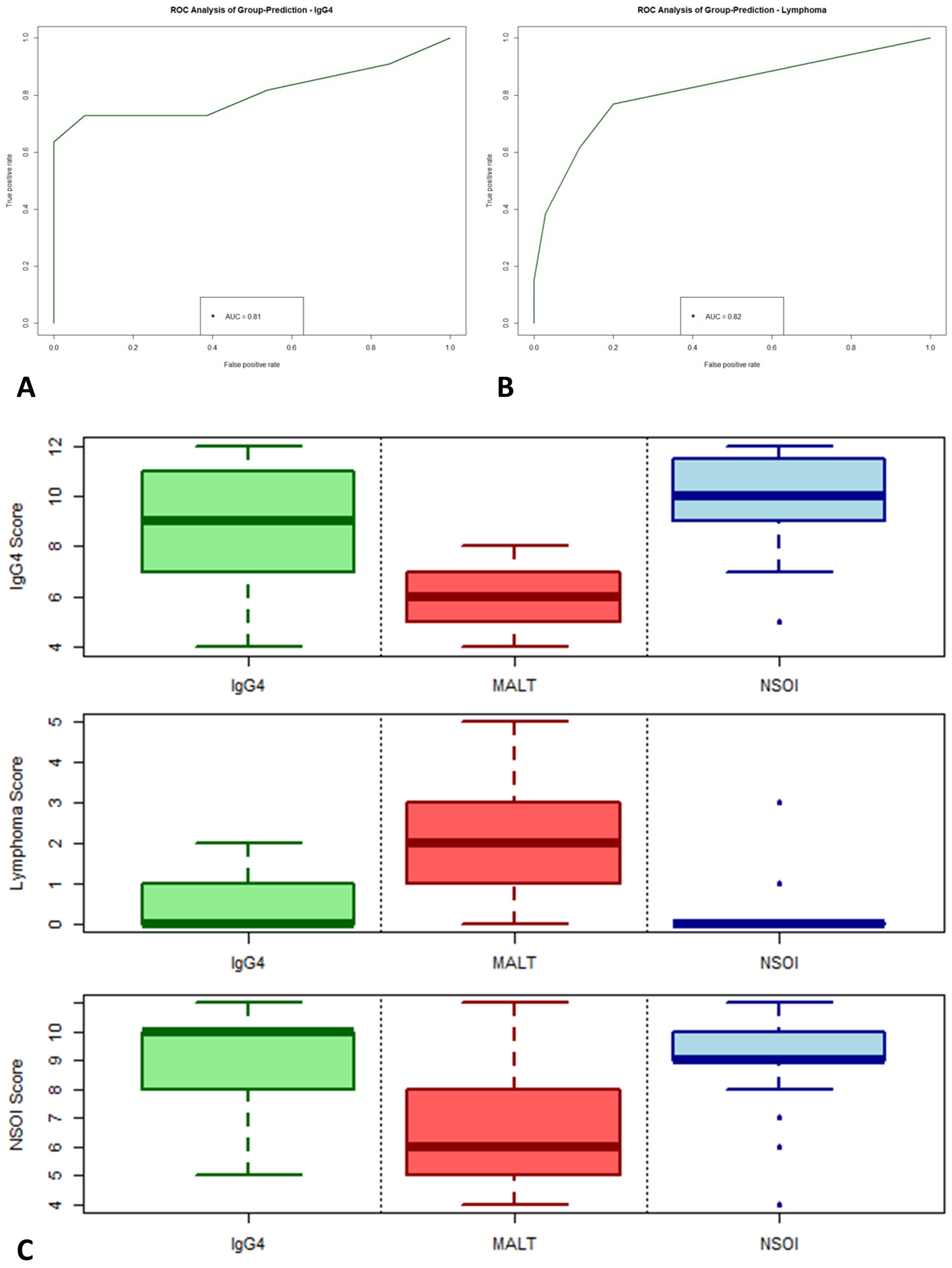
2.5. Calculation of Scores
2.6. Data Validation
3. Discussion
3.1. Clinical Examination
3.2. Gene expression Analysis as Diagnostic Tool
3.3. Progression to MALT Lymphoma
3.4. Limitations
4. Materials and Methods
4.1. Study Population
4.2. Clinical Examination
4.3. Tissue Samples
4.4. RNA Extraction
4.5. nCounter CodeSet Design and Expression Analysis
4.6. Nanostring Data Processing
4.7. Statistical Evaluation
4.8. Model Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, M.J.; Planck, S.R.; Choi, D.; Harrington, C.A.; Wilson, D.J.; Dailey, R.A.; Ng, J.D.; Steele, E.A.; Hamilton, B.E.; Khwarg, S.I.; et al. Non-specific orbital inflammation: Current understanding and unmet needs. Prog. Retin. Eye Res. 2021, 81, 100885. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A.; Shields, C.L. Orbital pseudotumor versus idiopathic nongranulomatous orbital inflammation. Ophthalmic Plast. Reconstr. Surg. 2013, 29, 349. [Google Scholar] [CrossRef] [PubMed]
- Gunalp, I.; Gunduz, K.; Yazar, Z. Idiopathic orbital inflammatory disease. Acta Ophthalmol. Scand. 1996, 74, 191–193. [Google Scholar] [CrossRef]
- Swamy, B.N.; McCluskey, P.; Nemet, A.; Crouch, R.; Martin, P.; Benger, R.; Ghabriel, R.; Wakefield, D. Idiopathic orbital inflammatory syndrome: Clinical features and treatment outcomes. Br. J. Ophthalmol. 2007, 91, 1667–1670. [Google Scholar] [CrossRef] [Green Version]
- Harris, G.J. Idiopathic orbital inflammation: A pathogenetic construct and treatment strategy: The 2005 ASOPRS Foundation Lecture. Ophthalmic Plast. Reconstr. Surg. 2006, 22, 79–86. [Google Scholar] [CrossRef]
- Mombaerts, I.; Bilyk, J.R.; Rose, G.E.; McNab, A.A.; Fay, A.; Dolman, P.J.; Allen, R.C.; Devoto, M.H.; Harris, G.J.; Expert Panel of the Orbital Society. Consensus on Diagnostic Criteria of Idiopathic Orbital Inflammation Using a Modified Delphi Approach. JAMA Ophthalmol. 2017, 135, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Haradome, K.; Haradome, H.; Usui, Y.; Ueda, S.; Kwee, T.C.; Saito, K.; Tokuuye, K.; Matsubayashi, J.; Nagao, T.; Goto, H. Orbital lymphoproliferative disorders (OLPDs): Value of MR imaging for differentiating orbital lymphoma from benign OPLDs. AJNR Am. J. Neuroradiol. 2014, 35, 1976–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrew, N.H.; Sladden, N.; Kearney, D.J.; Selva, D. An analysis of IgG4-related disease (IgG4-RD) among idiopathic orbital inflammations and benign lymphoid hyperplasias using two consensus-based diagnostic criteria for IgG4-RD. Br. J. Ophthalmol. 2015, 99, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wu, Z.; Li, Y. The differentiation of idiopathic inflammatory pseudotumor from lymphoid tumors of orbit: Analysis of 319 cases. Orbit 2004, 23, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Reis, P.P.; Waldron, L.; Goswami, R.S.; Xu, W.; Xuan, Y.; Perez-Ordonez, B.; Gullane, P.; Irish, J.; Jurisica, I.; Kamel-Reid, S. mRNA transcript quantification in archival samples using multiplexed, color-coded probes. BMC Biotechnol. 2011, 11, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, R.F.; Mairinger, F.D.; Wohlschlaeger, J.; Worm, K.; Ting, S.; Vollbrecht, C.; Schmid, K.W.; Hager, T. FFPE tissue as a feasible source for gene expression analysis—A comparison of three reference genes and one tumor marker. Pathol. Res. Pract. 2013, 209, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Goto, H.; Ueda, S.I.; Nemoto, R.; Ohshima, K.I.; Sogabe, Y.; Kitagawa, K.; Ogawa, Y.; Oyama, T.; Furuta, M.; Azumi, A.; et al. Clinical features and symptoms of IgG4-related ophthalmic disease: A multicenter study. Jpn. J. Ophthalmol. 2021, 65, 651–656. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Wang, N.; Li, J.; Ge, X.; Zhang, J.; Ma, J. Clinical Features and Prognoses of IgG4-Positive and IgG4-Negative Lacrimal Lymphomas. Front. Oncol. 2021, 11, 622847. [Google Scholar] [CrossRef] [PubMed]
- McNab, A.A. Orbital Myositis: A Comprehensive Review and Reclassification. Ophthalmic Plast. Reconstr. Surg. 2020, 36, 109–117. [Google Scholar] [CrossRef]
- Suimon, Y.; Kase, S.; Ishijima, K.; Kanno-Okada, H.; Ishida, S. A clinicopathological study on IgG4-related ophthalmic disease. Int. J. Ophthalmol. 2018, 11, 1539–1544. [Google Scholar] [CrossRef]
- Goto, H.; Takahira, M.; Azumi, A.; Japanese Study Group for IgG4-Related Ophthalmic Disease. Diagnostic criteria for IgG4-related ophthalmic disease. Jpn. J. Ophthalmol. 2015, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Usui, Y.; Nagai, T.; Diaz-Aguilar, D.; Nagao, T.; Goto, H. Immunophenotypic profiles for distinguishing orbital mucosa-associated lymphoid tissue lymphoma from benign lymphoproliferative tumors. Jpn. J. Ophthalmol 2017, 61, 354–360. [Google Scholar] [CrossRef] [PubMed]
- McKelvie, P.A. Ocular adnexal lymphomas: A review. Adv. Anat. Pathol. 2010, 17, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Stucke, E.M.; Grotjan, T.M.; Kemme, K.A.; Abonia, J.P.; Putnam, P.E.; Franciosi, J.P.; Garza, J.M.; Kaul, A.; King, E.C.; et al. Molecular diagnosis of eosinophilic esophagitis by gene expression profiling. Gastroenterology 2013, 145, 1289–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeremenko, N.; Noordenbos, T.; Cantaert, T.; van Tok, M.; van de Sande, M.; Canete, J.D.; Tak, P.P.; Baeten, D. Disease-specific and inflammation-independent stromal alterations in spondylarthritis synovitis. Arthritis Rheum. 2013, 65, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Lassner, D.; Kuhl, U.; Siegismund, C.S.; Rohde, M.; Elezkurtaj, S.; Escher, F.; Tschope, C.; Gross, U.M.; Poller, W.; Schultheiss, H.P. Improved diagnosis of idiopathic giant cell myocarditis and cardiac sarcoidosis by myocardial gene expression profiling. Eur. Heart J. 2014, 35, 2186–2195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.M.; Choi, D.; Planck, S.R.; Harrington, C.A.; Austin, C.R.; Lewis, J.A.; Diebel, T.N.; Martin, T.M.; Smith, J.R.; Rosenbaum, J.T. Insights in to the pathogenesis of axial spondyloarthropathy based on gene expression profiles. Arthritis Res. Ther. 2009, 11, R168. [Google Scholar] [CrossRef] [Green Version]
- Higgs, B.W.; Liu, Y.; Guo, J.; Sebastian, Y.; Morehouse, C.; Zhu, W.; Ren, L.; Liu, M.; Du, Y.; Yu, G.; et al. High-throughput RNA sequencing reveals distinct gene signatures in active IgG4-related disease. Sci. Rep. 2017, 7, 17567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, M.S.; Chougule, A.; MacLeay, A.R.; Kurzawa, P.; Chebib, I.; Le, L.; Deshpande, V. Morphologic Overlap Between Inflammatory Myofibroblastic Tumor and IgG4-related Disease: Lessons from Next-generation Sequencing. Am. J. Surg. Pathol. 2019, 43, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Asakage, M.; Usui, Y.; Nezu, N.; Shimizu, H.; Tsubota, K.; Umazume, K.; Yamakawa, N.; Umezu, T.; Suwanai, H.; Kuroda, M.; et al. Comprehensive Gene Analysis of IgG4-Related Ophthalmic Disease Using RNA Sequencing. J. Clin. Med. 2020, 9, 3458. [Google Scholar] [CrossRef]
- Rosenbaum, J.T.; Choi, D.; Harrington, C.A.; Wilson, D.J.; Grossniklaus, H.E.; Sibley, C.H.; Salek, S.S.; Ng, J.D.; Dailey, R.A.; Steele, E.A.; et al. Gene Expression Profiling and Heterogeneity of Nonspecific Orbital Inflammation Affecting the Lacrimal Gland. JAMA Ophthalmol. 2017, 135, 1156–1162. [Google Scholar] [CrossRef]
- Rousseau, G.G. Control of gene expression by glucocorticoid hormones. Biochem. J. 1984, 224, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Mizoguchi, F.; Slowikowski, K.; Wei, K.; Marshall, J.L.; Rao, D.A.; Chang, S.K.; Nguyen, H.N.; Noss, E.H.; Turner, J.D.; Earp, B.E.; et al. Functionally distinct disease-associated fibroblast subsets in rheumatoid arthritis. Nat. Commun. 2018, 9, 789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, S.M.; Chan, A.S.Y.; Jajeh, I.A.; Shen, S.; Seah, L.L.; Choo, C.T.; Lang, S.S.; Looi, A.L.G. Clinical Features and Treatment Outcomes of Orbital Inflammatory Disease in Singapore: A 10-Year Clinicopathologic Review. Ophthalmic Plast. Reconstr. Surg. 2017, 33, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Cheuk, W.; Yuen, H.K.; Chan, A.C.; Shih, L.Y.; Kuo, T.T.; Ma, M.W.; Lo, Y.F.; Chan, W.K.; Chan, J.K. Ocular adnexal lymphoma associated with IgG4+ chronic sclerosing dacryoadenitis: A previously undescribed complication of IgG4-related sclerosing disease. Am. J. Surg. Pathol. 2008, 32, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ohshima, K.; Ichimura, K.; Sato, M.; Yamadori, I.; Tanaka, T.; Takata, K.; Morito, T.; Kondo, E.; Yoshino, T. Ocular adnexal IgG4-related disease has uniform clinicopathology. Pathol. Int. 2008, 58, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Go, H.; Kim, J.E.; Kim, Y.A.; Chung, H.K.; Khwarg, S.I.; Kim, C.W.; Jeon, Y.K. Ocular adnexal IgG4-related disease: Comparative analysis with mucosa-associated lymphoid tissue lymphoma and other chronic inflammatory conditions. Histopathology 2012, 60, 296–312. [Google Scholar] [CrossRef]
- Deshpande, V.; Zen, Y.; Chan, J.K.; Yi, E.E.; Sato, Y.; Yoshino, T.; Kloppel, G.; Heathcote, J.G.; Khosroshahi, A.; Ferry, J.A.; et al. Consensus statement on the pathology of IgG4-related disease. Mod. Pathol. 2012, 25, 1181–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umehara, H.; Okazaki, K.; Masaki, Y.; Kawano, M.; Yamamoto, M.; Saeki, T.; Matsui, S.; Yoshino, T.; Nakamura, S.; Kawa, S.; et al. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod. Rheumatol. 2012, 22, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Usui, Y.; Wakita, R.; Aita, Y.; Tomita, A.; Tsubota, K.; Asakage, M.; Nezu, N.; Komatsu, H.; Umazume, K.; et al. Differential Tissue Metabolic Signatures in IgG4-Related Ophthalmic Disease and Orbital Mucosa-Associated Lymphoid Tissue Lymphoma. Investig. Ophthalmol. Vis. Sci. 2021, 62, 15. [Google Scholar] [CrossRef] [PubMed]
- Walter, R.F.H.; Werner, R.; Vollbrecht, C.; Hager, T.; Flom, E.; Christoph, D.C.; Schmeller, J.; Schmid, K.W.; Wohlschlaeger, J.; Mairinger, F.D. ACTB, CDKN1B, GAPDH, GRB2, RHOA and SDCBP Were Identified as Reference Genes in Neuroendocrine Lung Cancer via the nCounter Technology. PLoS ONE 2016, 11, e0165181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mairinger, F.; Bankfalvi, A.; Schmid, K.W.; Mairinger, E.; Mach, P.; Walter, R.F.; Borchert, S.; Kasimir-Bauer, S.; Kimmig, R.; Buderath, P. Digital Immune-Related Gene Expression Signatures in High-Grade Serous Ovarian Carcinoma: Developing Prediction Models for Platinum Response. Cancer Manag. Res. 2019, 11, 9571–9583. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Watts, D.G. Nonlinear Regression Analysis and Its Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1988. [Google Scholar]
- Bonate, P.L. Nonlinear Models and Regression. In Pharmacokinetic-Pharmacodynamic Modeling and Simulation; Springer: Boston, MA, USA, 2006; pp. 93–124. [Google Scholar]
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]

| NSOI | IgG4-ROD | MALT Lymphoma | p | |
|---|---|---|---|---|
| Number | 30 | 14 | 16 | |
| Age | 52 ± 16.89 | 63 ± 13.59 | 69 ± 11.5 | 0.0013 a |
| Females | 45% | 46% | 30% | 0.36 b |
| NSOI | IgG4-ROD | MALT Lymphoma |
|---|---|---|
| AQP1 *, ANG *, NR4A3 *, SDC4 *, NME4 *, ID1, ITGA7 *, PDCL3 *, ERBB2 *, CALD1 *, BMPR1A *, TNXB * and RPS27A * | ADAM9, CLEC2B *, RNH1 *, TLR4 *, RBX1 *, CLIC4. ANXA2P2 *, RHOA *, NRP1, FCGR3A/B, TNFRSF1A *, GPX1 *, TCEB1 *, PSMD7, HSP90B1, TNFSF12, GIMAP4, PTGIS, PNPLA6, TGFB1, FCGR2A * and ADAM17 * | CLIC4 *, JAG1 *, RAC1 *, TNS1 *, APP *, HSPB1 *, MEG3 *, NRP1 *, RTN4 *, LGALS3 *, DST, CD59 *, CFH, AQP1, ADAM15, AEBP1, ALDOA, ANPEP, ANXA2P2 * and BMPR1A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Ghazzawi, K.; Baum, S.H.; Pförtner, R.; Philipp, S.; Bechrakis, N.; Görtz, G.; Eckstein, A.; Mairinger, F.D.; Oeverhaus, M. Evaluation of Orbital Lymphoproliferative and Inflammatory Disorders by Gene Expression Analysis. Int. J. Mol. Sci. 2022, 23, 8609. https://doi.org/10.3390/ijms23158609
Al-Ghazzawi K, Baum SH, Pförtner R, Philipp S, Bechrakis N, Görtz G, Eckstein A, Mairinger FD, Oeverhaus M. Evaluation of Orbital Lymphoproliferative and Inflammatory Disorders by Gene Expression Analysis. International Journal of Molecular Sciences. 2022; 23(15):8609. https://doi.org/10.3390/ijms23158609
Chicago/Turabian StyleAl-Ghazzawi, Karim, Sven Holger Baum, Roman Pförtner, Svenja Philipp, Nikolaos Bechrakis, Gina Görtz, Anja Eckstein, Fabian D. Mairinger, and Michael Oeverhaus. 2022. "Evaluation of Orbital Lymphoproliferative and Inflammatory Disorders by Gene Expression Analysis" International Journal of Molecular Sciences 23, no. 15: 8609. https://doi.org/10.3390/ijms23158609
APA StyleAl-Ghazzawi, K., Baum, S. H., Pförtner, R., Philipp, S., Bechrakis, N., Görtz, G., Eckstein, A., Mairinger, F. D., & Oeverhaus, M. (2022). Evaluation of Orbital Lymphoproliferative and Inflammatory Disorders by Gene Expression Analysis. International Journal of Molecular Sciences, 23(15), 8609. https://doi.org/10.3390/ijms23158609







