Unique Features of River Lamprey (Lampetra fluviatilis) Myogenesis
Abstract
:1. Introduction
2. Results
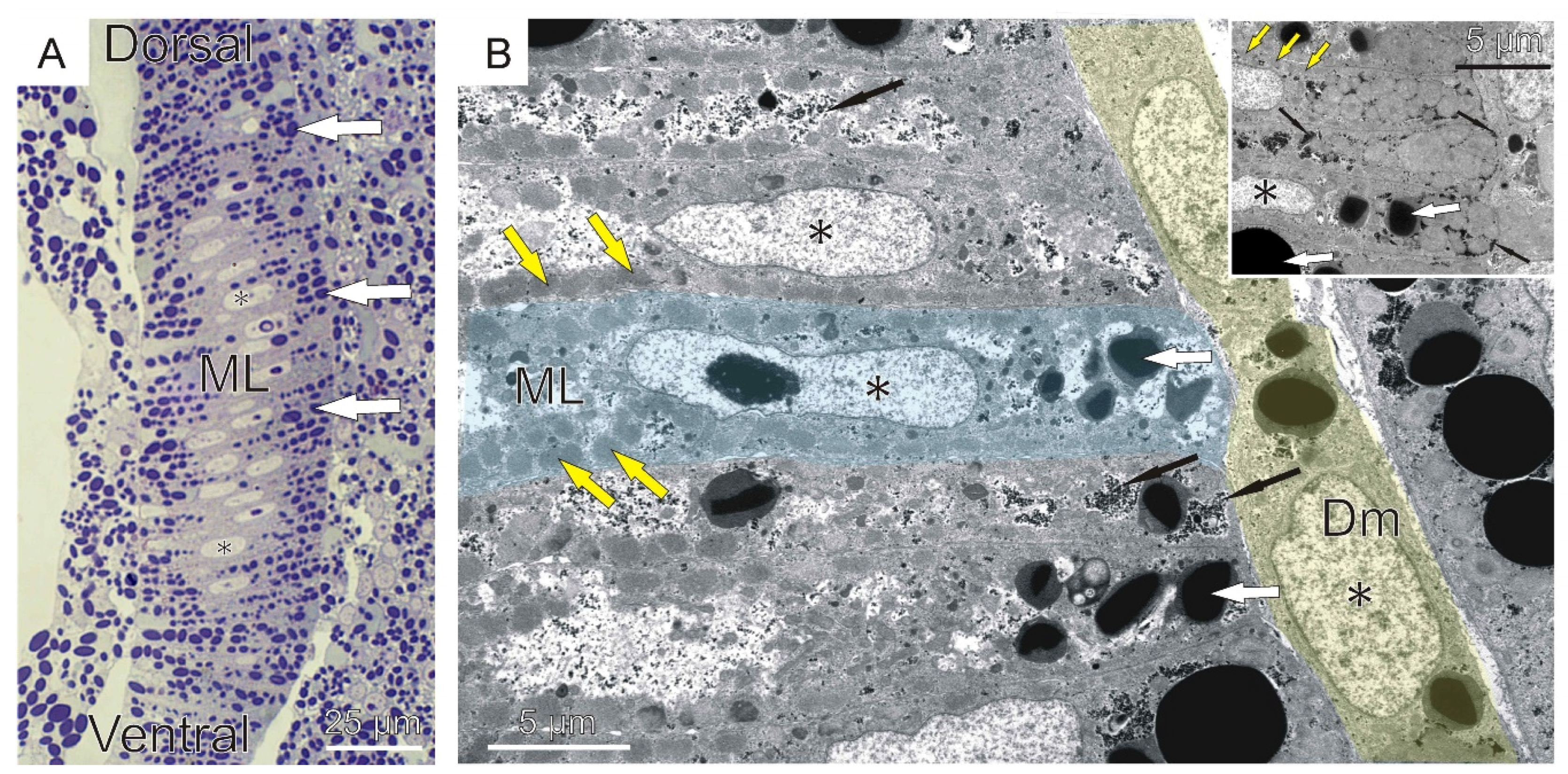
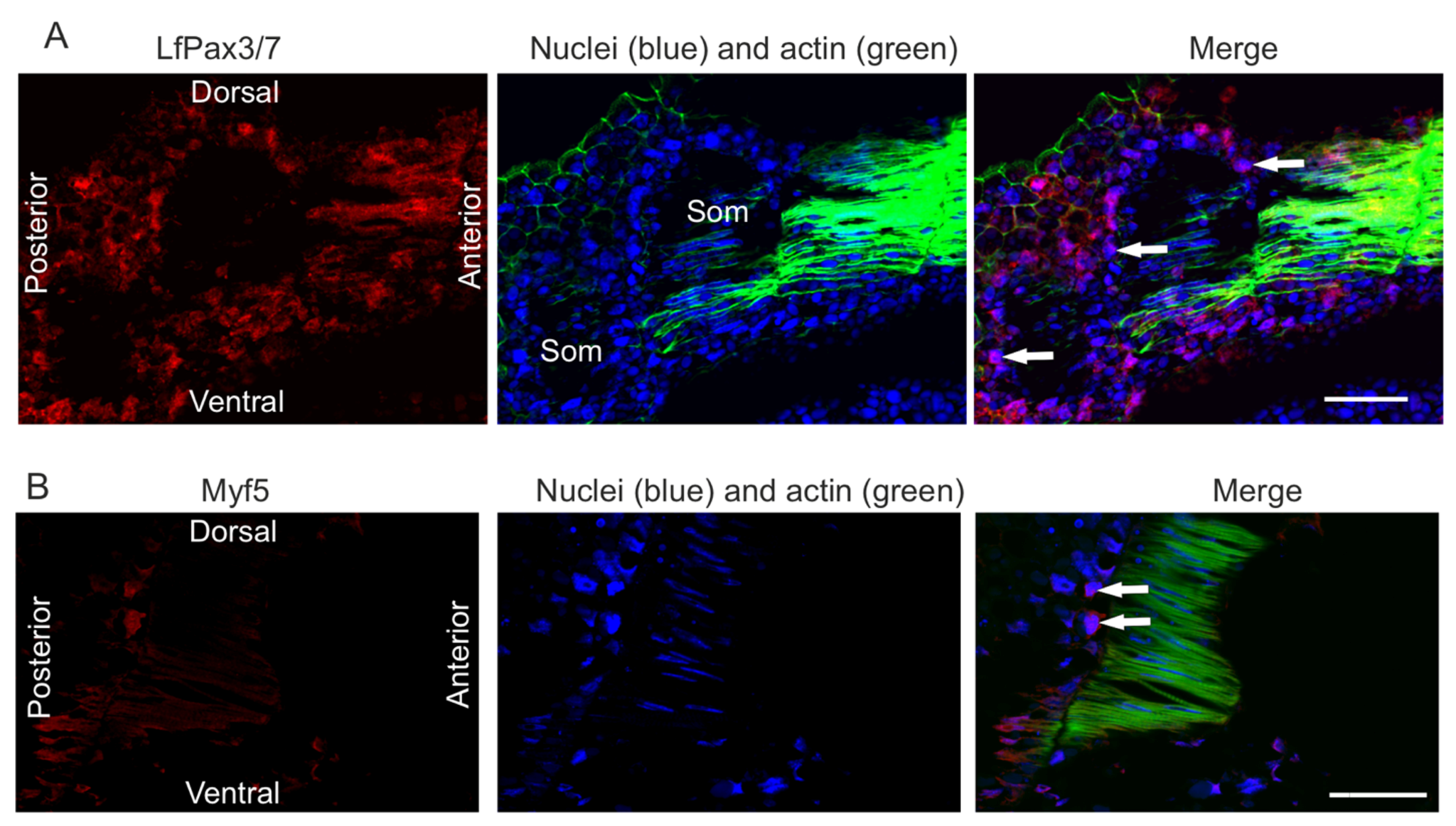
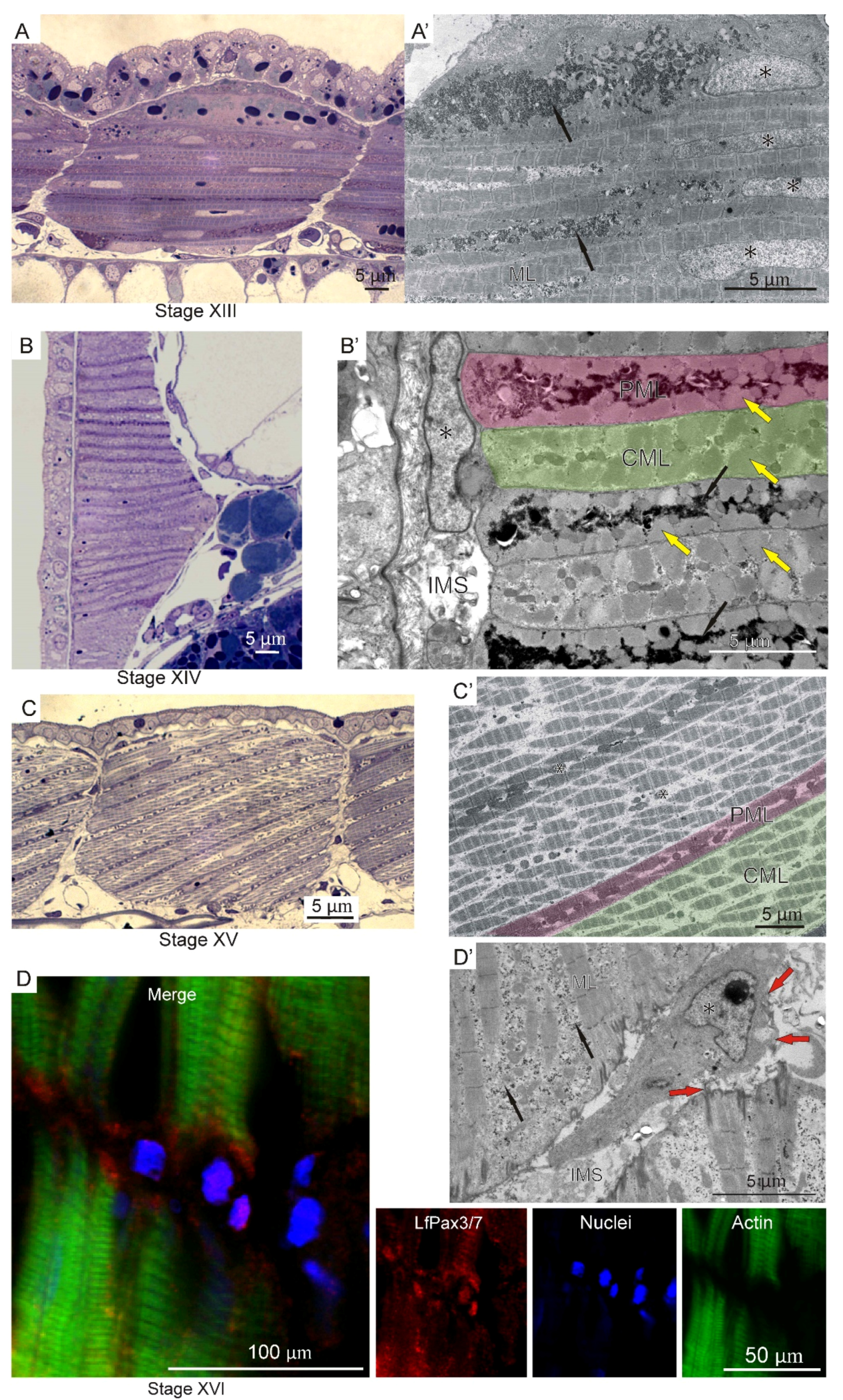
2.1. Embryonal and Larval Myogenesis
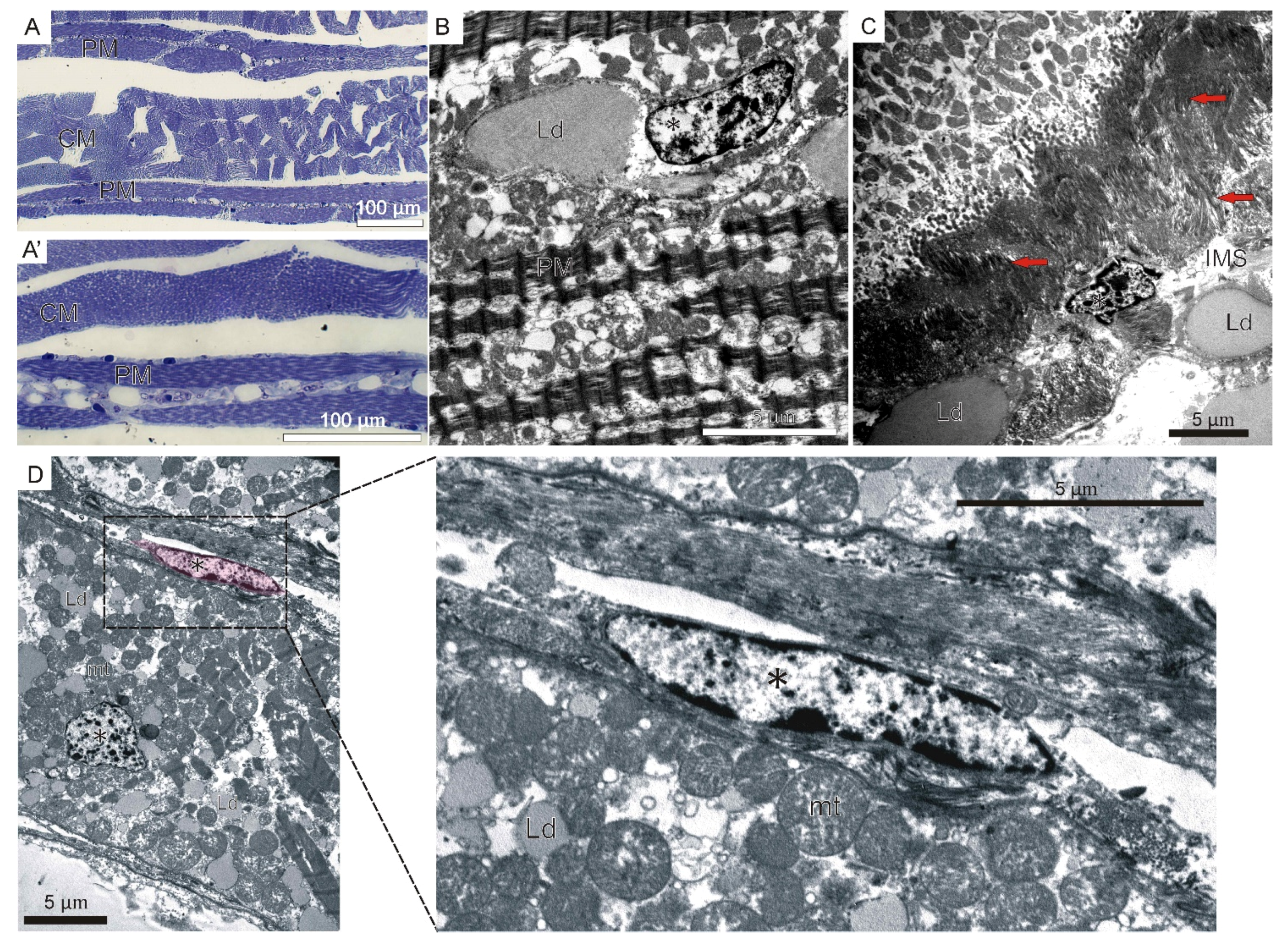
2.2. Adult Myogenesis
3. Discussion
3.1. Somitogenesis
3.2. Myogenesis
3.3. Adult Muscle Fibers
3.4. Ancestral Features in River Lamprey Myogenesis
4. Materials and Methods
4.1. Animals and Sampling Sites
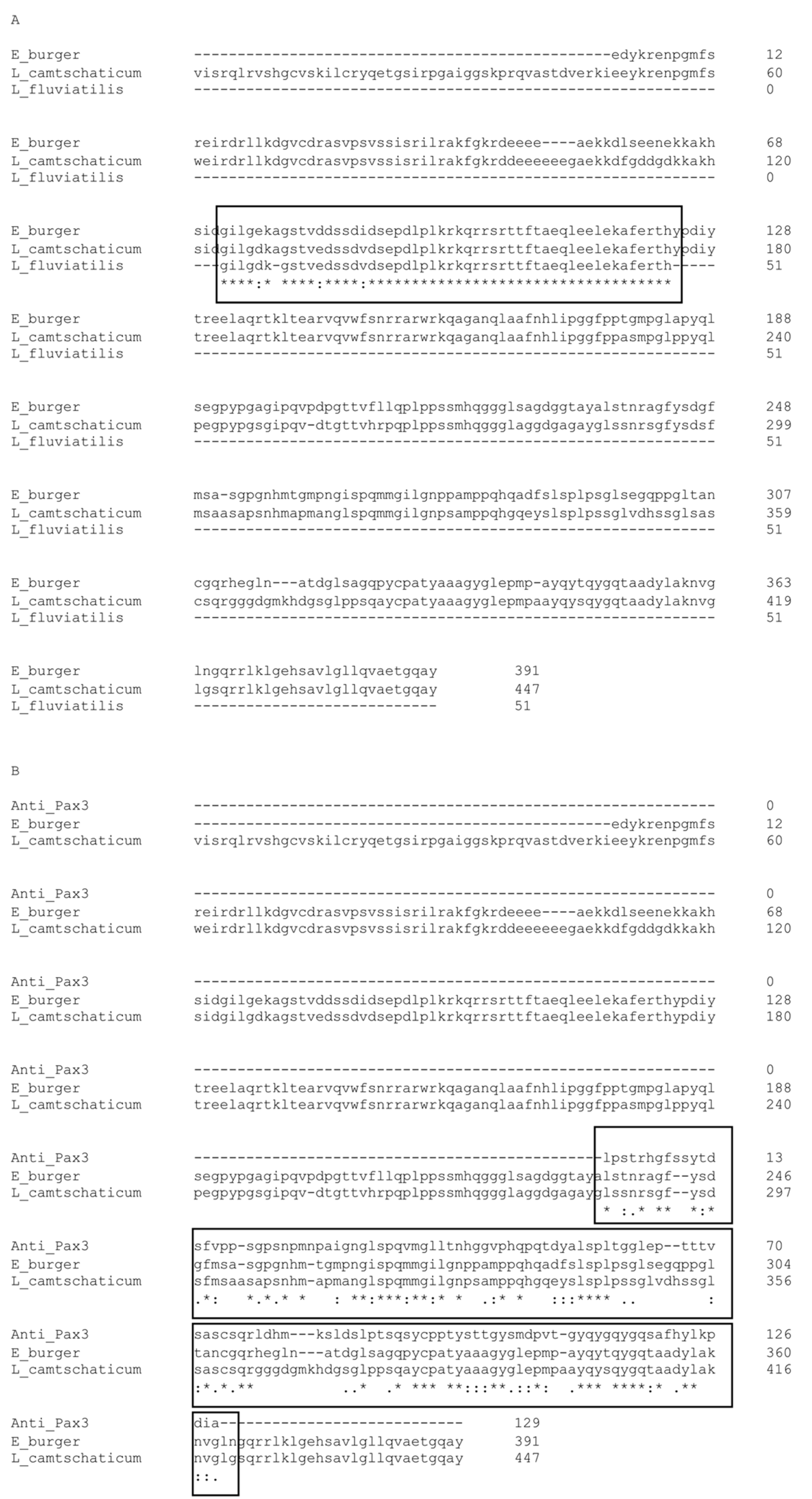
4.2. Aminoacidic Sequence Analysis of LfPax3/7 and Myf5
4.3. Light and Transmission Electron Microscopy
4.4. Immunofluorescence Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kujawa, R. River Lamprey [Pol. Minóg Rzeczny]; UWM: Olsztyn, Poland, 2017; ISBN 978-83-8100-053-6. [Google Scholar]
- Manzon, R.G.; Manzon, L.A. Lamprey Metamorphosis: Thyroid Hormone Signaling in a Basal Vertebrate. Mol. Cell. Endocrinol. 2017, 459, 28–42. [Google Scholar] [CrossRef]
- Borowiec, B.G.; Docker, M.F.; Johnson, N.S.; Moser, M.L.; Zielinski, B.; Wilkie, M.P. Exploiting the Physiology of Lampreys to Refine Methods of Control and Conservation. J. Great Lakes Res. 2021, 47, S723–S741. [Google Scholar] [CrossRef]
- Kusakabe, R.; Kuratani, S. Evolution and Developmental Patterning of the Vertebrate Skeletal Muscles: Perspectives from the Lamprey. Dev. Dyn. 2005, 234, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Christ, B.; Ordahl, C.P. Early Stages of Chick Somite Development. Anat. Embryol. 1995, 191, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Sato, Y. Somitogenesis as a Model to Study the Formation of Morphological Boundaries and Cell Epithelialization: Boundary Formation during Somitogenesis. Dev. Growth Differ. 2008, 50, S149–S155. [Google Scholar] [CrossRef] [PubMed]
- Gros, J.; Scaal, M.; Marcelle, C. A Two-Step Mechanism for Myotome Formation in Chick. Dev. Cell 2004, 6, 875–882. [Google Scholar] [CrossRef] [Green Version]
- Brent, A.E.; Schweitzer, R.; Tabin, C.J. A Somitic Compartment of Tendon Progenitors. Cell 2003, 113, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Relaix, F.; Rocancourt, D.; Mansouri, A.; Buckingham, M. A Pax3/Pax7-Dependent Population of Skeletal Muscle Progenitor Cells. Nature 2005, 435, 948–953. [Google Scholar] [CrossRef] [Green Version]
- Relaix, F.; Montarras, D.; Zaffran, S.; Gayraud-Morel, B.; Rocancourt, D.; Tajbakhsh, S.; Mansouri, A.; Cumano, A.; Buckingham, M. Pax3 and Pax7 Have Distinct and Overlapping Functions in Adult Muscle Progenitor Cells. J. Cell Biol. 2006, 172, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Daczewska, M.; Saczko, J. Myotomal Myogenesis of Axial Muscle in the Sturgeon Acipenser baeri (Chondrostei, Acipenseriformes). Folia Biol. 2005, 53, 29–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kacperczyk, A.; Daczewska, M. The Australian Lungfish (Neoceratodus forsteri)—Fish or Amphibian Pattern of Muscle Development? Int. J. Dev. Biol. 2008, 52, 279–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kacperczyk, A.; Daczewska, M. Mixed Mesodermal and Mesenchymal Origin of Myotomal Muscles in Pike (Esox lucius: Teleostei). Anat. Histol. Embryol. 2006, 35, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kacperczyk, A.; Jagla, T.; Daczewska, M. Pax-3 and Pax-7 Label Muscle Progenitor Cells During Myotomal Myogenesis in Coregonus lavaretus (Teleostei: Coregonidae). Anat. Histol. Embryol. 2009, 38, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Steinbacher, P.; Haslett, J.R.; Six, M.; Gollmann, H.P.; Sänger, A.M.; Stoiber, W. Phases of Myogenic Cell Activation and Possible Role of Dermomyotome Cells in Teleost Muscle Formation. Dev. Dyn. 2006, 235, 3132–3143. [Google Scholar] [CrossRef]
- Kacperczyk, A.; Jędrzejowska, I.; Daczewska, M. Differentiation and Growth of Myotomal Muscles in a Non-Model Tropical Fish Pterophyllum scalare (Teleostei: Cichlidae): Differentiation of Muscles in P. Scalare. Anat. Histol. Embryol. 2011, 40, 411–418. [Google Scholar] [CrossRef]
- Kiełbówna, L.; Daczewska, M. Onto-Phylogenetic Aspect of Myotomal Myogenesis in Chordata. Folia Biol. 2004, 52, 1–12. [Google Scholar]
- Kiełbówna, L.; Daczewska, M. The Origin of Syncytial Muscle Fibres in the Myotomes of Xenopus laevis—A Revision. Folia Biol. 2005, 53, 39–44. [Google Scholar] [CrossRef] [Green Version]
- Daczewska, M.; Pałucka, M. Development of Primary and Secondary Muscle Fibres in the Myotomes of Rana lessonae [Anura: Ranidae]. Zool. Pol. 1999, 44, 1–4. [Google Scholar]
- Daczewska, M.; Kiełbówna, L. Myotomal Myogenesis in Triturus vulgaris L. (Urodela) with Special Reference to the Role of Mesenchymal Cells. Folia Biol. 2000, 48, 37–42. [Google Scholar]
- Kiełbówna, L.; Migocka-Patrzałek, M. Models of Amphibian Myogenesis—The Case of Bombina variegata. Int. J. Dev. Biol. 2017, 61, 17–27. [Google Scholar] [CrossRef]
- Daczewska, M. Mechanism of Multinucleate Myotomal Muscle Fibre Formation in Hymenochirus boettgeri (Anura, Pipidae). Zoomorphology 2001, 121, 27–36. [Google Scholar] [CrossRef]
- Jacob, M.; Jacob, H.J.; Christ, B. Die frühe Differenzierung des chordanahen Bindegewebes. Raster- und transmissionselektronenmikroskopische Untersuchungen an Hühnerembryonen. Experientia 1975, 31, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Rupik, W.; Swadźba, E.; Dubińska-Magiera, M.; Jędrzejowska, I.; Daczewska, M. Reptilian Myotomal Myogenesis—Lessons from the Sand Lizard Lacerta agilis L. (Reptilia, Lacertidae). Zoology 2012, 115, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Khannoon, E.R.; Rupik, W.; Lewandowski, D.; Dubińska–Magiera, M.; Swadźba, E.; Daczewska, M. Unique Features of Myogenesis in Egyptian cobra (Naja haje) (Squamata: Serpentes: Elapidae). Protoplasma 2016, 253, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Lewandowski, D.; Dubińska-Magiera, M.; Posyniak, E.; Rupik, W.; Daczewska, M. Does the Grass snake (Natrix natrix) (Squamata: Serpentes: Natricinae) Fit the Amniotes-Specific Model of Myogenesis? Protoplasma 2017, 254, 1507–1516. [Google Scholar] [CrossRef] [Green Version]
- Braun, T.; Winter, B.; Bober, E.; Arnold, H.H. Transcriptional Activation Domain of the Muscle-Specific Gene-Regulatory Protein Myf5. Nature 1990, 346, 663–665. [Google Scholar] [CrossRef]
- Richardson, M.K.; Allen, S.P.; Wright, G.M.; Raynaud, A.; Hanken, J. Somite Number and Vertebrate Evolution. Development 1998, 125, 151–160. [Google Scholar] [CrossRef]
- Rhodes, S.J.; Konieczny, S.F. Identification of MRF4: A New Member of the Muscle Regulatory Factor Gene Family. Genes Dev. 1989, 3, 2050–2061. [Google Scholar] [CrossRef] [Green Version]
- Davis, R.L.; Weintraub, H.; Lassar, A.B. Expression of a Single Transfected CDNA Converts Fibroblasts to Myoblasts. Cell 1987, 51, 987–1000. [Google Scholar] [CrossRef]
- Hinterberger, T.J.; Sassoon, D.A.; Rhodes, S.J.; Konieczny, S.F. Expression of the Muscle Regulatory Factor MRF4 during Somite and Skeletal Myofiber Development. Dev. Biol. 1991, 147, 144–156. [Google Scholar] [CrossRef]
- Buckingham, M. Muscle Differentiation. Which Myogenic Factors Make Muscle? Curr. Biol. 1994, 4, 61–63. [Google Scholar] [CrossRef]
- Johnston, I.A.; Hoar, W.S.; Farrell, A.P. Fish Physiology: Muscle Development and Growth; Academic Press: Cambridge, MA, USA, 2020; Volume 18, ISBN 978-0-12-350442-5. [Google Scholar]
- Waterman, R.E. Development of the Lateral Musculature in the Teleost, Brachydanio Rerio: A Fine Structural Study. Am. J. Anat. 1969, 125, 457–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raamsdonk, W.; Stelt, A.; Diegenbach, P.C.; Berg, W.; Bruyn, H.; Dijk, J.; Mijzen, P. Differentiation of the Musculature of the Teleost Brachydanio Rerio: I. Myotome Shape and Movements in the Embryo. Z. Anat. Entwickl. Gesch. 1974, 145, 321–342. [Google Scholar] [CrossRef] [PubMed]
- Stoiber, W.; Sanger, A.M. An Electron Microscopic Investigation into the Possible Source of New Muscle Fibres in Teleost Fish. Anat. Embryol. 1996, 194, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, D.; Dubińska-Magiera, M.; Migocka-Patrzałek, M.; Niedbalska-Tarnowska, J.; Haczkiewicz-Leśniak, K.; Dzięgiel, P.; Daczewska, M. Everybody Wants to Move-Evolutionary Implications of Trunk Muscle Differentiation in Vertebrate Species. Semin. Cell Dev. Biol. 2020, 104, 3–13. [Google Scholar] [CrossRef]
- Devoto, S.H.; Stoiber, W.; Hammond, C.L.; Steinbacher, P.; Haslett, J.R.; Barresi, M.J.F.; Patterson, S.E.; Adiarte, E.G.; Hughes, S.M. Generality of Vertebrate Developmental Patterns: Evidence for a Dermomyotome in Fish. Evol. Dev. 2006, 8, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Hollway, G.E.; Bryson-Richardson, R.J.; Berger, S.; Cole, N.J.; Hall, T.E.; Currie, P.D. Whole-Somite Rotation Generates Muscle Progenitor Cell Compartments in the Developing Zebrafish Embryo. Dev. Cell 2007, 12, 207–219. [Google Scholar] [CrossRef] [Green Version]
- Stellabotte, F.; Dobbs-McAuliffe, B.; Fernández, D.A.; Feng, X.; Devoto, S.H. Dynamic Somite Cell Rearrangements Lead to Distinct Waves of Myotome Growth. Development 2007, 134, 1253–1257. [Google Scholar] [CrossRef] [Green Version]
- Modrell, M.S.; Hockman, D.; Uy, B.; Buckley, D.; Sauka-Spengler, T.; Bronner, M.E.; Baker, C.V.H. A Fate-Map for Cranial Sensory Ganglia in the Sea Lamprey. Dev. Biol. 2014, 385, 405–416. [Google Scholar] [CrossRef] [Green Version]
- McCauley, D.W.; Bronner-Fraser, M. Conservation of Pax Gene Expression in Ectodermal Placodes of the Lamprey. Gene 2002, 287, 129–139. [Google Scholar] [CrossRef]
- Osorio, J.; Mazan, S.; Rétaux, S. Organisation of the Lamprey (Lampetra fluviatilis) Embryonic Brain: Insights from LIM-Homeodomain, Pax and Hedgehog Genes. Dev. Biol. 2005, 288, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Paixão-Côrtes, V.R.; Salzano, F.M.; Bortolini, M.C. Evolutionary History of Chordate PAX Genes: Dynamics of Change in a Complex Gene Family. PLoS ONE 2013, 8, e73560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusakabe, R.; Kuraku, S.; Kuratani, S. Expression and Interaction of Muscle-Related Genes in the Lamprey Imply the Evolutionary Scenario for Vertebrate Skeletal Muscle, in Association with the Acquisition of the Neck and Fins. Dev. Biol. 2011, 350, 217–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peachey, L.D. Structure of The Longitudinal Body Muscles of Amphioxus. J. Biophys. Biochem. Cytol. 1961, 10, 159–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flood, P.R. Structure of the Segmental Trunk Muscle in Amphioxus. With Notes on the Course and “Endings” of the so-Called Ventral Root Fibres. Z. Zellforsch. Mikrosk. Anat. 1968, 84, 389–416. [Google Scholar] [CrossRef]
- Hammond, K.L.; Baxendale, S.; McCauley, D.W.; Ingham, P.W.; Whitfield, T.T. Expression of Patched, Prdm1 and Engrailed in the Lamprey Somite Reveals Conserved Responses to Hedgehog Signaling. Evol. Dev. 2009, 11, 27–40. [Google Scholar] [CrossRef]
- Meyer, W. Oxidative Enzymes and Myosin-ATPase in the Trunk Musculature of the River Lamprey (Lampetra fluviatilis). Histochem J 1979, 11, 187–195. [Google Scholar] [CrossRef]
- Kimura, T.; Watanabe, T.; Egawa, K.; Takiguchi, R. Architecture and Muscle Fiber Types of Lamprey Myotome. Biomed. Res. 1995, 16, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, D.; Ono, Y.; Hirano, S.; Kan-no, N.; Watabe, S. Lampreys Have a Single Gene Cluster for the Fast Skeletal Myosin Heavy Chain Gene Family. PLoS ONE 2013, 8, e85500. [Google Scholar] [CrossRef] [PubMed]
- Holland, P.W.; Koschorz, B.; Holland, L.Z.; Herrmann, B.G. Conservation of Brachyury (T) Genes in Amphioxus and Vertebrates: Developmental and Evolutionary Implications. Development 1995, 121, 4283–4291. [Google Scholar] [CrossRef]
- Jasper, D. Body muscles of the lamprey. J. Cell Biol. 1967, 32, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kujawa, R.; Kucharczyk, D.; Mamcarz, A. A Model System for Keeping Spawners of Wild and Domestic Fish before Artificial Spawning. Aquac. Eng. 1999, 20, 85–89. [Google Scholar] [CrossRef]
- Richardson, M.K.; Admiraal, J.; Wright, G.M. Developmental Anatomy of Lampreys. Biol. Rev. 2010, 85, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Goujon, M.; McWilliam, H.; Li, W.; Valentin, F.; Squizzato, S.; Paern, J.; Lopez, R. A New Bioinformatics Analysis Tools Framework at EMBL-EBI. Nucleic Acids Res. 2010, 38, W695–W699. [Google Scholar] [CrossRef]
- Altschul, S. Gapped BLAST and PSI-BLAST: A New Generation of Protein Database Search Programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Dubińska-Magiera, M.; Lewandowski, D.; Cysewski, D.; Pawlak, S.; Najbar, B.; Daczewska, M. Lipid Droplets in Skeletal Muscle during Grass Snake (Natrix natrix L.) Development. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2022, 1867, 159086. [Google Scholar] [CrossRef]
- Reynolds, E.S. The Use of Lead Citrate at High Ph as An Electron-Opaque Stain in Electron Microscopy. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Migocka-Patrzałek, M.; Kujawa, R.; Podlasz, P.; Juchno, D.; Haczkiewicz-Leśniak, K.; Daczewska, M. Unique Features of River Lamprey (Lampetra fluviatilis) Myogenesis. Int. J. Mol. Sci. 2022, 23, 8595. https://doi.org/10.3390/ijms23158595
Migocka-Patrzałek M, Kujawa R, Podlasz P, Juchno D, Haczkiewicz-Leśniak K, Daczewska M. Unique Features of River Lamprey (Lampetra fluviatilis) Myogenesis. International Journal of Molecular Sciences. 2022; 23(15):8595. https://doi.org/10.3390/ijms23158595
Chicago/Turabian StyleMigocka-Patrzałek, Marta, Roman Kujawa, Piotr Podlasz, Dorota Juchno, Katarzyna Haczkiewicz-Leśniak, and Małgorzata Daczewska. 2022. "Unique Features of River Lamprey (Lampetra fluviatilis) Myogenesis" International Journal of Molecular Sciences 23, no. 15: 8595. https://doi.org/10.3390/ijms23158595
APA StyleMigocka-Patrzałek, M., Kujawa, R., Podlasz, P., Juchno, D., Haczkiewicz-Leśniak, K., & Daczewska, M. (2022). Unique Features of River Lamprey (Lampetra fluviatilis) Myogenesis. International Journal of Molecular Sciences, 23(15), 8595. https://doi.org/10.3390/ijms23158595






