Effects of Exercise Training during Advanced Maternal Age on the Cognitive Function of Offspring
Abstract
1. Introduction
2. Results
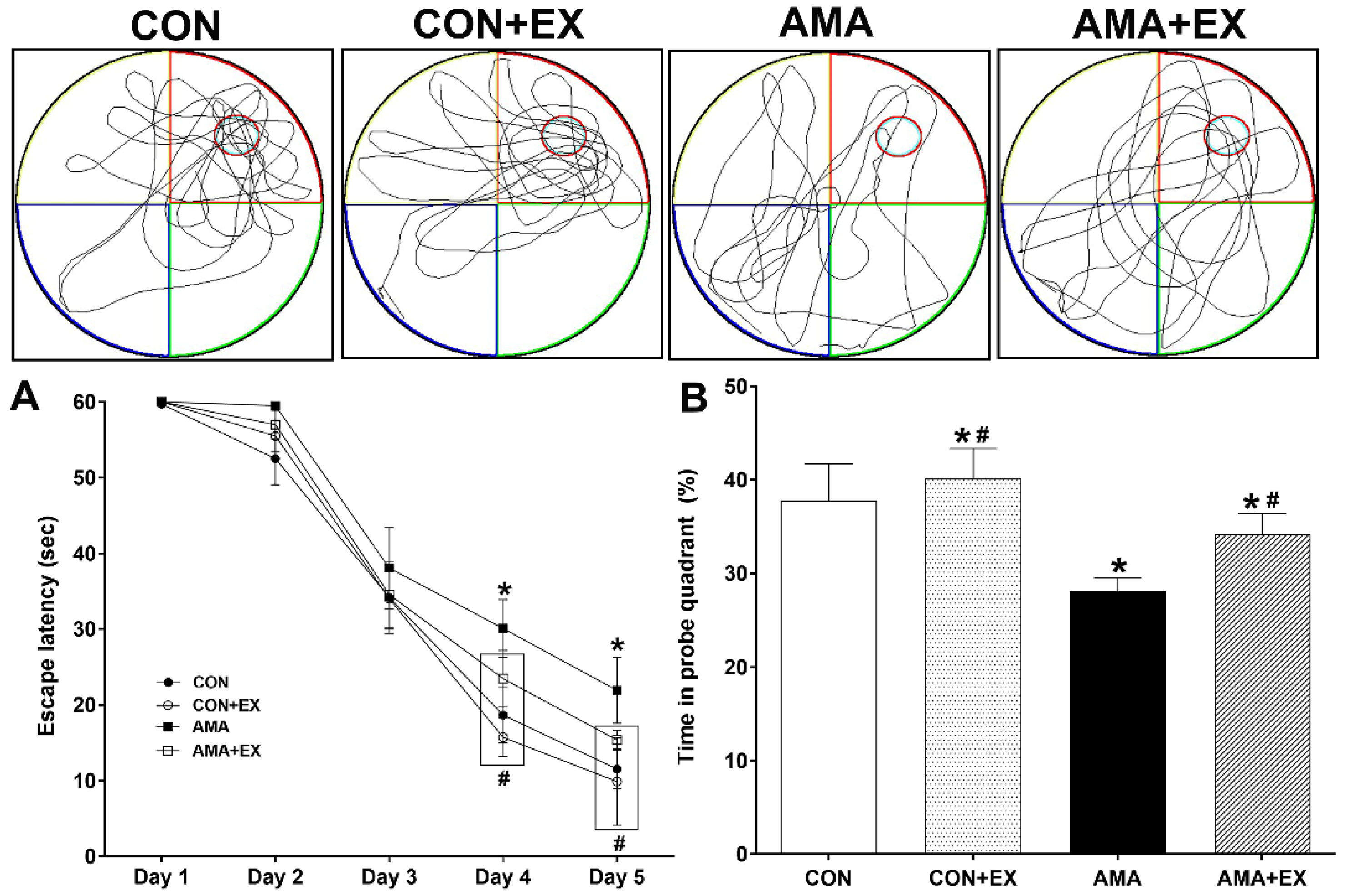
2.1. Effects of Exercise in Mothers with AMA on Cognitive Function in Offspring
2.2. Effect of Exercise in Mothers with AMA on Hippocampal BDNF and PSD-95 Levels in the Offspring
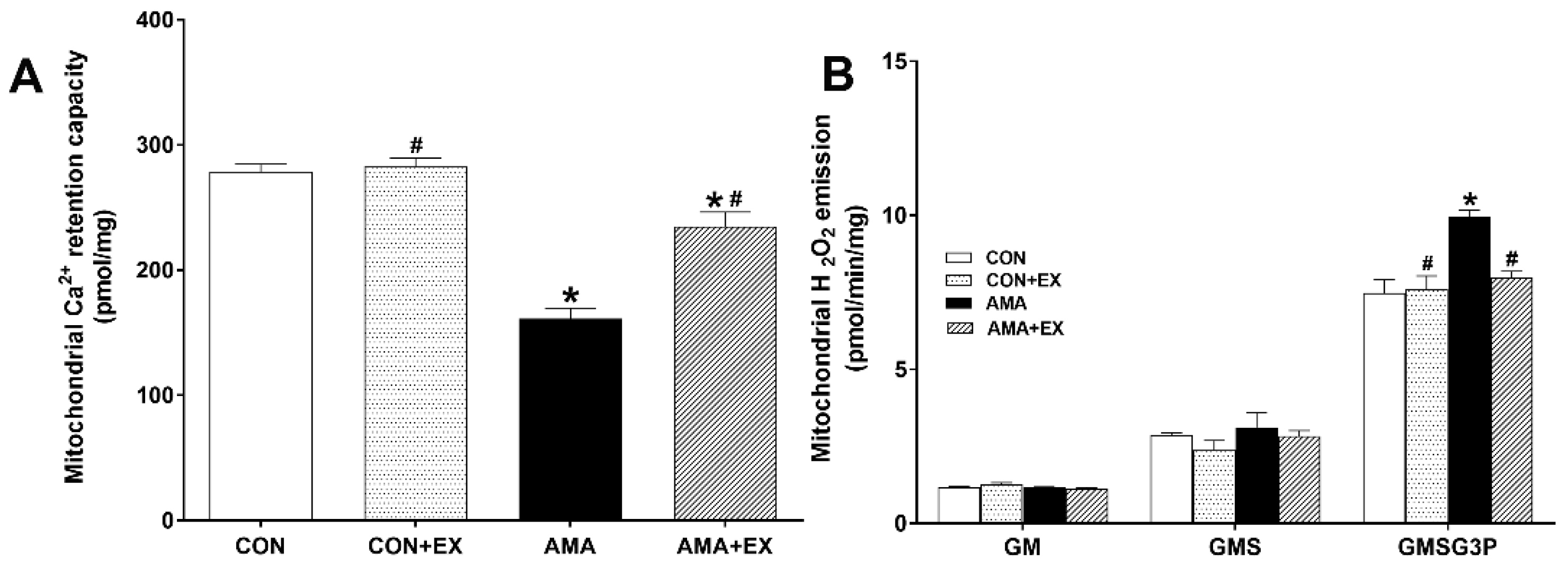
2.3. Effect of Exercise in Mothers with AMA on Mitochondrial Ca2+ Retention and H2O2 Emission in the Hippocampus of Offspring
2.4. Effect of Exercise in Mothers with AMA on Apoptosis and Cell Death in the Hippocampus of the Offspring
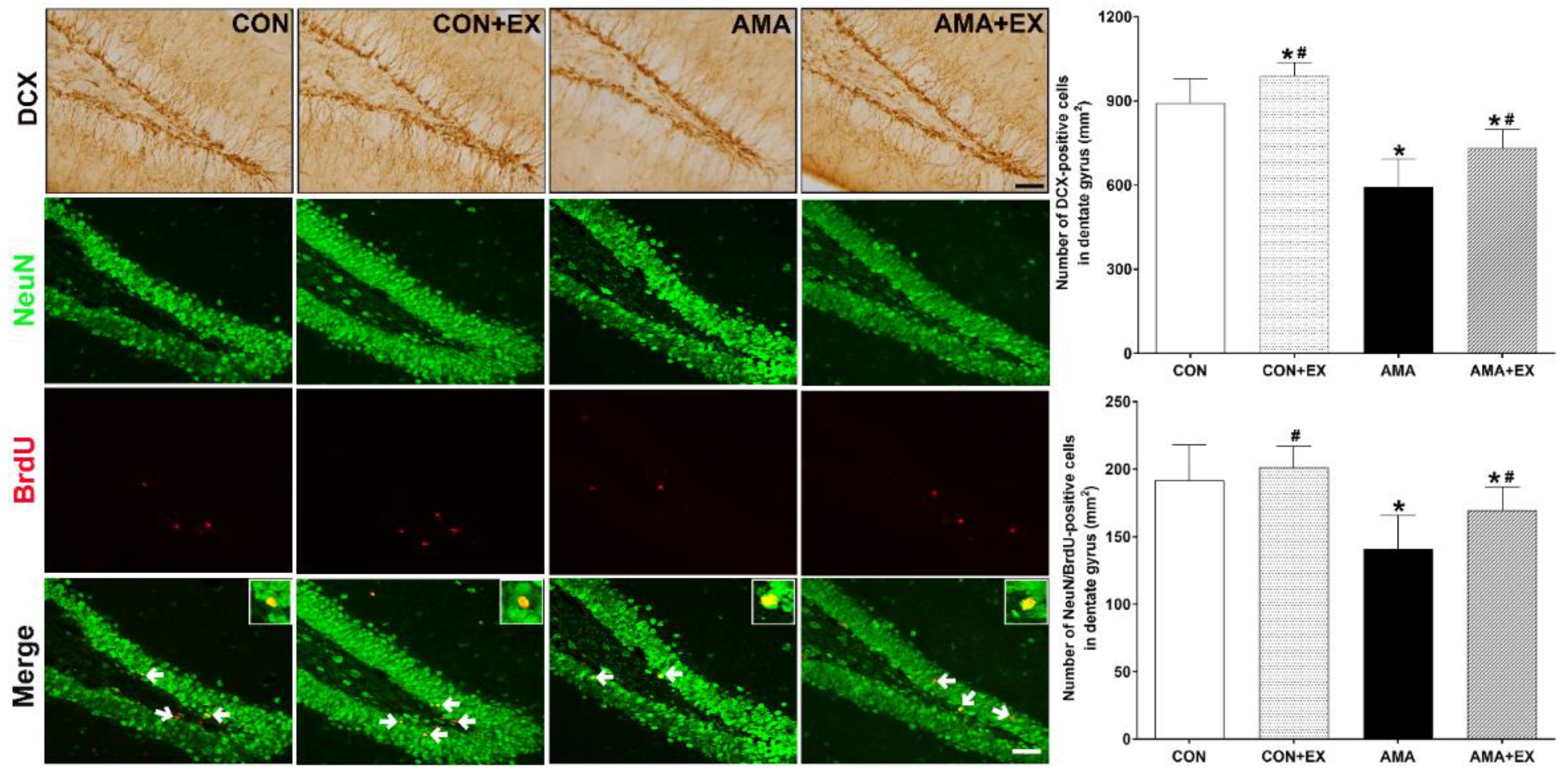
2.5. Effect of Exercise in Mothers with AMA on Cell Differentiation and Neurogenesis in the Hippocampus of Offspring
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Exercise Protocol
4.3. Preparation of the Tissue Samples
4.4. Behavioral Analysis
4.5. Immunohistochemistry
4.6. Immunofluorescence
4.7. Quantification of the Histology
4.8. TUNEL Staining
4.9. Western Blotting
4.10. Mitochondrial Ca2+ Retention Capacity
4.11. Mitochondrial H2O2 Emission
4.12. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, J.E.; Ahn, J.A.; Lee, S.K.; Roh, E.H. Factors related to low birth rate among married women in Korea. PLoS ONE 2018, 13, e0194597. [Google Scholar] [CrossRef] [PubMed]
- Chervenak, J.L.; Kardon, N.B. Advancing maternal age: The actual risks. Female Patient 1991, 16, 17–24. [Google Scholar] [PubMed]
- Mehta, S.; Tran, K.; Stewart, L.; Soutter, E.; Nauta, M.; Yoong, W. Pregnancy outcomes in women greater than 45 years: A cohort control study in a multi-ethnic inner city population. Arch. Gynecol. Obstet. 2014, 289, 1125–1128. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, K.; Urayama, K.Y.; Tanigaki, S.; Sago, H.; Sato, S.; Saito, S.; Morisaki, N. Association between very advanced maternal age and adverse pregnancy outcomes: A cross sectional Japanese study. BMC Pregnancy Childbirth 2017, 17, 349. [Google Scholar] [CrossRef] [PubMed]
- Wilding, M. Can we define maternal age as a genetic disease? Facts Views Vis. Obgyn. 2014, 6, 105–108. [Google Scholar]
- Niggeschulze, A.; Kast, A. Maternal age, reproduction and chromosomal aberrations in Wistar derived rats. Lab. Anim. 1994, 28, 55–62. [Google Scholar] [CrossRef]
- Wang, Y.; Tanbo, T.; Abyholm, T.; Henriksen, T. The impact of advanced maternal age and parity on obstetric and perinatal outcomes in singleton gestations. Arch. Gynecol. Obstet. 2011, 284, 31–37. [Google Scholar] [CrossRef]
- Tuzuka, Y.; Wada, E.; Wada, K. “Bio-communication” between mother and offspring: Lessons from animals and new perspective for brain science. J. Pharmacol. Sci. 2009, 110, 127–132. [Google Scholar] [CrossRef]
- Berglund, S.K.; Torres-Espínola, F.J.; García-Valdés, L.; Segura, M.T.; Martínez-Zaldívar, C.; Padilla, C.; Rueda, R.; Pérez García, M.; McArdle, H.J.; Campoy, C. The impacts of maternal iron deficiency and being overweight during pregnancy on neurodevelopment of the offspring. Br. J. Nutr. 2017, 118, 533–540. [Google Scholar] [CrossRef]
- Tuzuka, Y.; Wada, E.; Wada, K. Diet-induced obesity in female mice leads to peroxidized lipid accumulations and impairment of hippocampal neurogenesis during the early life of their offspring. FASEB J. 2009, 23, 1920–1934. [Google Scholar] [CrossRef]
- Tuzuka, Y.; Kumon, M.; Wada, E.; Onodera, M.; Mochizuki, H.; Wada, K. Maternal obesity impairs hippocampal BDNF production and spatial learning performance in young mouse offspring. Neurochem. Int. 2010, 57, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Basatemur, E.; Gardiner, J.; Williams, C.; Melhuish, E.; Barnes, J.; Sutcliffe, A. Maternal prepregnancy BMI and child cognition: A longitudinal cohort study. Pediatrics 2013, 13, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Stetic, L.; Belcic, I.; Sporis, G.; Stetic, L.; Starcevic, N. Influence of Physical Activity on the Regulation of Disease of Elderly Persons with Metabolic Syndrome. Int. J. Environ. Res. Public Health 2021, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, C.; Musumeci, G.; Castrogiovanni, P.; Fazio, F.; Li Volti, G.; Barbagallo, I.; Maugeri, G.; Ravalli, S.; Imbesi, R.; Di Rosa, M. Hippocampal transcriptome deconvolution reveals differences in cell architecture of not demented elderly subjects underwent late-life physical activity. J. Chem. Neuroanat. 2021, 113, 101934. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Lee, M.Y.; Getchell, K.M.; So, K.; Hirshman, M.F.; Goodyear, L.J. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 2015, 64, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Parnpiansil, P.; Jutapakdeegul, N.; Chentanez, T.; Kotchabhakdi, N. Exercise during pregnancy increases hippocampal brain-derived neurotrophic factor mRNA expression and spatial learning in neonatal rat pup. Neurosci. Lett. 2003, 352, 45–48. [Google Scholar] [CrossRef]
- Kim, H.; Lee, S.H.; Kim, S.S.; Yoo, J.H.; Kim, C.J. The influence of maternal treadmill running during pregnancy on short-term memory and hippocampal cell survival in rat pups. Int. J. Dev. Neurosci. 2007, 25, 243–249. [Google Scholar] [CrossRef]
- Gomes da Silva, S.; de Almeida, A.A.; Fernandes, J.; Lopim, G.M.; Cabral, F.R.; Scerni, D.A.; de Oliveira-Pinto, A.V.; Lent, R.; Arida, R.M. Maternal Exercise during Pregnancy Increases BDNF Levels and Cell Numbers in the Hippocampal Formation but Not in the Cerebral Cortex of Adult Rat Offspring. PLoS ONE 2016, 11, e0147200. [Google Scholar] [CrossRef]
- Malaspina, D.; Reichenberg, A.; Weiser, M.; Fennig, S.; Davidson, M.; Harlap, S.; Wolitzky, R.; Rabinowitz, J.; Susser, E.; Knobler, H.Y. Paternal age and intelligence: Implications for age-related genomic changes in male germ cells. Psychiatr. Genet. 2005, 15, 117–125. [Google Scholar] [CrossRef]
- Hultman, C.M.; Sandin, S.; Levine, S.Z.; Lichtenstein, P.; Reichenberg, A. Advancing paternal age and risk of autism: New evidence from a population-based study and a meta-analysis of epidemiological studies. Mol. Psychiatry 2011, 16, 1203–1212. [Google Scholar] [CrossRef]
- Myrskylä, M.; Fenelon, A. Maternal age and offspring adult health: Evidence from the health and retirement study. Demography 2012, 49, 1231–1257. [Google Scholar] [CrossRef] [PubMed]
- Larsson, H.J.; Eaton, W.W.; Madsen, K.M.; Vestergaard, M.; Olesen, A.V.; Agerbo, E.; Schendel, D.; Thorsen, P.; Mortensen, P.B. Risk factors for autism: Perinatal factors, parental psychiatric history, and socioeconomic status. Am. J. Epidemiol. 2005, 161, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Frans, E.M.; Sandin, S.; Reichenberg, A.; Lichtenstein, P.; Langstrom, N.; Hultman, C.M. Advancing paternal age and bipolar disorder. Arch. Gen. Psychiatry 2008, 65, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Barnett, A.G.; Foldi, C.; Burne, T.H.; Eyles, D.W.; Buka, S.L.; McGrath, J.J. Advanced paternal age is associated with impaired neurocognitive outcomes during infancy and childhood. PLoS Med. 2009, 6, e40. [Google Scholar] [CrossRef]
- Cannon, M. Contrasting effects of maternal and paternal age on offspring intelligence: The clock ticks for men too. PLoS Med. 2009, 6, e42. [Google Scholar] [CrossRef]
- Myrskylä, M.; Silventoinen, K.; Tynelius, P.; Rasmussen, F. Is later better or worse? Association of advanced parental age with offspring cognitive ability among half a million young Swedish men. Am. J. Epidemiol. 2013, 177, 649–655. [Google Scholar] [CrossRef]
- Han, W.; Dong, X.; Song, X.; Cheng, L.; Xie, L.; Chen, H.; Jiang, L. Effects of advanced maternal age on cognitive and emotional development in offspring rats. Behav. Brain Res. 2018, 353, 218–226. [Google Scholar] [CrossRef]
- Mao, W.J.; Wu, Z.Y.; Yang, Z.H.; Xum, Y.W.; Wang, S.Q. Advanced maternal age impairs spatial learning capacity in young adult mouse offspring. Am. J. Transl. Res. 2018, 10, 975–988. [Google Scholar]
- Li, D.; Wang, K.; Yang, Z.; Li, H.; Wang, S. Vitamin D supplementation in mice with advanced maternal age and cognitive function of the offspring. Am. J. Transl. Res. 2021, 13, 7641–7653. [Google Scholar]
- Staples, M.C.; Porch, M.W.; Savage, D.D. Impact of combined prenatal ethanol and prenatal stress exposures on markers of activity-dependent synaptic plasticity in rat dentate gyrus. Alcohol 2014, 48, 523–532. [Google Scholar] [CrossRef]
- Bielas, H.; Arck, P.; Bruenahl, C.A.; Walitzam, S.; Grünblatt, E. Prenatal stress increases the striatal and hippocampal expression of correlating c-FOS and serotonin transporters in murine offspring. Int. J. Dev. Neurosci. 2014, 38, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Korkmaz, K.S.; Braunm, K.; Bock, J. Early life stress-induced histone acetylations correlate with activation of the synaptic plasticity genes Arc and Egr1 in the mouse hippocampus. J. Neurochem. 2013, 125, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Sampino, S.; Stankiewicz, A.M.; Zacchinim, F.; Goscik, J.; Szostak, A.; Swiergiel, A.H.; Drago, G.; Modlinski, J.A.; Ptak, G.E. Pregnancy at Advanced Maternal Age Affects Behavior and Hippocampal Gene Expression in Mouse Offspring. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Wei, H.; Jie, L.; Chen, Y.; Wen-Jie, Z.; Hong, S.; Ya-Nan, P.; Heng-Sheng, C.; Li, C.; Li, J. Effect of advanced maternal age on development of hippocampal neural stem cells in offspring rats. Chin. J. Contemp. Pediatr. 2020, 22, 1017–1026. [Google Scholar]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Pesole, G.; Gissi, C.; De Chirico, A.; Saccone, C. Nucleotide substitution rate of mammalian mitochondrial genomes. J. Mol. Evol. 1999, 48, 427–434. [Google Scholar] [CrossRef]
- Cummins, J.M. Mitochondria: Potential roles in embryogenesis and nucleocytoplasmic transfer. Hum. Reprod. Update 2001, 7, 217–228. [Google Scholar] [CrossRef][Green Version]
- Cotman, C.W.; Berchtold, N.C. Exercise: A behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002, 25, 295–301. [Google Scholar] [CrossRef]
- van Praag, H.; Christi, B.R.; Sejnowski, T.J.; Gagem, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef]
- Wolfe, L.A.; Brennerm, I.K.; Mottolam, M.F. Maternal exercise, fetal well-being and pregnancy outcome. Exerc. Sport Sci. Rev. 1994, 22, 145–194. [Google Scholar] [CrossRef]
- Clapp, J.F., 3rd. Morphometric and neurodevelopmental outcome at age five years of the offspring of women who continued to exercise regularly throughout pregnancy. J. Pediatr. 1996, 129, 856–863. [Google Scholar] [CrossRef]
- Weissgerber, T.L.; Wolfe, L.A.; Davies, G.A.; Mottola, M.F. Exercise in the prevention and treatment of maternal-fetal disease: A review of the literature. Appl. Physiol. Nutr. Metab. 2006, 31, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Park, H.S. Physical exercise improves cognitive function by enhancing hippocampal neurogenesis and inhibiting apoptosis in male offspring born to obese mother. Behav. Brain Res. 2018, 347, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Bick-Sander, A.; Steiner, B.; Wolf, S.A.; Babu, H.; Kempermann, G. Running in pregnancy transiently increases postnatal hippocampal neurogenesis in the offspring. Proc. Natl. Acad. Sci. USA 2006, 103, 3852–3857. [Google Scholar] [CrossRef]
- Almeida, R.D.; Manadas, B.J.; Melo, C.V.; Gomes, J.R.; Mendes, C.S.; Grãos, M.M.; Carvalho, R.F.; Carvalho, A.P.; Duarte, C.B. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005, 12, 1329–1343. [Google Scholar] [CrossRef]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef]
- Markham, A.; Cameron, I.; Bains, R.; Franklin, P.; Kiss, J.P.; Schwendimann, L.; Gressens, P.; Spedding, M. Brain-derived neurotrophic factor-mediated effects on mitochondrial coupling and neuroprotection share the same molecular signaling pathways. Eur. J. Neurosci. 2012, 35, 366–374. [Google Scholar] [CrossRef]
- Markham, A.; Bains, R.; Franklin, P.; Spedding, M. Changes in mitochondrial function are pivotal in neurodegenerative and psychiatric disorders: How important is BDNF? Br. J. Pharmacol. 2014, 171, 2206–2229. [Google Scholar] [CrossRef]
- Kodomari, I.; Wada, E.; Nakamura, S.; Wada, K. Maternal supply of BDNF to mouse fetal brain through the placenta. Neurochem. Int. 2009, 54, 95–98. [Google Scholar] [CrossRef]
- Jin, J.J.; Ko, I.G.; Kim, S.E.; Hwang, L.K.; Lee, M.G.; Kim, D.Y.; Jung, S.Y. Age-dependent differences of treadmill exercise on spatial learning ability between young- and adult age rats. J. Exerc. Rehabil. 2017, 13, 381–386. [Google Scholar] [CrossRef]
- Shin, M.S.; Ko, I.G.; Kim, S.E.; Kim, B.K.; Kim, T.S.; Lee, S.H.; Hwang, D.S.; Kim, C.J.; Park, J.K.; Lim, B.V. Treadmill exercise ameliorates symptoms of methimazole-induced hypothyroidism through enhancing neurogenesis and suppressing apoptosis in the hippocampus of rat pups. Int. J. Dev. Neurosci. 2013, 31, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.W.; No, M.H.; Cho, J.K.; Choi, Y.J.; Cho, E.J.; Park, D.H.; Kim, T.W.; Kim, C.J.; Seo, D.Y.; Han, J.; et al. Moderate aerobic exercise training ameliorates impairment of mitochondrial function and dynamics in skeletal muscle of high-fat diet-induced obese mice. FASEB J. 2021, 35, e21340. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-W.; Park, S.-S.; Park, H.-S. Effects of Exercise Training during Advanced Maternal Age on the Cognitive Function of Offspring. Int. J. Mol. Sci. 2022, 23, 5517. https://doi.org/10.3390/ijms23105517
Kim T-W, Park S-S, Park H-S. Effects of Exercise Training during Advanced Maternal Age on the Cognitive Function of Offspring. International Journal of Molecular Sciences. 2022; 23(10):5517. https://doi.org/10.3390/ijms23105517
Chicago/Turabian StyleKim, Tae-Woon, Sang-Seo Park, and Hye-Sang Park. 2022. "Effects of Exercise Training during Advanced Maternal Age on the Cognitive Function of Offspring" International Journal of Molecular Sciences 23, no. 10: 5517. https://doi.org/10.3390/ijms23105517
APA StyleKim, T.-W., Park, S.-S., & Park, H.-S. (2022). Effects of Exercise Training during Advanced Maternal Age on the Cognitive Function of Offspring. International Journal of Molecular Sciences, 23(10), 5517. https://doi.org/10.3390/ijms23105517






