Investigation of Structural, Morphological and Magnetic Properties of MFe2O4 (M = Co, Ni, Zn, Cu, Mn) Obtained by Thermal Decomposition
Abstract
:1. Introduction
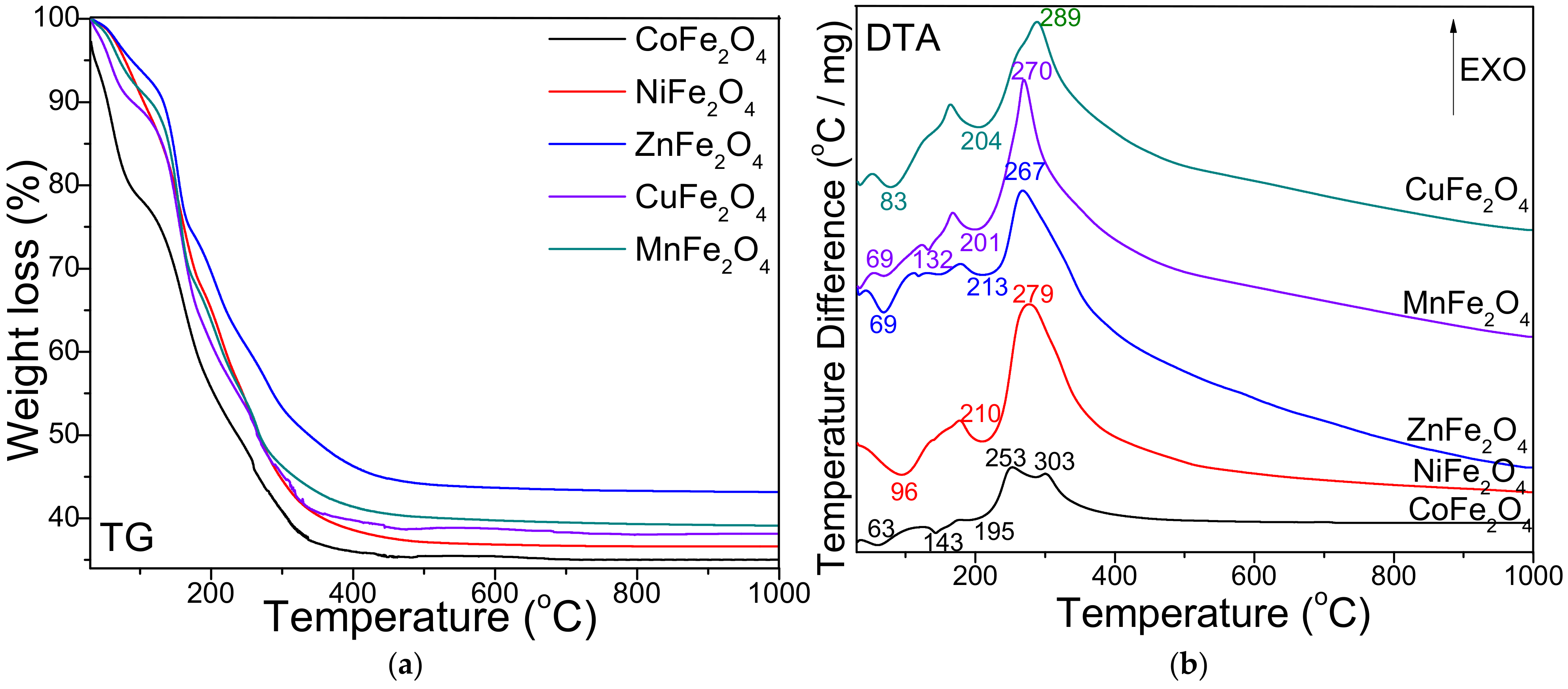
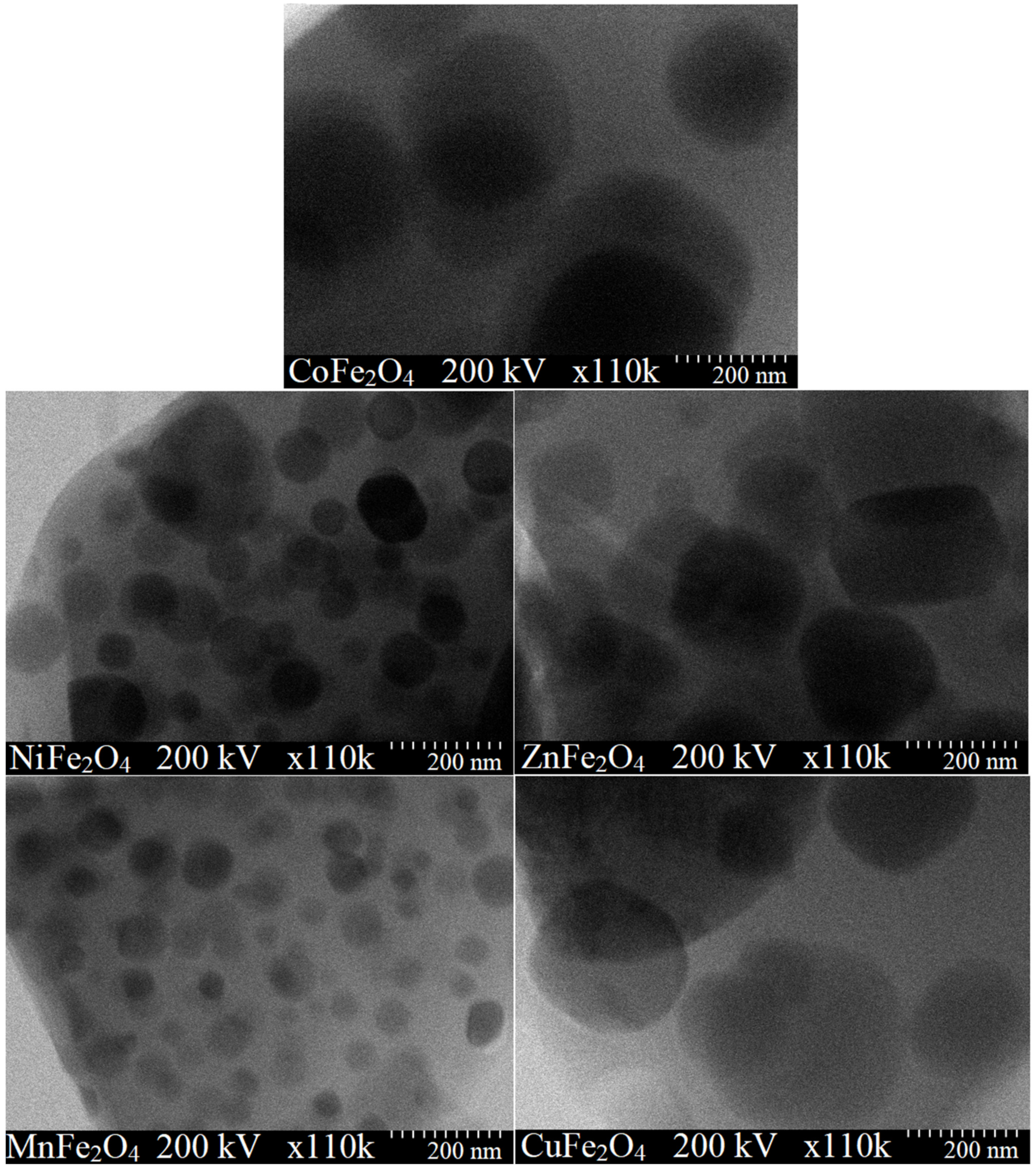
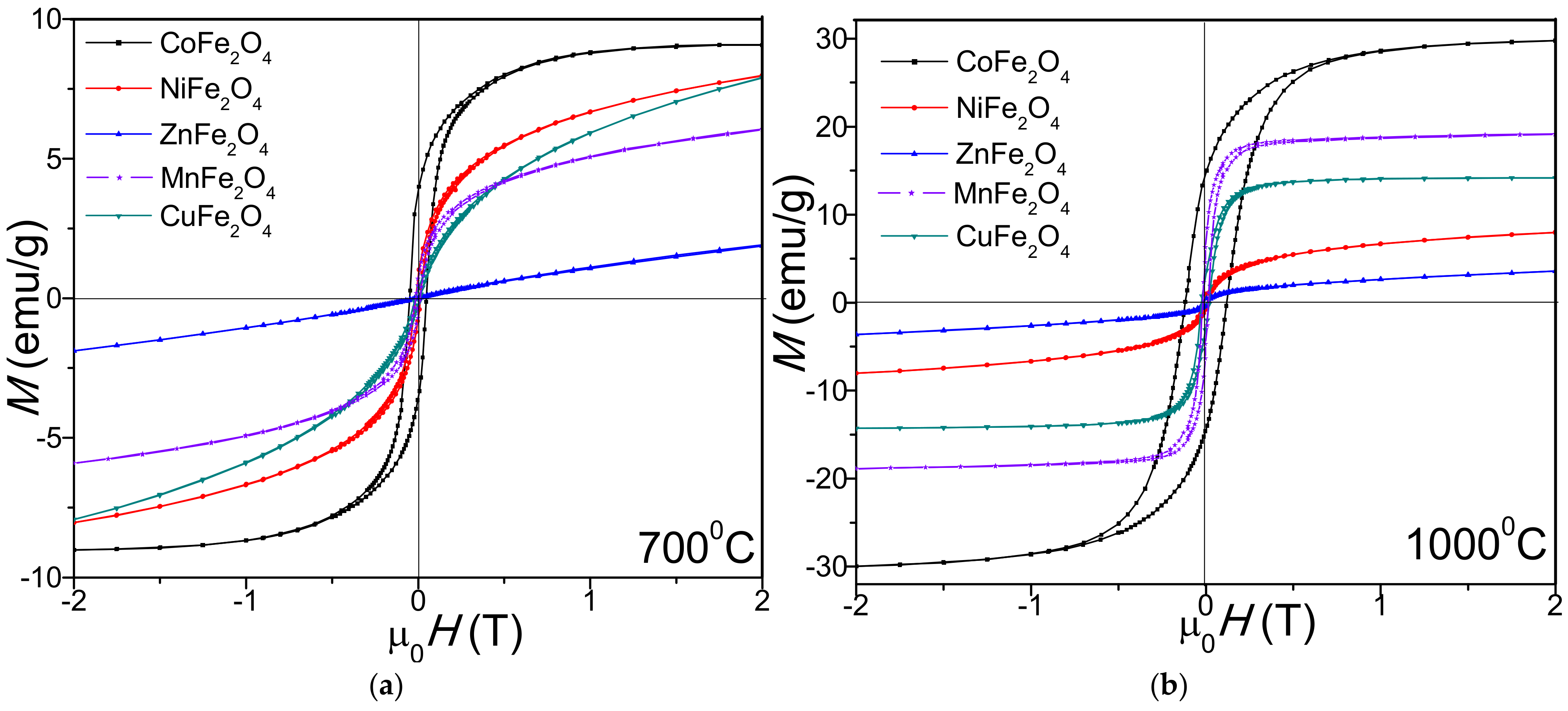
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mylarappa, M.; Lakshmi Venkata, V.; Mahesh Vishnu, K.R.; Raghavendra, N.; Nagaswarupa, H.P. Cyclic voltammetry, impedance and thermal properties of CoFe2O4 obtained from waste Li-Ion batteries. Mater. Today Proc. 2018, 5, 22425–22432. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Tu, J.P.; Shi, S.J.; Liu, X.J.; Wang, X.L.; Gu, C.D. Ascorbic acid-assisted synthesis of cobalt ferrite (CoFe2O4) hierarchical flower-like microspheres with enhanced lithium storage properties. J. Power Sources 2014, 256, 153–159. [Google Scholar] [CrossRef]
- Shetty, K.; Renuka, L.; Nagaswarupa, H.P.; Nagabhushana, H.; Anantharaju, K.S.; Rangappa, D.; Prashantha, S.C.; Ashwini, K. A comparative study on CuFe2O4, ZnFe2O4 and NiFe2O4: Morphology, impedance and photocatalytic studies. Mater. Today Proc. 2017, 4, 11806–11815. [Google Scholar] [CrossRef]
- Asghar, K.; Qasim, M.; Das, D. Preparation and characterization of mesoporous magnetic MnFe2O4@mSiO2 nanocomposite for drug delivery application. Mater. Today Proc. 2020, 26, 87–93. [Google Scholar] [CrossRef]
- Chand, P.; Vaish, S.; Kumar, P. Structural, optical and dielectric properties of transition metal (MFe2O4; M = Co, Ni and Zn) nanoferrites. Phys. B 2017, 524, 53–63. [Google Scholar] [CrossRef]
- Revathi, J.; Abel, M.J.; Archana, V.; Sumithra, T.; Thiruneelakandan, R. Synthesis and characterization of CoFe2O4 and Ni-doped CoFe2O4 nanoparticles by chemical Co-precipitation technique for photo-degradation of organic dyestuffs under direct sunlight. Phys. B 2020, 587, 412136. [Google Scholar] [CrossRef]
- Li, X.; Sun, Y.; Zong, Y.; Wei, Y.; Liu, X.; Li, X.; Peng, Y.; Zheng, X. Size-effect induced cation redistribution on the magnetic properties of well-dispersed CoFe2O4 nanocrystals. J. Alloy. Compd. 2020, 841, 155710. [Google Scholar] [CrossRef]
- Shilpa Amulya, M.A.; Nagaswarupta, H.P.; Anil Kumar, M.R.; Ravikumar, C.R.; Kusuma, K.B.; Prashantha, S.C. Evaluation of bifunctional applications of CuFe2O4 nanoparticles synthesized by a sonochemical method. J. Phys. Chem. Solids 2021, 148, 109756. [Google Scholar] [CrossRef]
- Peng, S.; Wang, S.; Liu, R.; Wu, J. Controlled oxygen vacancies of ZnFe2O4 with superior gas sensing properties prepared via a facile one-step self-catalyzed treatment. Sens. Actuators B Chem. 2019, 288, 649–655. [Google Scholar] [CrossRef]
- Cui, K.; Sun, M.; Zhang, J.; Xu, J.; Zhai, Z.; Gong, T.; Hou, L.; Yuan, C. Facile solid-state synthesis of tetragonal CuFe2O4 spinels with improved infrared radiation performance. Ceram. Int. 2022, 48, 10555–10561. [Google Scholar] [CrossRef]
- Sarkar, K.; Mondal, R.; Dey, S.; Kumar, S. Cation vacancy and magnetic properties of ZnFe2O4 microspheres. Phys. B Condens. Matter 2020, 583, 412015. [Google Scholar] [CrossRef]
- Soufi, A.; Hajjaoui, H.; Elmoubarki, R.; Abdennouri, M.; Qourzal, S.; Barka, N. Heterogeneous Fenton-like degradation of tartrazine using CuFe2O4 nanoparticles synthesized by sol-gel combustion. Appl. Surf. Sci. Adv. 2022, 9, 100251. [Google Scholar] [CrossRef]
- Rajini, R.; Ferdinand, A.C. Structural, morphological and magnetic properties of (c-ZnFe2O4 and t-CuFe2O4) ferrite nanoparticle synthesized by reactive ball milling. Chem. Data Collect. 2022, 38, 100825. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Yaqub, N.; Sepiol, B.; Ismail, B. A study of the influence of crystallite size on the electrical and magnetic properties of CuFe2O4. Mater. Res. Bull. 2011, 46, 1837–1842. [Google Scholar] [CrossRef]
- Dey, A.; Saini, B. Effect of various surfactant templates on the physicochemical properties of CoFe2O4 nanoparticles. Mater. Today Proc. 2022, 61, 351–355. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Lengauer, C.L.; Daniel, A.; Toloman, D.; Cadar, O. Investigation of thermal, structural, morphological and photocatalytic properties of CuxCo1-xFe2O4 (0 ≤ x ≤ 1) nanoparticles embedded in SiO2 matrix. Mater. Charact. 2020, 163, 110268. [Google Scholar] [CrossRef]
- Stefanescu, M.; Dippong, T.; Stoia, M.; Stefanescu, O. Study on the obtaining of cobalt oxides by thermal decomposition of some complex combinations, undispersed and dispersed in SiO2 matrix. J. Therm. Anal. Calorim. 2008, 94, 389–393. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Goga, F.; Petean, I.; Avram, A.; Cadar, O. The impact of polyol structure on the formation of Zn0.6Co0. 4Fe2O4 spinel-based pigments. J. Sol-Gel Sci. Technol. 2019, 93, 736–744. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O.; Mesaros, A.; Borodi, G. Sol-gel synthesis of CoFe2O4: SiO2 nanocomposites–insights into the thermal decomposition process of precursors. J. Anal. Appl. Pyrolysis 2017, 125, 169–177. [Google Scholar] [CrossRef]
- Sivakumar, A.; Dhas, S.S.J.; Dhas, S.A.M.B. Assessment of crystallographic and magnetic phase stabilities on MnFe2O4 nano crystalline materials at shocked conditions. Solid State Sci. 2020, 107, 106340. [Google Scholar] [CrossRef]
- Ozçelik, B.; Ozçelik, S.; Amaveda, H.; Santos, H.; Borrell, C.J.; Saez-Puche, R.; de la Fuente, G.F.; Angurel, L.A. High speed processing of NiFe2O4 spinel using laser furnance. J. Mater. 2020, 6, 661–670. [Google Scholar]
- Hoghoghifard, S.; Moradi, M. Influence of annealing temperature on structural, magnetic, and dielectric properties of NiFe2O4 nanorods synthesized by simple hydrothermal method. Ceram. Int. 2022, 48, 17768–17775. [Google Scholar] [CrossRef]
- Majid, F.; Rauf, J.; Ata, S.; Bibi, I.; Malik, A.; Ibrahim, S.M.; Ali, A.; Iqbal, M. Synthesis and characterization of NiFe2O4 ferrite: Sol–gel and hydrothermal synthesis routes effect on magnetic, structural and dielectric characteristics. Mater. Chem. Phys. 2021, 258, 123888. [Google Scholar] [CrossRef]
- Mohanty, D.; Satpathy, S.K.; Behera, B.; Mohapatra, R.K. Dielectric and frequency dependent transport properties in magnesium doped CuFe2O4 composite. Mater. Today Proc. 2020, 33, 5226–5231. [Google Scholar] [CrossRef]
- Priyadharsini, R.; Das, S.; Venkateshwarlu, M.; Deenadayalan, K.; Manoharan, C. The influence of reaction and annealing temperature on physical and magnetic properties of CuFe2O4 nanoparticles: Hydrothermal method. Inorg. Chem. Commun. 2022, 140, 109406. [Google Scholar] [CrossRef]
- Xu, P.; Xie, S.; Liu, X.; Wang, L.; Jia, X.; Yang, C. Electrochemical enhanced heterogenous activation of peroxymonosulfate using CuFe2O4 particle electrodes for the degradation of diclofenac. Chem. Eng. J. 2022, 446, 136941. [Google Scholar] [CrossRef]
- Ge, Y.-C.; Wang, Z.-L.; Yi, M.-Z.; Ran, L.-P. Fabrication and magnetic transformation from paramagnetic to ferrimagnetic of ZnFe2O4 hollow spheres. Trans. Nonferrous Met. Soc. China 2019, 29, 1503–1509. [Google Scholar] [CrossRef]
- Mohanty, D.; Mallick, P.; Biswall, S.K.; Behera, B.; Mohapatra, R.K.; Behera, A.; Satpathy, S.K. Investigation of structural, dielectric and electric properties of ZnFe2O4. Mater. Today Proc. 2020, 33, 4971–4975. [Google Scholar] [CrossRef]
- Junlabhut, P.; Nuthongkum, P.; Pechrapa, W. Influences of calcination temperature on structural properties of MnFe2O4 nanopowders synthesized by co-precipitation method for reusable absorbent materials. Mater. Today Proc. 2018, 5, 13857–13864. [Google Scholar] [CrossRef]
- Sivakumar, P.; Ramesh, R.; Ramanand, A.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and characterization of NiFe2O4 nanoparticles and nanorods. J. Alloys Compd. 2013, 563, 6–11. [Google Scholar] [CrossRef]
- Airimioaei, M.; Ciomaga, C.E.; Apostolescu, A.; Leonite, L.; Iordan, A.R.; Mitoseriu, L.; Palamaru, M.N. Synthesis and functional properties of the Ni1−xMnxFe2O4 ferrites. J. Alloys Compd. 2011, 509, 8065–8072. [Google Scholar] [CrossRef]
- Mathubala, G.; Manikandan, A.; Arul Antony, S.; Ramar, P. Photocatalytic degradation of methylene blue dye and magnetooptical studies of magnetically recyclable spinel NixMn1-xFe2O4 (x = 0.0 − 1.0) nanoparticles. J. Mol. Struct. 2016, 113, 79–87. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O. Formation, structure and magnetic properties of MFe2O4@SiO2 (M = Co, Mn, Zn, Ni, Cu) nanocomposites. Materials 2021, 14, 1139. [Google Scholar] [CrossRef]
- Alarifi, A.; Deraz, N.M.; Shaban, S. Structural, morphological and magnetic properties of NiFe2O4 nano-particles. J. Alloy. Compd. 2009, 486, 501–506. [Google Scholar] [CrossRef]
- Naseri, M.G.; Bin Saion, E.; Ahangar, H.A.; Hashim, M.; Shaari, A.H. Synthesis and characterization of manganese ferrite nanoparticles by thermal treatment method. J. Magn. Magn. Mater. 2011, 323, 1745–1749. [Google Scholar] [CrossRef]
- Joint Committee on Powder Diffraction Standards. International Center for Diffraction Data; ASTM: Philadelphia, PA, USA, 1999. [Google Scholar]
- Ati, M.A.; Othaman, Z.; Samavati, A. Influence of cobalt on structural and magnetic properties of nickel ferrite nanoparticles. J. Mol. Struct. 2013, 1052, 177–182. [Google Scholar] [CrossRef]
- Vinosha, P.A.; Xavier, B.; Krishnan, S.; Das, S.J. Investigation on zinc substituted highly porous improved catalytic activity of NiFe2O4 nanocrystal by co-precipitation method. Mater. Res. Bull. 2018, 101, 190–198. [Google Scholar] [CrossRef]
- Jadhav, J.; Biswas, S.; Yadav, A.K.; Jha, S.N.; Bhattacharyya, D. Structural and magnetic properties of nanocrystalline Ni-Zn ferrites: In the context of cationic distribution. J. Alloys Compd. 2017, 696, 28–41. [Google Scholar] [CrossRef]
- Salunkhe, A.B.; Khot, V.M.; Phadatare, M.R.; Thorat, N.D.; Joshi, R.S.; Yadav, H.M.; Pawar, S.H. Low temperature combustion synthesis and magnetostructural properties of Co-Mn nanoferrites. J. Magn. Magn. Mater. 2014, 352, 91–98. [Google Scholar] [CrossRef]
- Lemine, O.M.; Bououdina, M.; Sajieddine, M.; Al-Saie, A.M.; Shafi, M.; Khatab, A.; Al-Hilali, M.; Henini, M. Synthesis, structural, magnetic and optical properties of nanocrystalline ZnFe2O4. Phys. B 2011, 406, 1989–1994. [Google Scholar] [CrossRef]
- Yadav, S.P.; Shinde, S.S.; Bhatt, P.; Meena, S.S.; Rajpure, K.Y. Distribution of cations in Co1-xMnxFe2O4 using XRD, magnetization and Mossbauer spectroscopy. J. Alloys Compd. 2015, 646, 550–556. [Google Scholar] [CrossRef]
- Stefanescu, M.; Stoia, M.; Caizer, C.; Dippong, T.; Barvinschi, P. Preparation of CoxFe3− xO4 nanoparticles by thermal decomposition of some organo-metallic precursors. J. Therm. Anal. Calorim. 2009, 97, 245–250. [Google Scholar] [CrossRef]
- Sontu, U.B.; Yelasani, V.; Musugu, V.R.R. Structural, electrical and magnetic characteristics of nickel substituted cobalt ferrite nanoparticles, synthesized by self combustion method. J. Magn. Magn. Mater. 2015, 374, 376–380. [Google Scholar] [CrossRef]
- Bameri, I.; Saffari, J.; Baniyaghoob, S.; Ekrami-Kakhki, M.S. Synthesis of magnetic nano-NiFe2O4 with the assistance of ultrasound and its application for photocatalytic degradation of Titan Yellow: Kinetic and isotherm studies. Colloid Interface Sci. Commun. 2022, 48, 100610. [Google Scholar] [CrossRef]
- Belhadj, H.; Messaoudi, Y.; Khelladi, M.R.; Azizi, A. A facile synthesis of metal ferrites (MFe2O4, M = Co, Ni, Zn, Cu) as effective electrocatalysts toward electrochemical hydrogen evolution reaction. Int. J. Hydrog. Energy 2022, 47, 20129–20137. [Google Scholar] [CrossRef]

| Temperature (°C) | CoFe2O4 | NiFe2O4 | ZnFe2O4 | MnFe2O4 | CuFe2O4 | |
|---|---|---|---|---|---|---|
| DPS (nm) | 1000 | 78 | 52 | 68 | 32 | 85 |
| DCS (nm) | 700 1000 | 23 69 | 18 49 | 13 57 | 14 29 | 27 81 |
| a (Å) | 700 1000 | 8.275 8.334 | 8.258 8.365 | 8.278 8.342 | 8.269 8.318 | 8.207 8.302 |
| M/Fe molar ratio | 700 1000 | - 0.99/2.00 | 0.98/2.04 0.99/2.01 | 0.97/2.01 0.99/2.00 | - 0.98/1.99 | 0.98/2.03 1.00/2.01 |
| Temperature (°C) | CoFe2O4 | NiFe2O4 | ZnFe2O4 | CuFe2O4 | MnFe2O4 | |
|---|---|---|---|---|---|---|
| MS (emu/g) | 700 1000 | 9.20 29.7 | 8.08 11.2 | 1.89 2.45 | 7.91 14.2 | 6.08 19.1 |
| MR (emu/g) | 700 1000 | 3.98 14.1 | 1.55 8.14 | 0.142 0.695 | 1.11 9.82 | 0.98 1.55 |
| HC (Oe) | 700 1000 | 49.5 131 | 4.62 12.8 | 1.81 10.1 | 10.4 14.7 | 20.8 51.6 |
| S | 700 1000 | 0.437 0.475 | 0.192 0.727 | 0.075 0.284 | 0.143 0.692 | 0.161 0.081 |
| K × 103 (erg/cm3) | 700 1000 | 0.286 2.44 | 0.023 0.090 | 0.002 0.015 | 0.052 0.131 | 0.079 0.619 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dippong, T.; Levei, E.A.; Cadar, O. Investigation of Structural, Morphological and Magnetic Properties of MFe2O4 (M = Co, Ni, Zn, Cu, Mn) Obtained by Thermal Decomposition. Int. J. Mol. Sci. 2022, 23, 8483. https://doi.org/10.3390/ijms23158483
Dippong T, Levei EA, Cadar O. Investigation of Structural, Morphological and Magnetic Properties of MFe2O4 (M = Co, Ni, Zn, Cu, Mn) Obtained by Thermal Decomposition. International Journal of Molecular Sciences. 2022; 23(15):8483. https://doi.org/10.3390/ijms23158483
Chicago/Turabian StyleDippong, Thomas, Erika Andrea Levei, and Oana Cadar. 2022. "Investigation of Structural, Morphological and Magnetic Properties of MFe2O4 (M = Co, Ni, Zn, Cu, Mn) Obtained by Thermal Decomposition" International Journal of Molecular Sciences 23, no. 15: 8483. https://doi.org/10.3390/ijms23158483
APA StyleDippong, T., Levei, E. A., & Cadar, O. (2022). Investigation of Structural, Morphological and Magnetic Properties of MFe2O4 (M = Co, Ni, Zn, Cu, Mn) Obtained by Thermal Decomposition. International Journal of Molecular Sciences, 23(15), 8483. https://doi.org/10.3390/ijms23158483








