Tracking Immature Testicular Tissue after Vitrification In Vitro and In Vivo for Pre-Pubertal Fertility Preservation: A Translational Transgenic Mouse Model
Abstract
1. Introduction
2. Results
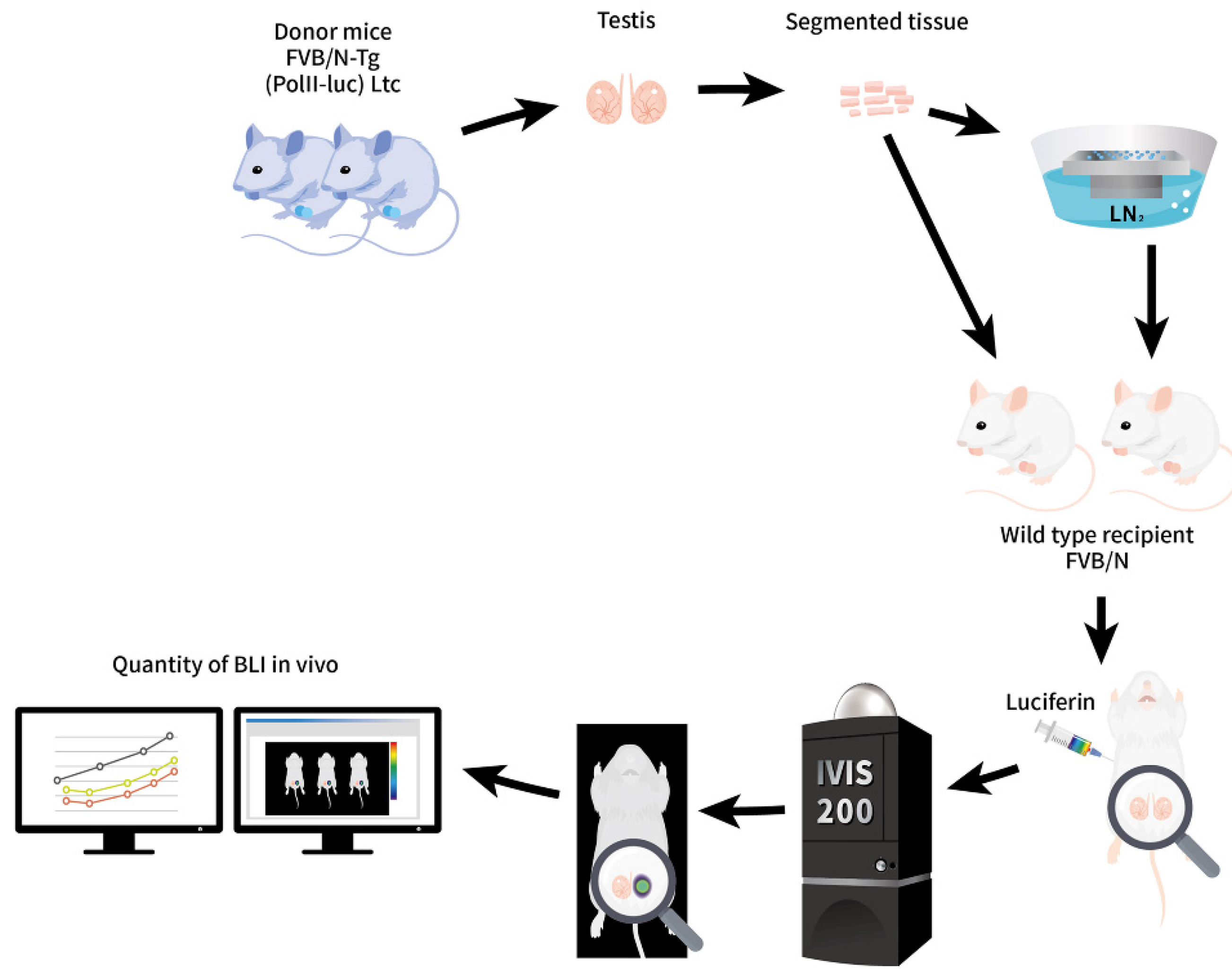
2.1. Tracking ITT Grafts after SSV and Rewarming In Vitro and In Vivo until Adulthood for Pre-Pubertal Fertility Preservation
2.2. ITT Fragments of 3-Week-Old FVB/N-Tg (PolII-luc) Ltc Mice Used as Donor Grafts
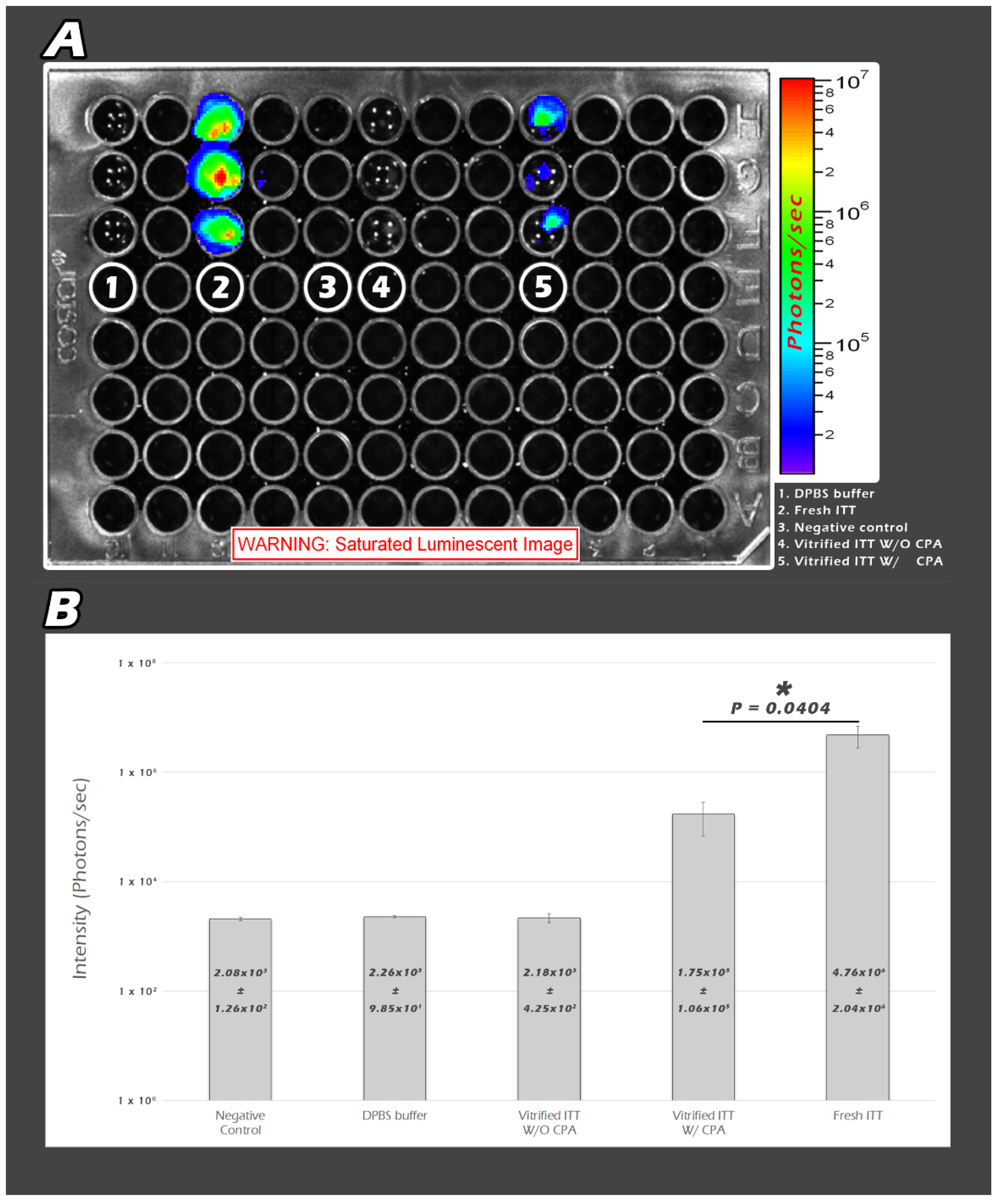
2.3. In Vitro Quantity-Based BLI of ITT Fragments by Quantum Yields (QYs) in the Culture Plate
2.4. The Ultrastructure of Fresh ITT, Vitrfied ITT without and with CPA by SEM
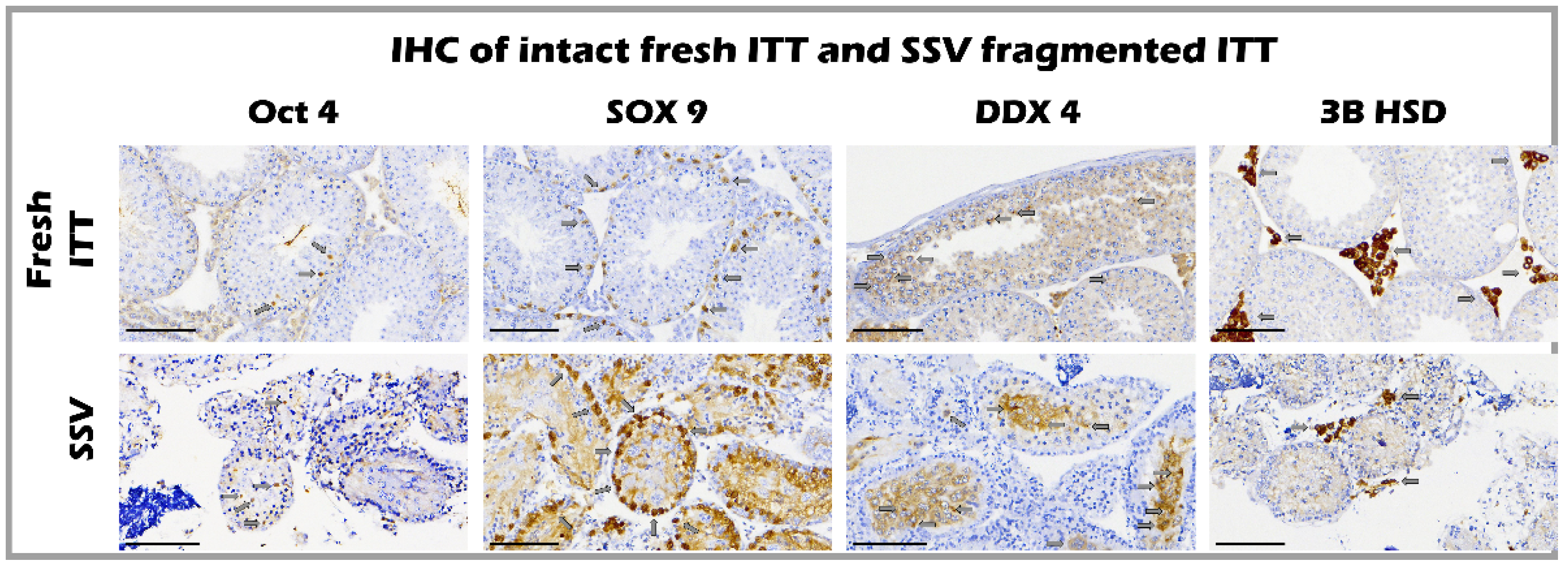
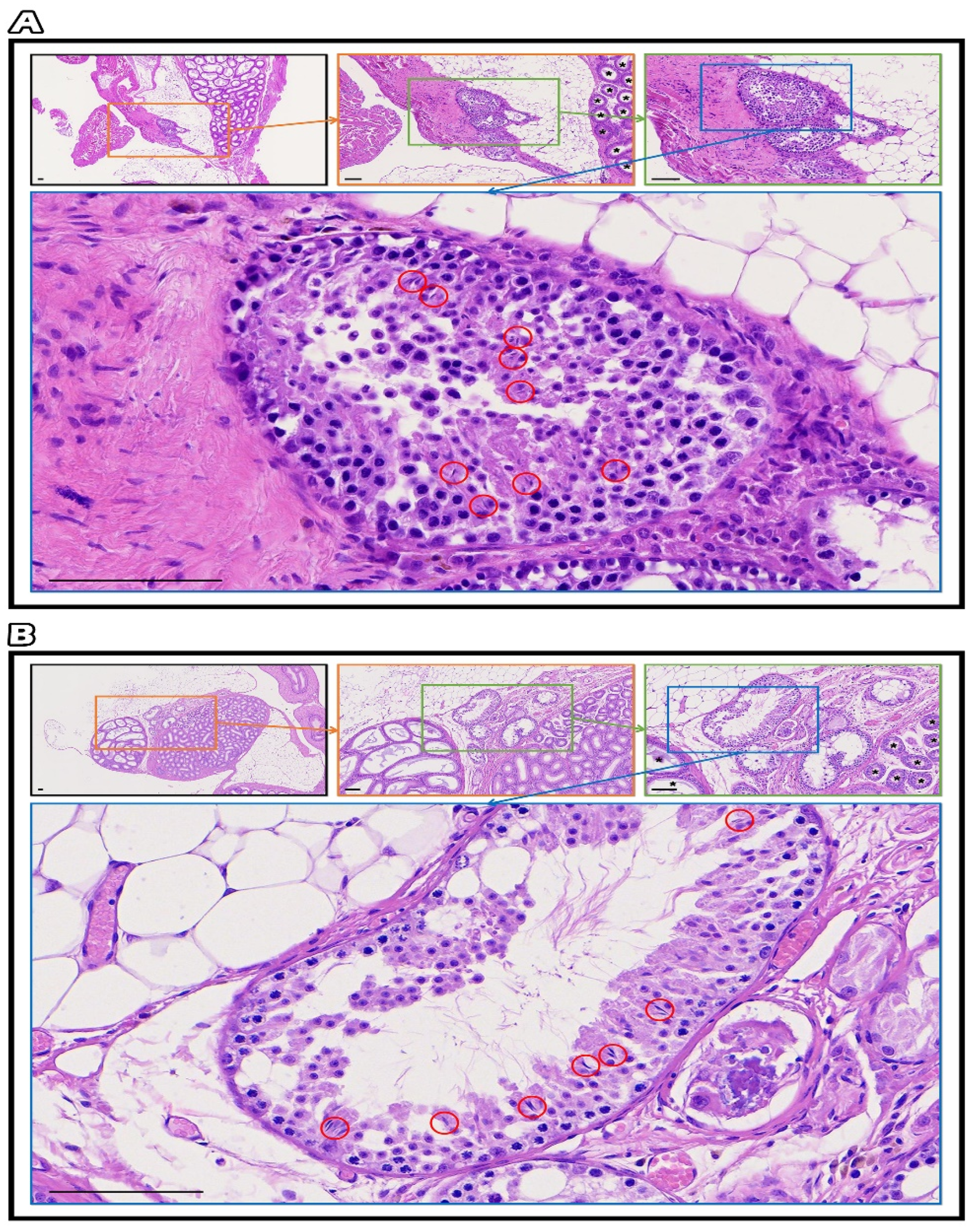
2.5. The Ultrastructure of Seminiferous Tubules of Fresh and SSV Groups by IHC
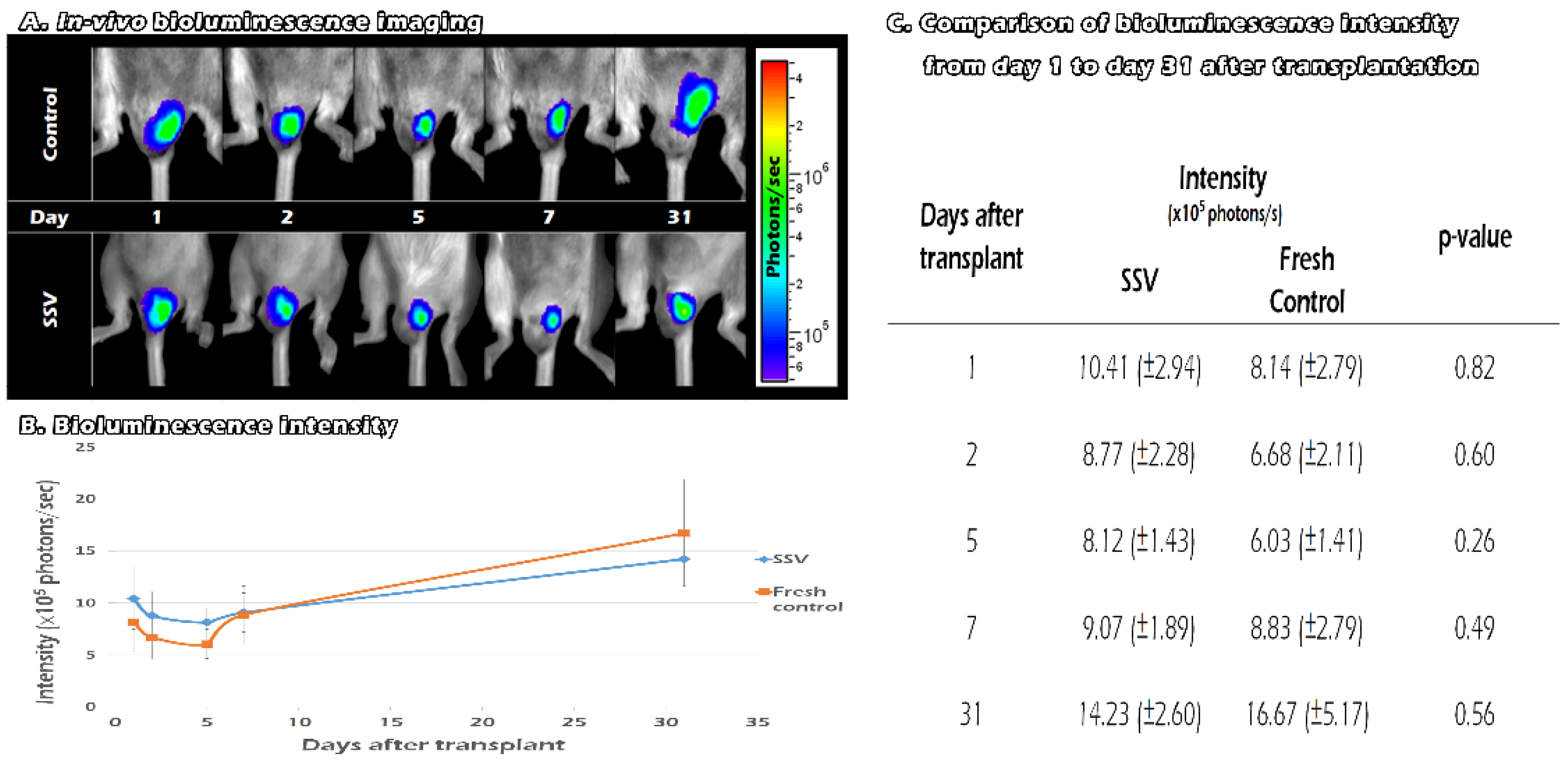
2.6. Tracking the In Vivo BLI of Transgenic Mouse ITT Grafts in Fresh and SSV Group over Time
2.7. Testicular Grafts of Hematoxylin and Eosin Staining
3. Discussion
4. Materials and Methods
4.1. Bioethics and Animals
4.2. Study Design and ITT Collection
4.3. ITT Transplantation after Solid Surface Vitrification and Rewarming of ITT
4.4. Assessment of Thawed ITT Recovery In Vitro
4.5. Scanning Electron Microscopy of the Seminiferous Tubule Ultrastructure of Fresh ITT, ITT without CPA, and ITT with CPA
4.6. Immunohistochemistry Staining
4.7. In Vivo Tracking the Development of Vitrified ITT Grafts until 31 Days after Transplantation
4.8. Testicular Grafts of Hematoxylin and Eosin Staining
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Wyns, C.; Kanbar, M.; Giudice, M.G.; Poels, J. Fertility preservation for prepubertal boys: Lessons learned from the past and update on remaining challenges towards clinical translation. Hum. Reprod. Update 2021, 27, 433–459. [Google Scholar] [CrossRef] [PubMed]
- Oktay, K.; Harvey, B.E.; Loren, A.W. Fertility Preservation in Patients with Cancer: ASCO Clinical Practice Guideline Update Summary. J. Oncol. Pract. 2018, 14, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Demeestere, I.; Simon, P.; Dedeken, L.; Moffa, F.; Tsépélidis, S.; Brachet, C.; Delbaere, A.; Devreker, F.; Ferster, A. Live birth after autograft of ovarian tissue cryopreserved during childhood. Hum. Reprod. 2015, 30, 2107–2109. [Google Scholar] [CrossRef]
- Ladanyi, C.; Mor, A.; Christianson, M.S.; Dhillon, N.; Segars, J.H. Recent advances in the field of ovarian tissue cryopreservation and opportunities for research. J. Assist. Reprod. Genet. 2017, 34, 709–722. [Google Scholar] [CrossRef]
- Fayomi, A.P.; Peters, K.; Sukhwani, M.; Valli-Pulaski, H.; Shetty, G.; Meistrich, M.L.; Houser, L.; Robertson, N.; Roberts, V.; Ramsey, C.; et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science 2019, 363, 1314–1319. [Google Scholar] [CrossRef]
- Goossens, E.; Jahnukainen, K.; Mitchell, R.T.; van Pelt, A.; Pennings, G.; Rives, N.; Poels, J.; Wyns, C.; Lane, S.; Rodriguez-Wallberg, K.A.; et al. Fertility preservation in boys: Recent developments and new insights (dagger). Hum. Reprod. Open 2020, 2020, hoaa016. [Google Scholar] [CrossRef] [PubMed]
- Curaba, M.; Poels, J.; van Langendonckt, A.; Donnez, J.; Wyns, C. Can prepubertal human testicular tissue be cryopreserved by vitrification? Fertil. Steril. 2011, 95, 2123.e9–2123.e12. [Google Scholar] [CrossRef]
- Martino, A.; Songsasen, N.; Leibo, S.P. Development into blastocysts of bovine oocytes cryopreserved by ultra-rapid cooling. Biol. Reprod. 1996, 54, 1059–1069. [Google Scholar] [CrossRef]
- Vajta, G.; Holm, P.; Kuwayama, M.; Booth, P.J.; Jacobsen, H.; Greve, T.; Callesen, H. Open Pulled Straw (OPS) vitrification: A new way to reduce cryoinjuries of bovine ova and embryos. Mol. Reprod. Dev. 1998, 51, 53–58. [Google Scholar] [CrossRef]
- Lane, M.; Bavister, B.D.; Lyons, E.A.; Forest, K.T. Containerless vitrification of mammalian oocytes and embryos. Nat. Biotechnol. 1999, 17, 1234–1236. [Google Scholar] [CrossRef]
- Al-aghbari, A.M.; Menino, A.R. Survival of oocytes recovered from vitrified sheep ovarian tissues. Anim. Reprod. Sci. 2002, 71, 101–110. [Google Scholar] [CrossRef]
- Papis, K.; Shimizu, M.; Izaike, Y. Factors affecting the survivability of bovine oocytes vitrified in droplets. Theriogenology 2000, 54, 651–658. [Google Scholar] [CrossRef]
- Abrishami, M.; Anzar, M.; Yang, Y.; Honaramooz, A. Cryopreservation of immature porcine testis tissue to maintain its developmental potential after xenografting into recipient mice. Theriogenology 2010, 73, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Curaba, M.; Verleysen, M.; Amorim, C.A.; Dolmans, M.M.; Van Langendonckt, A.; Hovatta, O.; Wyns, C.; Donnez, J. Cryopreservation of prepubertal mouse testicular tissue by vitrification. Fertil. Steril. 2011, 95, 1229–1234.e1. [Google Scholar] [CrossRef]
- Dumont, L.; Arkoun, B.; Jumeau, F.; Milazzo, J.P.; Bironneau, A.; Liot, D.; Wils, J.; Rondanino, C.; Rives, N. Assessment of the optimal vitrification protocol for pre-pubertal mice testes leading to successful in vitro production of flagellated spermatozoa. Andrology 2015, 3, 611–625. [Google Scholar] [CrossRef]
- Poels, J.; Van Langendonckt, A.; Many, M.C.; Wese, F.X.; Wyns, C. Vitrification preserves proliferation capacity in human spermatogonia. Hum. Reprod. 2013, 28, 578–589. [Google Scholar] [CrossRef]
- Baert, Y.; Van Saen, D.; Haentjens, P.; In’t Veld, P.; Tournaye, H.; Goossens, E. What is the best cryopreservation protocol for human testicular tissue banking? Hum. Reprod. 2013, 28, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.C.; Tzeng, C.R.; Wang, C.W.; Hsu, M.I.; Tan, S.J.; Chen, C.H. Antiapoptotic agent sphingosine-1-phosphate protects vitrified murine ovarian grafts. Reprod. Sci. 2014, 21, 236–243. [Google Scholar] [CrossRef]
- Zinn, K.R.; Chaudhuri, T.R.; Szafran, A.A.; O’Quinn, D.; Weaver, C.; Dugger, K.; Lamar, D.; Kesterson, R.A.; Wang, X.; Frank, S.J. Noninvasive bioluminescence imaging in small animals. Ilar. J. 2008, 49, 103–115. [Google Scholar] [CrossRef]
- Lin, Y.H.; Yeh, Y.C.; Tzeng, C.R.; Shang, W.J.; Liu, J.Y.; Chen, C.H. Evaluating the effects of immunosuppression by in-vivo bioluminescence imaging after allotransplantation of ovarian grafts. Reprod. Biomed. Online 2011, 22, 220–227. [Google Scholar] [CrossRef]
- Chen, C.H.; Tan, S.J.; Tzeng, C.R. In vivo fate mapping of cryopreserved murine ovarian grafts. J. Ovarian. Res. 2014, 7, 81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, C.H.; Wang, C.W.; Hsu, M.I.; Huang, Y.H.; Lai, W.F.; Tzeng, C.R. Bioluminescence imaging as a tool to evaluate germ cells in vitro and transplantation in vivo as fertility preservation of prepubertal male mice. Fertil. Steril. 2012, 97, 1192–1198. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.; Liu, Y.L.; Lu, B.J.; Chen, C.H. Immature Testicular Tissue Engineered from Weaned Mice to Adults for Prepubertal Fertility Preservation-An In Vivo Translational Study. Int. J. Mol. Sci. 2022, 23, 2042. [Google Scholar] [CrossRef] [PubMed]
- Varner, D.D.; Johnson, L. From a sperm’s eye view: Revisiting our perception of this intriguing cell. In Proceedings of the AAEP, 2007, Orlando, FL, USA, 5 December 2007; pp. 104–177. [Google Scholar]
- Dann, C.T.; Alvarado, A.L.; Molyneux, L.A.; Denard, B.S.; Garbers, D.L.; Porteus, M.H. Spermatogonial stem cell self-renewal requires OCT4, a factor downregulated during retinoic acid-induced differentiation. Stem. Cells 2008, 26, 2928–2937. [Google Scholar] [CrossRef] [PubMed]
- Hemendinger, R.A.; Gores, P.; Blacksten, L.; Harley, V.; Halberstadt, C. Identification of a specific Sertoli cell marker, Sox9, for use in transplantation. Cell Transpl. 2002, 11, 499–505. [Google Scholar] [CrossRef]
- Haider, S.G. Cell biology of Leydig cells in the testis. Int. Rev. Cytol. 2004, 233, 181–241. [Google Scholar]
- Azizi, H.; NiaziTabar, A.; Mohammadi, A.; Skutella, T. Characterization of DDX4 Gene Expression in Human Cases with Non-Obstructive Azoospermia and in Sterile and Fertile Mice. J. Reprod. Infertil. 2021, 22, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Dumont, L.; Chalmel, F.; Oblette, A.; Berby, B.; Rives, A.; Duchesne, V.; Rondanino, C.; Rives, N. Evaluation of apoptotic- and autophagic-related protein expressions before and after IVM of fresh, slow-frozen and vitrified pre-pubertal mouse testicular tissue. Mol. Hum. Reprod. 2017, 23, 738–754. [Google Scholar] [CrossRef]
- Yokonishi, T.; Sato, T.; Komeya, M.; Katagiri, K.; Kubota, Y.; Nakabayashi, K.; Hata, K.; Inoue, K.; Ogonuki, N.; Ogura, A.; et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat. Commun. 2014, 5, 4320. [Google Scholar] [CrossRef] [PubMed]
- Dissen, G.A.; Lara, H.E.; Fahrenbach, W.H.; Costa, M.E.; Ojeda, S.R. Immature Rat Ovaries Become Revascularized Rapidly after Autotransplantation and Show a Gonadotropin-Dependent Increase in Angiogenic Factor Gene-Expression. Endocrinology 1994, 134, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Eslahi, N.; Hadjighassem, M.R.; Joghataei, M.T.; Mirzapour, T.; Bakhtiyari, M.; Shakeri, M.; Pirhajati, V.; Shirinbayan, P.; Koruji, M. The effects of poly L-lactic acid nanofiber scaffold on mouse spermatogonial stem cell culture. Int. J. Nanomed. 2013, 8, 4563–4576. [Google Scholar]
- Baert, Y.; Goossens, E.; van Saen, D.; Ning, L.; in’t Veld, P.; Tournaye, H. Orthotopic grafting of cryopreserved prepubertal testicular tissue: In search of a simple yet effective cryopreservation protocol. Fertil. Steril. 2012, 97, 1152–1157.e2. [Google Scholar] [CrossRef] [PubMed]
- Gouk, S.S.; Loh, Y.F.; Kumar, S.D.; Watson, P.F.; Kuleshova, L.L. Cryopreservation of mouse testicular tissue: Prospect for harvesting spermatogonial stem cells for fertility preservation. Fertil. Steril. 2011, 95, 2399–2403. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, T.; Inoue, K.; Ogonuki, N.; Kanatsu-Shinohara, M.; Miki, H.; Nakata, K.; Kurome, M.; Nagashima, H.; Toyokuni, S.; Kogishi, K.; et al. Birth of offspring following transplantation of cryopreserved immature testicular pieces and in-vitro microinsemination. Hum. Reprod. 2002, 17, 3039–3045. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Kikuchi, K.; Nakai, M.; Somfai, T.; Noguchi, J.; Tanihara, F.; Ito, J.; Kashiwazaki, N. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PLoS ONE 2013, 8, e70989. [Google Scholar] [CrossRef]
- Wyns, C.; Curaba, M.; Vanabelle, B.; Van Langendonckt, A.; Donnez, J. Options for fertility preservation in prepubertal boys. Hum. Reprod. Update 2010, 16, 312–328. [Google Scholar] [CrossRef]
- Sato, T.; Katagiri, K.; Gohbara, A.; Inoue, K.; Ogonuki, N.; Ogura, A.; Kubota, Y.; Ogawa, T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 2011, 471, 504–507. [Google Scholar] [PubMed]
- Patra, T.; Gupta, M.K. Solid surface vitrification of goat testicular cell suspension enriched for spermatogonial stem cells. Cryobiology 2022, 104, 8–14. [Google Scholar] [CrossRef]
- Patra, T.; Gupta, M.K. Cryopreservation of murine testicular Leydig cells by modified solid surface vitrification with supplementation of antioxidants. Cryobiology 2019, 88, 38–46. [Google Scholar] [CrossRef]
- Allen, C.M.; Lopes, F.; Mitchell, R.T.; Spears, N. How does chemotherapy treatment damage the prepubertal testis? Reproduction 2018, 156, R209–R233. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Moreira, M.; Halbert, J.; Valloton, D.; Velten, B.; Chen, C.; Shao, Y.; Liechti, A.; Ascencao, K.; Rummel, C.; Ovchinnikova, S.; et al. Gene expression across mammalian organ development. Nature 2019, 571, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Sarropoulos, I.; Marin, R.; Cardoso-Moreira, M.; Kaessmann, H. Developmental dynamics of lncRNAs across mammalian organs and species. Nature 2019, 571, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Leushkin, E.; Liechti, A.; Ovchinnikova, S.; Mossinger, K.; Bruning, T.; Rummel, C.; Grutzner, F.; Cardoso-Moreira, M.; Janich, P.; et al. Transcriptome and translatome co-evolution in mammals. Nature 2020, 588, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Dapson, R.W.; Horobin, R.W. Dyes from a twenty-first century perspective. Biotech. Histochem. 2009, 84, 135–137. [Google Scholar] [CrossRef]


| Year | Author | Donor | Recipient/Culture | V Protocol | Evaluation | Conclusion |
|---|---|---|---|---|---|---|
| 2010 | Abrishami et al. [14] | Piglet | Mice | SSV | In vitro histology | SSV and SF presented normal spermatogenesis |
| 2011 | Curaba et al. [15] | Mice | In vitro short-term oganotypic culture | Cryo-straws | In vitro apoptotic marker and histology | V and SF preserve survival, development, and integrity of ITT |
| 2011 | Curaba et al. [8] | Human | 10-day of organoty-pic culture | Cryo-straws | In vitro histology and IHC stain | V shows promise as an alternative strategy to SF |
| 2011 | Gouk et al. [35] | Mice | SSCs suspen-sions | Straw-in-straw method | In vitro evaluation and flow cytometry | V of testicular tissue is a time- and cost-efficient strategy to preserve SCC |
| 2012 | Baert et al. [34] | Mice | Mice | SSV | In vitro histology and IHC TEM | SSV resulted in success rates similar to SF |
| 2013 | Poels et al. [17] | Human | Mice | SSV | In vitro histology and IHC | SSV is able to maintain proliferation capacity in SSCs after 6 months of xenografting |
| 2013 | Baert et al. [18] | Huamn | No transplan-tation | SSV; Direct cover V | In vitro histology TEM | SSV may have a negative impact on spermatogonial number |
| 2015 | Dumont et al. [16] | Mice | In vitro culture | SSV; Open pulled straws; Single drop | In vitro histology and IHC Testosterone level Cell death assessment | SSV resulted in success rates better than SF for immediate frozen/thawed tissues but also after a long-term in vitro culture. |
| Our study 2022 | Lu et al. | Trans-genic mice | Mice | SSV | In vitro histology; IHC; SEM In vivo and in vitro BLI imaging | SSV presented similar BLI intensity, intact cells and ultrastructures for spermatogenesis after transplantation with the fresh control |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, B.-J.; Huang, Y.-L.; Liu, Y.-L.; Chen, B.S.; Lin, B.-Z.; Chen, C.-H. Tracking Immature Testicular Tissue after Vitrification In Vitro and In Vivo for Pre-Pubertal Fertility Preservation: A Translational Transgenic Mouse Model. Int. J. Mol. Sci. 2022, 23, 8425. https://doi.org/10.3390/ijms23158425
Lu B-J, Huang Y-L, Liu Y-L, Chen BS, Lin B-Z, Chen C-H. Tracking Immature Testicular Tissue after Vitrification In Vitro and In Vivo for Pre-Pubertal Fertility Preservation: A Translational Transgenic Mouse Model. International Journal of Molecular Sciences. 2022; 23(15):8425. https://doi.org/10.3390/ijms23158425
Chicago/Turabian StyleLu, Buo-Jia, Ya-Li Huang, Yung-Liang Liu, Brian Shiian Chen, Bou-Zenn Lin, and Chi-Huang Chen. 2022. "Tracking Immature Testicular Tissue after Vitrification In Vitro and In Vivo for Pre-Pubertal Fertility Preservation: A Translational Transgenic Mouse Model" International Journal of Molecular Sciences 23, no. 15: 8425. https://doi.org/10.3390/ijms23158425
APA StyleLu, B.-J., Huang, Y.-L., Liu, Y.-L., Chen, B. S., Lin, B.-Z., & Chen, C.-H. (2022). Tracking Immature Testicular Tissue after Vitrification In Vitro and In Vivo for Pre-Pubertal Fertility Preservation: A Translational Transgenic Mouse Model. International Journal of Molecular Sciences, 23(15), 8425. https://doi.org/10.3390/ijms23158425





