Therapeutic Effects of Quetiapine and 5-HT1A Receptor Agonism on Hyperactivity in Dopamine-Deficient Mice
Abstract
:1. Introduction
2. Results
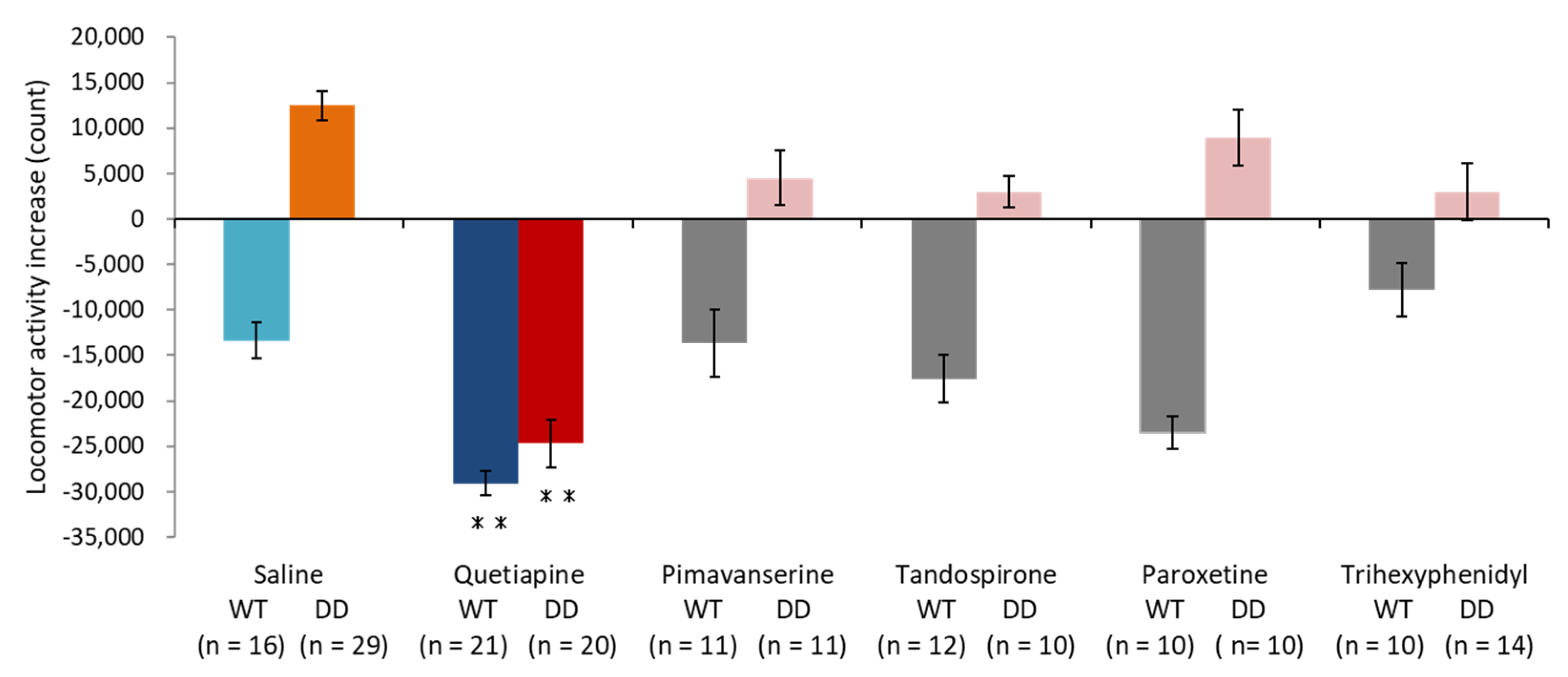
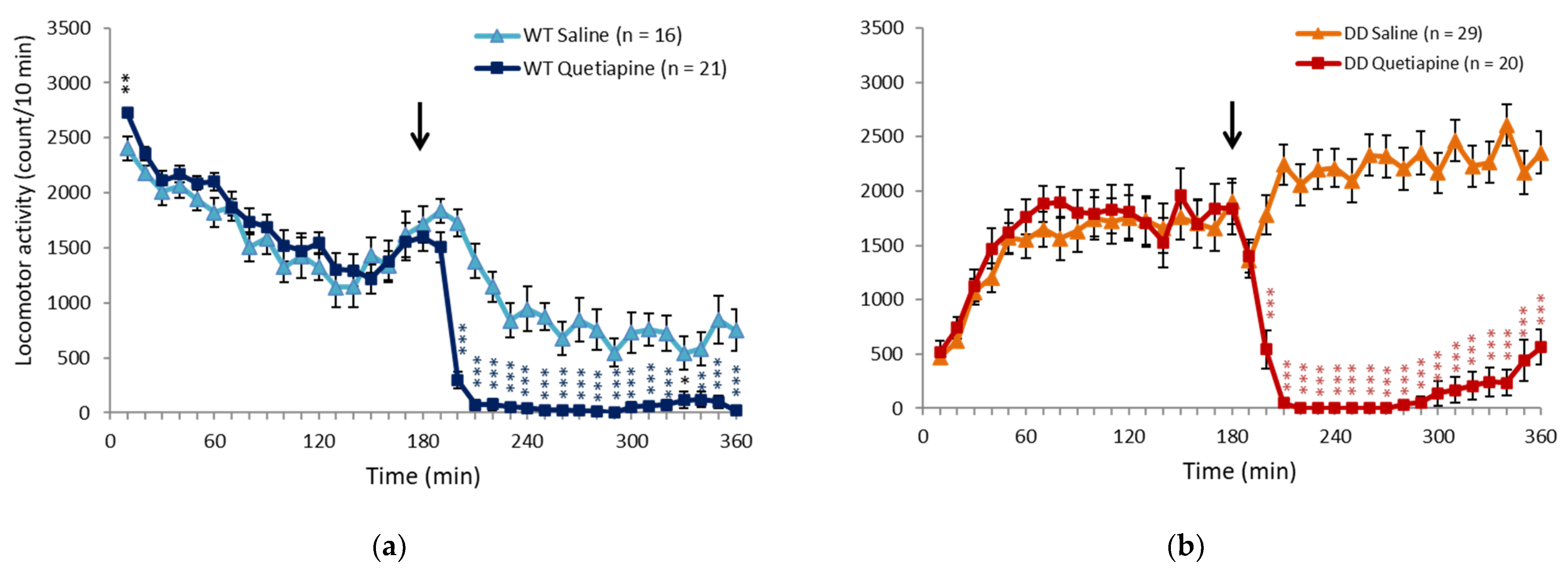
2.1. Quetiapine Ameliorated Hyperactivity in DD Mice
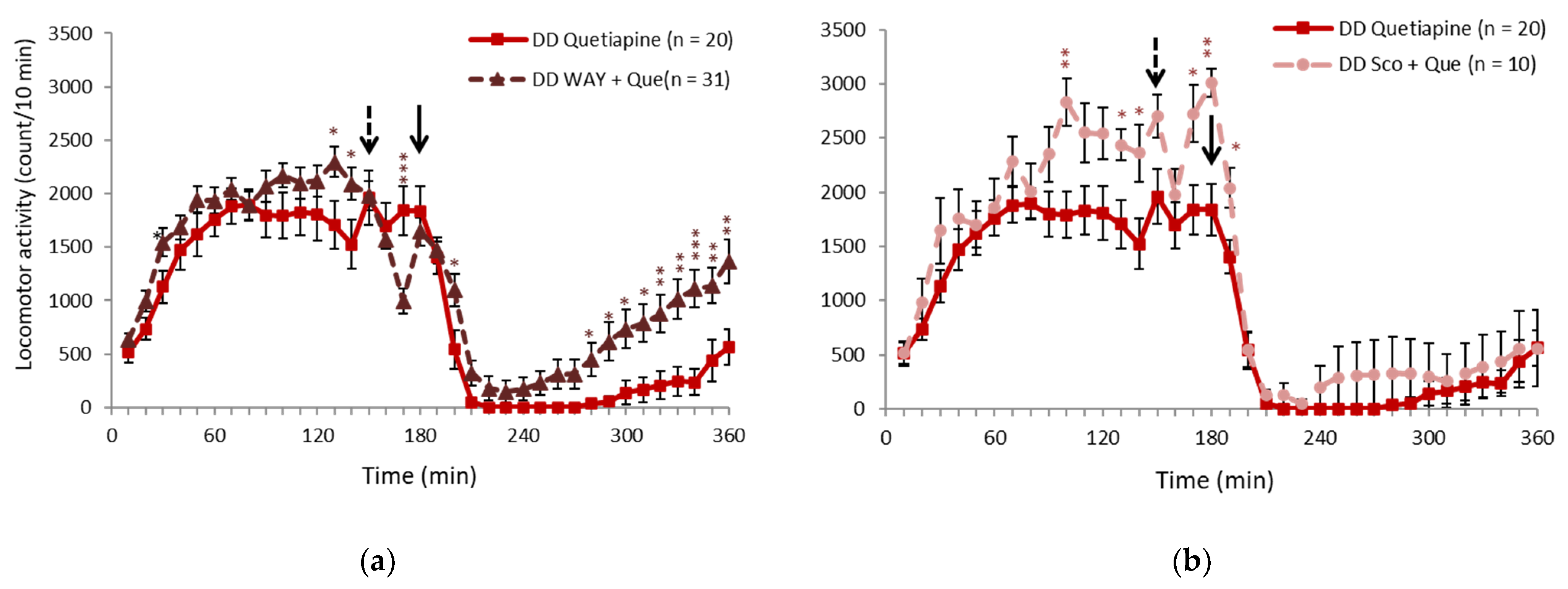
2.2. 5-HT1A Receptor Antagonist Partially Inhibited the Effect of Quetiapine
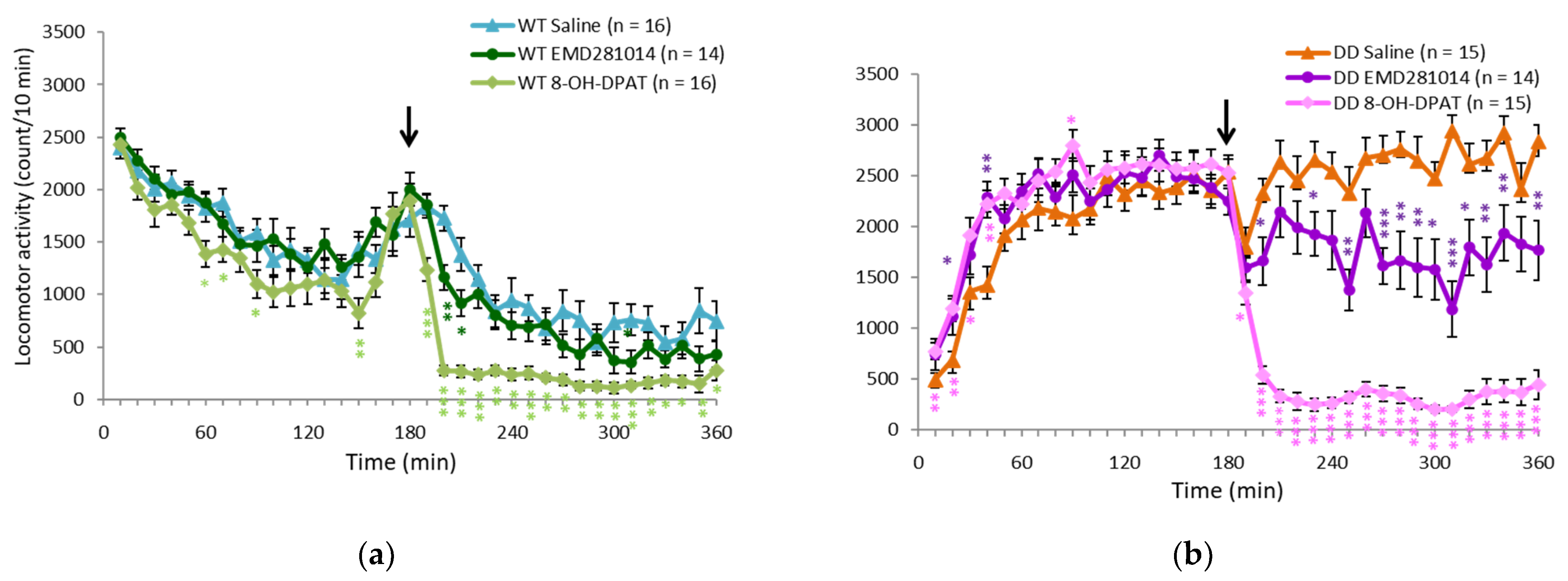
2.3. 5-HT1A Receptor Agonist Ameliorated Hyperactivity in DD Mice
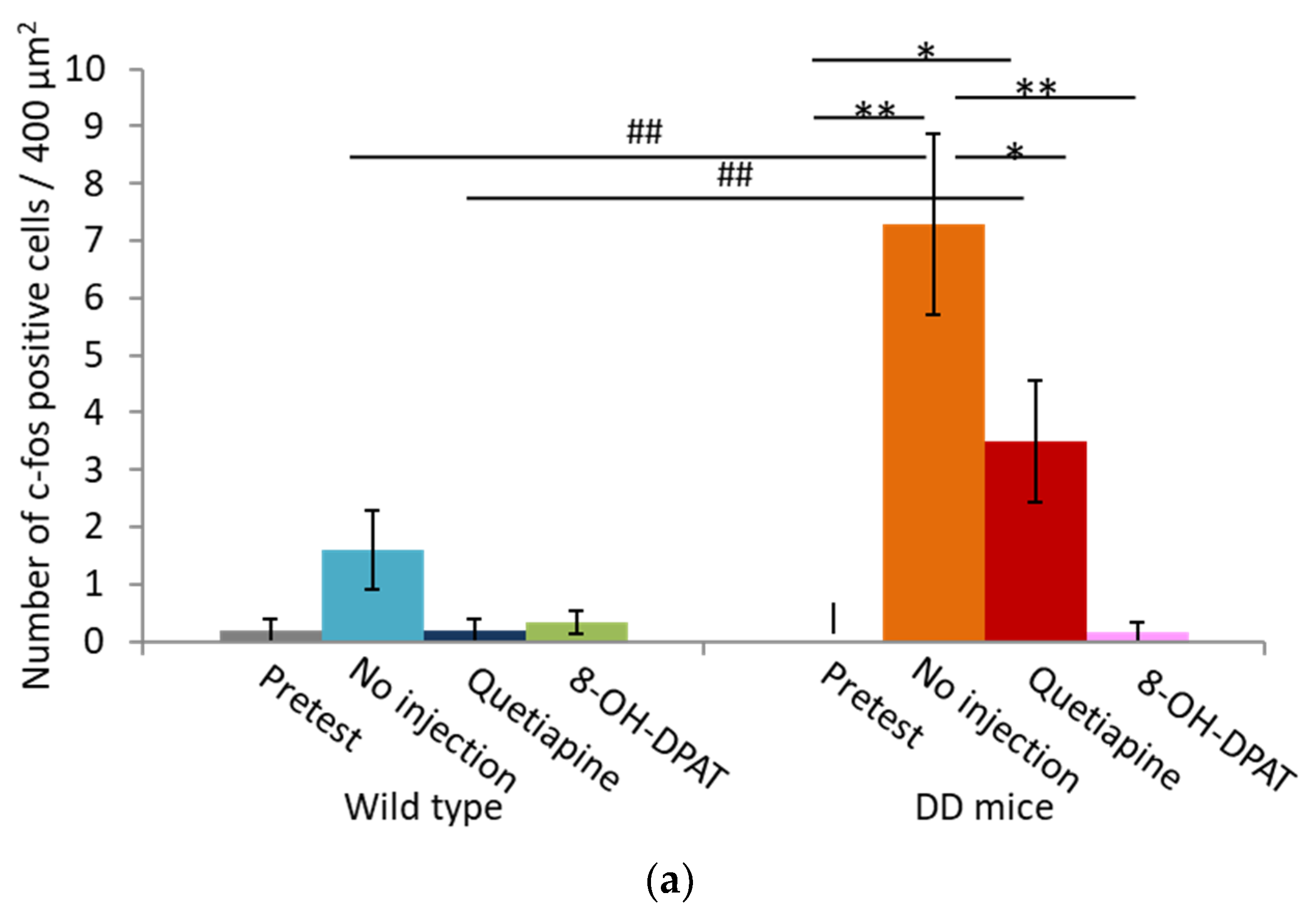
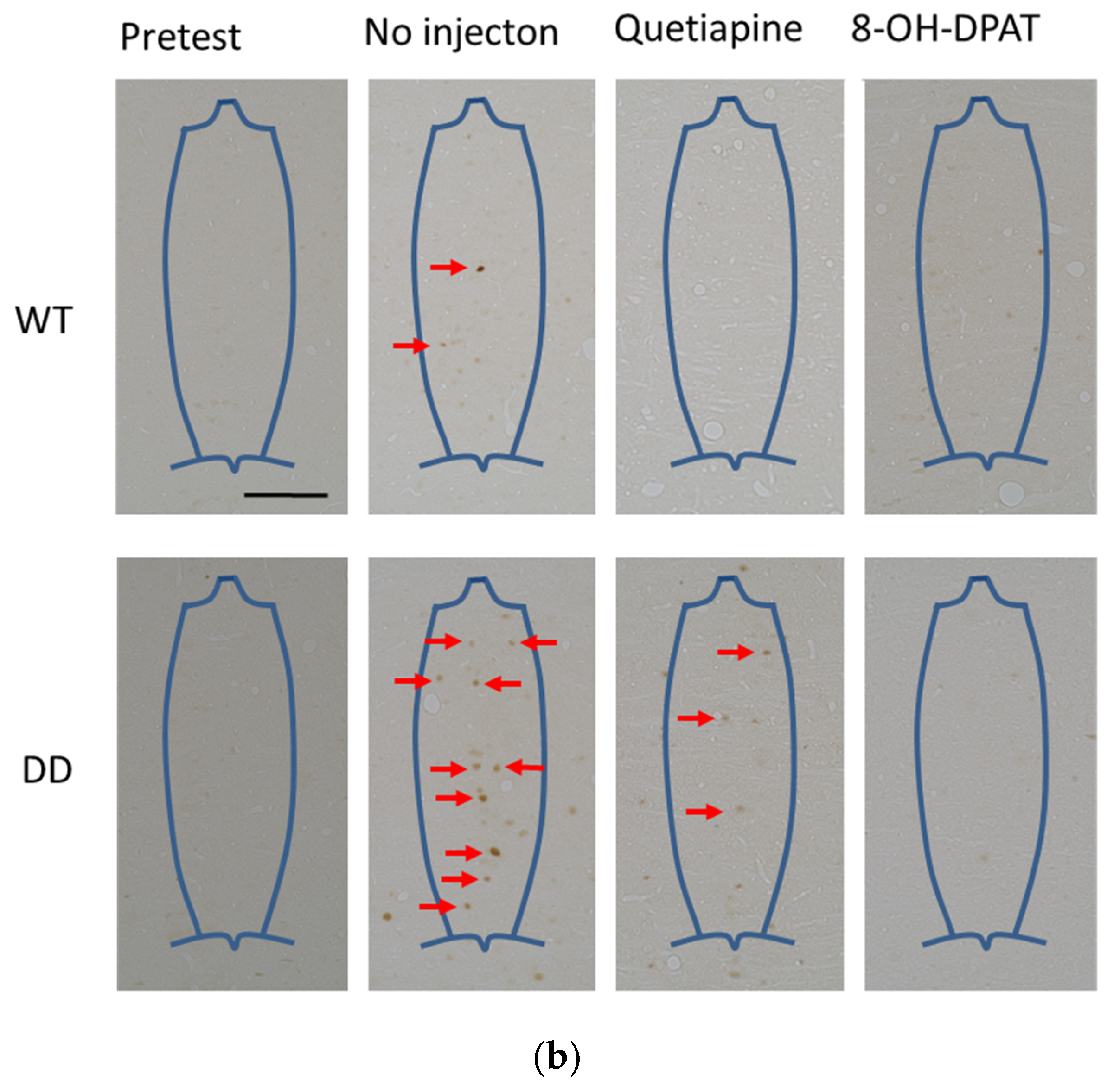
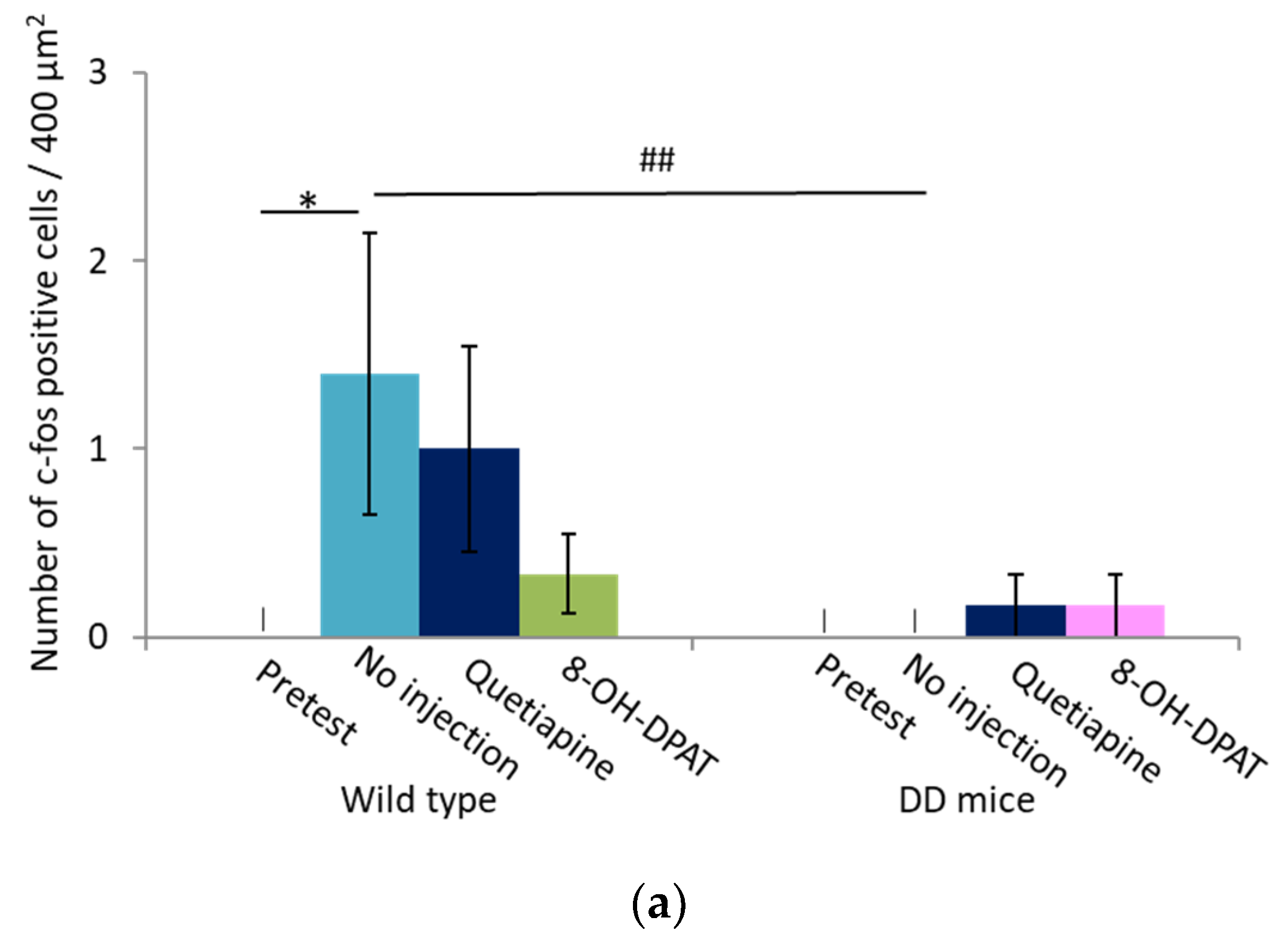
2.4. Number of Fos-Positive Cells was Reduced by Quetiapine and 8-OH-DPAT in the MRN
3. Discussion
4. Materials and Methods
4.1. Drugs
4.2. Dopamine-Deficient Mice
4.3. Open-Field Test
4.4. Tissue Preparation
4.5. Immunohistochemistry
4.6. Cell Counting
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, signaling, and association with neurological diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef]
- Delong, M.R.; Wichmann, T. Basal Ganglia Circuits as Targets for Neuromodulation in Parkinson Disease. JAMA Neurol. 2015, 72, 1354–1360. [Google Scholar] [CrossRef]
- Keener, A.M.; Bordelon, Y.M. Parkinsonism. Semin. Neurol. 2016, 36, 330–334. [Google Scholar]
- Höllerhage, M. Secondary parkinsonism due to drugs, vascular lesions, tumors, trauma, and other insults. Int. Rev. Neurobiol. 2019, 149, 377–418. [Google Scholar] [CrossRef]
- Surguchov, A. Biomarkers in Parkinson’s disease. In Neurodegenerative Diseases Biomarkers: Towards Translating Research to Clinical Practice; Peplow, P.V., Martinez, B., Gennarelli, T.A., Eds.; Springer: New York, NY, USA, 2022; pp. 155–180. [Google Scholar]
- Levin, O.S.; Chimagomedova, A.S.; Skripkina, N.A.; Lyashenko, E.A.; Babkina, O.V. Nonmotor Symptoms in Vascular and Other Secondary Parkinsonism. Int. Rev. Neurobiol. 2017, 134, 1303–1334. [Google Scholar] [CrossRef]
- Lin, M.; Colon-Perez, L.M.; Sambo, D.O.; Miller, D.R.; Lebowitz, J.J.; Jimenez-Rondan, F.; Cousins, R.J.; Horenstein, N.; Aydemir, T.B.; Febo, M.; et al. Mechanism of Manganese Dysregulation of Dopamine Neuronal Activity. J. Neurosci. 2020, 40, 5871–5891. [Google Scholar] [CrossRef]
- Bowler, R.M.; Nakagawa, S.; Drezgic, M.; Roels, H.A.; Park, R.M.; Diamond, E.; Mergler, D.; Bouchard, M.; Bowler, R.P.; Koller, W. Sequelae of fume exposure in confined space welding: A neurological and neuropsychological case series. NeuroToxicology 2007, 28, 298–311. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Liu, L.; Zhang, L.; Ma, L.; Wu, H.; He, X.; Zhu, M.; Wang, L.; Mei, F. Case Report of a pathologically confirmed vascular parkinsonism with early cognitive impairment and Behavioral disturbance. BMC Neurol. 2021, 21, 15. [Google Scholar] [CrossRef]
- Guptha, S.H.; Han, T.; Arafat, Q.; Deyo, O. Orange and green monkeys jumping around the room. Lancet 2007, 370, 1588. [Google Scholar] [CrossRef]
- Bishnoi, R.; Badir, M.C.; Surya, S.; Youssef, N.A. Predicting Neuropsychiatric Symptoms of Parkinson’s Disease with Measures of Striatal Dopaminergic Deficiency. Curr. Alzheimer Res. 2021, 18, 499–504. [Google Scholar] [CrossRef]
- Factor, S.A.; McDonald, W.M.; Goldstein, F.C. The role of neurotransmitters in the development of Parkinson’s disease-related psychosis. Eur. J. Neurol. 2017, 24, 1244–1254. [Google Scholar] [CrossRef]
- Kyle, K.; Bronstein, J.M. Treatment of psychosis in Parkinson’s disease and dementia with Lewy bodies: A review. Parkinsonism Relat. Disord. 2020, 75, 55–62. [Google Scholar] [CrossRef]
- Nishii, K.; Matsushita, N.; Sawada, H.; Sano, H.; Noda, Y.; Mamiya, T.; Nabeshima, T.; Nagatsu, I.; Hata, T.; Kiuchi, K.; et al. Motor and learning dysfunction during postnatal development in mice defective in dopamine neuronal transmission. J. Neurosci. Res. 1998, 54, 450–464. [Google Scholar] [CrossRef]
- Zhou, Q.-Y.; Palmiter, R.D. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell 1995, 83, 1197–1209. [Google Scholar] [CrossRef] [Green Version]
- Hagino, Y.; Kasai, S.; Fujita, M.; Setogawa, S.; Yamaura, H.; Yanagihara, D.; Hashimoto, M.; Kobayashi, K.; Meltzer, H.Y.; Ikeda, K. Involvement of Cholinergic System in Hyperactivity in Dopamine-Deficient Mice. Neuropsychopharmacology 2014, 40, 1141–1150. [Google Scholar] [CrossRef]
- White, I.M.; Whitaker, C.; White, W. Amphetamine-induced hyperlocomotion in rats: Hippocampal modulation of the nucleus accumbens. Hippocampus 2006, 16, 596–603. [Google Scholar] [CrossRef]
- Polissidis, A.; Koronaiou, M.; Kollia, V.; Koronaiou, E.; Nakos-Bimpos, M.; Bogiongko, M.; Vrettou, S.; Karali, K.; Casadei, N.; Riess, O.; et al. Psychosis-like behavior and hyperdopaminergic dysregulation in human α-synuclein BAC transgenic rats. Mov. Disord. 2021, 36, 716–728. [Google Scholar] [CrossRef]
- Albert, P.R.; Vahid-Ansari, F. The 5-HT1A receptor: Signaling to behavior. Biochimie 2018, 161, 34–45. [Google Scholar] [CrossRef]
- Matsubara, K.; Shimizu, K.; Suno, M.; Ogawa, K.; Awaya, T.; Yamada, T.; Noda, T.; Satomi, M.; Ohtaki, K.-I.; Chiba, K.; et al. Tandospirone, a 5-HT1A agonist, ameliorates movement disorder via non-dopaminergic systems in rats with unilateral 6-hydroxydopamine-generated lesions. Brain Res. 2006, 1112, 126–133. [Google Scholar] [CrossRef]
- Tanaka, H.; Tatsuno, T.; Shimizu, H.; Hirose, A.; Kumasaka, Y.; Nakamura, M. Effects of tandospirone on second messenger systems and neurotransmitter release in the rat brain. Gen. Pharmacol. Vasc. Syst. 1995, 26, 1765–1772. [Google Scholar] [CrossRef]
- Winsauer, P.J.; Rodriguez, F.H.; Cha, A.E.; Moerschbaecher, J.M. Full and partial 5-HT1A receptor agonists disrupt learning and performance in rats. J. Pharmacol. Exp. Ther. 1999, 288, 335–347. [Google Scholar] [PubMed]
- Depoortère, R.; Bardin, L.; Varney, M.A.; Newman-Tancredi, A. Serotonin 5-HT1A Receptor Biased Agonists Display Differential Anxiolytic Activity in a Rat Social Interaction Model. ACS Chem. Neurosci. 2019, 10, 3101–3107. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Shi, S.; Luo, H. The therapeutic effect of quetiapine on cognitive impairment associated with 5-HT1A presynaptic receptor involved schizophrenia. J. Integr. Neurosci. 2019, 18, 245–251. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, A.; Kasai, S.; Hagino, Y.; Kotajima-Murakami, H.; Kashii, H.; Takamatsu, Y.; Nishito, Y.; Inagaki, M.; Mizuguchi, M.; et al. Brain hyperserotonemia causes autism-relevant social deficits in mice. Mol. Autism 2018, 9, 60. [Google Scholar] [CrossRef]
- Politis, M.; Niccolini, F. Serotonin in Parkinson’s disease. Behav. Brain Res. 2015, 277, 136–145. [Google Scholar] [CrossRef]
- Fox, S.H.; Visanji, N.P.; Johnston, T.H.; Gomez-Ramirez, J.; Voon, V.; Brotchie, J.M. Dopamine Receptor Agonists and Levodopa and Inducing Psychosis-Like Behavior in the MPTP Primate Model of Parkinson Disease. Arch. Neurol. 2006, 63, 1343–1344. [Google Scholar] [CrossRef]
- McFarland, K.; Price, D.L.; Bonhaus, D.W. Pimavanserin, a 5-HT2A inverse agonist, reverses psychosis-like behaviors in a rodent model of Parkinson’s disease. Behav. Pharmacol. 2011, 22, 681–692. [Google Scholar] [CrossRef]
- Beaudoin-Gobert, M.; Epinat, J.; Météreau, E.; Duperrier, S.; Neumane, S.; Ballanger, B.; Lavenne, F.; Liger, F.; Tourvielle, C.; Bonnefoi, F.; et al. Behavioural impact of a double dopaminergic and serotonergic lesion in the non-human primate. Brain 2015, 138 Pt 9, 2632–2647. [Google Scholar] [CrossRef] [Green Version]
- Shim, I.; Stratford, T.R.; Wirtshafter, D. Dopamine is differentially involved in the locomotor hyperactivity produced by manipulations of opioid, GABA and glutamate receptors in the median raphe nucleus. Behav. Brain Res. 2013, 261, 65–70. [Google Scholar] [CrossRef] [Green Version]
- da Silva, W.J.; Silva, J.R.S.; Quintans, J.D.S.S.; Junior, W.D.L. Increased Accuracy to c-Fos-Positive Neuron Counting. BioMed Res. Int. 2021, 2021, 3060983. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochiai, Y.; Fujita, M.; Hagino, Y.; Kobayashi, K.; Okiyama, R.; Takahashi, K.; Ikeda, K. Therapeutic Effects of Quetiapine and 5-HT1A Receptor Agonism on Hyperactivity in Dopamine-Deficient Mice. Int. J. Mol. Sci. 2022, 23, 7436. https://doi.org/10.3390/ijms23137436
Ochiai Y, Fujita M, Hagino Y, Kobayashi K, Okiyama R, Takahashi K, Ikeda K. Therapeutic Effects of Quetiapine and 5-HT1A Receptor Agonism on Hyperactivity in Dopamine-Deficient Mice. International Journal of Molecular Sciences. 2022; 23(13):7436. https://doi.org/10.3390/ijms23137436
Chicago/Turabian StyleOchiai, Yukiko, Masayo Fujita, Yoko Hagino, Kazuto Kobayashi, Ryoichi Okiyama, Kazushi Takahashi, and Kazutaka Ikeda. 2022. "Therapeutic Effects of Quetiapine and 5-HT1A Receptor Agonism on Hyperactivity in Dopamine-Deficient Mice" International Journal of Molecular Sciences 23, no. 13: 7436. https://doi.org/10.3390/ijms23137436
APA StyleOchiai, Y., Fujita, M., Hagino, Y., Kobayashi, K., Okiyama, R., Takahashi, K., & Ikeda, K. (2022). Therapeutic Effects of Quetiapine and 5-HT1A Receptor Agonism on Hyperactivity in Dopamine-Deficient Mice. International Journal of Molecular Sciences, 23(13), 7436. https://doi.org/10.3390/ijms23137436





