Evolutionary Insight into Immunothrombosis as a Healing Mechanism
Abstract
1. Introduction
2. Convergence in Function of Invertebrate Immunoclotting and Vertebrate Immunothrombosis
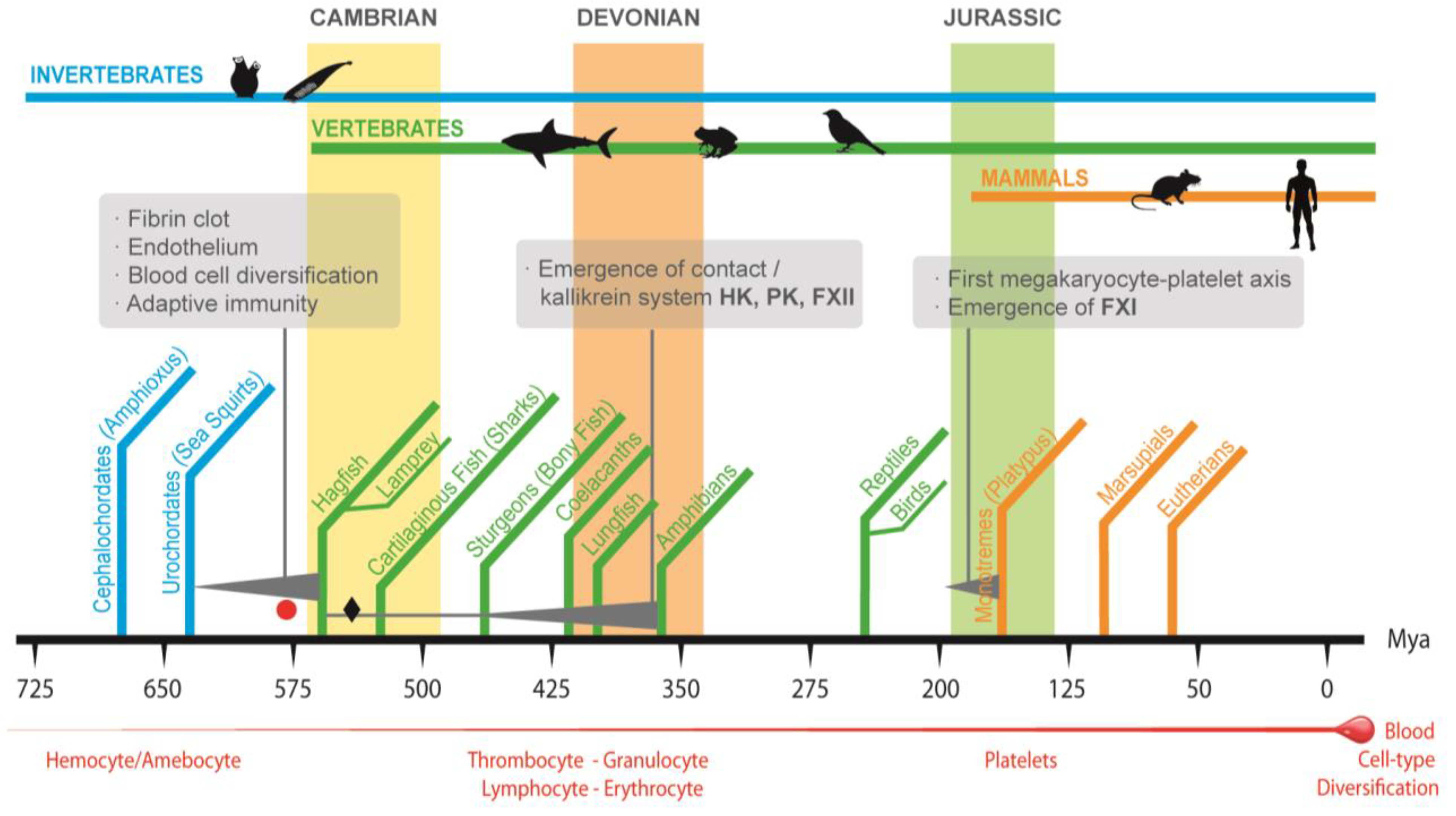
3. The Emergence of Vertebrate Coagulation System and Hemostasis
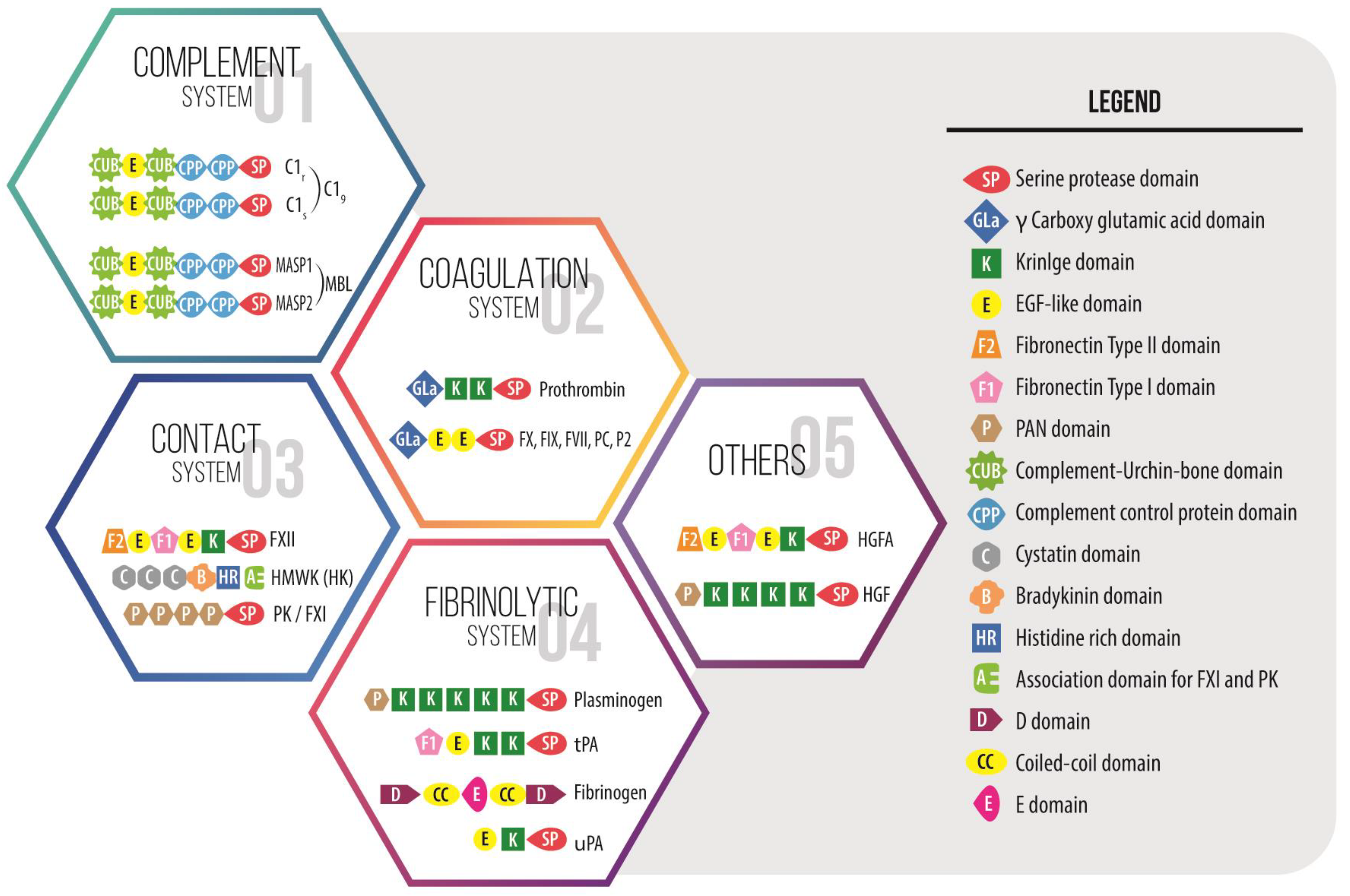
4. Pleiotropic Effects of Proteins of the Coagulation System: From Coagulation and Inflammation to Healing
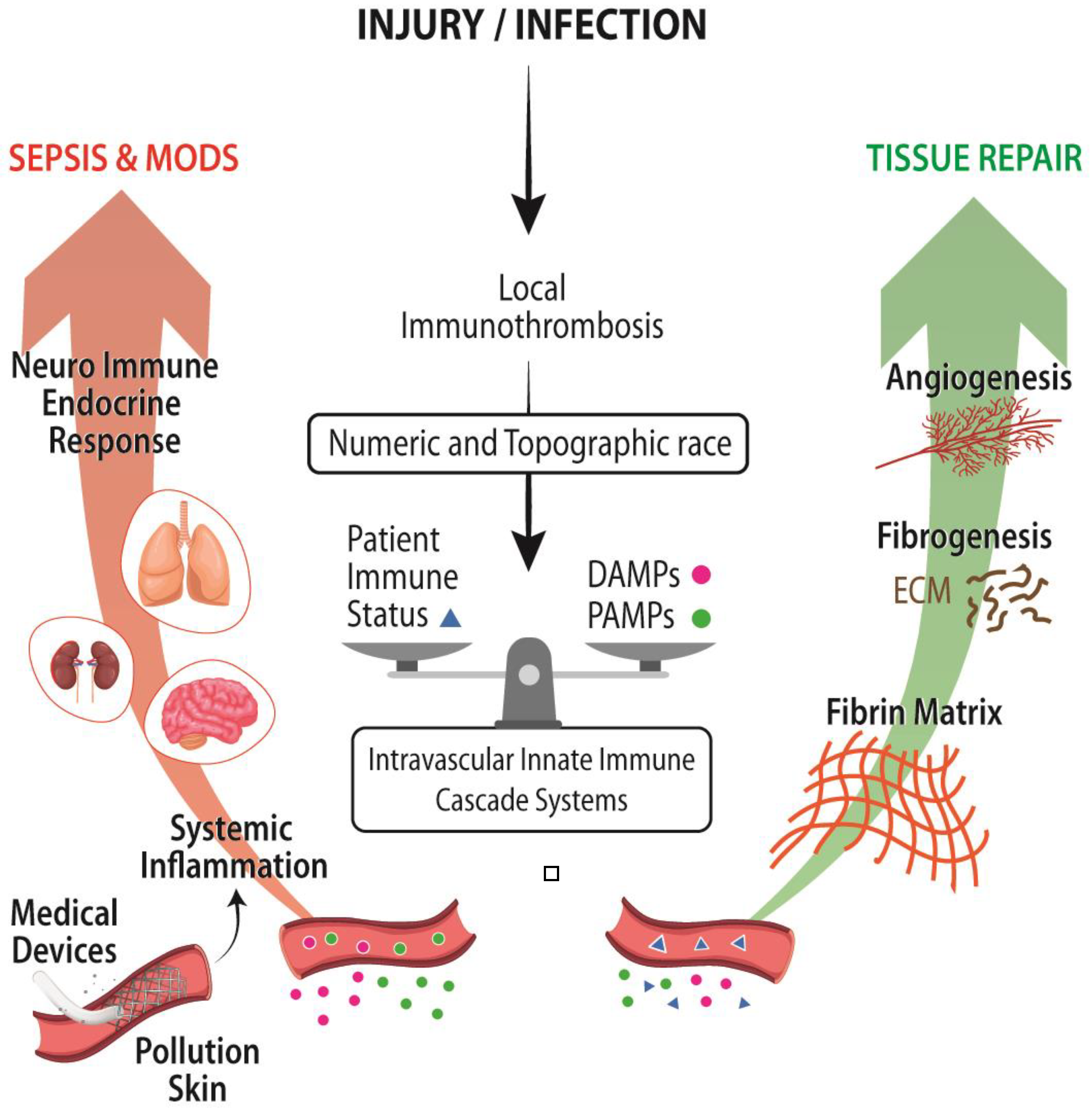
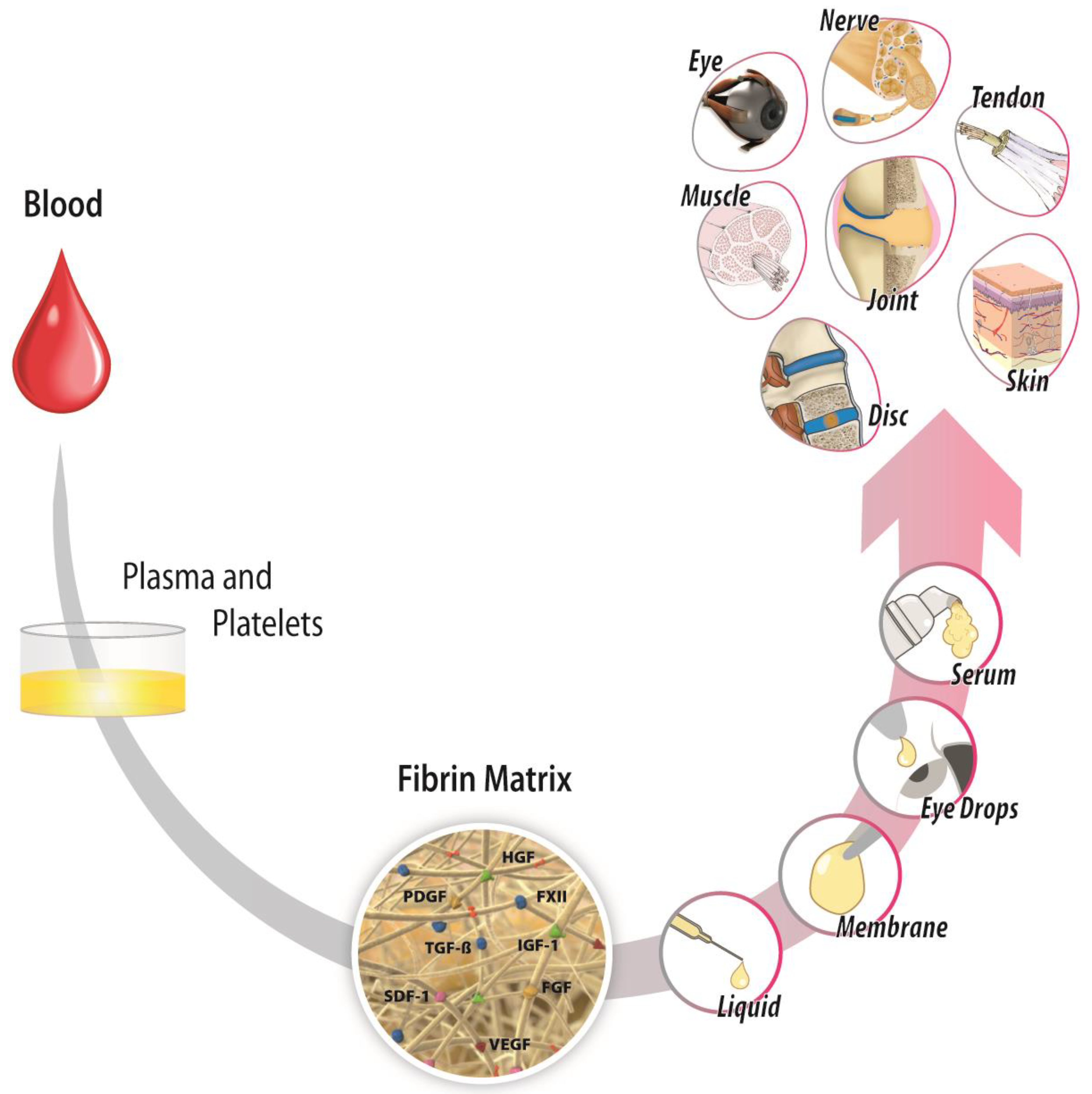
5. Vulnerabilities and Strengths of Immunothrombosis in Our Modern Medicine
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | autologous fibrin matrix |
| CAS | contact activation/kallikrein system |
| HGF | hepatocyte growth factor |
| HGFAC | pro-HGF activator |
| M/Pa | megakaryocyte/platelet axis |
| MODS | multiple organ dysfunction syndrome |
| Mya | million years ago |
| NLR | Nod-like receptor |
| PK | prekallikrein |
| PKa | kallikrein protease |
| PLG | plasminogen |
| PT | prothrombin |
| RAGE | receptors for advanced glycation end products |
| SIRS | systemic inflammatory response syndrome |
| TLR | toll-like receptor |
Appendix A
References
- Gaertner, F.; Massberg, S. Blood coagulation in immunothrombosis-At the frontline of intravascular immunity. Semin. Immunol. 2016, 28, 561–569. [Google Scholar] [CrossRef]
- Doolittle, R.F. The Evolution of Vertebrate Blood Clotting; University Science Books: Melville, NY, USA, 2012. [Google Scholar]
- Krem, M.M.; Di Cera, E. Evolution of enzyme cascades from embryonic development to blood coagulation. Trends Biochem. Sci. 2002, 27, 67–74. [Google Scholar] [CrossRef]
- Patthy, L. Evolution of the proteases of blood coagulation and fibrinolysis by assembly from modules. Cell 1985, 41, 657–663. [Google Scholar] [CrossRef]
- Anitua, E.; Nurden, P.; Prado, R.; Nurden, A.T.; Padilla, S. Autologous fibrin scaffolds: When platelet- and plasma-derived biomolecules meet fibrin. Biomaterials 2019, 192, 440–460. [Google Scholar] [CrossRef]
- Levin, J. The Evolution of Mammalian Platelets. In Platelets, 4th ed.; Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 1–23. [Google Scholar]
- Cerenius, L.; Söderhäll, K. Coagulation in invertebrates. J. Innate Immun. 2011, 3, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ponczek, M.B.; Shamanaev, A.; LaPlace, A.; Dickeson, S.K.; Srivastava, P.; Sun, M.F.; Gruber, A.; Kastrup, C.; Emsley, J.; Gailani, D. The evolution of factor XI and the kallikrein-kinin system. Blood Adv. 2020, 4, 6135–6147. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, R.F. Bioinformatic Characterization of Genes and Proteins Involved in Blood Clotting in Lampreys. J. Mol. Evol. 2015, 81, 121–130. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hoffmann, F.G.; Storz, J.F.; Kuraku, S.; Vandewege, M.W.; Opazo, J.C. Whole-Genome Duplications and the Diversification of the Globin-X Genes of Vertebrates. Genome Biol. Evol. 2021, 13, evab205. [Google Scholar] [CrossRef] [PubMed]
- Simakov, O.; Marletaz, F.; Yue, J.X.; O’Connell, B.; Jenkins, J.; Brandt, A.; Calef, R.; Tung, C.H.; Huang, T.K.; Schmutz, J.; et al. Deeply conserved synteny resolves early events in vertebrate evolution. Nat. Ecol. Evol. 2020, 4, 820–830. [Google Scholar] [CrossRef]
- Grant, M.A.; Aird, W.C. Molecular Evolution of the Vertebrate Blood Coagulation System. In Hemostasis and Thrombosis: Basic Principles and Clinical Practice; Marder, V.J., Aird, W.C., Bennett, J.S., Schulman, S., White, G.C., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 11–25. [Google Scholar]
- Carroll, S.B.; Grenier, J.K.; Weatherbee, S.D. From DNA to Diversity: Molecular Genetics and the Evolution of Animal Design, 2nd ed.; John Wiley & Sons: Malden, MA, USA, 2004. [Google Scholar]
- Monahan-Earley, R.; Dvorak, A.M.; Aird, W.C. Evolutionary origins of the blood vascular system and endothelium. J. Thromb. Haemost. 2013, 11, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Kolkman, J.A.; Stemmer, W.P. Directed evolution of proteins by exon shuffling. Nat. Biotechnol. 2001, 19, 423–428. [Google Scholar] [CrossRef]
- Patthy, L. Exon Shuffling Played a Decisive Role in the Evolution of the Genetic Toolkit for the Multicellular Body Plan of Metazoa. Genes 2021, 12, 382. [Google Scholar] [CrossRef] [PubMed]
- Nakao, M.; Somamoto, T. The Evolution of Complement System Functions and Pathways in Vertebrates. In The Evolution of the Immune System; Malagoli, D., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 151–171. [Google Scholar]
- Di Cera, E. Thrombin. Mol. Aspects Med. 2008, 29, 203–254. [Google Scholar] [CrossRef] [PubMed]
- Ponczek, M.B.; Bijak, M.Z.; Nowak, P.Z. Evolution of thrombin and other hemostatic proteases by survey of protochordate, hemichordate, and echinoderm genomes. J. Mol. Evol. 2012, 74, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, R.F.; McNamara, K.; Lin, K. Correlating structure and function during the evolution of fibrinogen-related domains. Protein Sci. 2012, 21, 1808–1823. [Google Scholar] [CrossRef]
- Hanington, P.C.; Zhang, S.M. The primary role of fibrinogen-related proteins in invertebrates is defense, not coagulation. J. Innate Immun. 2011, 3, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Kairies, N.; Beisel, H.G.; Fuentes-Prior, P.; Tsuda, R.; Muta, T.; Iwanaga, S.; Bode, W.; Huber, R.; Kawabata, S. The 2.0-A crystal structure of tachylectin 5A provides evidence for the common origin of the innate immunity and the blood coagulation systems. Proc. Natl. Acad. Sci. USA 2001, 98, 13519–13524. [Google Scholar] [CrossRef]
- Loof, T.G.; Deicke, C.; Medina, E. The role of coagulation/fibrinolysis during infection. Front. Cell. Infect. Microbiol. 2014, 4, 128. [Google Scholar] [CrossRef]
- Theopold, U.; Schmidt, O.; Soderhall, K.; Dushay, M.S. Coagulation in arthropods: Defence, wound closure and healing. Trends Immunol. 2004, 25, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Chana-Muñoz, A.; Jendroszek, A.; Sønnichsen, M.; Wang, T.; Ploug, M.; Jensen, J.K.; Andreasen, P.A.; Bendixen, C.; Panitz, F. Origin and diversification of the plasminogen activation system among chordates. BMC Evol. Biol. 2019, 19, 27. [Google Scholar] [CrossRef]
- Kearney, K.J.; Butler, J.; Posada, O.M.; Wilson, C.; Heal, S.; Ali, M.; Hardy, L.; Ahnstrom, J.; Gailani, D.; Foster, R.; et al. Kallikrein directly interacts with and activates Factor IX, resulting in thrombin generation and fibrin formation independent of Factor XI. Proc. Natl. Acad. Sci. USA 2021, 118, e2014810118. [Google Scholar] [CrossRef] [PubMed]
- Padilla, S.; Nurden, A.T.; Prado, R.; Nurden, P.; Anitua, E. Healing through the lens of immunothrombosis: Biology-inspired, evolution-tailored, and human-engineered biomimetic therapies. Biomaterials 2021, 279, 121205. [Google Scholar] [CrossRef] [PubMed]
- Yeaman, M.R. The Role of Platelets in Antimicrobial Host Defense. In Platelets, Fourth Edition; Michelson, A.D., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 523–546. [Google Scholar]
- Martin, J.F.; Wagner, G.P. The origin of platelets enabled the evolution of eutherian placentation. Biol. Lett. 2019, 15, 20190374. [Google Scholar] [CrossRef]
- Miyazawa, K. Hepatocyte growth factor activator (HGFA): A serine protease that links tissue injury to activation of hepatocyte growth factor. FEBS J. 2010, 277, 2208–2214. [Google Scholar] [CrossRef]
- Burzynski, L.C.; Humphry, M.; Pyrillou, K.; Wiggins, K.A.; Chan, J.N.E.; Figg, N.; Kitt, L.L.; Summers, C.; Tatham, K.C.; Martin, P.B.; et al. The Coagulation and Immune Systems Are Directly Linked through the Activation of Interleukin-1α by Thrombin. Immunity 2019, 50, 1033–1042.e6. [Google Scholar] [CrossRef] [PubMed]
- Macrae, F.L.; Duval, C.; Papareddy, P.; Baker, S.R.; Yuldasheva, N.; Kearney, K.J.; McPherson, H.R.; Asquith, N.; Konings, J.; Casini, A.; et al. A fibrin biofilm covers blood clots and protects from microbial invasion. J. Clin. Investig. 2018, 128, 3356–3368. [Google Scholar] [CrossRef] [PubMed]
- Huber-Lang, M.; Lambris, J.D.; Ward, P.A. Innate immune responses to trauma. Nat. Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, E.X.; Fang, C.; Bane, K.L.; Long, A.T.; Naudin, C.; Kucukal, E.; Gandhi, A.; Brett-Morris, A.; Mumaw, M.M.; Izadmehr, S.; et al. Factor XII and uPAR upregulate neutrophil functions to influence wound healing. J. Clin. Investig. 2018, 128, 944–959. [Google Scholar] [CrossRef]
- Juang, L.J.; Mazinani, N.; Novakowski, S.K.; Prowse, E.N.P.; Haulena, M.; Gailani, D.; Lavkulich, L.M.; Kastrup, C.J. Coagulation factor XII contributes to hemostasis when activated by soil in wounds. Blood Adv. 2020, 4, 1737–1745. [Google Scholar] [CrossRef]
- Deppermann, C.; Cherpokova, D.; Nurden, P.; Schulz, J.N.; Thielmann, I.; Kraft, P.; Vogtle, T.; Kleinschnitz, C.; Dutting, S.; Krohne, G.; et al. Gray platelet syndrome and defective thrombo-inflammation in Nbeal2-deficient mice. J. Clin. Investig. 2013, 123, 3331–3342. [Google Scholar] [CrossRef]
- Gawaz, M.; Vogel, S. Platelets in tissue repair: Control of apoptosis and interactions with regenerative cells. Blood 2013, 122, 2550–2554. [Google Scholar] [CrossRef] [PubMed]
- Talbot, S.; Foster, S.L.; Woolf, C.J. Neuroimmunity: Physiology and Pathology. Annu. Rev. Immunol. 2016, 34, 421–447. [Google Scholar] [CrossRef]
- Ekdahl, K.N.; Fromell, K.; Mohlin, C.; Teramura, Y.; Nilsson, B. A human whole-blood model to study the activation of innate immunity system triggered by nanoparticles as a demonstrator for toxicity. Sci. Technol. Adv. Mater. 2019, 20, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Seddon, H.J.; Medawar, P.B. Fibrin Suture of Human Nerves. Lancet 1942, 240, 87–88. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anitua, E.; Prado, R.; Padilla, S. Evolutionary Insight into Immunothrombosis as a Healing Mechanism. Int. J. Mol. Sci. 2022, 23, 8346. https://doi.org/10.3390/ijms23158346
Anitua E, Prado R, Padilla S. Evolutionary Insight into Immunothrombosis as a Healing Mechanism. International Journal of Molecular Sciences. 2022; 23(15):8346. https://doi.org/10.3390/ijms23158346
Chicago/Turabian StyleAnitua, Eduardo, Roberto Prado, and Sabino Padilla. 2022. "Evolutionary Insight into Immunothrombosis as a Healing Mechanism" International Journal of Molecular Sciences 23, no. 15: 8346. https://doi.org/10.3390/ijms23158346
APA StyleAnitua, E., Prado, R., & Padilla, S. (2022). Evolutionary Insight into Immunothrombosis as a Healing Mechanism. International Journal of Molecular Sciences, 23(15), 8346. https://doi.org/10.3390/ijms23158346





