GTP Binding Protein Gtr1 Cooperating with ASF1 Regulates Asexual Development in Stemphylium eturmiunum
Abstract
:1. Introduction
2. Results
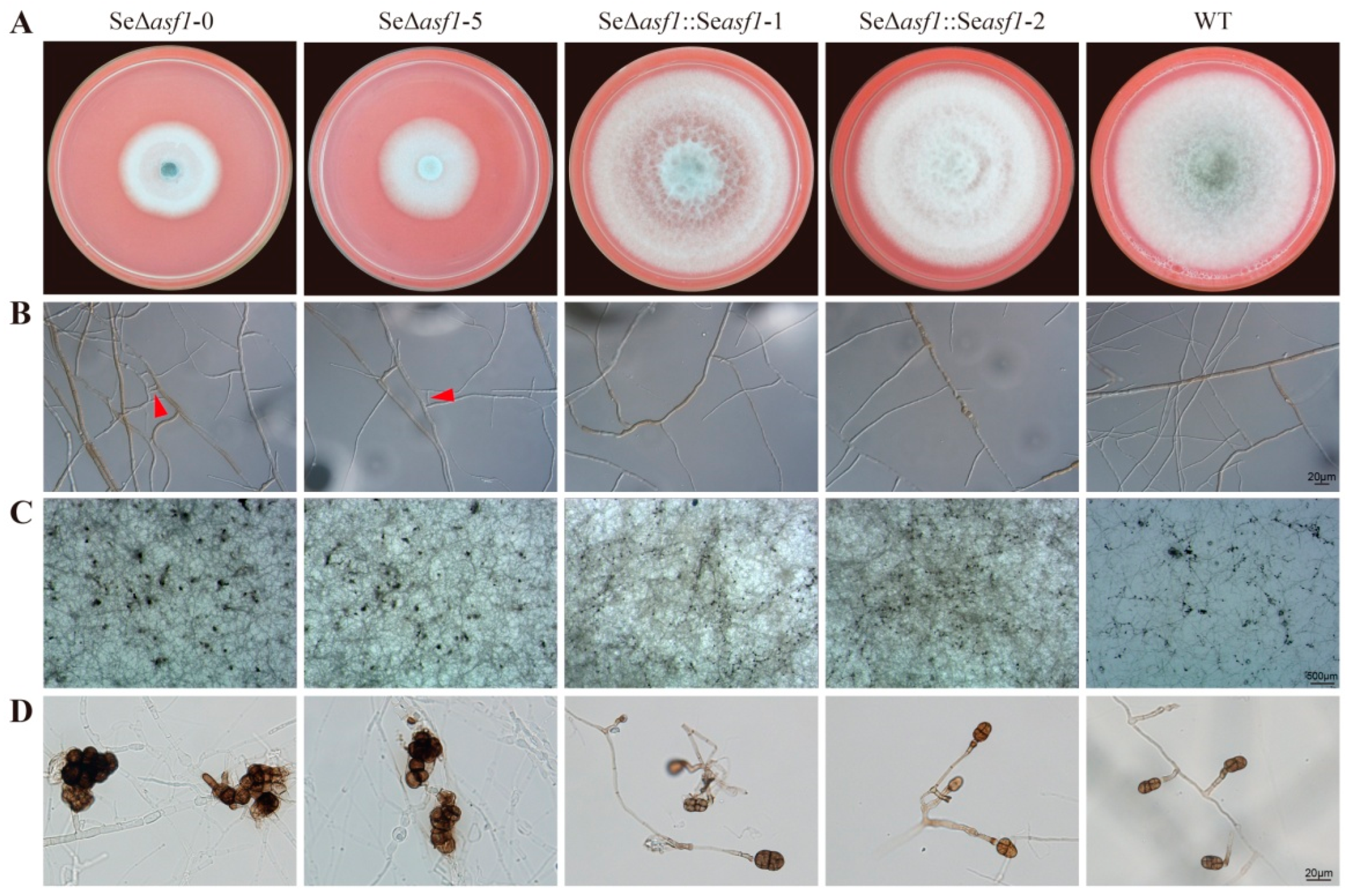
2.1. SeASF1 Regulates Asexual Development in S. eturmiunum
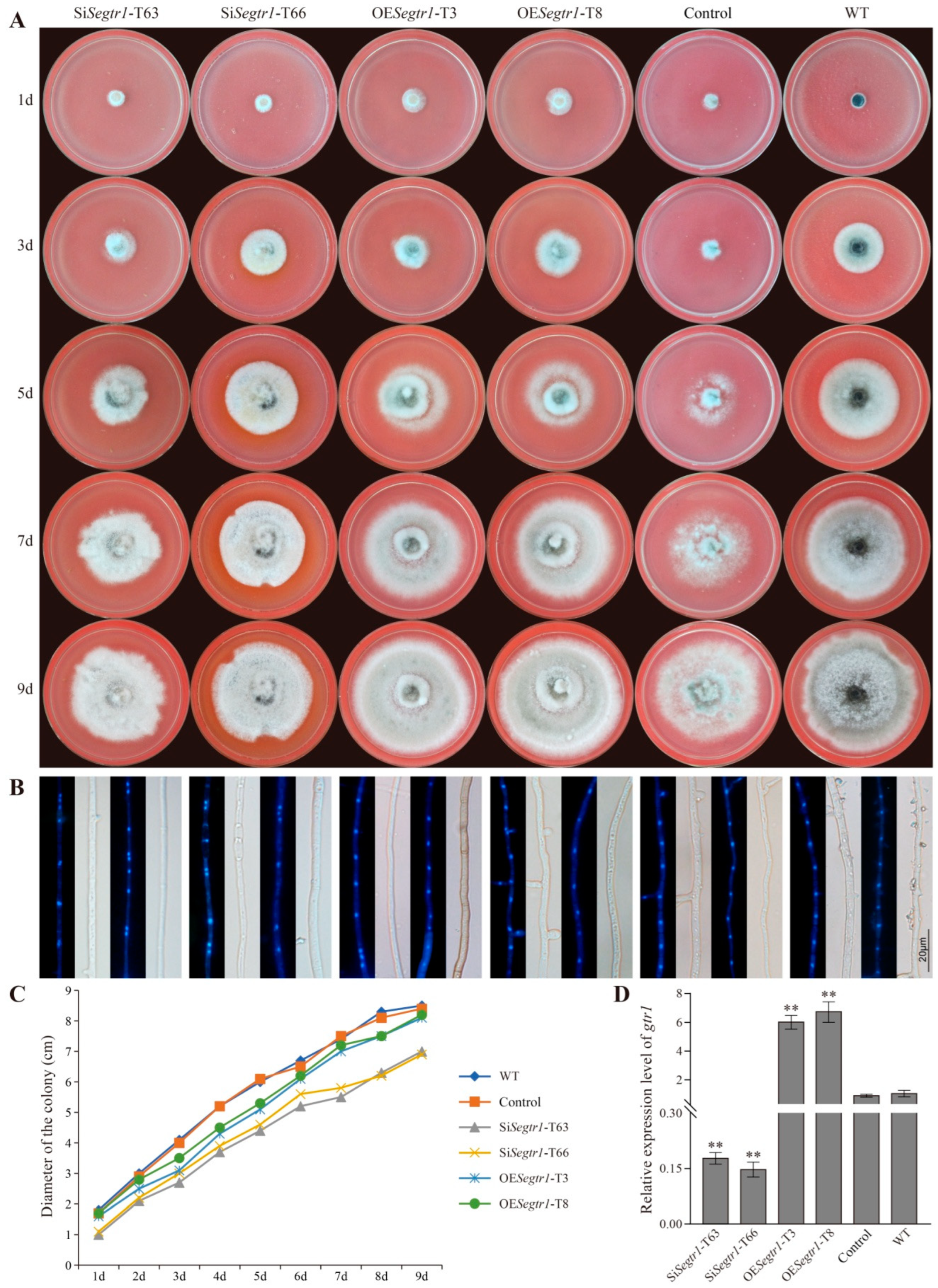
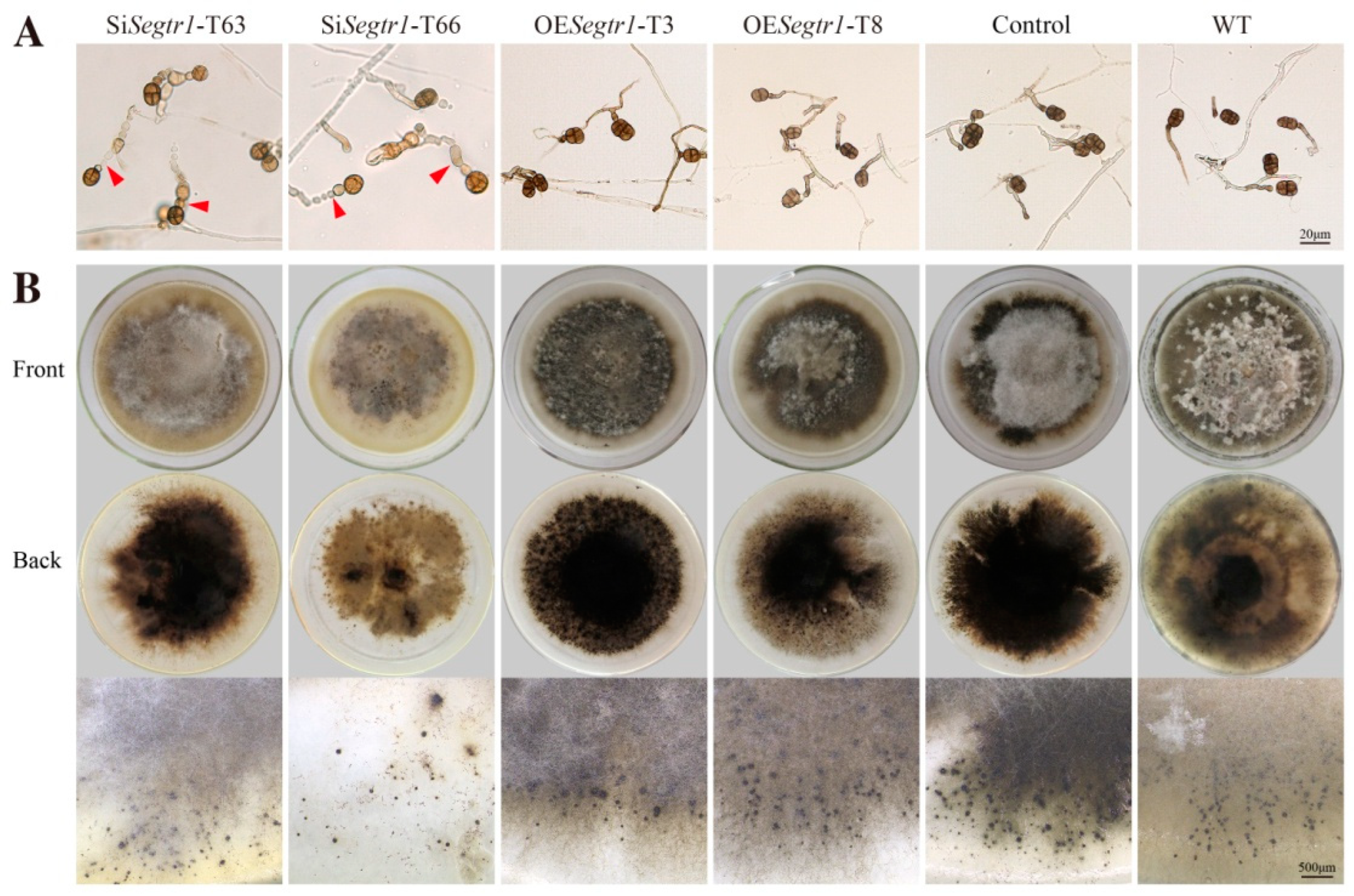
2.2. SeGtr1 Plays a Role in Asexual Development
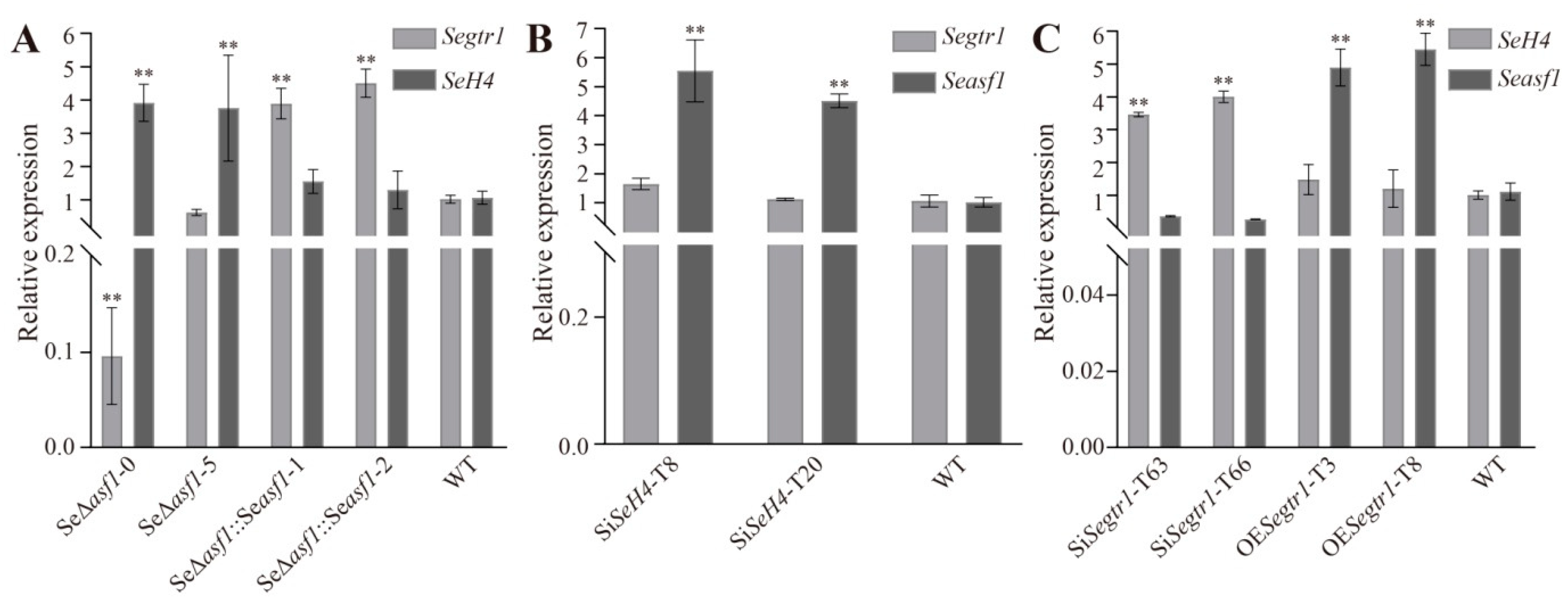
2.3. The Expression Patterns of Seasf1, SeH4, and Segtr1 in Knockout Mutants, Silenced Lines or Overexpression Strains
2.4. SeASF1 Interaction with SeH4, and SeGtr1 Interaction with SeASF1 and SeH4
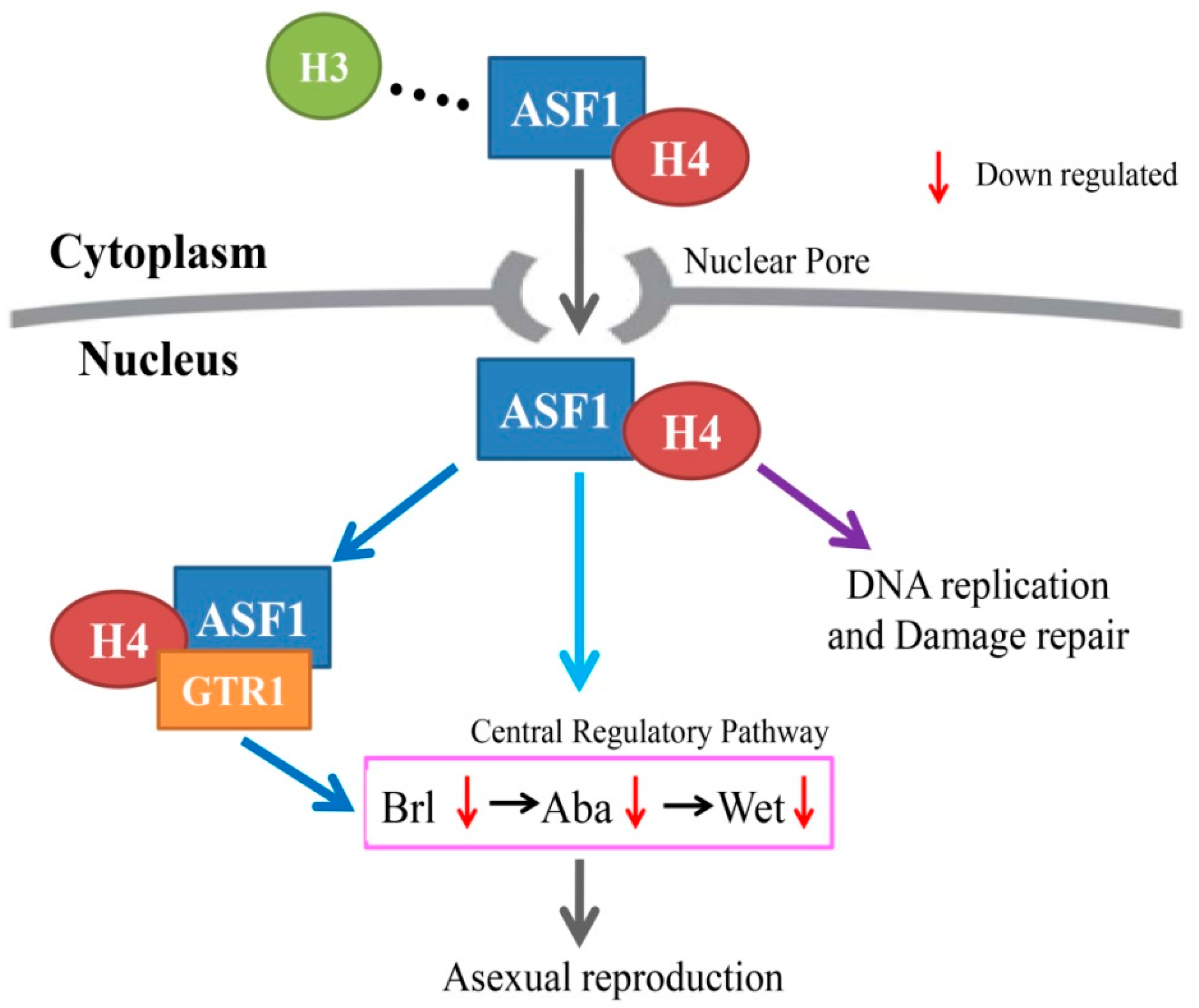
3. Discussion
4. Materials and Methods
4.1. Strains and Culture Conditions
4.2. Plasmid Construction
4.3. RNA Extraction and qRT-PCR
4.4. Yeast Two-Hybrid
4.5. GST Pull-Down
4.6. Co-IP
4.7. Western Blot
4.8. Microscopy
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, M.Y.; Mead, M.E.; Lee, M.K.; Neuhaus, G.F.; Adpressa, D.A.; Martien, J.I.; Son, Y.E.; Moon, H.; Amador-Noguez, D.; Han, K.H.; et al. Transcriptomic, protein-DNA interaction, and metabolomic studies of VosA, VelB, and WetA in Aspergillus nidulans asexual spores. Mol. Biol. 2021, 12, e03128-20. [Google Scholar] [CrossRef] [PubMed]
- Dyer, P.S.; Ingram, D.S.; Johnstone, K. The control of sexual morphogenesis in the Ascomycotina. Biol. Rev. 1992, 67, 421–458. [Google Scholar] [CrossRef]
- Milgroom, M.G. Recombination and the multilocus structure of fungal populations. Annu. Rev. Phytopathol. 1996, 34, 457–477. [Google Scholar] [CrossRef]
- Grigsby, I.F.; Rutledge, E.M.; Morton, C.A.; Finger, F.P. Functional redundancy of two C. elegans homologs of the histone chaperone Asf1 in germline DNA replication. Dev. Biol. 2009, 329, 64–79. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.; Jacobson, D.; Fisher, M. The volution of asexual fungi: Reproduction, speciation and classification. Annu. Rev. Phytopathol. 1999, 37, 197–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, E.G. Typification of Alternaria, Stemphylium, and Ulocladium. Mycologia 1967, 59, 67–92. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.; Li, Z.; Xia, L.Y.; Wang, Q.; Hu, X.M.; Zhang, X.G. Characterization and phylogenetic analysis of the mating-type loci in the asexual ascomycete genus Ulocladium. Mycologia 2014, 106, 649–665. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Xiong, C.L.; James, T.Y.; Zhang, X.G. Mating-type genes of the anamorphic fungus Ulocladium botrytis affect both asexual sporulation and sexual reproduction. Sci. Rep. 2017, 7, 7932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, E.G. Perfect states of Stemphylium. Mycologia 1969, 61, 1–26. [Google Scholar] [CrossRef]
- Bayram, O.; Krappmann, S.; Ni, M.; Bok, J.W.; Helmstaedt, K.; Valerius, O.; Braus-Stromeyer, S.; Kwon, N.J.; Keller, N.P.; Yu, J.H.; et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 2008, 320, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Sarikaya Bayram, O.; Bayram, O.; Valerius, O.; Park, H.S.; Irniger, S.; Gerke, J.; Ni, M.; Han, K.H.; Yu, J.H.; Braus, G.H. LaeA control of velvet family regulatory proteins for light-dependent development and fungal cell-type specificity. PLoS Genet. 2010, 6, e1001226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blumenstein, A.; Vienken, K.; Tasler, R.; Purschwitz, J.; Veith, D.; Frankenberg-Dinkel, N.; Fischer, R. The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr. Biol. 2005, 15, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Schürmann, A.; Brauers, A.; Massmann, S.; Becker, W.; Joost, H.G. Cloning of a novel family of mammalian GTP-binding proteins (RagA, RagBs, RagB1) with remote similarity to the Ras-related GTPases. J. Biol. Chem. 1995, 270, 28982–28988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masanori, B.Y.; Harashima, S.; Oshima, Y. Putative GTP-Binding protein, Gtr1, associated with the function of the Pho84 inorganic-phosphate transporter in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992, 12, 2958–2966. [Google Scholar]
- Sekiguchi, T.; Hayashi, N.; Wang, Y.; Kobayashi, H. Genetic evidence that Raslike GTPases, Gtr1p, and Gtr2p, are involved in epigenetic control of gene expression in Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2008, 368, 748–754. [Google Scholar] [CrossRef]
- Saito, H.; Oikawa, T.; Hamamoto, S.; Ishimaru, Y.; Kanamori-Sato, M.; Sasaki-Sekimoto, Y.; Utsumi, T.; Chen, J.; Kanno, Y.; Masuda, S.; et al. The jasmonate-responsive GTR1 transporter is required for gibberellin-mediated stamen development in Arabidopsis. Nat. Commun. 2015, 6, 6095. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, C.; Liu, W.; Wang, G.; Kang, Z.; Kistler, H.C.; Xu, J.R. The HDF1 histone deacetylase gene is important for conidiation, sexual reproduction, and pathogenesis in Fusarium graminearum. Mol. Plant Microbe Interact. 2011, 24, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramamoorthy, V.; Shantappa, S.; Dhingra, S.; Calvo, A.M. VeA-dependent RNApol II transcription elongation factor-like protein, RtfA, is associated with secondary metabolism and morphological development in Aspergillus nidulans. Mol. Microbiol. 2012, 85, 795–814. [Google Scholar] [CrossRef] [Green Version]
- Sarikaya Bayram, O.; Bayram, O.; Feussner, K.; Kim, J.H.; Kim, H.S.; Kaever, A.; Feussner, I.; Chae, K.S.; Han, D.M.; Han, K.H.; et al. Membrane-bound methyltransferase complex VapA-VipC-VapB guides epigenetic control of fungal development. Dev. Cell 2014, 29, 406–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, S.; Davis, C.; Konopka, J.B.; Sternglanz, R. Two new S-phase-specific genes from Saccharomyces cerevisiae. Yeast 1997, 13, 1029–1042. [Google Scholar] [CrossRef]
- Eitoku, M.; Sato, L.; Senda, T.; Horikoshi, M. Histone chaperones: 30 years from isolation to elucidation of the mechanisms of nucleosome assembly and disassembly. Cell. Mol. Life Sci. 2008, 65, 414–444. [Google Scholar] [CrossRef]
- Min, Y.; Frost, J.M.; Choi, Y. Gametophytic abortion in heterozygotes but not in homozygotes: Implied chromosome rearrangement during T-DNA insertion at the ASF1 locus in Arabidopsis. Mol. Cells 2020, 43, 448–458. [Google Scholar]
- Sanematsu, F.; Takami, Y.; Barman, H.K.; Fukagawa, T.; Ono, T.; Shibahara, K.I.; Nakayama, T. Asf1 is required for viability and chromatin assembly during DNA replication in vertebrate cells. J. Biol. Chem. 2006, 281, 13817–13827. [Google Scholar] [CrossRef] [Green Version]
- Mousson, F.; Ochsenbein, F.; Mann, C. The histone chaperone Asf1 at the crossroads of chromatin and DNA checkpoint pathways. Chromosoma 2007, 116, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Wallroth, F.G. Flora Cryptogamica Germaniae, pars. Post. Nurenberg; J. L. Schrag: Madison, WI, USA, 1833; 923p. [Google Scholar]
- Câmara, M.P.; O’Neill, N.R.; van Berkum, P. Phylogeny of Stemphylium spp. based on ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 2002, 94, 660–672. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.G. Three new species of Stemphylium from China. Mycotaxon 2006, 96, 77–81. [Google Scholar]
- Wang, Y.; Geng, Y.; Pei, Y.F.; Zhang, X.G. Molecular and morphological description of two new species of Stemphylium from China and France. Mycologia 2010, 102, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, H.B.; O’Neill, N.R.; Zhang, X.G. Two new species of Stemphylium from Northwest China. Mycol. Prog. 2009, 8, 301–304. [Google Scholar] [CrossRef]
- Gesing, S.; Schindler, D.; Fränzel, B.; Wolters, D.; Nowrousian, M. The histone chaperone ASF1 is essential for sexual development in the filamentous fungus Sordaria macrospora. Mol. Microbiol. 2012, 84, 748–765. [Google Scholar]
- Lennox, E.S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1995, 1, 190–206. [Google Scholar] [CrossRef]
- Bernardi Wenzel, J.; Quecine, M.C.; Azevedo, J.L.; Pamphile, J.A. Agrobacterium-mediated transformation of Fusarium proliferatum. Genet. Mol. Res. 2016, 15, 1–12. [Google Scholar] [CrossRef]
- Zhao, Y.; He, M.; Ding, J.; Xi, Q.; Loake, G.J.; Zheng, W. Regulation of anticancer styrylpyrone biosynthesis in the medicinal mushroom Inonotus obliquus requires thioredoxin mediated transnitrosylation of S-nitrosoglutathione reductase. Sci. Rep. 2016, 6, 37601. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Li, G.; Xu, J.R. Efficient approaches for generating GFP fusion and epitope-tagging constructs in filamentous fungi. Methods Mol. Biol. 2011, 722, 199–212. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]

| Primer | Sequence(5′-3′) | Application |
|---|---|---|
| Seasf1-F | ATGTCTGTCGTTTCGCTTC | Amplify Seasf1 sequence |
| Seasf1-R | CTAGTGAACCATGACATCTGC | |
| Seasf1-5f | CCGCTCGAGCAATCCAGGGGCGATAAAG | Amplify Seasf1 upstream sequence |
| Seasf1-5r | GGAAGATCTGCTTGGCGGGGTAGATAGAG | |
| Seasf1-3f | CGCGGATCCGCCGCCGTCTGTTAGTCTT | Amplify Seasf1 downstream sequence |
| Seasf1-3r | CTAGTCTAGACAGAGGAGATGCTTGCTTGTC | |
| Seasf1-f | TCTGTCGTTTCGCTTCTCG | For identification of Seasf1 deletion strains |
| Seasf1-r | TGAACCATGACATCTGCGC | |
| Seasf1-3D | CCTCGTCGTCTTCCTGATCACT | For identification of Hph in deletion transformants |
| Hph-3D | GAGATTCTTCGCCCTCCGAG | |
| Hph-5D | AATTTCGATGATGCAGCTTGGG | |
| Seasf1-5D | CAAGACTGCTTCCTTCTCATCGT | |
| Hph-F | CGACAGCGTCTCCGACCTGA | For identification of Hph For identification of |
| Hph-R | CGCCCAAGCTGCATCATCGAA | |
| Seasf1-PHDT-F | CCGCTCGAGATGTCTGTCGTTTCGCTTCTCG | Seasf1complementation expression |
| Seasf1-PHDT-R | GCTCTAGACTAGTGAACCATGACATCTGCGC | |
| PHDT-F | GATCACATGGTCCTGCTG | Vector construction of sequencing primer |
| PHDT-R | CACCAACGATCTTATATCCAG | |
| Segtr1-pCIT-F(BamHI/ClaI) | CGCGGATCCATCGATGTAGTGCACCTGTTGA ATGAGAGC | Primer for Segtr1 silence |
| Segtr1-pCIT-R(PstI/EcoRV) | AACTGCAG GATATCGCCCAGCC TACGTAC ATCCTT | |
| Seasf1-QRT-F | ACAACGAGTACACAGATGAGG | QRT-PCR for Seasf1 |
| Seasf1-QRT-R | TCGTCAGAGTCCCATTTGATAG | |
| SeH4-QRT-F | AGCCATGATCTACGAGGAAAC | QRT-PCR for SeH4 |
| SeH4-QRT-R | CGAGAGAAGTGACAGTCTTACG | |
| Segtr1-QRT-F | AAGCTAGCGAGGGTTTCAAG | QRT-PCR for Segtr1 |
| Segtr1-QRT-R | GGCGTTTGGTATCAGGTAGTAG | |
| Seactin-F | GTCGATTGGAGAAGGAGCTAAA | QRT-PCR for Seactin |
| Seactin-R | GTTCTCCTTGTCGGCCATAAT | |
| Seasf1-BD-F(NdeI) | GGAATTCCATATGATGTCTGTCGTTTCGCTTCTCG | Seasf1 recombined into pGBKT7 |
| Seasf1-BD-R(BamHI) | CGCGGATCCCTAGTGAACCATGACATCTGCG | |
| SeH4-BD-F(SmaI) | TCCCCCGGGATGACTGGTCGCGGTAAAGGT | SeH4 recombined into pGBKT7 |
| SeH4-BD-R(BamHI) | CGCGGATCCCTAACCACCGAAACCGTAAAGGG | |
| SeH4-AD-F(SmaI) | TCCCCCGGGATGACTGGTCGCGGTAAAGGT | SeH4 recombined into pGADT7 |
| SeH4-AD-R(BamHI) | CGGGATCCCTAACCACCGAAACCGTAAAGGG | |
| SeH3-AD-F(SmaI) | TCCCCCGGGATGCCGCCAAAATCCCCTACCAG | SeH3 recombined into pGADT7 |
| SeH3-AD-R(BamHI) | CGCGGATCCTCAGACAGGCGCCCCCCAAG | |
| Segtr1-AD-F(SmaI) | TCCCCCGGGATGAATTCAGTCAAGCGTCAGA | Segtr1 recombined into pGADT7 |
| Segtr1-AD-R(BamHI) | CGCGGATCCCTACATTCCAGAGCCATGC | |
| Seasf1-pET28a-Flag-F(NdeI) | GGAATTCCATATGGATTACAAGGACGACG ATGACAAGATGTCTGTCGTTTCGCTTCTC | Seasf1 recombined into pET28a |
| Seasf1-pET28a-Flag-R(HindIII) | CCCAAGCTTCTAGTGAACCATGACATCTGC | |
| Seasf1-pGEX-F(BamHI) | CGCGGATCCATGTCTGTCGTTTCGCTTCTCG | Seasf1 recombined into pGEX |
| Seasf1-pGEX-R(NotI) | ATAAGAATGCGGCCGCCTAGTGAACCATG ACATCTGCG | |
| Segtr1-pGEX-F(NdeI) | GGAATTCCATATGATGAATTCAGTCAAGCG TCAGA | Segtr1 recombined into pGEX |
| Segtr1-pGEX-R(NotI) | ATAAGAATGCGGCCGCCTACATTCCAGAG CCATGC | |
| Seasf1-pDL2-GFP-F | CAGATCTTGGCTTTCGTAGGAACCCAATCTTCA ATGTCTGTCGTTTCGCTTCTCG | Seasf1 recombined into pDL2 |
| Seasf1-pDL2-GFP-R | CACCACCCCGGTGAACAGCTCCTCGCCCTTGCT CACGTGAACCATGACATCTGCGC | |
| SeH4-pDL2-GFP-F | CAGATCTTGGCTTTCGTAGGAACCCAATCTTCA ATGACTGGTCGCGGTAAAGGT | SeH4 recombined into pDL2 |
| SeH4-pDL2-GFP-R | CACCACCCCGGTGAACAGCTCCTCGCCCTTGCT CACACCACCGAAACCGTAAAGGG | |
| SeH4-pFL7-Flag-F | CAGATCTTGGCTTTCGTAGGAACCCAATCTTCA ATGACTGGTCGCGGTAAAGGT | SeH4 recombined into pFL7 |
| SeH4-pFL7-Flag-R | CTTTATAATCACCGTCATGGTCTTTGTAGT CACCACCGAAACCGTAAAGGG | |
| Segtr1-pFL7-Flag-F | CAGATCTTGGCTTTCGTAGGAACCCAATCTTCA ATGAATTCAGTCAAGCGTCAGA | Segtr1 recombined into pFL7 |
| Segtr1-pFL7-Flag-R | CTTTATAATCACCGTCATGGTCTTTGTAGT CATTCCAGAGCCATGC | |
| Segtr1-PHDT-F(SnaBI) | CCTACGTA ATGAATTCAGTCAAGCGTCAGA | Primer for Segtr1 overexpression |
| Segtr1-PHDT-R(XbaI) | GCTCTAGACATTCCAGAGCCATGC |
| Name | Origin of Target Genes | Construction Path | Purpose |
|---|---|---|---|
| Seasf1-pXEH | S. eturmiunum | Seasf1 recombined into pXEH | Knockout asf1 |
| Seasf1-pHDT | S. eturmiunum | Seasf1 recombined into pHDT | asf1complementation expression |
| Segtr1-pCIT | S. eturmiunum | Segtr1 recombined into pCIT | Segtr1 silence |
| Seasf1-pGBKT7 | S. eturmiunum | Seasf1 recombined into pGBKT7 | Yeast two-hybrid assays |
| SeH4-pGBKT7 | S. eturmiunum | SeH4 recombined into pGBKT7 | Yeast two-hybrid assays |
| Segtr1-pGADT7 | S. eturmiunum | Segtr1 recombined into pGADT7 | Yeast two-hybrid assays |
| SeH4-pGADT7 | S. eturmiunum | SeH4 recombined into pGADT7 | Yeast two-hybrid assays |
| SeH3-pGADT7 | S. eturmiunum | SeH3 recombined into pGADT7 | Yeast two-hybrid assays |
| Seasf1-pET28a | S. eturmiunum | Seasf1 recombined into pET28a | Pull-down assays |
| Segtr1-pGEX-6P-1 | S. eturmiunum | Segtr1 recombined into pGEX-6P-1 | Pull-down assays |
| Seasf1-pDL2 | S. eturmiunum | Seasf1 recombined into pDL2 | CO-IP assays |
| SeH4-pDL2 | S. eturmiunum | SeH4 recombined into pDL2 | CO-IP assays |
| Segtr1-pFL7 | S. eturmiunum | Segtr1 recombined into pFL7 | CO-IP assays |
| SeH4-pFL7 | S. eturmiunum | SeH4 recombined into pFL7 | CO-IP assays |
| Segtr1-pHDT | S. eturmiunum | Segtr1 recombined into pHDT | Segtr1 over expression |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Song, C.; Zhao, L.; Xu, W.; Li, Z.; Liu, X.; Zhang, X. GTP Binding Protein Gtr1 Cooperating with ASF1 Regulates Asexual Development in Stemphylium eturmiunum. Int. J. Mol. Sci. 2022, 23, 8355. https://doi.org/10.3390/ijms23158355
Wang S, Song C, Zhao L, Xu W, Li Z, Liu X, Zhang X. GTP Binding Protein Gtr1 Cooperating with ASF1 Regulates Asexual Development in Stemphylium eturmiunum. International Journal of Molecular Sciences. 2022; 23(15):8355. https://doi.org/10.3390/ijms23158355
Chicago/Turabian StyleWang, Shi, Chunyan Song, Lili Zhao, Wenmeng Xu, Zhuang Li, Xiaoyong Liu, and Xiuguo Zhang. 2022. "GTP Binding Protein Gtr1 Cooperating with ASF1 Regulates Asexual Development in Stemphylium eturmiunum" International Journal of Molecular Sciences 23, no. 15: 8355. https://doi.org/10.3390/ijms23158355
APA StyleWang, S., Song, C., Zhao, L., Xu, W., Li, Z., Liu, X., & Zhang, X. (2022). GTP Binding Protein Gtr1 Cooperating with ASF1 Regulates Asexual Development in Stemphylium eturmiunum. International Journal of Molecular Sciences, 23(15), 8355. https://doi.org/10.3390/ijms23158355







