Generation of Leukaemia-Derived Dendritic Cells (DCleu) to Improve Anti-Leukaemic Activity in AML: Selection of the Most Efficient Response Modifier Combinations
Abstract
1. Introduction
2. Results
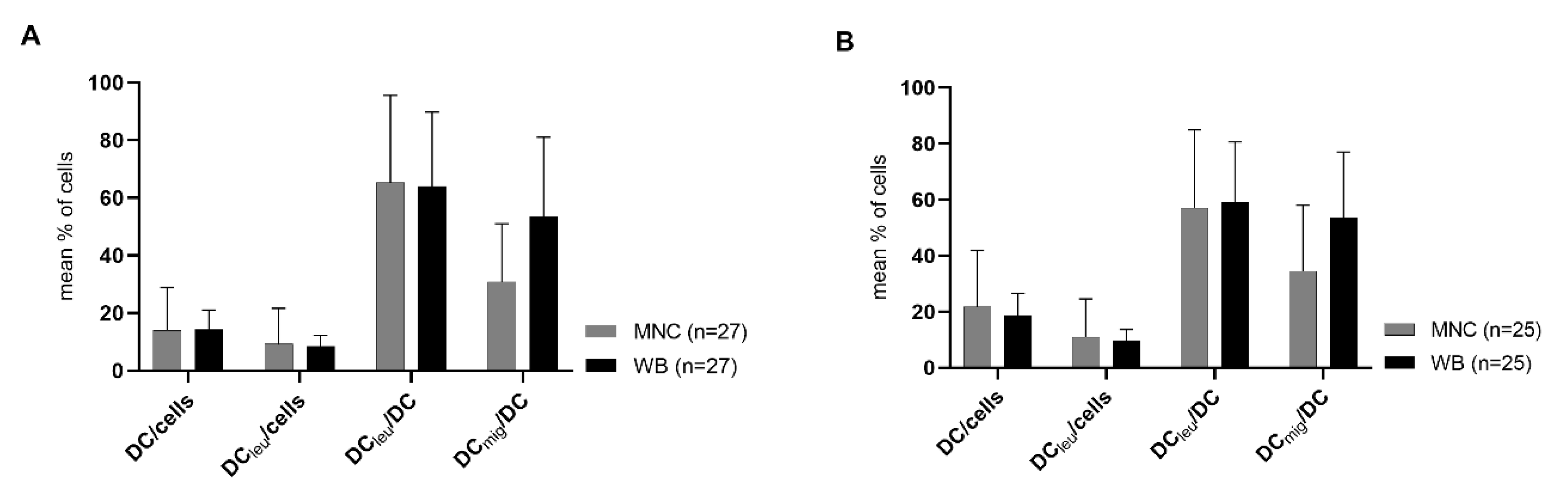
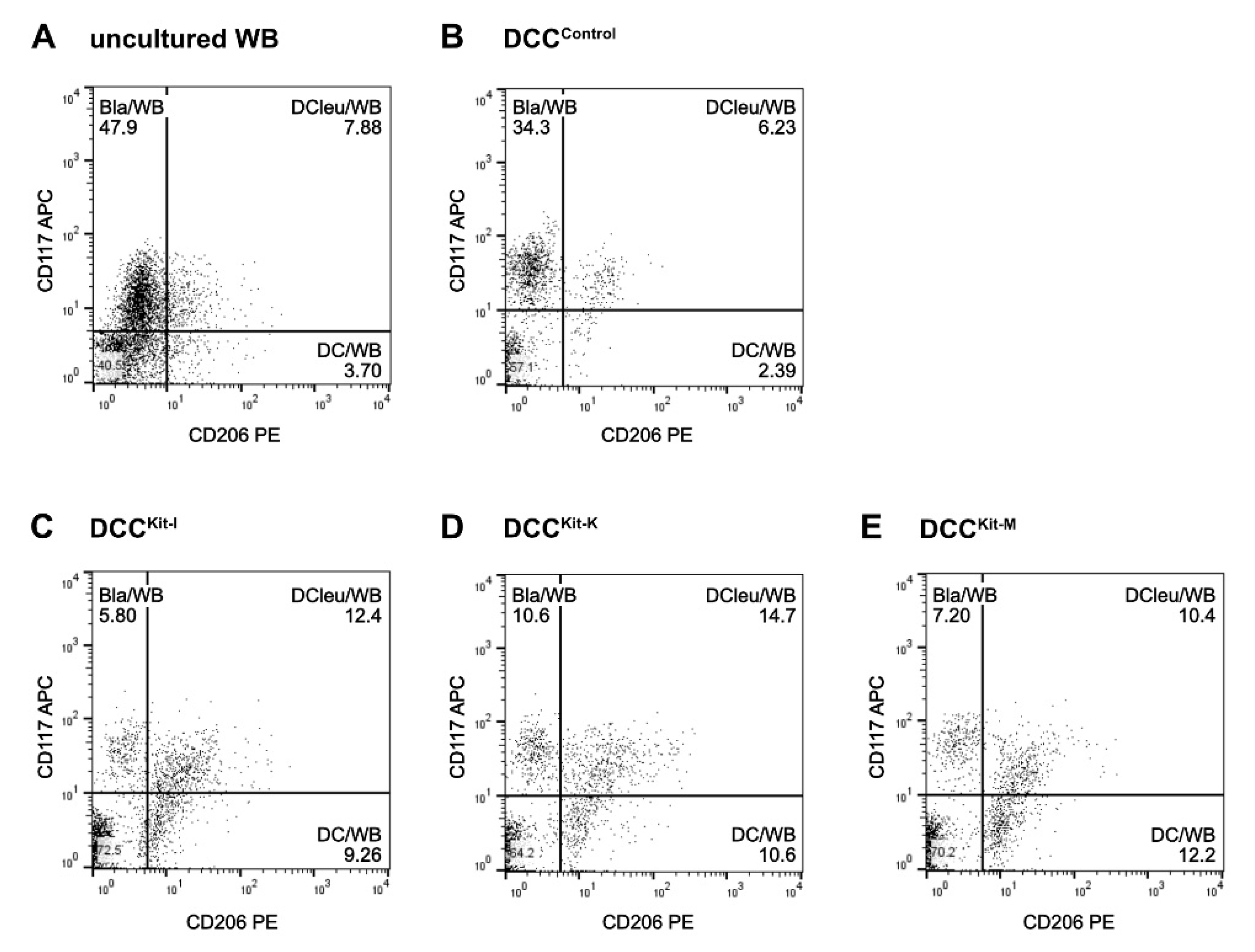
2.1. DC/DCleu-Generation Using Standard Protocols Is Comparable from MNC and WB
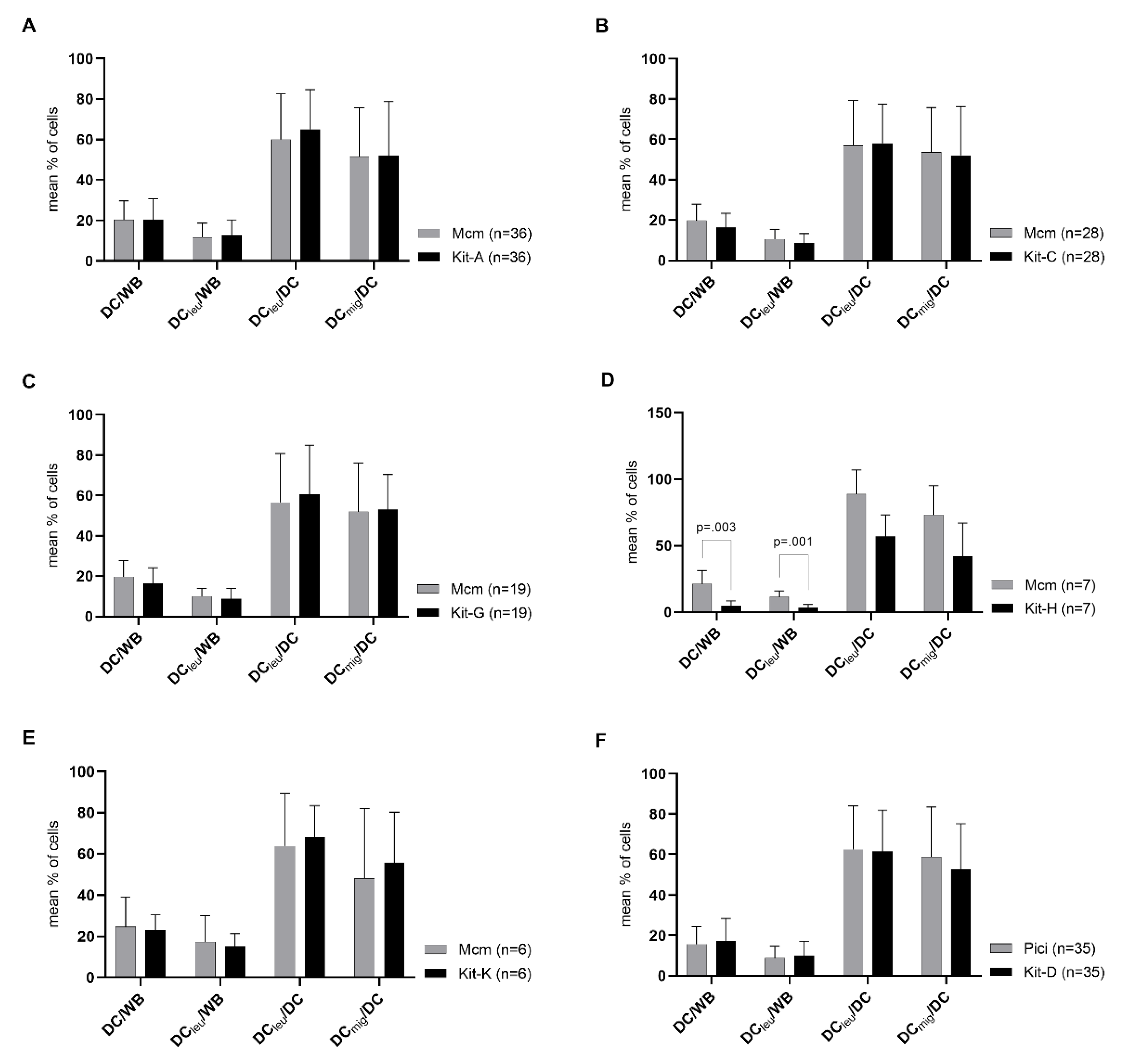
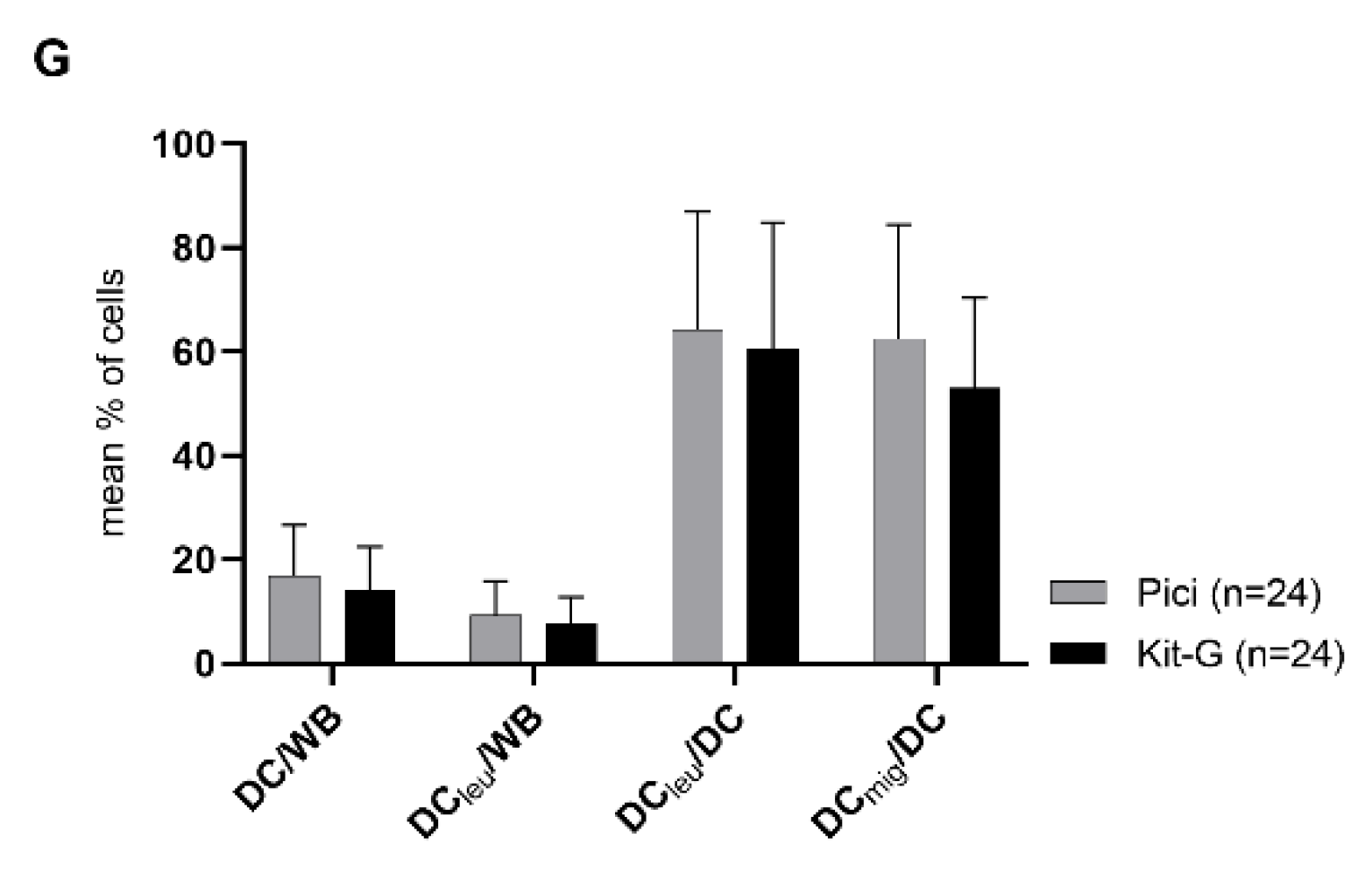
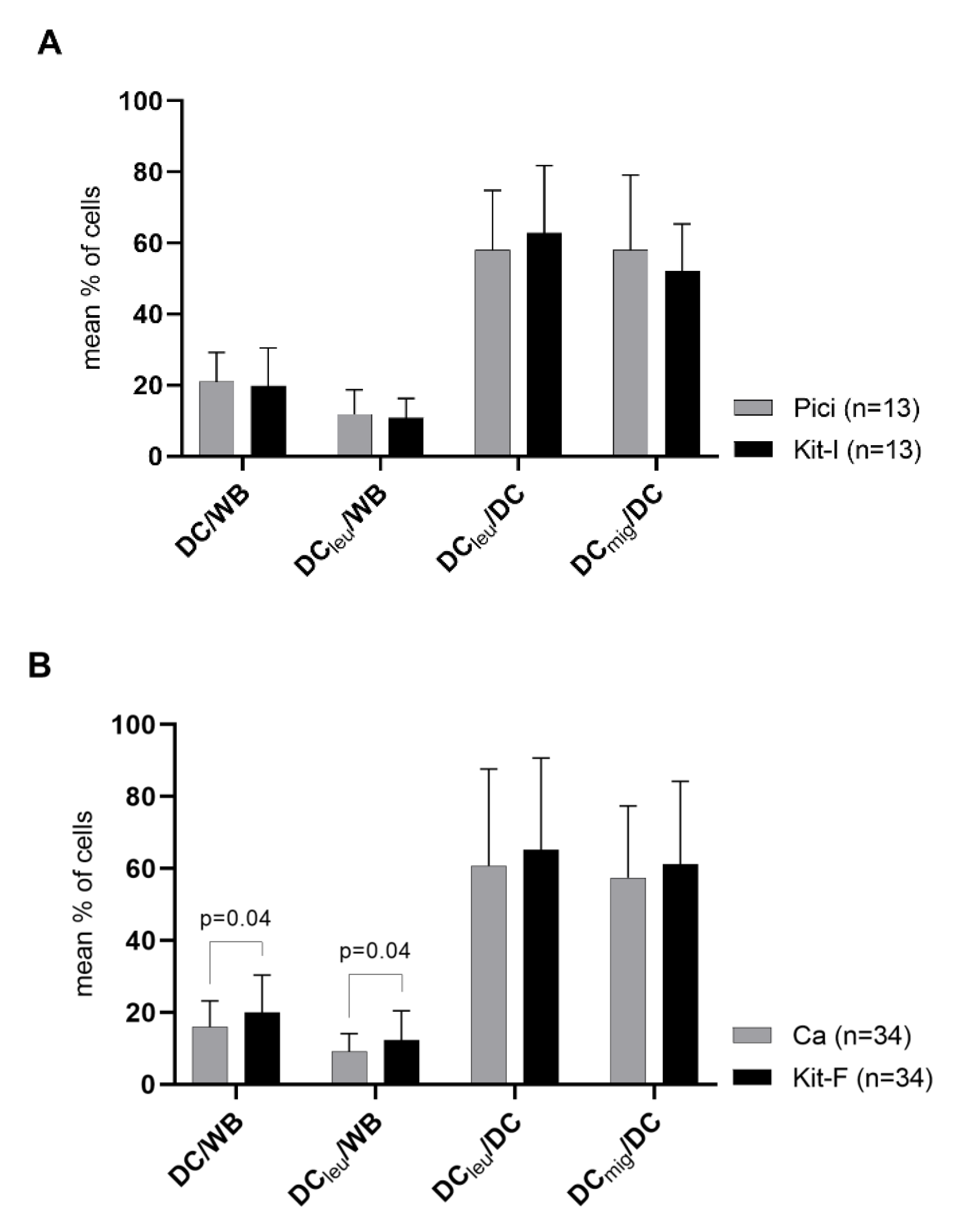
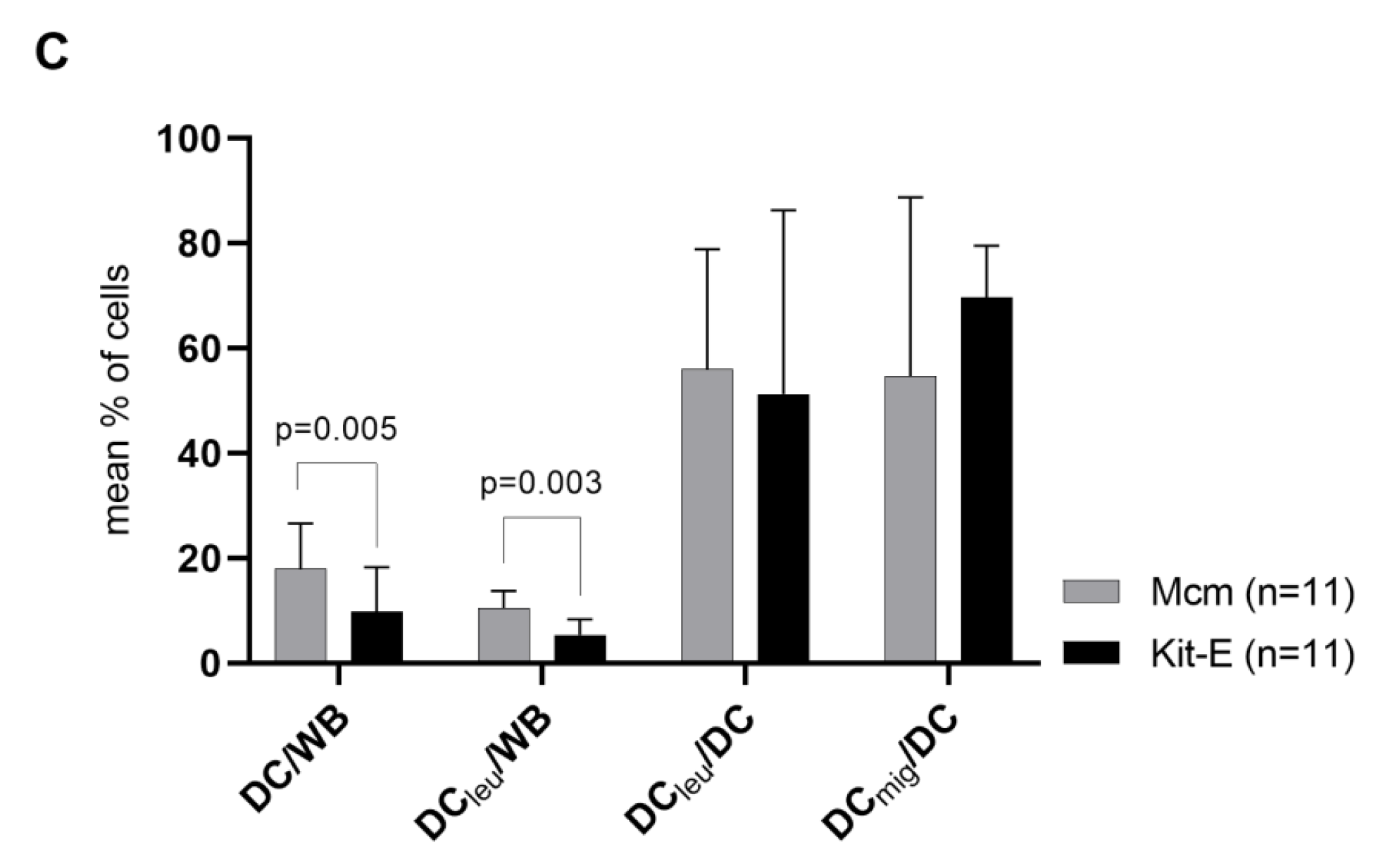
2.2. DC/DCleu-Generation from WB Is Comparable Using New Protocols (Kits) and Standard Protocols
2.3. Ranking of Kits
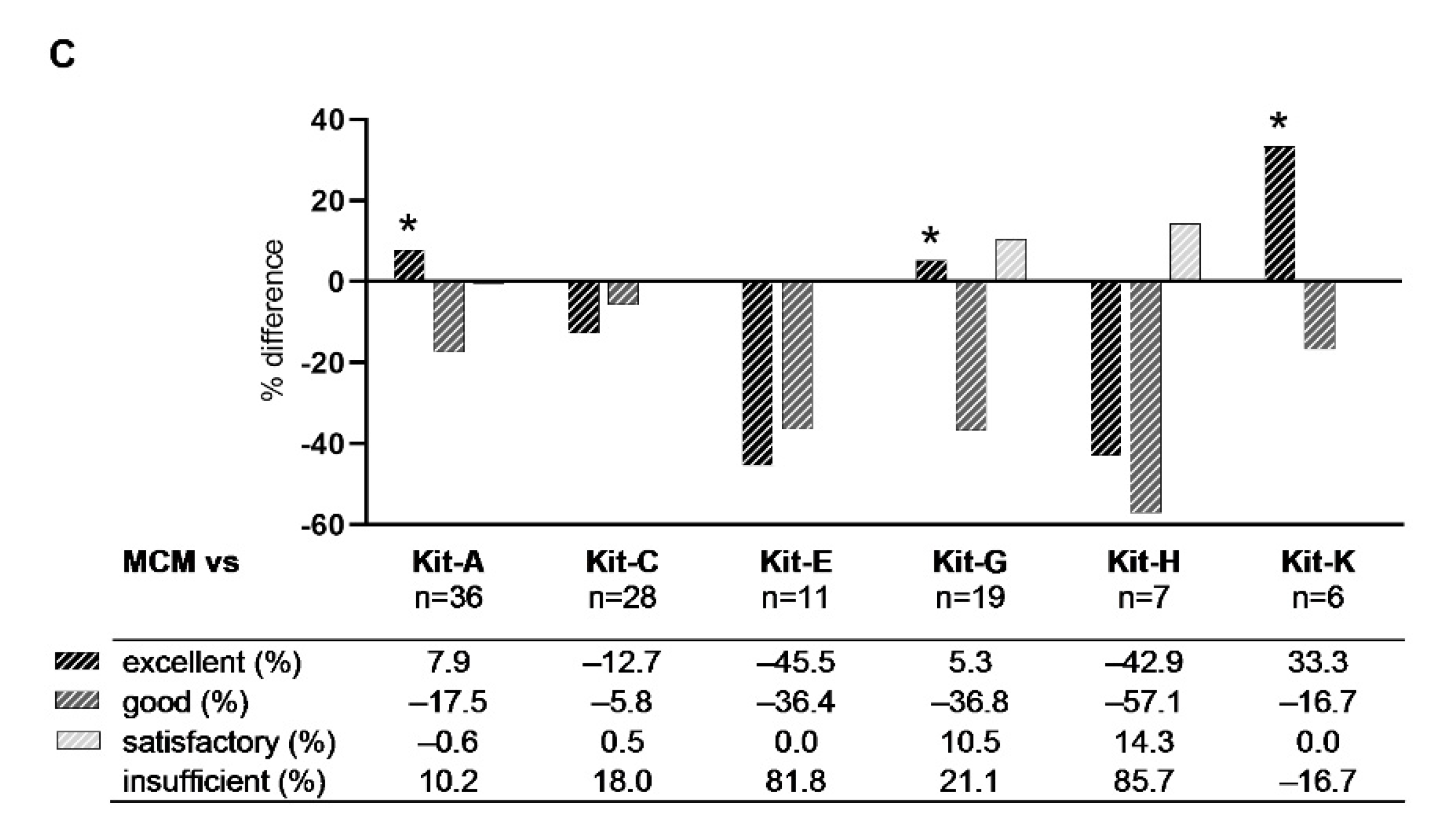
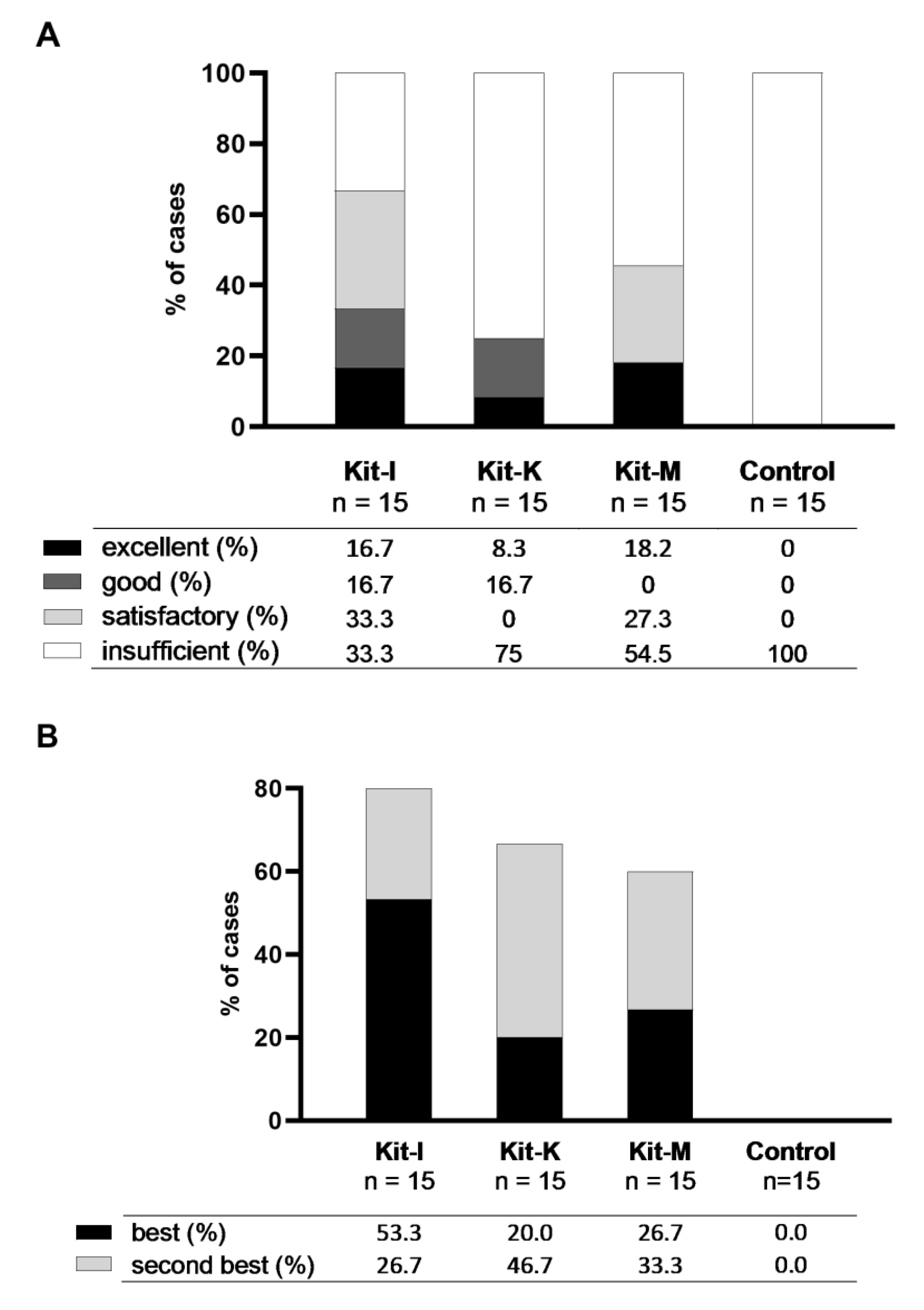
2.3.1. Class-Ranking of Kits
2.3.2. Best-Ranking of Kits
2.4. Selection of Best Kits and Evaluation of Their DC/DCleu-Generating and Anti-Leukaemic Performance
2.4.1. Selection of Best Kits
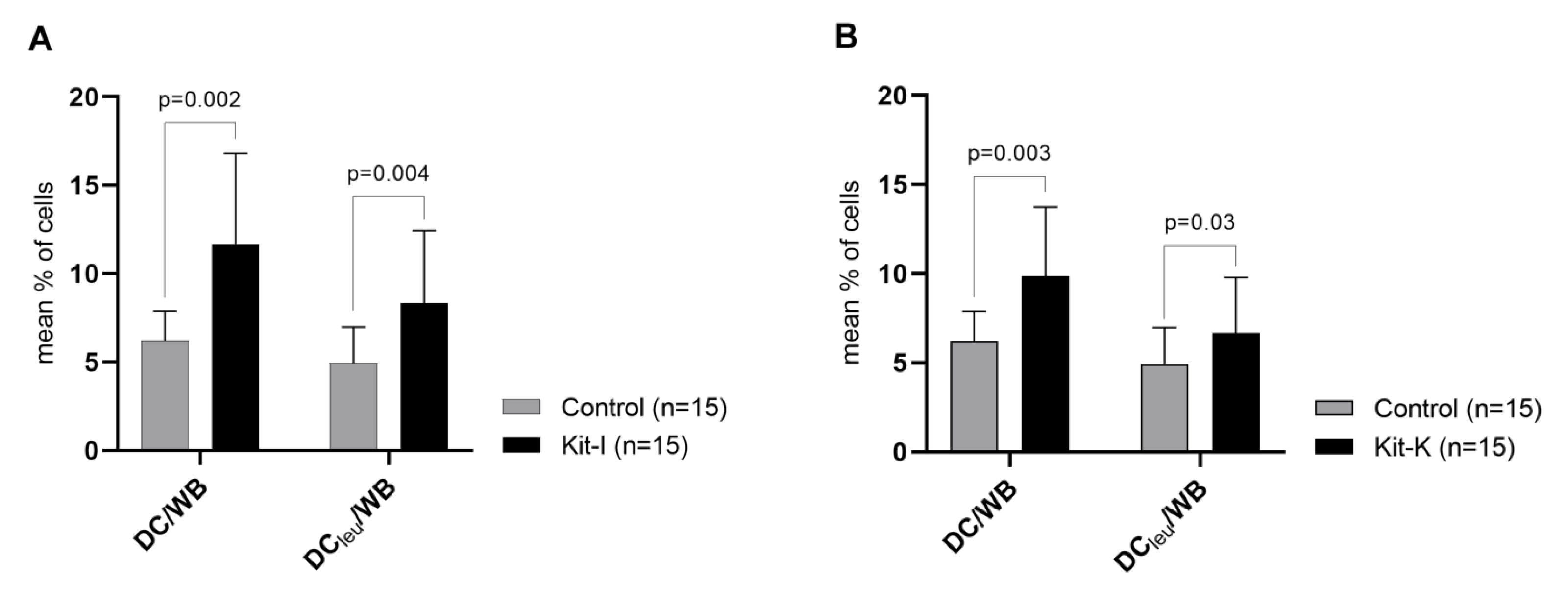
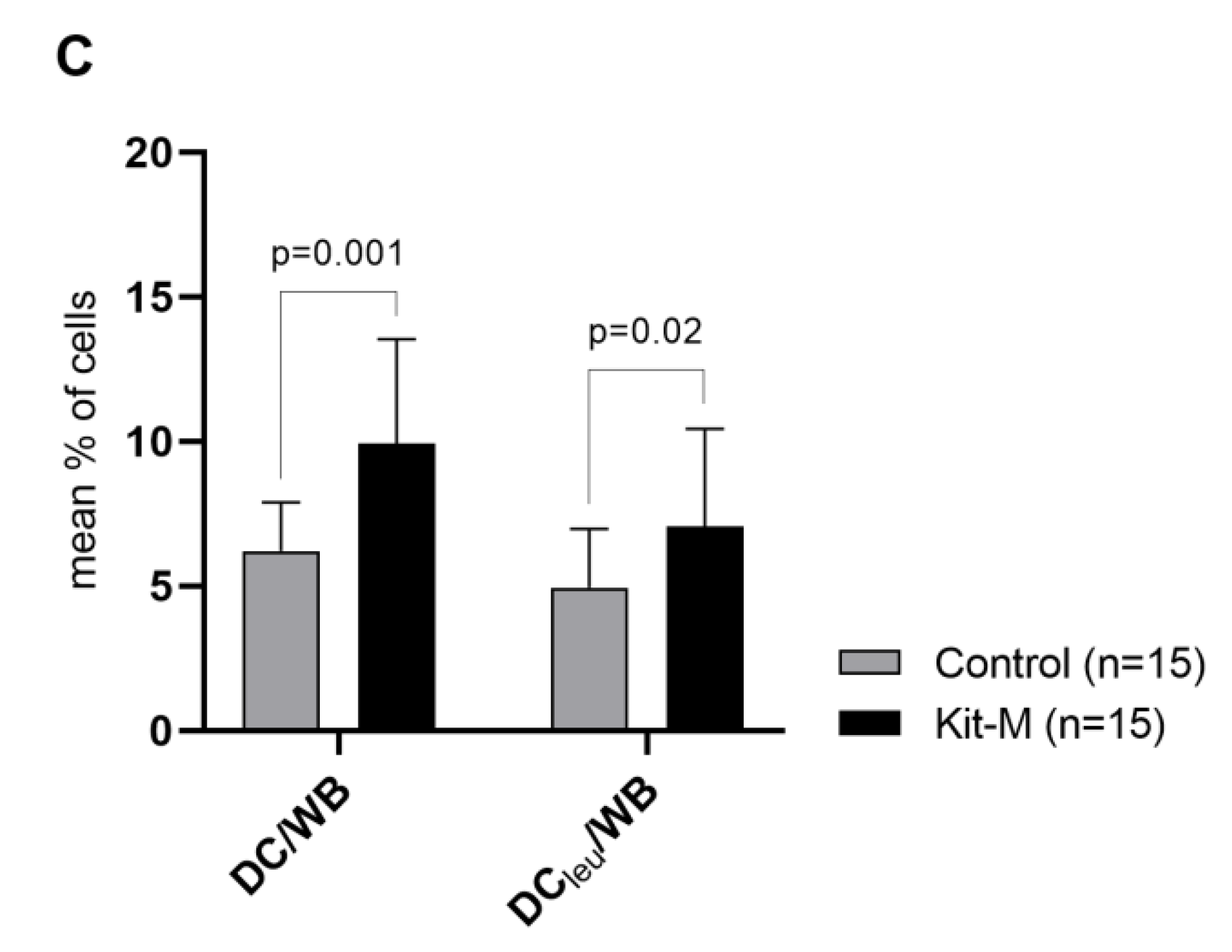
2.4.2. Kit-I, -K and -M Generate Significantly Higher Frequencies of DC/DCleu Compared to Control
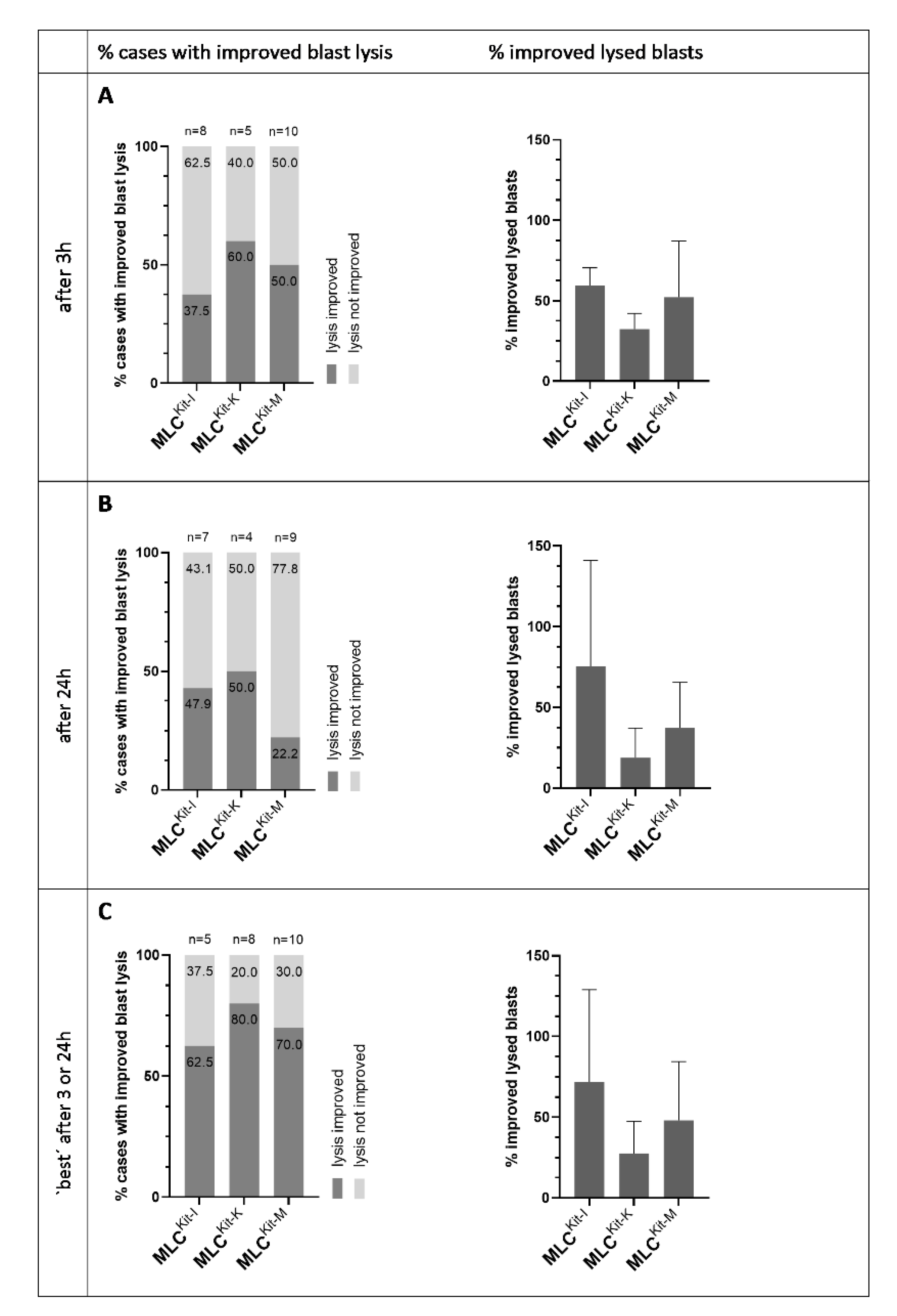
2.4.3. DC/DCleu Generated with Kit-I, -K and -M Stimulate Anti-Leukaemic Activity
3. Discussion
3.1. DCleu-Based Immunotherapy
3.2. Standard Protocols—DC/DCleu Generation from Leukaemic MNC and WB
3.3. New Kits Specifically Designed for WB
3.4. Ranking of Kits
3.5. Selection of Kits for (Potential) Treatment of Patients
3.6. Ongoing Kit Studies
4. Materials and Methods
4.1. Sample Collection and Preparation
4.2. Patients’ Characteristics
4.3. Flow Cytometry
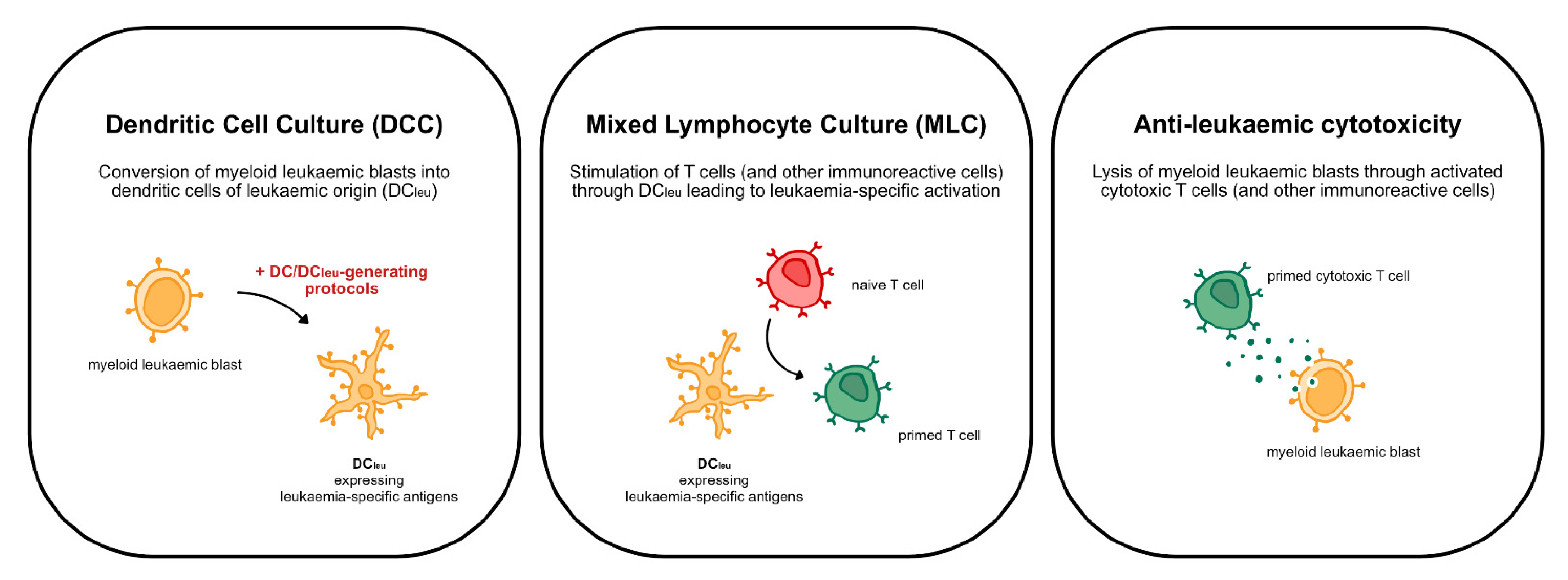
4.4. Dendritic Cell Culture (DCC)
4.5. Mixed Lymphocyte Culture (MLC)
4.6. Cytotoxicity Fluorolysis Assay (CTX)
4.7. Statistical Methods
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Sawyers, C.L.; Denny, C.T.; Witte, O.N. Leukemia and the disruption of normal hematopoiesis. Cell 1991, 64, 337–350. [Google Scholar] [CrossRef]
- Lowenberg, B.; Downing, J.R.; Burnett, A. Acute myeloid leukemia. N. Engl. J. Med. 1999, 341, 1051–1062. [Google Scholar] [CrossRef]
- Shallis, R.M.; Wang, R.; Davidoff, A.; Ma, X.; Zeidan, A.M. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019, 36, 70–87. [Google Scholar] [CrossRef]
- Döhner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Büchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar]
- Short, N.J.; Rytting, M.E.; Cortes, J.E. Acute myeloid leukaemia. Lancet 2018, 392, 593–606. [Google Scholar] [CrossRef]
- Cancer Stat Facts: Leukaemia—Acute Myeloid Leukaemia (AML). 2020. Available online: https://seer.cancer.gov/statfacts/html/amyl.html (accessed on 11 February 2021).
- Moeinafshar, A.; Hemmati, S.; Rezaei, N. Immunotherapy in AML: A brief review on emerging strategies. Clin. Transl. Oncol. 2021, 23, 2431–2447. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar]
- Mellman, I. Dendritic Cells: Master Regulators of the Immune Response. Cancer Immunol. Res. 2013, 1, 145–149. [Google Scholar] [CrossRef]
- Ogasawara, M.; Miyashita, M.; Yamagishi, Y.; Ota, S. Wilms’ tumor 1 peptide-loaded dendritic cell vaccination in patients with relapsed or refractory acute leukemia. Ther. Apher. Dial. 2022, 26, 537–547. [Google Scholar] [CrossRef]
- Lee, J.-J.; Kook, H.; Park, M.-S.; Nam, J.-H.; Choi, B.-H.; Song, W.-H.; Park, K.-S.; Lee, I.-K.; Chung, I.-J.; Hwang, T.-J.; et al. Immunotherapy using autologous monocyte-derived dendritic cells pulsed with leukemic cell lysates for acute myeloid leukemia relapse after autologous peripheral blood stem cell transplantation. J. Clin. Apher. 2004, 19, 66–70. [Google Scholar] [CrossRef]
- Kitawaki, T.; Kadowaki, N.; Fukunaga, K.; Kasai, Y.; Maekawa, T.; Ohmori, K.; Itoh, T.; Shimizu, A.; Kuzushima, K.; Kondo, T.; et al. Cross-priming of CD8(+) T cells in vivo by dendritic cells pulsed with autologous apoptotic leukemic cells in im-munotherapy for elderly patients with acute myeloid leukemia. Exp. Hematol. 2011, 39, 424–433.e2. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, J.; Stone, R.M.; Uhl, L.; Neuberg, D.; Joyce, R.; Levine, J.D.; Arnason, J.; McMasters, M.; Luptakova, K.; Jain, S.; et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci. Transl. Med. 2016, 8, 368ra171. [Google Scholar] [CrossRef]
- Anguille, S.; Van de Velde, A.L.; Smits, E.L.; Van Tendeloo, V.F.; Juliusson, G.; Cools, N.; Nijs, G.; Stein, B.; Lion, E.; Van Driessche, A.; et al. Dendritic cell vaccination as postremission treatment to prevent or delay relapse in acute myeloid leukemia. Blood 2017, 130, 1713–1721. [Google Scholar] [CrossRef]
- Li, L.; Giannopoulos, K.; Reinhardt, P.; Tabarkiewicz, J.; Schmitt, A.; Greiner, J.; Rolinski, J.M.; Hus, I.; Dmoszynska, A.; Wiesneth, M.; et al. Immunotherapy for patients with acute myeloid leukemia using autologous dendritic cells generated from leukemic blasts. Int. J. Oncol. 2006, 28, 855–861. [Google Scholar] [CrossRef]
- Amberger, D.C.; Schmetzer, H.M. Dendritic Cells of Leukemic Origin: Specialized Antigen-Presenting Cells as Potential Treat-ment Tools for Patients with Myeloid Leukemia. Transfus. Med. Hemotherapy 2020, 47, 432–443. [Google Scholar] [CrossRef]
- Schmetzer, H.M.; Kremser, A.; Loibl, J.; Kroell, T.; Kolb, H.-J. Quantification of ex vivo generated dendritic cells (DC) and leukemia-derived DC contributes to estimate the quality of DC, to detect optimal DC-generating methods or to optimize DC-mediated T-cell-activation-procedures ex vivo or in vivo. Leukemia 2007, 21, 1338–1341. [Google Scholar] [CrossRef]
- Narita, M.; Takahashi, M.; Liu, A.; Ayres, F.; Satoh, N.; Abe, T.; Nikkuni, K.; Furukawa, T.; Toba, K.; Aizawa, Y. Generation of Dendritic Cells from Leukaemia Cells of a Patient with Acute Promyelocytic Leukaemia by Culture with GM-CSF, IL-4 and TNF-α. Acta Haematol. 2001, 106, 89–94. [Google Scholar] [CrossRef]
- Cignetti, A.; Vallario, A.; Roato, I.; Circosta, P.; Allione, B.; Casorzo, L.; Ghia, P.; Caligaris-Cappio, F. Leukemia-derived immature dendritic cells differentiate into functionally competent mature dendritic cells that effi-ciently stimulate T cell responses. J. Immunol. 2004, 173, 2855–2865. [Google Scholar]
- Kremser, A.; Dreyig, J.; Grabrucker, C.; Liepert, A.; Kroell, T.; Scholl, N.; Schmid, C.; Tischer, J.; Kufner, S.; Salih, H.; et al. Dendritic cells (DCs) can be successfully generated from leukemic blasts in individual patients with AML or MDS: An evaluation of different methods. J. Immunother. 2010, 33, 185–199. [Google Scholar] [CrossRef]
- Grabrucker, C.; Liepert, A.; Dreyig, J.; Kremser, A.; Kroell, T.; Freudenreich, M.; Schmid, C.; Schweiger, C.; Tischer, J.; Kolb, H.-J.; et al. The Quality and Quantity of Leukemia-derived Dendritic Cells from Patients with Acute Myeloid Leukemia and Myelodysplastic Syndrome Are a Predictive Factor for the Lytic Potential of Dendritic Cells-primed Leukemia-Specific T Cells. J. Immunother. 2010, 33, 523–537. [Google Scholar] [CrossRef]
- Liepert, A.; Grabrucker, C.; Kremser, A.; Dreyßig, J.; Ansprenger, C.; Freudenreich, M.; Kroell, T.; Reibke, R.; Tischer, J.; Schweiger, C.; et al. Quality of T-cells after stimulation with leukemia-derived dendritic cells (DC) from patients with acute myeloid leu-kemia (AML) or myeloid dysplastic syndrome (MDS) is predictive for their leukemia cytotoxic potential. Cell. Immunol. 2010, 265, 23–30. [Google Scholar] [CrossRef]
- Boeck, C.L.; Amberger, D.C.; Doraneh-Gard, F.; Sutanto, W.; Guenther, T.; Schmohl, J.; Schuster, F.; Salih, H.; Babor, F.; Borkhardt, A.; et al. Significance of Frequencies, Compositions, and/or Antileukemic Activity of (DC-stimulated) Invariant NKT, NK and CIK Cells on the Outcome of Patients With AML, ALL and CLL. J. Immunother. 2017, 40, 224–248. [Google Scholar] [CrossRef]
- Klauer, L.K.; Schutti, O.; Ugur, S.; Doraneh-Gard, F.; Amberger, D.C.; Rogers, N.; Krämer, D.; Rank, A.; Schmid, C.; Eiz-Vesper, B.; et al. Interferon Gamma Secretion of Adaptive and Innate Immune Cells as a Parameter to Describe Leukaemia-Derived Dendritic-Cell-Mediated Immune Responses in Acute Myeloid Leukaemia in vitro. Transfus. Med. Hemotherapy 2021, 49, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Hirn-Lopez, A.; Deen, D.; Fischer, Z.; Rabe, A.; Ansprenger, C.; Stein, K.; Vogt, V.; Schick, J.; Kroell, T.; Kraemer, D.; et al. Role of Interferon (IFN)α in “Cocktails” for the Generation of (Leukemia-derived) Dendritic Cells (DCleu) From Blasts in Blood from Patients (pts) With Acute Myeloid Leukemia (AML) and the Induction of Antileukemic Reactions. J. Immunother. 2019, 42, 143–161. [Google Scholar] [CrossRef]
- Yan, W.-L.; Shen, K.-Y.; Tien, C.-Y.; Chen, Y.-A.; Liu, S.-J. Recent progress in GM-CSF-based cancer immunotherapy. Immunotherapy 2017, 9, 347–360. [Google Scholar] [CrossRef] [PubMed]
- van de Laar, L.; Coffer, P.J.; Woltman, A.M. Regulation of dendritic cell development by GM-CSF: Molecular control and impli-cations for immune homeostasis and therapy. Blood 2012, 119, 3383–3393. [Google Scholar] [CrossRef] [PubMed]
- Tough, D.F. Type I Interferon as a Link Between Innate and Adaptive Immunity through Dendritic Cell Stimulation. Leuk. Lymphoma 2004, 45, 257–264. [Google Scholar] [CrossRef]
- Koski, G.K.; Schwartz, G.N.; Weng, D.E.; Gress, R.E.; Engels, F.H.; Tsokos, M.; Czerniecki, B.J.; Cohen, P.A. Calcium ionophore-treated myeloid cells acquire many dendritic cell characteristics independent of prior differentiation state, transformation status, or sensitivity to biologic agents. Blood 1999, 94, 1359–1371. [Google Scholar] [CrossRef]
- Czerniecki, B.J.; Carter, C.; Rivoltini, L.; Koski, G.K.; Kim, H.I.; Weng, D.E.; Roros, J.G.; Hijazi, Y.M.; Xu, S.; Rosenberg, S.A.; et al. Calcium ionophore-treated peripheral blood monocytes and dendritic cells rapidly display characteristics of acti-vated dendritic cells. J. Immunol. 1997, 159, 3823–3837. [Google Scholar]
- Ryoma, Y.; Moriya, Y.; Okamoto, M.; Kanaya, I.; Saito, M.; Sato, M. Biological effect of OK-432 (picibanil) and possible application to dendritic cell therapy. Anticancer Res. 2004, 24, 3295–3302. [Google Scholar]
- Sato, M.; Takayama, T.; Tanaka, H.; Konishi, J.; Suzuki, T.; Kaiga, T.; Tahara, H. Generation of mature dendritic cells fully capable of T helper type 1 polarization using OK-432 combined with prosta-glandin E2. Cancer Sci. 2003, 94, 1091–1098. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of Immune Responses by Prostaglandin E2. J. Immunol. 2012, 188, 21–28. [Google Scholar] [CrossRef]
- Jonuleit, H.; Kühn, U.; Müller, G.; Steinbrink, K.; Paragnik, L.; Schmitt, E.; Knop, J.; Enk, A.H. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur. J. Immunol. 1997, 27, 3135–3142. [Google Scholar] [CrossRef] [PubMed]
- Kuroki, H.; Morisaki, T.; Matsumoto, K.; Onishi, H.; Baba, E.; Tanaka, M.; Katano, M. Streptococcal preparation OK-432: A new maturation factor of monocyte-derived dendritic cells for clinical use. Cancer Immunol. Immunother. 2003, 52, 561–568. [Google Scholar] [CrossRef]
- Ritter, U.; Meissner, A.; Ott, J.; Korner, H. Analysis of the maturation process of dendritic cells deficient for TNF and lymphotoxin-α reveals an essential role for TNF. J. Leukoc. Biol. 2003, 74, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Amberger, D.C.; Doraneh-Gard, F.; Gunsilius, C.; Weinmann, M.; Möbius, S.; Kugler, C.; Rogers, N.; Böck, C.; Ködel, U.; Werner, J.-O.; et al. PGE1-Containing Protocols Generate Mature (Leukemia-Derived) Dendritic Cells Directly from Leukemic Whole Blood. Int. J. Mol. Sci. 2019, 20, 4590. [Google Scholar] [CrossRef]
- Ogihara, T.; Iinuma, H.; Okinaga, K. Usefulness of immunomodulators for maturation of dendritic cells. Int. J. Oncol. 2004, 25, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Platt, A.M.; Randolph, G.J. Dendritic Cell Migration Through the Lymphatic Vasculature to Lymph Nodes. Adv. Immunol. 2013, 120, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef]
- Tiberio, L.; Del Prete, A.; Schioppa, T.; Sozio, F.; Bosisio, D.; Sozzani, S. Chemokine and chemotactic signals in dendritic cell migration. Cell. Mol. Immunol. 2018, 15, 346–352. [Google Scholar] [CrossRef]
- Bedrosian, I.; Roros, J.G.; Xu, S.; Nguyen, H.Q.; Engels, F.; Faries, M.B.; Koski, G.K.; Cohen, P.A.; Czerniecki, B.J. Granulocyte-macrophage colony-stimulating factor, interleukin-2, and interleukin-12 synergize with calcium ion-ophore to enhance dendritic cell function. J. Immunother. 2000, 23, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.K.; Harui, A.; Stolina, M.; Sharma, S.; Mitani, K.; Dubinett, S.M.; Roth, M.D. Increased dendritic cell number and function following continuous in vivo infusion of granulocyte macrophage–colony-stimulating factor and interleukin-4. Blood 2002, 99, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Romani, N.; Gruner, S.; Brang, D.; Kämpgen, E.; Lenz, A.; Trockenbacher, B.; Konwalinka, G.; Fritsch, P.O.; Steinman, R.M.; Schuler, G. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 1994, 180, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Naito, K.; Ueda, Y.; Itoh, T.; Fuji, N.; Shimizu, K.; Yano, Y.; Yamamoto, Y.; Imura, K.; Kohara, J.; Iwamoto, A.; et al. Mature dendritic cells generated from patient-derived peripheral blood monocytes in one-step culture using streptococcal preparation OK-432 exert an enhanced antigen-presenting capacity. Int. J. Oncol. 2006, 28, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Rieser, C.; Böck, G.; Klocker, H.; Bartsch, G.; Thurnher, M. Prostaglandin E2 and Tumor Necrosis Factor α Cooperate to Activate Human Dendritic Cells: Synergistic Activation of Interleukin 12 Production. J. Exp. Med. 1997, 186, 1603–1608. [Google Scholar] [CrossRef]
- Waclavicek, M.; Berer, A.; Oehler, L.; Stöckl, J.; Schloegl, E.; Majdic, O.; Knapp, W. Calcium ionophore: A single reagent for the differentiation of primary human acute myelogenous leukaemia cells towards dendritic cells. Br. J. Haematol. 2001, 114, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Westers, T.M.; Houtenbos, I.; Snoijs, N.C.L.; A Van De Loosdrecht, A.; Ossenkoppele, G.J. Leukemia-derived dendritic cells in acute myeloid leukemia exhibit potent migratory capacity. Leukemia 2005, 19, 1270–1272. [Google Scholar] [CrossRef]
- Kaliński, P.; Hilkens, C.M.; Snijders, A.; Snijdewint, F.G.; Kapsenberg, M.L. Dendritic cells, obtained from peripheral blood precursors in the presence of PGE 2, promote Th2 responses. In Dendritic Cells in Fundamental and Clinical Immunology; Springer: Berlin/Heidelberg, Germany, 1997; pp. 363–367. [Google Scholar]
- Quesada, J.R.; Rios, A.; Swanson, D.; Trown, P.; Gutterman, J.U. Antitumor activity of recombinant-derived interferon alpha in metastatic renal cell carcinoma. J. Clin. Oncol. 1985, 3, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Smits, E.L.; Anguille, S.; Berneman, Z.N. Interferon α may be back on track to treat acute myeloid leukemia. OncoImmunology 2013, 2, e23619. [Google Scholar] [CrossRef][Green Version]
- Jiang, H.; Liu, X.H.; Kong, J.; Wang, J.; Jia, J.S.; Lu, S.Y.; Gong, L.Z.; Zhao, X.S.; Jiang, Q.; Chang, Y.J.; et al. Interferon-α as maintenance therapy can significantly reduce relapse in patients with favorable-risk acute myeloid leukemia. Leuk Lymphoma 2021, 62, 2949–2956. [Google Scholar] [CrossRef] [PubMed]
- Ives, N.J.; Suciu, S.; Eggermont, A.M.; Kirkwood, J.; Lorigan, P.; Markovic, S.N.; Garbe, C.; Wheatley, K.; Bufalino, R.; Cameron, D.; et al. Adjuvant interferon-α for the treatment of high-risk melanoma: An individual patient data meta-analysis. Eur. J. Cancer 2017, 82, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Anguille, S.; Lion, E.; Willemen, Y.; Van Tendeloo, V.F.I.; Berneman, Z.N.; Smits, E.L.J.M. Interferon-α in acute myeloid leukemia: An old drug revisited. Leukemia 2011, 25, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Lowin, B.; Hahne, M.; Mattmann, C.; Tschopp, J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 1994, 370, 650–652. [Google Scholar] [CrossRef]
- Hassin, D.; Garber, O.G.; Meiraz, A.; Schiffenbauer, Y.S.; Berke, G. Cytotoxic T lymphocyte perforin and Fas ligand working in concert even when Fas ligand lytic action is still not detectable. Immunology 2011, 133, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Gard, F.D.; Amberger, D.; Weinmann, M.; Boeck, C.; Gunsilius, C.; Kugler, C.; Werner, J.; Kraemer, D.; Rank, A.; Schmid, C.; et al. Standard normoxic versus physiological hypoxic culture of AML patients’ (pts) whole blood (WB) samples with immune modulatory kits yields comparable proportions of dendritic cells and functional results. Eur. J. Cancer 2018, 92, S10–S11. [Google Scholar] [CrossRef]
- Pepeldjiyska, E.; Li, L.; Gao, J.; Seidel, C.L.; Blasi, C.; Özkaya, E.; Schmohl, J.; Kraemer, D.; Schmid, C.; Rank, A.; et al. Leukemia derived dendritic cell (DCleu) mediated immune response goes along with reduced (leukemia-specific) regulatory T-cells. Immunobiology 2022, 227, 152237. [Google Scholar] [CrossRef]
- Plett, C.; Amberger, D.C.; Rabe, A.; Deen, D.; Stankova, Z.; Hirn Lopez, A.; Vokac, Y.; Werner, J.O.; Krämer, D.; Rank, A. Kits do not induce AML-blasts’ proliferation ex vivo. IPO-38 is an appropriate and reliable marker to detect and quantify proliferating blasts. Eur. J. Cancer 2017, 5, 3–4. [Google Scholar]
- Schmid, C.; Atzler, M.; Rank, A.; Inngjerdingen, M.; Rabe, A.; Deen, D.; Wang, R.; Eiz-Vesper, B.; Schmetzer, H. Immune modulation of AML-blasts in therapy refractory AML-patient in vivo with clinically approved response modifiers improves clinical status, blood cell regeneration and gives rise to leukaemia specific adaptive and innate immune reactive cells. Eur. J. Cancer 2018, 92, S15. [Google Scholar] [CrossRef]
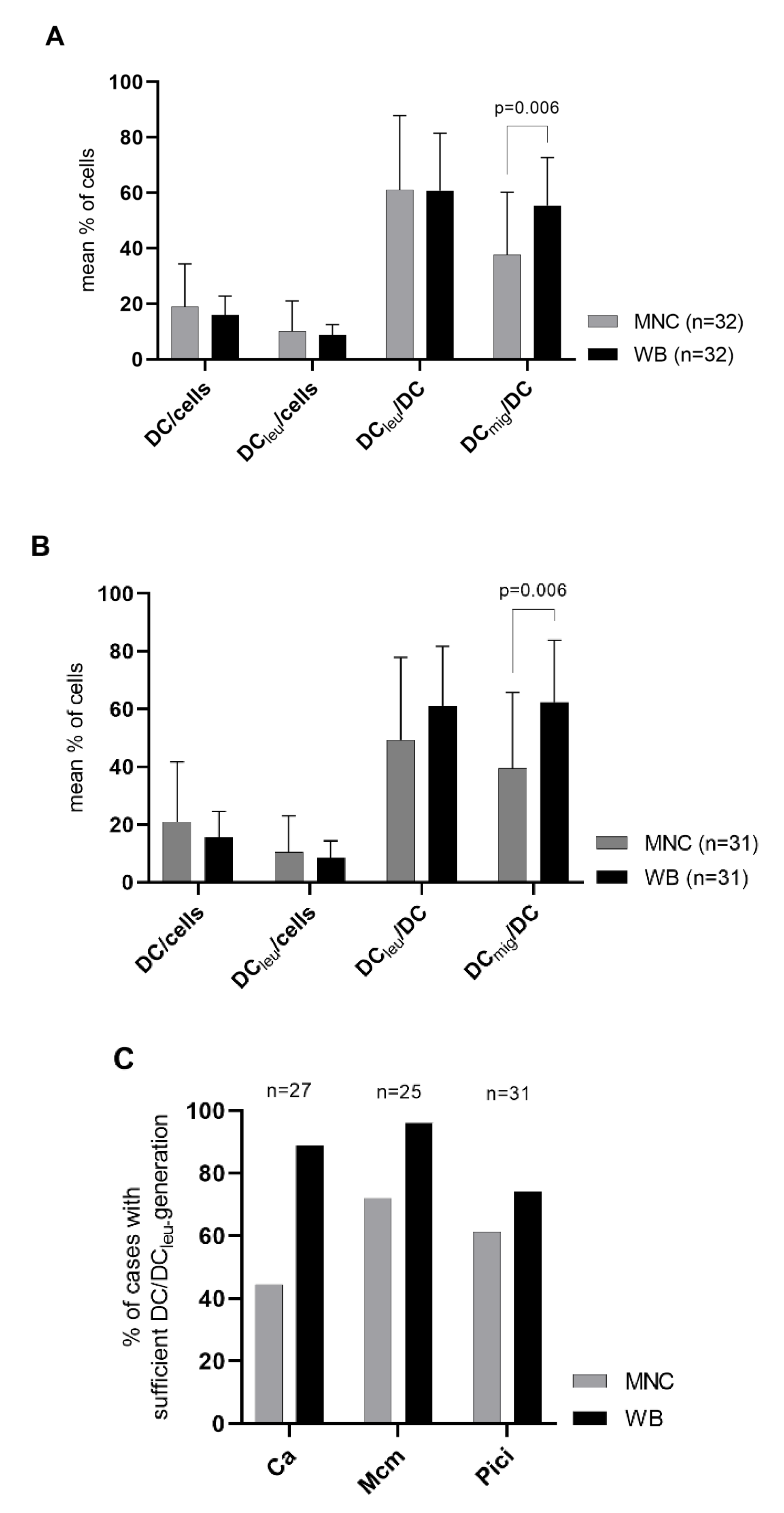


| Subcategories | DC/Cells | DCleu/Cells | ||
|---|---|---|---|---|
| Sufficient | Excellent | ≥15% | and | ≥10% |
| Good | ≥15% | and | <10% and ≥5% | |
| or | ||||
| <15% and ≥10% | and | ≥ 10% | ||
| Satisfactory | <15% and ≥10% | and | <10% and ≥5% | |
| Insufficient | <10% | and/or | <5% |
| Rank | Ranking 1 Excellent | Ranking 2 Excellent, Good | Ranking 3 Excellent, Good, Satisfactory | Ranking 4 Combined | |
|---|---|---|---|---|---|
| 1 | I | K | K | → | K |
| 2 | K | I | I | → | I |
| 3 | F | A | A | → | A |
| 4 | A | F | F | → | F |
| 5 | D | D | D | → | D |
| 6 | G | C | C | → | C |
| 7 | C | G | G | → | G |
| 8 | E | E | E | → | E |
| 9 | H | H | H | → | H |
| Rank | Ranking 1 Best | Ranking 2 Best, Second-Best | Ranking 3 Combined | |
|---|---|---|---|---|
| 1 | F | F | → | F |
| 2 | A | I | → | A = D = I |
| 3 | D | D | → | |
| 4 | I | A | → | |
| 5 | E | E | → | E |
| 6 | C | K | → | K = C |
| 7 | K | C | → | |
| 8 | G | G | → | G |
| 9 | H | H | → | H |
| Rank | Ranking 1 Excellent | Ranking 2 Excellent, Good | Ranking 3 Excellent, Good, Satisfactory | Ranking 4 Combined | |
|---|---|---|---|---|---|
| 1 | I | I | → | I | |
| 2 | I, M | K | M | → | M |
| 3 | K | M | K | → | K |
| Rank | Ranking 1 Best | Ranking 2 Best, Second-Best | Ranking 3 Combined | |
|---|---|---|---|---|
| 1 | I | I | → | I |
| 2 | M | K | → | M = K |
| 3 | K | M | → |
| Patient No. | Age, Sex | Stage | FAB Type | Blast Phenotype (CD) | IC Blasts (%) | Source | Conducted Experiments |
|---|---|---|---|---|---|---|---|
| AML | |||||||
| 1172 | 24, m | dgn | p-M0 | 117,13,33,34 | 92 | MNC, WB | DCC |
| 993 | 71, m | dgn | p-M1 | 34,13,33,117 | 95 | MNC, WB | DCC |
| 1289 | 65, w | dgn | p-M1 | 34,33, 56,117 | 60 | WB | DCC |
| 1300 | 24, w | dgn | p-M1 | 34,13,33,65,117 | 75 | WB | DCC, CTX |
| 1190 | 72, m | dgn | p-M2 | 117,33,34 | 28 | MNC, WB | DCC |
| 1292 | 44, w | dgn | p-M2 | 117,13,33,34 | 78 | WB | DCC, CTX |
| 1295 | 40, m | dgn | p-M2 | 34,13,33,117 | 45 | WB | DCC |
| 1225 | 48, m | dgn | p-M3 | 117,13,33 | 77 | MNC, WB | DCC |
| 1327 | 61, m | dgn | p-M4 | 117,7,13,33,65 | 75 | WB | DCC |
| 1453 | 54, w | dgn | p-M4 | 15,14,33,64,56 | 52 | WB | DCC, CTX |
| 1459 | 54, m | dgn | p-M4 | 56,4,11c,11b,33,38,64 | 14 | WB | DCC, CTX |
| 1460 | 78, w | dgn | p-M4 | 15,34,117 | 68 | WB | DCC, CTX |
| 1430 | 79, m | dgn | p-M5 | 34,117,13,33 | 70 | WB | DCC, CTX |
| 1432 | 34, m | dgn | p-M5 | 34,13,33,64 | 81 | WB | DCC, CTX |
| 1447 | 21, m | dgn | p-M5 | 33,56,45 | 65 | WB | DCC, CTX |
| 1443 | 64, m | dgn | p-M? | 34,117,13,33 | 50 | WB | DCC |
| 1452 | 44, m | dgn | p-M? | 34,117,33,13,45 | 55 | WB | DCC, CTX |
| 1050 | 32, w | dgn | s-M1 | 117,14,13,33 | 90 | MNC, WB | DCC |
| 1144 | 32, m | dgn | s-M1 | 34,13,19,56,117 | 60 | MNC, WB | DCC |
| 1204 | 72, m | dgn | s-M2 | 117,13,56 | 43 | MNC, WB | DCC |
| 1131 | 53, w | dgn | s-M4 | 117,33 | 15 | WB | DCC |
| 1207 | 56, w | dgn | s-M4 | 117,4,13,15,33,34 | 10 | MNC, WB | DCC |
| 1442 | 73, w | dgn | s-M4 | 117,33,61,138 | 14 | WB | DCC, CTX |
| 1426 | 61, w | dgn | s-M5 | 34,117,13,33,64 | 93 | WB | DCC, CTX |
| 1439 | 61, w | dgn | s-M5 | 34,117,13,33 | 17 | WB | DCC |
| 999 | 51, w | dgn | s-M? | 34,13,33,65,117 | 14 | MNC, WB | DCC |
| 1056 | 27, m | dgn | s-M? | 117,13,15,33,34 | 42 | MNC, WB | DCC |
| 1165 | 59, m | dgn | s-M? | 34,13,33,117 | 46 | MNC, WB | DCC |
| 1194 | 72, m | dgn | s-M? | 34,33,117 | 18 | MNC, WB | DCC |
| 1196 | 39, w | dgn | s-M? | 117,33 | 11 | MNC, WB | DCC |
| 1226 | 69, m | dgn | s-M? | 117,33,34 | 65 | MNC, WB | DCC |
| 1385 | 82, m | dgn | s-M? | 34,13,56,117 | 23 | WB | DCC, CTX |
| 1434 | 61, w | dgn | s-M? | 34,117,7,13,33,56,64 | 61 | WB | DCC, CTX |
| 1449 | 78, m | dgn | s-M? | 15,65,4,45,64 | 62 | WB | DCC, CTX |
| 1454 | 60, w | dgn | s-M? | 34,117,20,61 | 33 | WB | DCC |
| 1024 | 39, m | pers | p-M2 | 34,33,117 | 88 | MNC, WB | DCC |
| 1218 | 54, m | pers | p-M2 | 33,56,13 | 30 | MNC, WB | DCC |
| 1201 | 60, w | pers | p-M5b | 34,4,13,14,15,33,56,6,5 | 63 | WB | DCC |
| 1320 | 66, m | pers | s-M6 | 34,65,117 | 15 | WB | DCC, CTX |
| 1310 | 77, w | pers | s-M? | 117,33,34,56 | 52 | WB | DCC |
| 1011 | 88, w | rel | p-M1 | 34,13,33,65,117 | 88 | MNC, WB | DCC |
| 1127 | 61, w | rel | p-M1 | 34,13,33,117 | 46 | MNC, WB | DCC |
| 1138 | 24, m | rel | p-M1 | 34,4,14,33,56,65,117 | 50 | MNC, WB | DCC |
| 1222 | 40, m | rel | p-M1 | 117,33,34,56 | 50 | MNC, WB | DCC |
| 1080 | 37, w | rel | p-M2 | 34,4,13,33,117 | 34 | MNC, WB | DCC |
| 1123 | 70, m | rel | p-M2 | 34,13,33,117 | 47 | MNC, WB | DCC |
| 1205 | 60, m | rel | p-M2 | 34,2,13,33,117 | 67 | MNC, WB | DCC |
| 1206 | 74, w | rel | p-M2 | 34,4,33,117 | 27 | MNC, WB | DCC |
| 1243 | 34, m | rel | p-M2 | 34,2,13,33,117 | 32 | MNC, WB | DCC |
| 1376 | 52, w | rel | p-M2 | 34,33,117 | 60 | WB | DCC, CTX |
| 1387 | 63, m | rel | p-M2 | 34,13,33,117 | 37 | WB | DCC, CTX |
| 1001 | 60, w | rel | p-M4 | 34,33,117 | 35 | MNC, WB | DCC |
| 1017 | 67, m | rel | p-M4 | 34,33,117 | 34 | MNC, WB | DCC |
| 1018 | 40, w | rel | p-M4 | 34,15,33,64,117 | 17 | MNC, WB | DCC |
| 1143 | 46, w | rel | p-M4 | 117,2,7,13,34,65 | 75 | MNC, WB | DCC |
| 998 | 67, m | rel | p-M5a | 34,4,64,117 | 92 | MNC, WB | DCC |
| 1263 | 40, m | rel | p-M5 | 34,19,33,117 | 18 | WB | DCC |
| 1375 | 44, w | rel | p-M5 | 34,15,33,117 | 35 | WB | DCC, CTX |
| 1303 | 64, w | rel | s-M4 | 34,4,13,33,14,56,65 | 65 | WB | DCC, CTX |
| 1286 | 21, m | rel | s-M5 | 117,33,34 | 35 | WB | DCC, CTX |
| 987 | 61, m | rel | s-M? | 34,13,33,117 | 21 | MNC, WB | DCC |
| 1183 | 36, w | rel | s-M? | 34,33,117 | 33 | MNC, WB | DCC |
| 1307 | 54, m | rel | s-M? | 117,13,15,19,33,34,65 | 30 | WB | DCC, CTX |
| 1386 | 57, m | rel | s-M? | 34,33,117 | 78 | WB | DCC, CTX |
| MDS | |||||||
| 1433 | 59, m | 34,117,14,33 | 14 | WB | DCC, CTX | ||
| 1306 | 42, m | 117,13,33,34 | 11 | WB | DCC, CTX | ||
| Cell Types | Cell Abbreviation | Surface Marker | Referred To | Abbreviation | Reference |
|---|---|---|---|---|---|
| Blasts | Bla | Bla+ (CD15+, CD34+, CD65+, CD117+) | MNC or WB WB | Bla/cells Bla/WB | [10] |
| Dendritic Cells | DC | DC+ (CD80+, CD83+, CD86+, CD206+, CD209+,) | MNC or WB WB | DC/cells DC/WB | [10] |
| Leukaemia Derived Dendritic Cells | DCleu | DC+Bla+ | MNC or WB WB DC | DCleu/cells DCleu/WB DCleu/DC | [10] |
| Mature Migratory Dendritic Cells | DCmig | DC+CCR7+ | DC | DCmig/DC | [12] |
| DC/DCleu-Protocol | DC/DCleu-Source | Composition (Total) and Time Added (d) | Culture Time (d) | References |
|---|---|---|---|---|
| Ca | MNC WB | Ca-Iono 375 ng/mL (d1) IL-4 250 U/mL (d1) | 3–4 | [11,19] |
| Mcm | MNC WB | GM-CSF 800 U/mL (d1, d4-5) TNFa 200 U/mL (d7-8) PGE2 1 μg/mL (d7-8) IL-1b 5 ng/mL (d7-8) IL-4 500 U/mL (d1, d4-5) IL-6 150 ng/mL (d7-8) FL 40 ng/mL (d, d4-5) | 10–14 | [11,19] |
| Pici | MNC WB | GM-CSF 500 U/mL (d1) OK-432 10 μg/mL (d7-8) IL-4 250 U/mL (d1) | 9–11 | [11] |
| Kit-A | WB | GM-CSF 800 U/mL (d1, d2-3) TNFa 200 U/mL (d1, d2-3) | 7–10 | European Patent EP 3217975 B1 |
| Kit-C | WB | GM-CSF 800 U/mL (d1, d2-3) TNFa 200 U/mL (d, d2-3) PGE2 1 µg/mL (d1, d2-3) | 7–10 | |
| Kit-D | WB | GM-CSF 800 U/mL (d1, d2-3) OK-432 10 µg/mL (d1, d2-3) PGE2 1 µg/mL (d1, d2-3) | 7–10 | |
| Kit-E | WB | GM-CSF 800 U/mL (d1, d2-4, d7-8) IFNa 500 U/mL (d1, d2-4, d7-8) | 8–11 | [19] |
| Kit-F | WB | Ca-Iono 250 ng/mL (d1, d2-3) GM-CSF 800 U/mL (d1, d2-3) | 3–4 | European Patent EP 3217975 B1 |
| Kit-G | WB | GM-CSF 800 U/mL (d1, d2-3) | 7–10 | |
| Kit-H | WB | IFNa 500 U/mL (d1, d2-4, d7-8) | 7–10 | [19] |
| Kit-I | WB | GM-CSF 800 U/mL (d1, d2-3) OK-432 10 μg/mL (d1, d2-3) | 7–10 | European Patent EP 3217975 B1 US Patent US 10,912,820 |
| Kit-K | WB | GM-CSF 800 U/mL (d1, d2-3) PGE2 1 μg/mL (d1, d2-3) | 7–10 | European Patent EP 3217975 B1 |
| Kit-M | WB | GM-CSF 800 U/mL (d1, d2-3) PGE1 1 μg/mL (d1, d2-3) | 7–10 | European Patent EP 3217975 B1 US Patent US 10,912,820 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwepcke, C.; Klauer, L.K.; Deen, D.; Amberger, D.C.; Fischer, Z.; Doraneh-Gard, F.; Gunsilius, C.; Hirn-Lopez, A.; Kroell, T.; Tischer, J.; et al. Generation of Leukaemia-Derived Dendritic Cells (DCleu) to Improve Anti-Leukaemic Activity in AML: Selection of the Most Efficient Response Modifier Combinations. Int. J. Mol. Sci. 2022, 23, 8333. https://doi.org/10.3390/ijms23158333
Schwepcke C, Klauer LK, Deen D, Amberger DC, Fischer Z, Doraneh-Gard F, Gunsilius C, Hirn-Lopez A, Kroell T, Tischer J, et al. Generation of Leukaemia-Derived Dendritic Cells (DCleu) to Improve Anti-Leukaemic Activity in AML: Selection of the Most Efficient Response Modifier Combinations. International Journal of Molecular Sciences. 2022; 23(15):8333. https://doi.org/10.3390/ijms23158333
Chicago/Turabian StyleSchwepcke, Christoph, Lara Kristina Klauer, Diana Deen, Daniel Christoph Amberger, Zuzana Fischer, Fatemeh Doraneh-Gard, Carina Gunsilius, Annika Hirn-Lopez, Tanja Kroell, Johanna Tischer, and et al. 2022. "Generation of Leukaemia-Derived Dendritic Cells (DCleu) to Improve Anti-Leukaemic Activity in AML: Selection of the Most Efficient Response Modifier Combinations" International Journal of Molecular Sciences 23, no. 15: 8333. https://doi.org/10.3390/ijms23158333
APA StyleSchwepcke, C., Klauer, L. K., Deen, D., Amberger, D. C., Fischer, Z., Doraneh-Gard, F., Gunsilius, C., Hirn-Lopez, A., Kroell, T., Tischer, J., Weinmann, M., Werner, J.-O., Rank, A., Schmid, C., & Schmetzer, H. M. (2022). Generation of Leukaemia-Derived Dendritic Cells (DCleu) to Improve Anti-Leukaemic Activity in AML: Selection of the Most Efficient Response Modifier Combinations. International Journal of Molecular Sciences, 23(15), 8333. https://doi.org/10.3390/ijms23158333






