The Acute Effects of Leptin on the Contractility of Isolated Rat Atrial and Ventricular Cardiomyocytes
Abstract
1. Introduction
2. Results
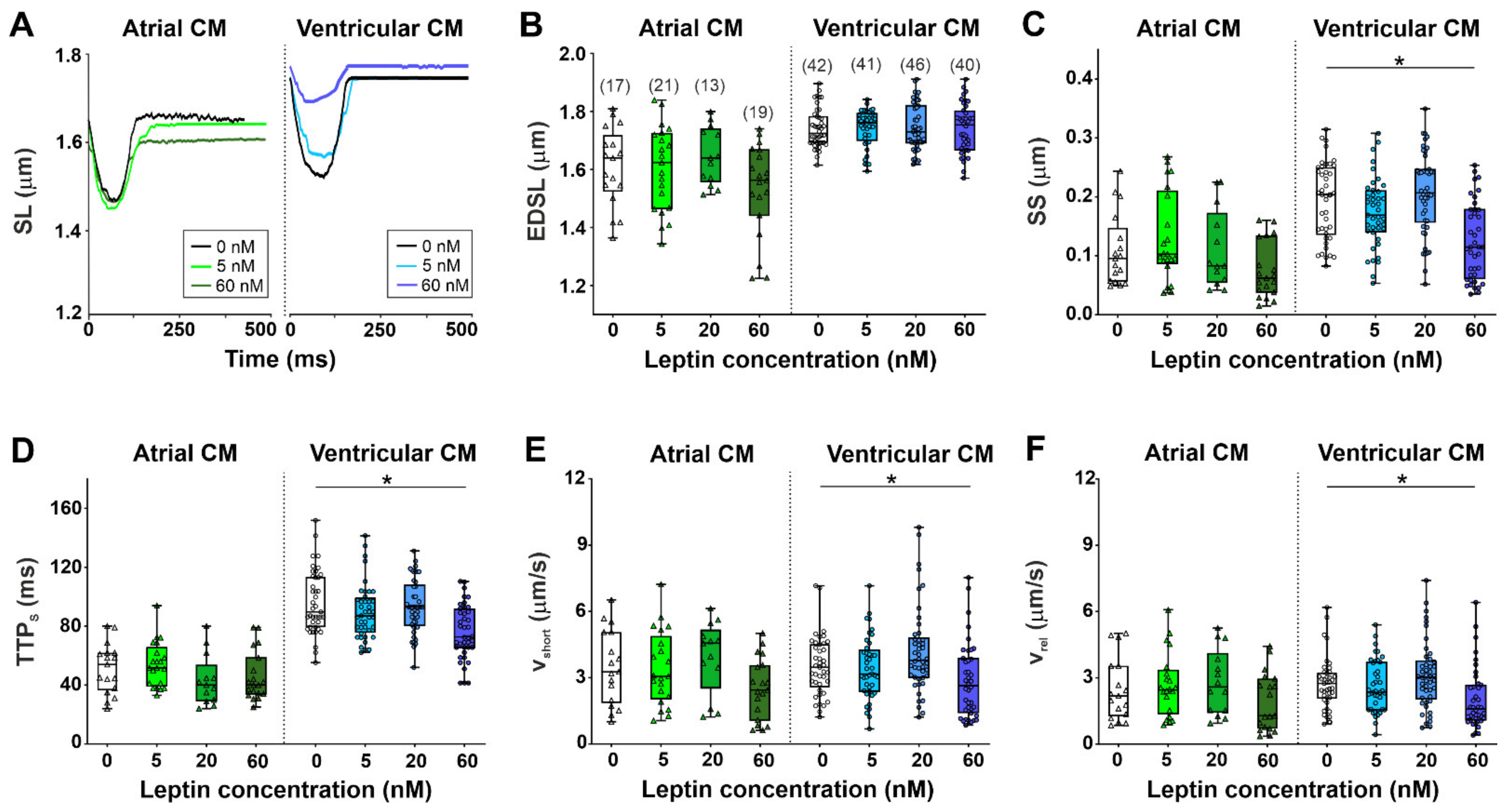
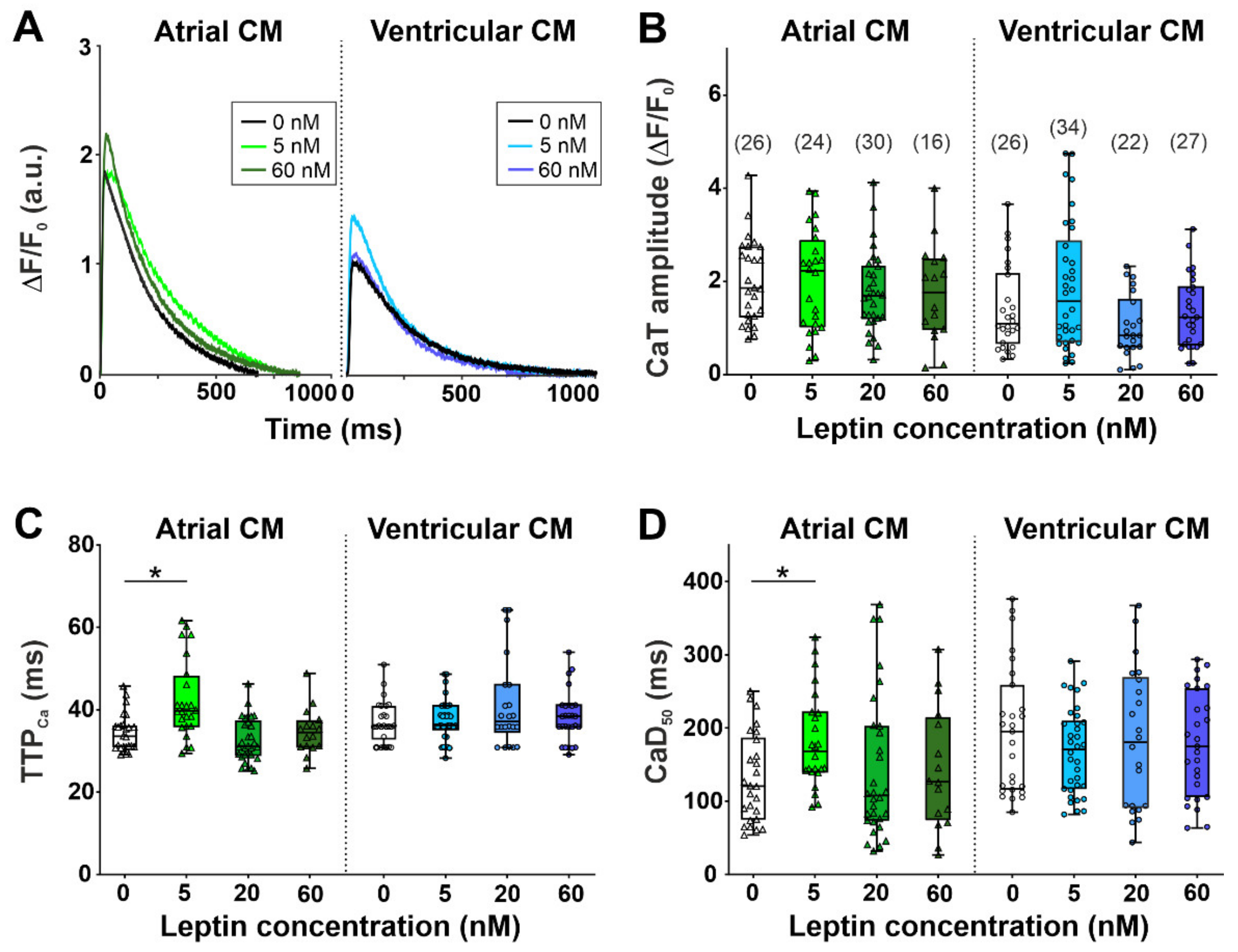
2.1. The Effects of Leptin on Sarcomere Dynamics and [Ca2+]i Transients in Single Atrial and Ventricular Cardiomyocytes
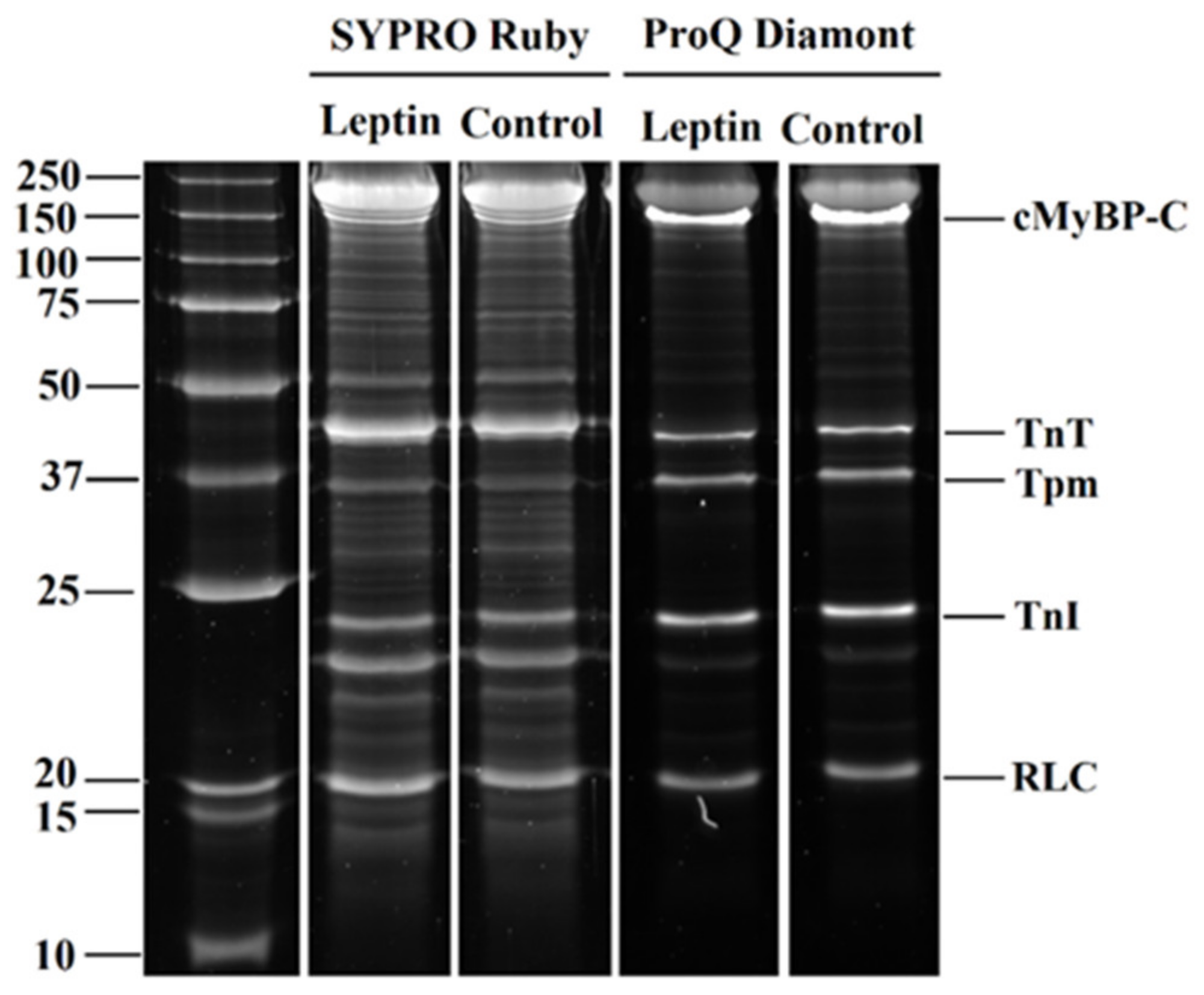
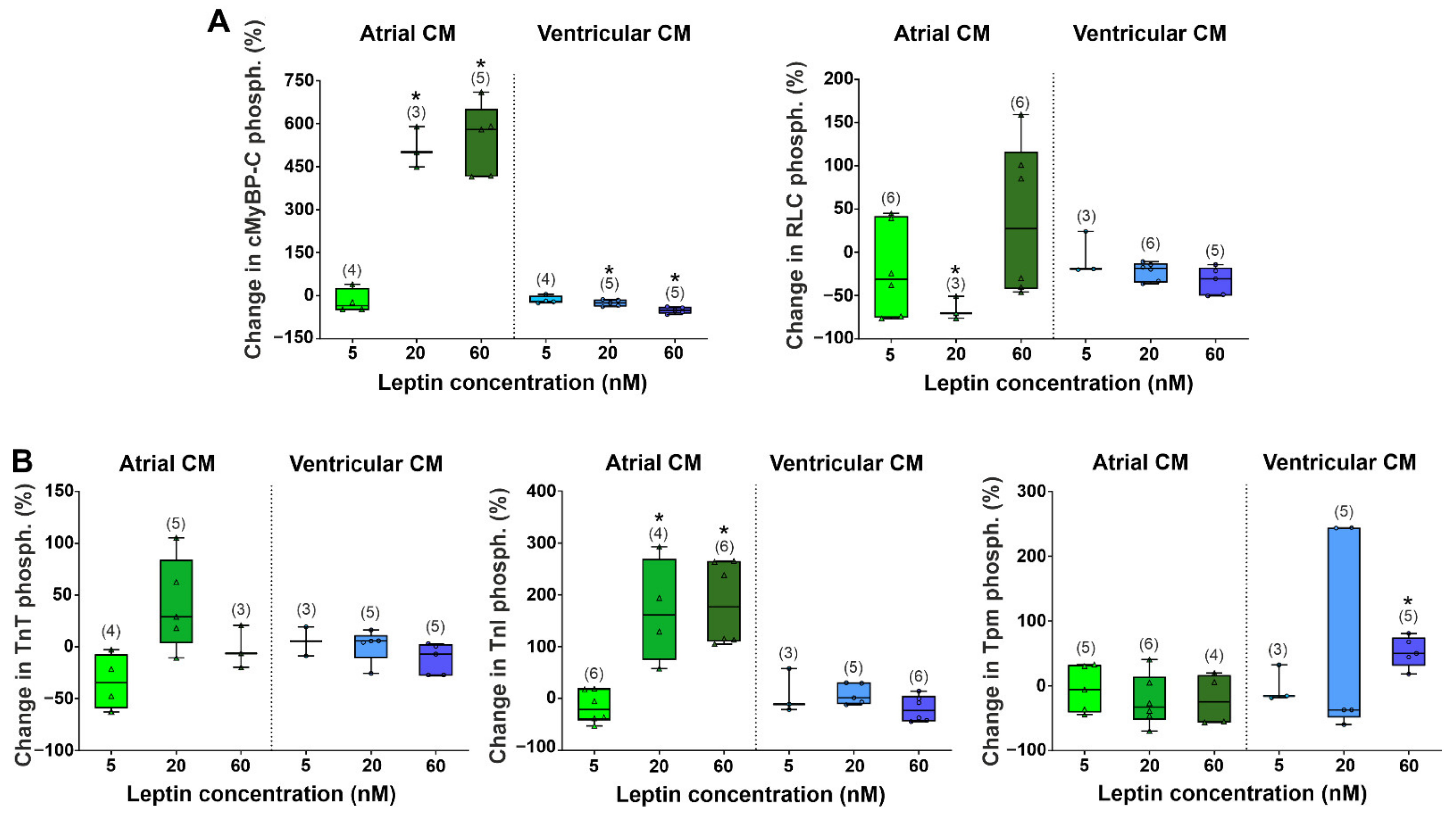
2.2. The Effects of Leptin on Actin-Myosin Interaction and Sarcomeric Protein Phosphorylation in Atrial and Ventricular Cardiomyocytes
3. Discussion
3.1. Effects of Leptin on the Contractility of Single Ventricular Cardiomyocytes
3.2. Effects of Leptin on the Contractility of Single Atrial Cardiomyocytes
4. Materials and Methods
4.1. Isolation of Rat Atrial and Ventricular Cardiomyocytes
4.2. Experimental Protocol of Leptin Treatment
4.3. Measurements of Sarcomere Dynamics
4.4. Measurements of [Ca2+]i Transients
4.5. Protein Preparation and Phosphorylation Analysis
4.6. In Vitro Motility Assay
4.7. Statistical Analysis
5. Summary
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Feijóo-Bandín, S.; Portolés, M.; Roselló-Lletí, E.; Rivera, M.; González-Juanatey, J.; Lago, F. 20 years of leptin: Role of leptin in cardiomyocyte physiology and physiopathology. Life Sci. 2015, 140, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, C.; Azrak, Z.; Hanache, S.; Abou-Kheir, W.; Zeidan, A. Differential role of leptin and adiponectin in cardiovascular system. Int. J. Endocrinol. 2015, 2015, 534320. [Google Scholar] [CrossRef] [PubMed]
- Bravo, P.E.; Morse, S.; Borne, D.M.; Aguilar, E.A.; Reisin, E. Leptin and hypertension in obesity. Vasc. Health Risk Manag. 2006, 2, 163. [Google Scholar] [CrossRef] [PubMed]
- Leifheit-Nestler, M.; Wagner, N.-M.; Gogiraju, R.; Didié, M.; Konstantinides, S.; Hasenfuss, G.; Schäfer, K. Importance of leptin signaling and signal transducer and activator of transcription-3 activation in mediating the cardiac hypertrophy associated with obesity. J. Transl. Med. 2013, 11, 170. [Google Scholar] [CrossRef]
- Kain, D.; Simon, A.J.; Greenberg, A.; Ben Zvi, D.; Gilburd, B.; Schneiderman, J. Cardiac leptin overexpression in the context of acute MI and reperfusion potentiates myocardial remodeling and left ventricular dysfunction. PLoS ONE 2018, 13, e0203902. [Google Scholar] [CrossRef]
- Fonfara, S.; Kitz, S.; Hetzel, U.; Kipar, A. Myocardial leptin transcription in feline hypertrophic cardiomyopathy. Res. Vet. Sci. 2017, 112, 105–108. [Google Scholar] [CrossRef]
- Barbosa-Ferreira, J.M.; Fernandes, F.; Dabarian, A.; Mady, C. Leptin in heart failure. Expert Opin. Med. Diagn. 2013, 7, 113–117. [Google Scholar] [CrossRef]
- Zeidan, A.; Purdham, D.M.; Rajapurohitam, V.; Javadov, S.; Chakrabarti, S.; Karmazyn, M. Leptin induces vascular smooth muscle cell hypertrophy through angiotensin II-and endothelin-1-dependent mechanisms and mediates stretch-induced hypertrophy. J. Pharmacol. Exp. Ther. 2005, 315, 1075–1084. [Google Scholar] [CrossRef]
- Matsui, H.; Motooka, M.; Koike, H.; Inoue, M.; Iwasaki, T.; Suzuki, T.; Kurabayashi, M.; Yokoyama, T. Ischemia/reperfusion in rat heart induces leptin and leptin receptor gene expression. Life Sci. 2007, 80, 672–680. [Google Scholar] [CrossRef]
- Rajapurohitam, V.; Gan, X.T.; Kirshenbaum, L.A.; Karmazyn, M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ. Res. 2003, 93, 277–279. [Google Scholar] [CrossRef]
- Purdham, D.M.; Zou, M.-X.; Rajapurohitam, V.; Karmazyn, M. Rat heart is a site of leptin production and action. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2877–H2884. [Google Scholar] [CrossRef]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of leptin in cardiovascular diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef]
- Nickola, M.W.; Wold, L.E.; Colligan, P.B.; Wang, G.-J.; Samson, W.K.; Ren, J. Leptin attenuates cardiac contraction in rat ventricular myocytes: Role of NO. Hypertension 2000, 36, 501–505. [Google Scholar] [CrossRef][Green Version]
- Hintz, K.; Aberle, N.; Ren, J. Insulin resistance induces hyperleptinemia, cardiac contractile dysfunction but not cardiac leptin resistance in ventricular myocytes. Int. J. Obes. 2003, 27, 1196–1203. [Google Scholar] [CrossRef]
- Ren, J.; Relling, D.P. Leptin-induced suppression of cardiomyocyte contraction is amplified by ceramide. Peptides 2006, 27, 1415–1419. [Google Scholar] [CrossRef]
- Dong, F.; Zhang, X.; Yang, X.; Esberg, L.; Yang, H.; Zhang, Z.; Culver, B.; Ren, J. Impaired cardiac contractile function in ventricular myocytes from leptin-deficient ob/ob obese mice. J. Endocrinol. 2006, 188, 25–36. [Google Scholar] [CrossRef]
- Purdham, D.M.; Rajapurohitam, V.; Zeidan, A.; Huang, C.; Gross, G.J.; Karmazyn, M. A neutralizing leptin receptor antibody mitigates hypertrophy and hemodynamic dysfunction in the postinfarcted rat heart. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H441–H446. [Google Scholar] [CrossRef]
- Hall, M.E.; Smith, G.; Hall, J.E.; Stec, D.E. Cardiomyocyte-specific deletion of leptin receptors causes lethal heart failure in Cre-recombinase-mediated cardiotoxicity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 303, R1241–R1250. [Google Scholar] [CrossRef][Green Version]
- Hou, N.; Luo, J.D. Leptin and cardiovascular diseases. Clin. Exp. Pharmacol. Physiol. 2011, 38, 905–913. [Google Scholar] [CrossRef]
- Lee, Y.; Naseem, R.H.; Duplomb, L.; Park, B.-H.; Garry, D.J.; Richardson, J.A.; Schaffer, J.E.; Unger, R.H. Hyperleptinemia prevents lipotoxic cardiomyopathy in acyl CoA synthase transgenic mice. Proc. Natl. Acad. Sci. USA 2004, 101, 13624–13629. [Google Scholar] [CrossRef]
- Gómez-Hurtado, N.; Domínguez-Rodríguez, A.; Mateo, P.; Fernández-Velasco, M.; Val-Blasco, A.; Aizpún, R.; Sabourin, J.; Gómez, A.M.; Benitah, J.P.; Delgado, C. Beneficial effects of leptin treatment in a setting of cardiac dysfunction induced by transverse aortic constriction in mouse. J. Physiol. 2017, 595, 4227–4243. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhang, X.; Ren, J. Leptin regulates cardiomyocyte contractile function through endothelin-1 receptor–NADPH oxidase pathway. Hypertension 2006, 47, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Wold, L.E.; Relling, D.P.; Duan, J.; Norby, F.L.; Ren, J. Abrogated leptin-induced cardiac contractile response in ventricular myocytes under spontaneous hypertension: Role of Jak/STAT pathway. Hypertension 2002, 39, 69–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rahmouni, K.; Haynes, W.G. Leptin and the central neural mechanisms of obesity hypertension. Drugs Today 2002, 38, 807–817. [Google Scholar] [CrossRef]
- Barouch, L.A.; Berkowitz, D.E.; Harrison, R.W.; O’Donnell, C.P.; Hare, J.M. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation 2003, 108, 754–759. [Google Scholar] [CrossRef]
- Lüss, I.; Boknik, P.; Jones, L.R.; Kirchhefer, U.; Knapp, J.; Linck, B.; Lüss, H.; Meissner, A.; Müller, F.U.; Schmitz, W. Expression of cardiac calcium regulatory proteins in atrium v ventricle in different species. J. Mol. Cell. Cardiol. 1999, 31, 1299–1314. [Google Scholar] [CrossRef]
- Bootman, M.D.; Higazi, D.R.; Coombes, S.; Roderick, H.L. Calcium signalling during excitation-contraction coupling in mammalian atrial myocytes. J. Cell Sci. 2006, 119, 3915–3925. [Google Scholar] [CrossRef]
- Khokhlova, A.; Myachina, T.; Butova, X.; Volzhaninov, D.; Berg, V.; Kochurova, A.; Kuznetsov, D.; Mukhlynina, E.; Kopylova, G.; Shchepkin, D. Differing effects of estrogen deficiency on the contractile function of atrial and ventricular myocardium. Biochem. Biophys. Res. Commun. 2021, 541, 30–35. [Google Scholar] [CrossRef]
- Koss, K.L.; Ponniah, S.; Jones, W.K.; Grupp, I.L.; Kranias, E.G. Differential phospholamban gene expression in murine cardiac compartments: Molecular and physiological analyses. Circ. Res. 1995, 77, 342–353. [Google Scholar] [CrossRef]
- Walden, A.; Dibb, K.; Trafford, A. Differences in intracellular calcium homeostasis between atrial and ventricular myocytes. J. Mol. Cell. Cardiol. 2009, 46, 463–473. [Google Scholar] [CrossRef]
- Minajeva, A.; Kaasik, A.; Paju, K.; Seppet, E.; Lompré, A.-M.; Veksler, V.; Ventura-Clapier, R. Sarcoplasmic reticulum function in determining atrioventricular contractile differences in rat heart. Am. J. Physiol. Heart Circ. Physiol. 1997, 273, H2498–H2507. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Govindan, S.; Zhang, M.; Khairallah, R.J.; Martin, J.L.; Sadayappan, S.; de Tombe, P.P. Cardiac myosin-binding protein C and troponin-I phosphorylation independently modulate myofilament length-dependent activation. J. Biol. Chem. 2015, 290, 29241–29249. [Google Scholar] [CrossRef] [PubMed]
- Hanft, L.M.; Fitzsimons, D.P.; Hacker, T.A.; Moss, R.L.; McDonald, K.S. Cardiac MyBP-C phosphorylation regulates the Frank–Starling relationship in murine hearts. J. Gen. Physiol. 2021, 153, e202012770. [Google Scholar] [CrossRef] [PubMed]
- El-Armouche, A.; Pohlmann, L.; Schlossarek, S.; Starbatty, J.; Yeh, Y.-H.; Nattel, S.; Dobrev, D.; Eschenhagen, T.; Carrier, L. Decreased phosphorylation levels of cardiac myosin-binding protein-C in human and experimental heart failure. J. Mol. Cell. Cardiol. 2007, 43, 223–229. [Google Scholar] [CrossRef]
- Kumar, M.; Haghighi, K.; Kranias, E.G.; Sadayappan, S. Phosphorylation of cardiac myosin–binding protein-C contributes to calcium homeostasis. J. Biol. Chem. 2020, 295, 11275–11291. [Google Scholar] [CrossRef]
- Rao, V.S.; Clobes, A.M.; Guilford, W.H. Force spectroscopy reveals multiple “closed states” of the muscle thin filament. J. Biol. Chem. 2011, 286, 24135–24141. [Google Scholar] [CrossRef]
- Nixon, B.R.; Liu, B.; Scellini, B.; Tesi, C.; Piroddi, N.; Ogut, O.; Solaro, R.J.; Ziolo, M.T.; Janssen, P.M.; Davis, J.P. Tropomyosin Ser-283 pseudo-phosphorylation slows myofibril relaxation. Arch. Biochem. Biophys. 2013, 535, 30–38. [Google Scholar] [CrossRef]
- Rajan, S.; Jagatheesan, G.; Petrashevskaya, N.; Biesiadecki, B.J.; Warren, C.M.; Riddle, T.; Liggett, S.; Wolska, B.M.; Solaro, R.J.; Wieczorek, D.F. Tropomyosin pseudo-phosphorylation results in dilated cardiomyopathy. J. Biol. Chem. 2019, 294, 2913–5835. [Google Scholar] [CrossRef]
- Imerbtham, T.; Thitiwuthikiat, P.; Jongjitwimol, J.; Nuamchit, T.; Yingchoncharoen, T.; Siriwittayawan, D. Leptin levels are associated with subclinical cardiac dysfunction in obese adolescents. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 925. [Google Scholar] [CrossRef]
- Gruber, T.; Pan, C.; Contreras, R.E.; Wiedemann, T.; Morgan, D.A.; Skowronski, A.A.; Lefort, S.; Murat, C.D.B.; Le Thuc, O.; Legutko, B. Obesity-associated hyperleptinemia alters the gliovascular interface of the hypothalamus to promote hypertension. Cell Metab. 2021, 33, 1155–1170.e1110. [Google Scholar] [CrossRef]
- Polyakova, E.A.; Mikhaylov, E.N.; Galagudza, M.M.; Shlyakhto, E.V. Hyperleptinemia results in systemic inflammation and the exacerbation of ischemia-reperfusion myocardial injury. Heliyon 2021, 7, e08491. [Google Scholar] [CrossRef]
- Kamareddine, L.; Ghantous, C.M.; Allouch, S.; Al-Ashmar, S.A.; Anlar, G.; Kannan, S.; Djouhri, L.; Korashy, H.M.; Agouni, A.; Zeidan, A. Between inflammation and autophagy: The role of leptin-adiponectin axis in cardiac remodeling. J. Inflamm. Res. 2021, 14, 5349. [Google Scholar] [CrossRef]
- Larson, J.; Rainer, P.; Watts, V.; Yang, R.; Miller, K.; Phan, A.; Barouch, L. Dependence of β3-adrenergic signaling on the adipokine leptin in cardiac myocytes. Int. J. Obes. 2012, 36, 876–879. [Google Scholar] [CrossRef]
- Steinberg, S.F. Oxidative stress and sarcomeric proteins. Circ. Res. 2013, 112, 393–405. [Google Scholar] [CrossRef]
- Cuello, F.; Wittig, I.; Lorenz, K.; Eaton, P. Oxidation of cardiac myofilament proteins: Priming for dysfunction? Mol. Asp. Med. 2018, 63, 47–58. [Google Scholar] [CrossRef]
- Dellas, C.; Schäfer, K.; Rohm, I.K.; Lankeit, M.; Leifheit, M.; Loskutoff, D.J.; Hasenfuss, G.; Konstantinides, S.V. Leptin signalling and leptin-mediated activation of human platelets: Importance of JAK2 and the phospholipases Cγ2 and A2. Thromb. Haemost. 2007, 98, 1063–1071. [Google Scholar] [CrossRef]
- Nanjappa, V.; Raju, R.; Muthusamy, B.; Sharma, J.; Thomas, J.K.; Nidhina, P.; Harsha, H.; Pandey, A.; Anilkumar, G.; Prasad, T.K. A comprehensive curated reaction map of leptin signaling pathway. J. Proteom. Bioinform. 2011, 4, 181–189. [Google Scholar] [CrossRef]
- Layland, J.; Cave, A.C.; Warren, C.; Grieve, D.J.; Sparks, E.; Kentish, J.C.; Solaro, R.J.; Shah, A.M. Protection against endotoxemia-induced contractile dysfunction in mice with cardiac-specific expression of slow skeletal troponin I. FASEB J. 2005, 19, 1137–1139. [Google Scholar] [CrossRef]
- Messer, A.E.; Gallon, C.E.; McKenna, W.J.; Dos Remedios, C.G.; Marston, S.B. The use of phosphate-affinity SDS-PAGE to measure the cardiac troponin I phosphorylation site distribution in human heart muscle. PROTEOMICS Clin. Appl. 2009, 3, 1371–1382. [Google Scholar] [CrossRef]
- Barefield, D.; Sadayappan, S. Phosphorylation and function of cardiac myosin binding protein-C in health and disease. J. Mol. Cell. Cardiol. 2010, 48, 866–875. [Google Scholar] [CrossRef]
- Hanft, L.M.; Cornell, T.D.; McDonald, C.A.; Rovetto, M.J.; Emter, C.A.; McDonald, K.S. Molecule specific effects of PKA-mediated phosphorylation on rat isolated heart and cardiac myofibrillar function. Arch. Biochem. Biophys. 2016, 601, 22–31. [Google Scholar] [CrossRef][Green Version]
- Brandenburg, S.; Arakel, E.C.; Schwappach, B.; Lehnart, S.E. The molecular and functional identities of atrial cardiomyocytes in health and disease. Biochim. Biophys. Acta BBA Mol. Cell Res. 2016, 1863, 1882–1893. [Google Scholar] [CrossRef]
- Lin, Y.-K.; Chen, Y.-C.; Huang, J.-H.; Lin, Y.-J.; Huang, S.-S.; Chen, S.-A.; Chen, Y.-J. Leptin modulates electrophysiological characteristics and isoproterenol-induced arrhythmogenesis in atrial myocytes. J. Biomed. Sci. 2013, 20, 94. [Google Scholar] [CrossRef]
- Fukui, A.; Takahashi, N.; Nakada, C.; Masaki, T.; Kume, O.; Shinohara, T.; Teshima, Y.; Hara, M.; Saikawa, T. Role of leptin signaling in the pathogenesis of angiotensin II–mediated atrial fibrosis and fibrillation. Circ. Arrhythmia Electrophysiol. 2013, 6, 402–409. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, M.; Wang, M.; Wang, Z.; Wang, S.; Hu, H.; Ma, K.; Jiang, H. Association between adiponectin-to-leptin ratio and heart rate variability in new-onset paroxysmal atrial fibrillation: A retrospective cohort study. Ann. Noninvasive Electrocardiol. 2022, 27, e12896. [Google Scholar] [CrossRef]
- Butova, X.; Myachina, T.; Khokhlova, A. A combined Langendorff-injection technique for simultaneous isolation of single cardiomyocytes from atria and ventricles of the rat heart. MethodsX 2021, 8, 101189. [Google Scholar] [CrossRef]
- Eiden, S.; Preibisch, G.; Schmidt, I. Leptin responsiveness of juvenile rats: Proof of leptin function within the physiological range. J. Physiol. 2001, 530, 131–139. [Google Scholar] [CrossRef]
- Khokhlova, A.; Myachina, T.; Volzhaninov, D.; Butova, X.; Kochurova, A.; Berg, V.; Gette, I.; Moroz, G.; Klinova, S.; Minigalieva, I. Type 1 Diabetes Impairs Cardiomyocyte Contractility in the Left and Right Ventricular Free Walls but Preserves It in the Interventricular Septum. Int. J. Mol. Sci. 2022, 23, 1719. [Google Scholar] [CrossRef]
- Myachina, T.; Butova, K.; Lookin, O. Development and program implementation of an algorithm to estimate the mean sarcomere length of a cardiomyocyte. Biophysics 2019, 64, 732–737. [Google Scholar] [CrossRef]
- Lookin, O.; Khokhlova, A.; Myachina, T.; Butova, X.; Cazorla, O.; de Tombe, P. Contractile State Dependent Sarcomere Length Variability in Isolated Guinea-Pig Cardiomyocytes. Front. Physiol. 2022, 13, 584. [Google Scholar] [CrossRef]
- Margossian, S.S.; Lowey, S. Preparation of Myosin and Its Subfragments from Rabbit Skeletal Muscle. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1982; Volume 85, pp. 55–71. [Google Scholar]
- Pardee, J.D.; Aspudich, J. Purification of Muscle Actin. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1982; Volume 85, pp. 164–181. [Google Scholar]
- Potter, J.D. Preparation of Troponin and Its Subnits. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1982; Volume 85, pp. 241–263. [Google Scholar]
- Matyushenko, A.M.; Shchepkin, D.V.; Kopylova, G.V.; Popruga, K.E.; Artemova, N.V.; Pivovarova, A.V.; Bershitsky, S.Y.; Levitsky, D.I. Structural and functional effects of cardiomyopathy-causing mutations in the troponin T-binding region of cardiac tropomyosin. Biochemistry 2017, 56, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Mashanov, G.; Molloy, J. Automatic detection of single fluorophores in live cells. Biophys. J. 2007, 92, 2199–2211. [Google Scholar] [CrossRef] [PubMed]
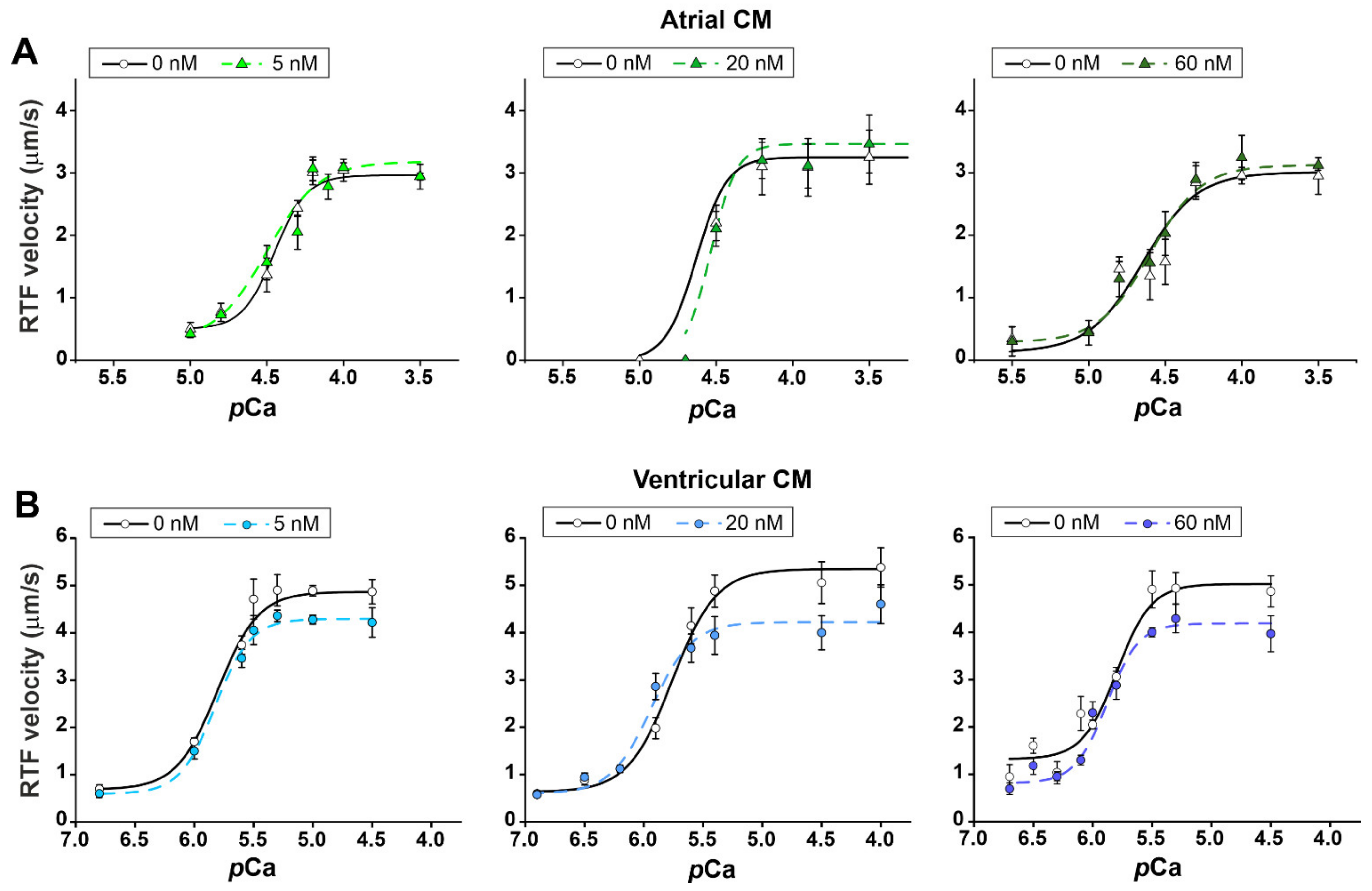
| Origin of Myosin | Leptin Concentration (nM) | vmax (µm/s) | v0 (µm/s) | pCa50 |
|---|---|---|---|---|
| Atrial CM | 0 | 3.0 ± 0.1 | 0.5 ± 0.1 | 4.45 ± 0.04 |
| 5 | 3.2 ± 0.2 | 0.3 ± 0.2 | 4.50 ± 0.06 | |
| 0 | 3.3 ± 0.1 | 0 | 4.63 ± 0.03 | |
| 20 | 3.5 ± 0.1 | 0 | 4.54 ± 0.05 | |
| 0 | 3.0 ± 0.3 | 0.1 ± 0.1 | 4.64± 0.10 | |
| 60 | 3.1 ± 0.1 | 0.3 ± 0.2 | 4.60 ± 0.04 | |
| Ventricular CM | 0 | 4.9 ± 0.1 | 0.7 ± 0.1 | 5.80 ± 0.03 |
| 5 | 4.3 ± 0.1 * | 0.6 ± 0.1 | 5.82 ± 0.06 | |
| 0 | 5.3 ± 0.2 | 0.6 ± 0.1 | 5.76 ± 0.06 | |
| 20 | 4.2 ± 0.2 * | 0.6 ± 0.1 | 5.92 ± 0.08 | |
| 0 | 5.0 ± 0.2 | 1.3 ± 0.2 | 5.80 ± 0.06 | |
| 60 | 4.2 ± 0.2 * | 0.8 ± 0.1 | 5.88 ± 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khokhlova, A.; Myachina, T.; Butova, X.; Kochurova, A.; Polyakova, E.; Galagudza, M.; Solovyova, O.; Kopylova, G.; Shchepkin, D. The Acute Effects of Leptin on the Contractility of Isolated Rat Atrial and Ventricular Cardiomyocytes. Int. J. Mol. Sci. 2022, 23, 8356. https://doi.org/10.3390/ijms23158356
Khokhlova A, Myachina T, Butova X, Kochurova A, Polyakova E, Galagudza M, Solovyova O, Kopylova G, Shchepkin D. The Acute Effects of Leptin on the Contractility of Isolated Rat Atrial and Ventricular Cardiomyocytes. International Journal of Molecular Sciences. 2022; 23(15):8356. https://doi.org/10.3390/ijms23158356
Chicago/Turabian StyleKhokhlova, Anastasia, Tatiana Myachina, Xenia Butova, Anastasia Kochurova, Ekaterina Polyakova, Michael Galagudza, Olga Solovyova, Galina Kopylova, and Daniil Shchepkin. 2022. "The Acute Effects of Leptin on the Contractility of Isolated Rat Atrial and Ventricular Cardiomyocytes" International Journal of Molecular Sciences 23, no. 15: 8356. https://doi.org/10.3390/ijms23158356
APA StyleKhokhlova, A., Myachina, T., Butova, X., Kochurova, A., Polyakova, E., Galagudza, M., Solovyova, O., Kopylova, G., & Shchepkin, D. (2022). The Acute Effects of Leptin on the Contractility of Isolated Rat Atrial and Ventricular Cardiomyocytes. International Journal of Molecular Sciences, 23(15), 8356. https://doi.org/10.3390/ijms23158356







