Niacin Improves Intestinal Health through Up-Regulation of AQPs Expression Induced by GPR109A
Abstract
:1. Introduction
2. Results
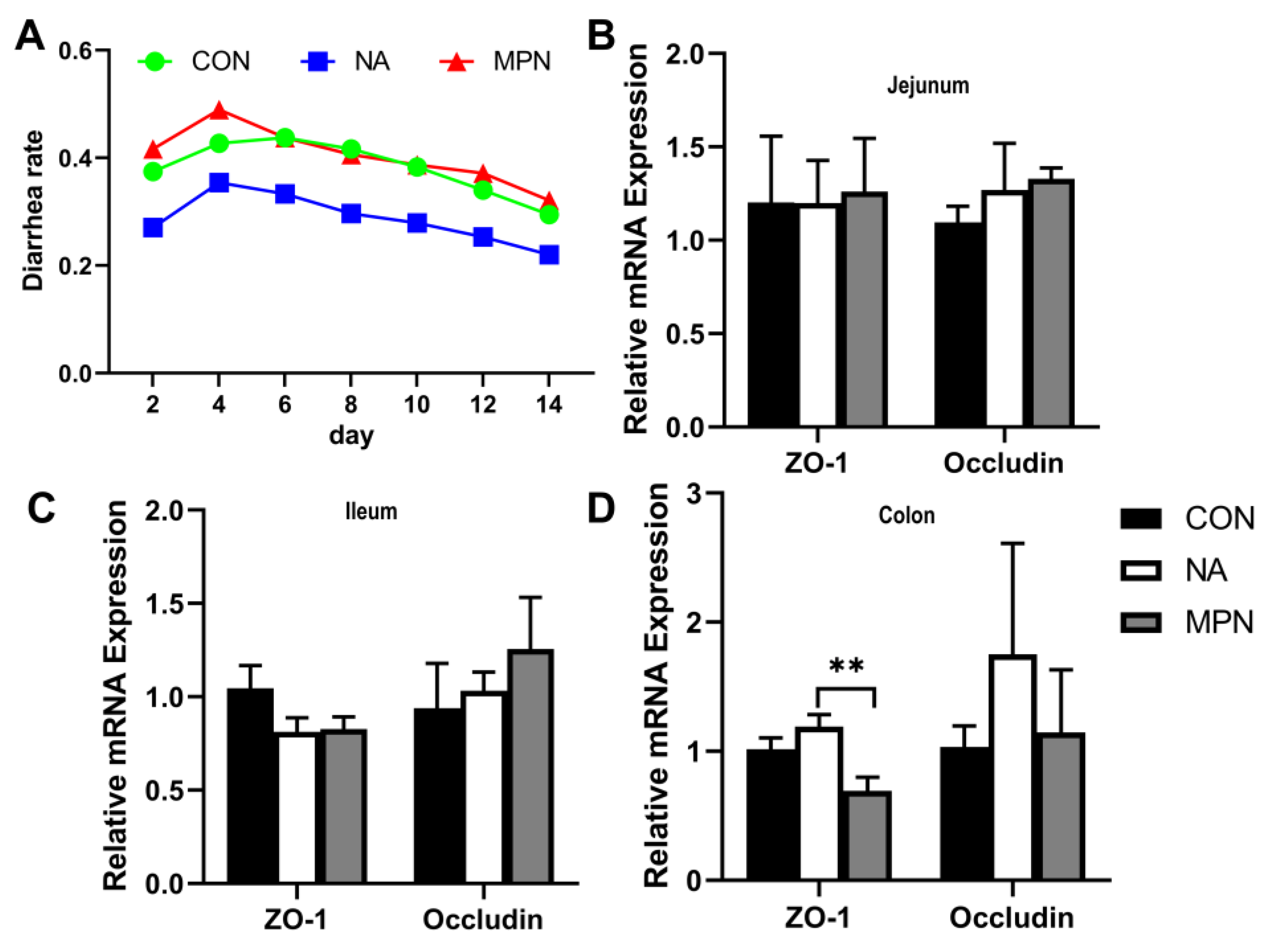
2.1. Effect of Niacin on Intestinal Barrier Function in Weaned Piglet
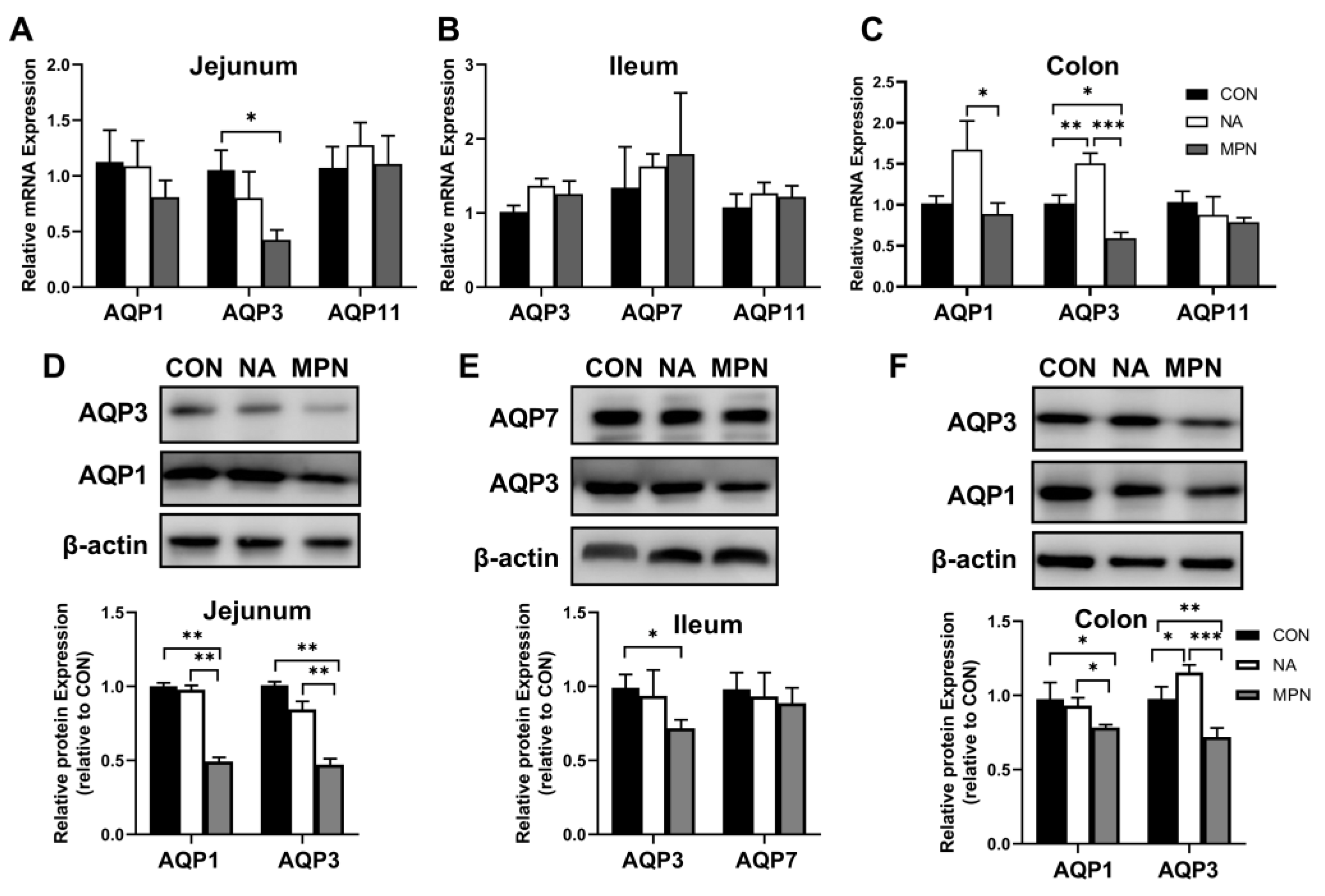
2.2. Effect of Niacin on Intestinal Water Transport in Weaned Piglets
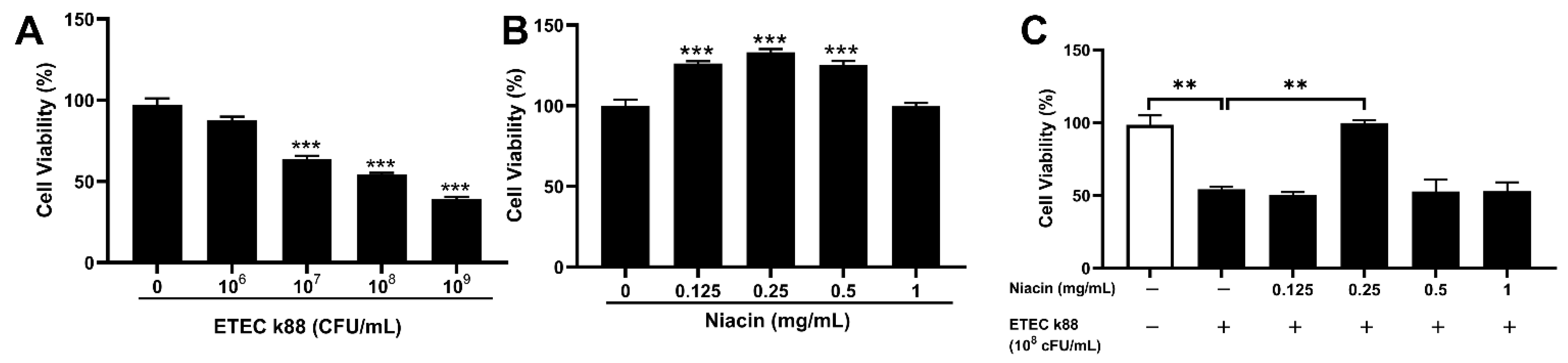
2.3. Effect of Niacin on Cell Viability in ETEC-Challenged in IPEC-J2 Cells
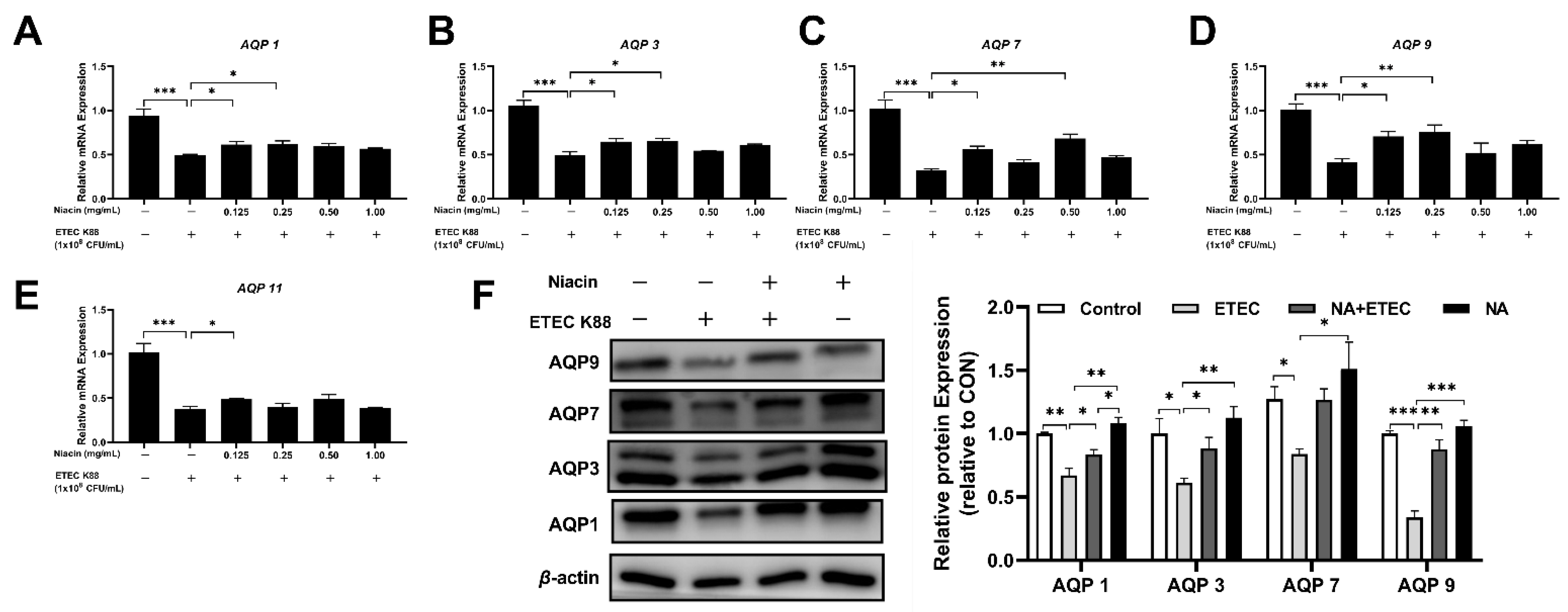
2.4. Effect of Niacin on the mRNA and Protein Expression of Water Transport Proteins in IPEC-J2 Cells
2.5. Niacin Ameliorated ETEC-Induced Cell Apoptosis and Water Transport Impairment via GPR109A in IPEC-J2 Cells
3. Discussion
4. Materials and Methods
4.1. Animal Trail Design and Sample Collection
4.2. Cell Culture
4.3. Bacterial Strains
4.4. Cell Viability
4.5. Effect of Niacin on Water Transport Proteins in K88-Challenged IPEC-J2 Cells
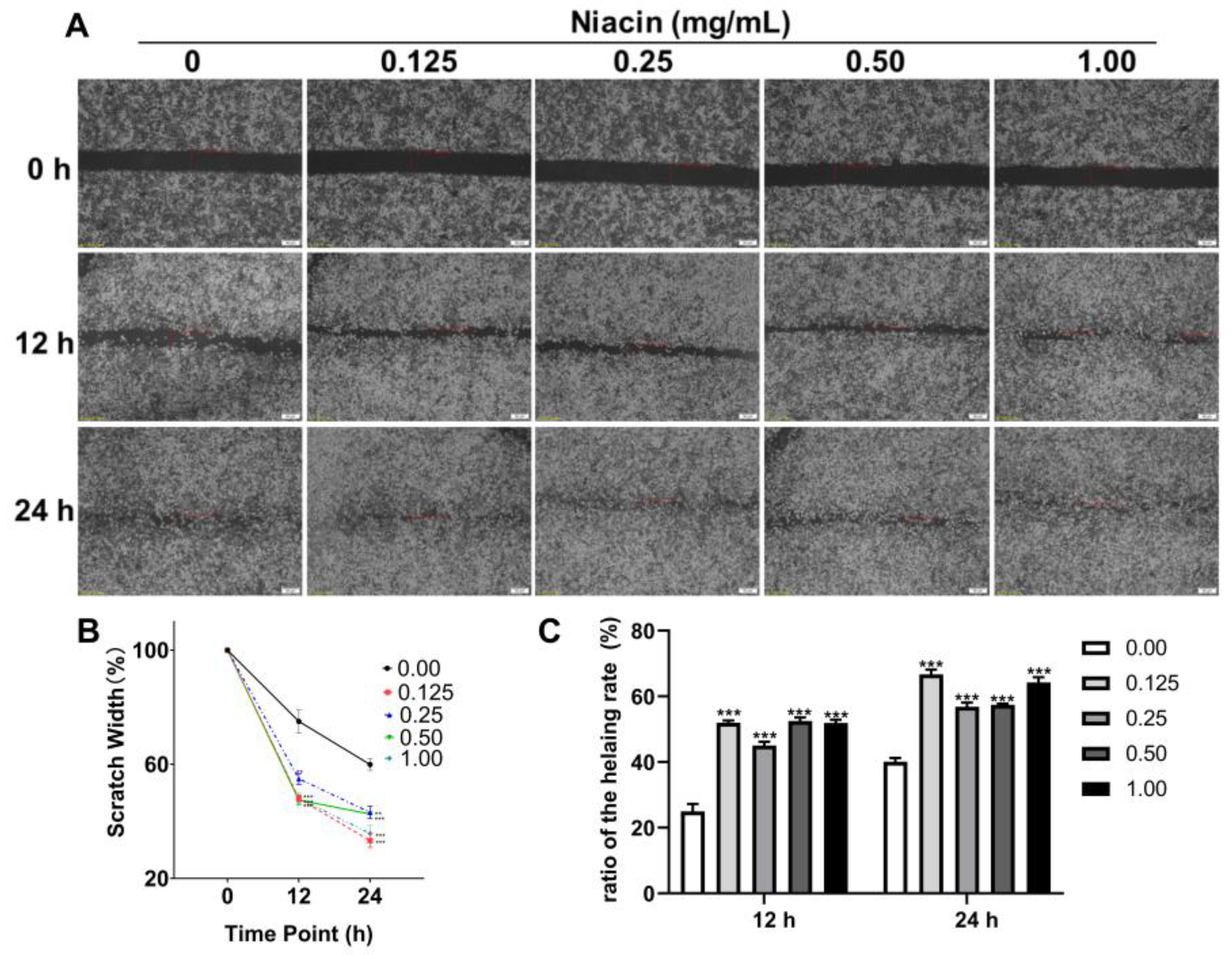
4.6. Cell Migration
4.7. siRNA and Transfection
4.8. Quantitative Real-Time PCR (qPCR)
4.9. Western Blot Analysis
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Preston, G.M.; Agre, P. Isolation of the cDNA for erythrocyte integral membrane protein of 28 kilodaltons: Member of an ancient channel family. Proc. Natl. Acad. Sci. USA 1991, 88, 11110–11114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denker, B.M.; Smith, B.L.; Kuhajda, F.P.; Agre, P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from erythrocytes and renal tubules. J. Biol. Chem. 1988, 263, 15634–15642. [Google Scholar] [CrossRef]
- Kreida, S.; Törnroth-Horsefield, S. Structural insights into aquaporin selectivity and regulation. Curr. Opin. Struct. Biol. 2015, 33, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Aikman, B.; de Almeida, A.; Meier-Menches, S.M.; Casini, A. Aquaporins in cancer development: Opportunities for bioinorganic chemistry to contribute novel chemical probes and therapeutic agents. Metallomics 2018, 10, 696–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Shao, C.; Fang, Y.; Wang, J.; Dong, N.; Shan, A. Binding loop of sunflower trypsin inhibitor 1 serves as a design motif for proteolysis-resistant antimicrobial peptides. Acta Biomater. 2021, 124, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.K.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, C.; Wang, W. Molecular aspects of aquaporins. Vitam. Horm. 2019, 113, 129–181. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Li, Y.; Xue, C.; Dong, N.; Bi, C.; Shan, A. Aquaporin: Targets for dietary nutrients to regulate intestinal health. J. Anim. Physiol. Anim. Nutr. 2021, 106, 167–180. [Google Scholar] [CrossRef]
- Marchbank, T.; Playford, R.J. Trefoil factor family peptides enhance cell migration by increasing cellular osmotic permeability and aquaporin 3 levels. FASEB J. 2018, 32, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Pimpão, C.; da Silva, I.V.; Mósca, A.F.; Pinho, J.O.; Gaspar, M.M.; Gumerova, N.I.; Rompel, A.; Aureliano, M.; Soveral, G. The Aquaporin-3-Inhibiting Potential of Polyoxotungstates. Int. J. Mol. Sci. 2020, 21, 2467. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.; Ye, J.L.; Yang, J.; Yang, K.M.; Chen, Z.; Liang, R.; Wu, X.J.; Wang, L.; Jiang, Z.Y. Differential expression of intestinal ion transporters and water channel aquaporins in young piglets challenged with enterotoxigenic Escherichia coli K881. J. Anim. Sci. 2017, 95, 5240–5252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischbarg, J. Fluid Transport Across Leaky Epithelia: Central Role of the Tight Junction and Supporting Role of Aquaporins. Physiol. Rev. 2010, 90, 1271–1290. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.; Verkman, A.S. Aquaporin water channels in gastrointestinal physiology. J. Physiol. 1999, 517, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhang, K.; Su, W.; Zhao, Y.; Ma, X.; Qian, G.; Qu, G.; Pei, Z.; Liu, S.; Ma, H. Aquaporin-3 is down-regulated in jejunum villi epithelial cells during enterotoxigenic Escherichia coli -induced diarrhea in mice. Microb. Pathog. 2017, 107, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Yde, J.; Keely, S.; Wu, Q.; Borg, J.F.; Lajczak, N.; O’Dwyer, A.; Dalsgaard, P.; Fenton, R.A.; Moeller, H.B. Characterization of AQPs in Mouse, Rat, and Human Colon and Their Selective Regulation by Bile Acids. Front. Nutr. 2016, 3, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guignot, J.; Chaplais, C.; Coconnier-Polter, M.-H.; Servin, A.L. The secreted autotransporter toxin, Sat, functions as a virulence factor in Afa/Dr diffusely adhering Escherichia coli by promoting lesions in tight junction of polarized epithelial cells. Cell. Microbiol. 2006, 9, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Kim, S.W. Intestinal challenge with enterotoxigenic Escherichia coli in pigs, and nutritional intervention to prevent postweaning diarrhea. Anim. Nutr. 2017, 3, 322–330. [Google Scholar] [CrossRef]
- Sakai, H.; Sagara, A.; Matsumoto, K.; Hasegawa, S.; Sato, K.; Nishizaki, M.; Shoji, T.; Horie, S.; Nakagawa, T.; Tokuyama, S.; et al. 5-Fluorouracil Induces Diarrhea with Changes in the Expression of Inflammatory Cytokines and Aquaporins in Mouse Intestines. PLoS ONE 2013, 8, e54788. [Google Scholar] [CrossRef]
- Kunzelmann, K.; Mall, M. Electrolyte Transport in the Mammalian Colon: Mechanisms and Implications for Disease. Physiol. Rev. 2002, 82, 245–289. [Google Scholar] [CrossRef] [Green Version]
- Guttman, J.A.; Samji, F.N.; Li, Y.; Deng, W.; Lin, A.; Finlay, B.B. Aquaporins contribute to diarrhoea caused by attaching and effacing bacterial pathogens. Cell. Microbiol. 2006, 9, 131–141. [Google Scholar] [CrossRef]
- Viswanathan, V.K.; Hodges, K.; Hecht, G. Enteric infection meets intestinal function: How bacterial pathogens cause diarrhoea. Nat. Rev. Genet. 2008, 7, 110–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, J.; Wang, L.; Chen, Y.; Xiong, Y.; Wu, Q.; Jiang, Z.; Yi, H. Effects of niacin on intestinal immunity, microbial community and intestinal barrier in weaned piglets during starvation. Int. Immunopharmacol. 2021, 95, 107584. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhu, X.; Qiu, Y.; Wang, L.; Shang, X.; Gao, K.; Yang, X.; Jiang, Z. Effect of Niacin on Growth Performance, Intestinal Morphology, Mucosal Immunity and Microbiota Composition in Weaned Piglets. Animals 2021, 11, 2186. [Google Scholar] [CrossRef]
- Soga, T.; Kamohara, M.; Takasaki, J.; Matsumoto, S.-I.; Saito, T.; Ohishi, T.; Hiyama, H.; Matsuo, A.; Matsushime, H.; Furuichi, K. Molecular identification of nicotinic acid receptor. Biochem. Biophys. Res. Commun. 2003, 303, 364–369. [Google Scholar] [CrossRef]
- Macia, L.; Tan, J.; Vieira, A.T.; Leach, K.; Stanley, D.; Luong, S.; Maruya, M.; McKenzie, C.l.; Hijikata, A.; Wong, C.; et al. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 2015, 6, 6734. [Google Scholar] [CrossRef] [Green Version]
- Taggart, A.K.; Kero, J.; Gan, X.; Cai, T.Q.; Cheng, K.; Ippolito, M.; Ren, N.; Kaplan, R.; Wu, K.; Wu, T.J.; et al. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 2005, 280, 26649–26652. [Google Scholar] [CrossRef] [Green Version]
- Salem, H.A.; Wadie, W. Effect of Niacin on Inflammation and Angiogenesis in a Murine Model of Ulcerative Colitis. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhen, R.; Liu, C.; Wei, C.; Luo, Y.; Hu, X.; Liu, G.; Yi, H.; Huang, Y. Effect of different dosages of sodium butyrate and niacin on growth, faecal microbiota and Vitamin B metabolism in weaned piglets. J. Appl. Microbiol. 2022, 132, 4466–4475. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Tajika, Y.; Ablimit, A.; Aoki, T.; Hagiwara, H.; Takata, K. Aquaporins in the digestive system. Med. Electron Microsc. Off. J. Clin. Electron Microsc. Soc. Jpn. 2004, 37, 71–80. [Google Scholar] [CrossRef]
- Laforenza, U. Water channel proteins in the gastrointestinal tract. Mol. Asp. Med. 2012, 33, 642–650. [Google Scholar] [CrossRef]
- Ikarashi, N.; Kon, R.; Iizasa, T.; Suzuki, N.; Hiruma, R.; Suenaga, K.; Toda, T.; Ishii, M.; Hoshino, M.; Ochiai, W.; et al. Inhibition of Aquaporin-3 Water Channel in the Colon Induces Diarrhea. Biol. Pharm. Bull. 2012, 35, 957–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, G.; Zhang, S. Aquaporins 1, 3 and 8 expression and cytokines in irritable bowel syndrome rats’ colon via cAMP-PKA pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 4117–4123. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Huang, N.; Li, H.; Tian, J.; Zhou, X.; Li, T.; Yao, K.; Wu, G.; Yin, Y. AMPK/α-Ketoglutarate Axis Regulates Intestinal Water and Ion Homeostasis in Young Pigs. J. Agric. Food Chem. 2017, 65, 2287–2298. [Google Scholar] [CrossRef] [PubMed]
- Thiagarajah, J.R.; Zhao, D.; Verkman, A.S. Impaired enterocyte proliferation in aquaporin-3 deficiency in mouse models of colitis. Gut 2007, 56, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Otero-Viñas, M.; Prieto-Castrillo, F.P.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, N.; Feng, X.; He, C.; Gao, H.; Yang, L.; Ma, Q.; Guo, L.; Qiao, Y.; Yang, H.; Ma, T. Defective macrophage function in aquaporin-3 deficiency. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 4233–4239. [Google Scholar] [CrossRef]
- Tyteca, D.; Nishino, T.; Debaix, H.; Smissen, P.; N’Kuli, F.; Hoffmann, D.; Cnops, Y.; Rabolli, V.; Loo, G.V.; Beyaert, R.J. Regulation of Macrophage Motility by the Water Channel Aquaporin-1: Crucial Role of M0/M2 Phenotype Switch. PLoS ONE 2015, 10, e0117398. [Google Scholar] [CrossRef] [Green Version]
- Hara-Chikuma, M.; Chikuma, S.; Sugiyama, Y.; Kabashima, K.; Verkman, A.S.; Inoue, S.; Miyachi, Y. Chemokine-dependent T cell migration requires aquaporin-3–mediated hydrogen peroxide uptake. J. Exp. Med. 2012, 209, 1743–1752. [Google Scholar] [CrossRef]
- Holm, A.; Karlsson, T.; Vikström, E. Pseudomonas aeruginosa lasI/rhlI quorum sensing genes promote phagocytosis and aquaporin 9 redistribution to the leading and trailing regions in macrophages. Front. Microbiol. 2015, 6, 915. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Hanson, J.; Gille, A.; Zwykiel, S.; Lukasova, M.; Clausen, B.E.; Ahmed, K.; Tunaru, S.; Wirth, A.; Offermanns, S. Nicotinic acid– and monomethyl fumarate–induced flushing involves GPR109A expressed by keratinocytes and COX-2–dependent prostanoid formation in mice. J. Clin. Investig. 2010, 120, 2910–2919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Hu, N.; Jiang, Q.; Zhu, L.; Zhang, M.; Jiang, J.; Xiong, M.; Yang, M.; Yang, J.; Shen, L.; et al. Protective effects of sodium butyrate on rotavirus inducing endoplasmic reticulum stress-mediated apoptosis via PERK-eIF2alpha signaling pathway in IPEC-J2 cells. J. Anim. Sci. Biotechnol. 2021, 12, 69. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [Green Version]
- Rask-Andersen, M.; Almén, M.S.; Schiöth, H.B. Trends in the exploitation of novel drug targets. Nat. Rev. Drug Discov. 2011, 10, 579–590. [Google Scholar] [CrossRef]
- Singh, V.; Jamwal, S.; Jain, R.; Verma, P.; Gokhale, R.; Rao, K.V. Mycobacterium tuberculosis-Driven Targeted Recalibration of Macrophage Lipid Homeostasis Promotes the Foamy Phenotype. Cell Host Microbe 2012, 12, 669–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Yang, X.; Wang, L.; Gao, K.; Jiang, Z. L-Arginine Inhibited Inflammatory Response and Oxidative Stress Induced by Lipopolysaccharide via Arginase-1 Signaling in IPEC-J2 Cells. Int. J. Mol. Sci. 2019, 20, 1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Ingredient | % | Energy and Nutrient Composition | |
|---|---|---|---|
| Corn | 34.00 | DE, kcal/kg | 3526.50 |
| Expanded corn | 18.00 | ME, kcal/kg | 3395.50 |
| Soybean meal | 9.50 | NE, kcal/kg | 2611.30 |
| Expanded soybean | 15.00 | CP (analyzed), % | 19.00 |
| Lactose | 2.00 | CF (analyzed), % | 2.65 |
| Whey powder | 8.00 | Total phosphorus (analyzed), % | 0.68 |
| Fish meal | 5.00 | Methionine + Cystine, % | 0.91 |
| Soybean oil | 2.00 | Valine, % | 1.00 |
| Soybean hull | 0.31 | Calcium (analyzed), % | 0.88 |
| Limestone | 0.60 | STTD of P, % | 0.47 |
| Monocalcium phosphate | 1.50 | ||
| Lysine 98.5% | 0.80 | ||
| Methionine | 0.30 | ||
| L-Threonine | 0.30 | ||
| Valine | 0.13 | ||
| L-Tryptophan | 0.10 | ||
| Salt | 0.25 | ||
| 60% choline chloride | 0.15 | ||
| Premix 2 | 2.00 | ||
| Acidifier | 0.05 | ||
| Phytase | 0.01 | ||
| Total | 100.00 |
| Primer | Sequence (5′-3′) |
|---|---|
| Occludin-F | GCACCCAGCAACGACAT |
| Occludin-R | CATAGACAGAATCCGAATCAC |
| ZO-1-F | GACTCCTTGCTGAATCTGA |
| ZO-1-R | GCACCTCATCATCTTCCAT |
| AQP1-F | TTGGGCTGAGCATTGCCACGC |
| AQP1-R | CAGCGAGTTCAGGCCAAGGGAGTT |
| AQP3-F | CACCTCCATGGGCTTCAACT |
| AQP3-R | TGCCCATTCGCATCTACTCC |
| AQP7-F | AGGCACTTCAGCAGACATCTAA |
| AQP7-R | TGGCGTGATCATCTTGGAGG |
| AQP9-F | TGTCATTGGCCTCCTGATTG |
| AQP9-R | TGGCACAGCCACTGTTCATC |
| AQP11-F | CGTCTTGGAGTTTCTGGCTACC |
| AQP11-R | CCTGTCCCTGACGTGATACTTG |
| GPR109A-F | CCGTCCCACCAGCAGAATCA |
| GPR109A-R | ACCCAGGAGCCCGAACACAA |
| β-actin-F | CACGCCATCCTGCGTCTGGA |
| β-actin-R | AGCACCGTGTTGGCGTAGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Qiu, Y.; Gu, F.; Xu, X.; Wu, S.; Jin, Z.; Wang, L.; Gao, K.; Zhu, C.; Yang, X.; et al. Niacin Improves Intestinal Health through Up-Regulation of AQPs Expression Induced by GPR109A. Int. J. Mol. Sci. 2022, 23, 8332. https://doi.org/10.3390/ijms23158332
Liu S, Qiu Y, Gu F, Xu X, Wu S, Jin Z, Wang L, Gao K, Zhu C, Yang X, et al. Niacin Improves Intestinal Health through Up-Regulation of AQPs Expression Induced by GPR109A. International Journal of Molecular Sciences. 2022; 23(15):8332. https://doi.org/10.3390/ijms23158332
Chicago/Turabian StyleLiu, Shilong, Yueqin Qiu, Fang Gu, Xiaoming Xu, Shansen Wu, Zhenhao Jin, Li Wang, Kaiguo Gao, Cui Zhu, Xuefen Yang, and et al. 2022. "Niacin Improves Intestinal Health through Up-Regulation of AQPs Expression Induced by GPR109A" International Journal of Molecular Sciences 23, no. 15: 8332. https://doi.org/10.3390/ijms23158332
APA StyleLiu, S., Qiu, Y., Gu, F., Xu, X., Wu, S., Jin, Z., Wang, L., Gao, K., Zhu, C., Yang, X., & Jiang, Z. (2022). Niacin Improves Intestinal Health through Up-Regulation of AQPs Expression Induced by GPR109A. International Journal of Molecular Sciences, 23(15), 8332. https://doi.org/10.3390/ijms23158332







