Evaluation of the Impact of Pregnancy-Associated Factors on the Quality of Wharton’s Jelly-Derived Stem Cells Using SOX2 Gene Expression as a Marker
Abstract
1. Introduction
2. Results
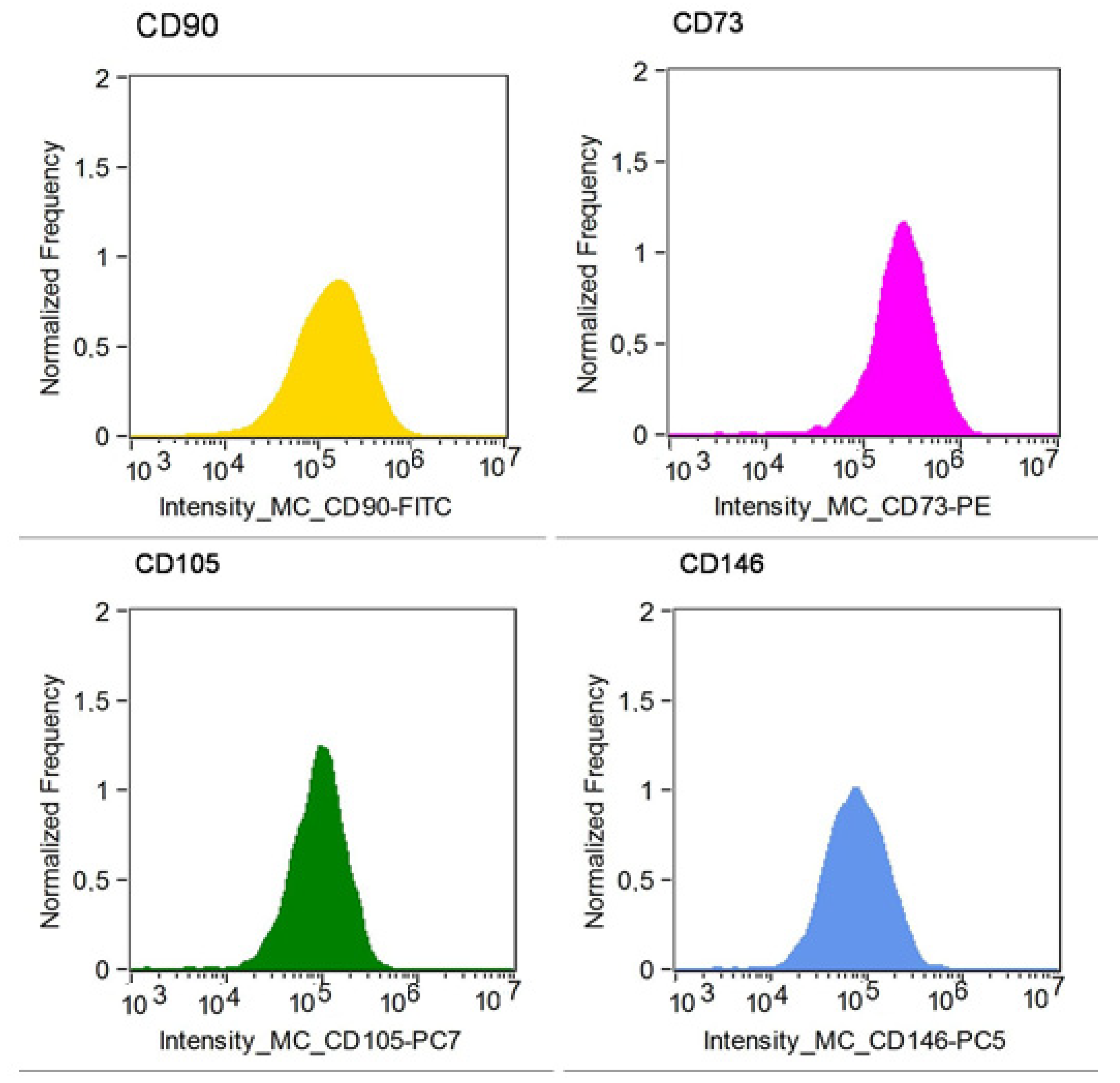
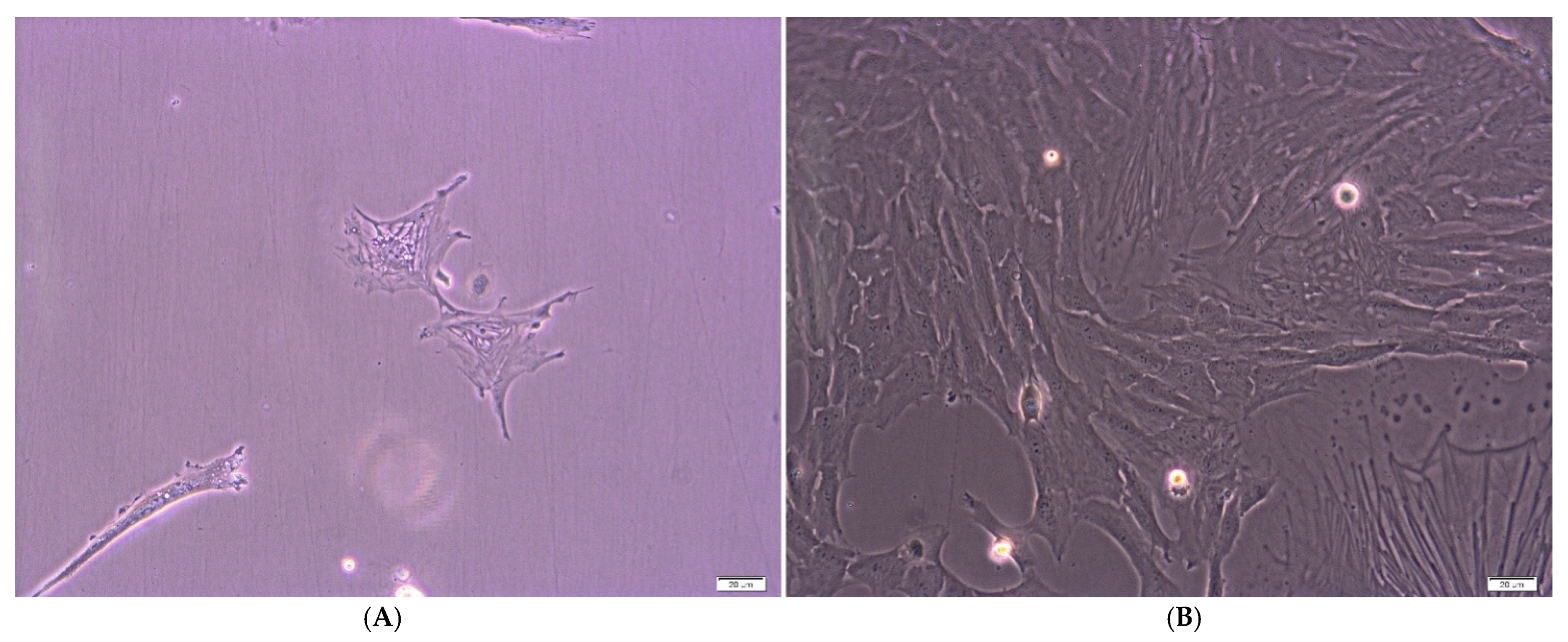
2.1. Cell Culture and Cytometric Analysis
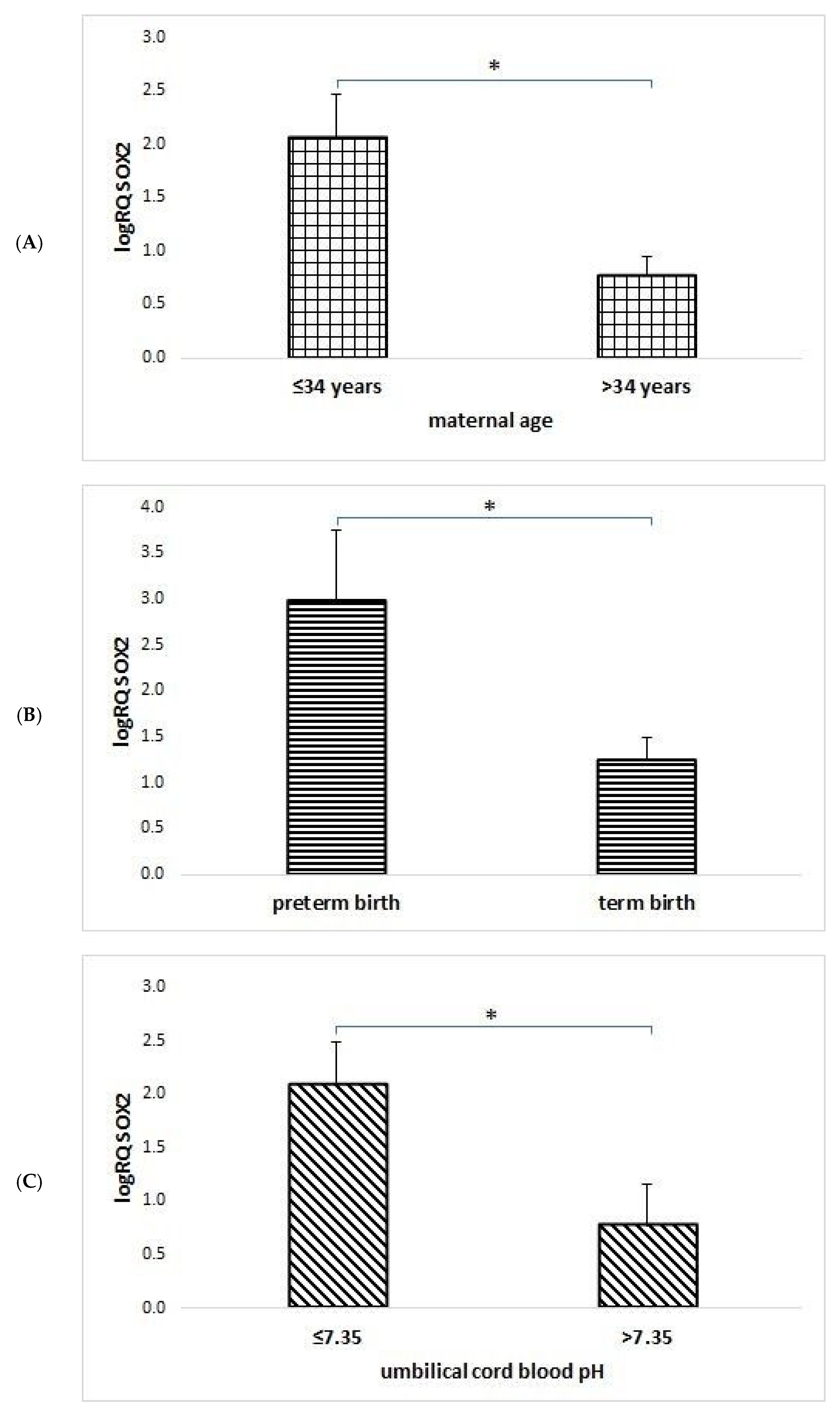
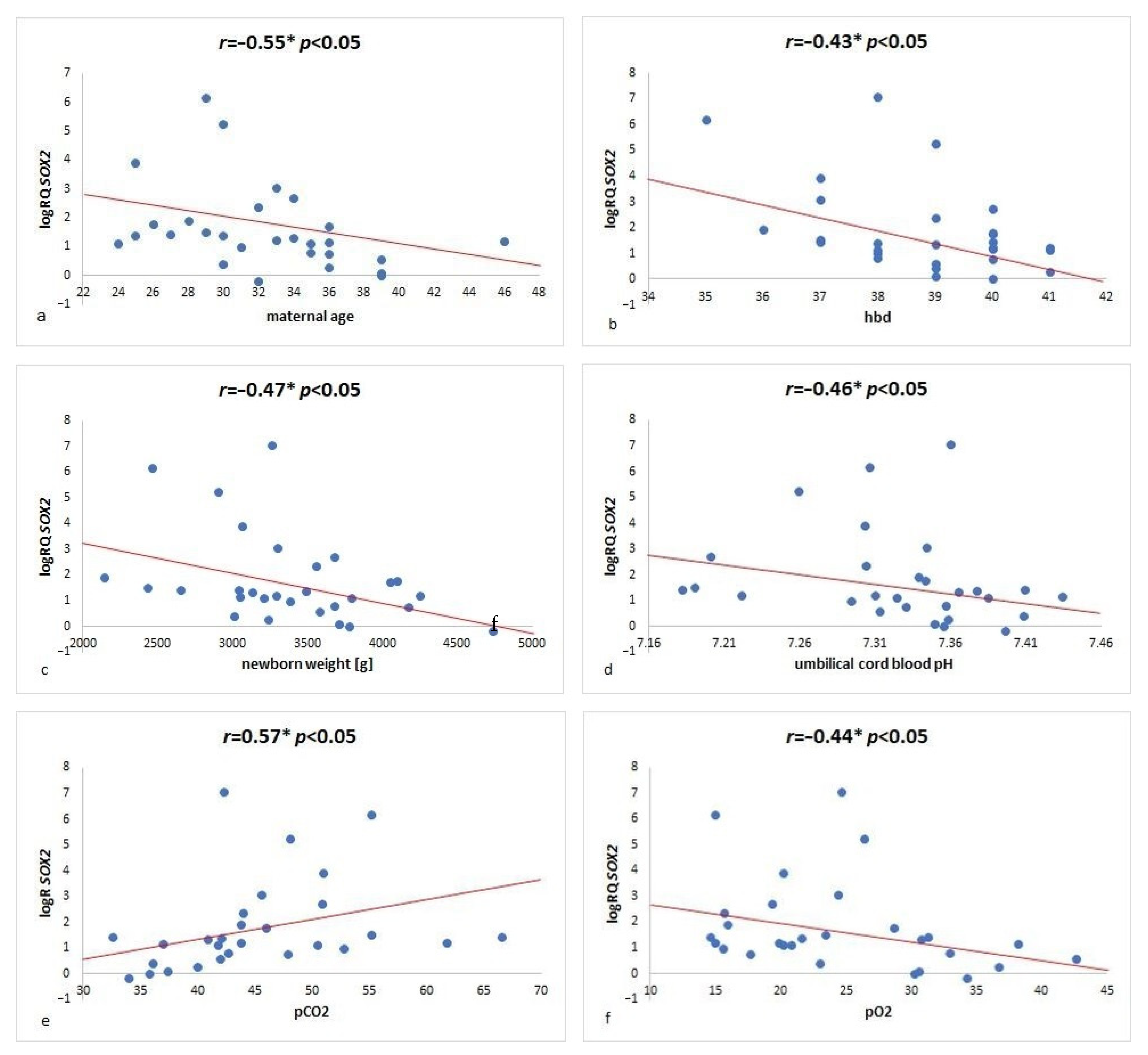
2.2. SOX2 Gene Expression Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Watson, N.; Divers, R.; Kedar, R.; Mehindru, A.; Mehindru, A.; Borlongan, M.; Borlongan, C.V. Discarded Wharton jelly of the human umbilical cord: A viable source for mesenchymal stromal cells. Cytotherapy 2015, 17, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E.; Walker, J.T.; Keating, A. Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Transl. Med. 2017, 6, 1620–1630. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, M.; Zuffardi, O.; Collignon, J.; Lovell-Badge, R.; Goodfellow, P.N. The cDNA sequence and chromosomal location of the human SOX2 gene. Mamm. Genome 1994, 5, 640–642. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Yu, X.; Liu, S. Pluripotency transcription factors and cancer stem cells: Small genes make a big difference. Chin. J. Cancer 2013, 32, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.A. Floppy SOX: Mutual induced fit in hmg (high-mobility group) box-DNA recognition. Mol. Endocrinol. 2001, 15, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Badis, G.; Berger, M.F.; Philippakis, A.A.; Talukder, S.; Gehrke, A.R.; Jaeger, S.A.; Chan, E.T.; Metzler, G.; Vedenko, A.; Chen, X.; et al. Diversity and Complexity in DNA Recognition by Transcription Factors. Science 2009, 324, 1720–1723. [Google Scholar] [CrossRef]
- Kondoh, H.; Kamachi, Y. SOX–partner code for cell specification: Regulatory target selection and underlying molecular mechanisms. Int. J. Biochem. Cell Biol. 2010, 42, 391–399. [Google Scholar] [CrossRef]
- Chambers, I.; Silva, J.C.R.; Colby, D.; Nichols, J.; Nijmeijer-Winter, B.; Robertson, M.; Vrana, J.; Jones, K.; Grotewold, L.; Smith, A. Nanog safeguards pluripotency and mediates germline development. Nature 2007, 450, 1230–1234. [Google Scholar] [CrossRef]
- Masui, S.; Nakatake, Y.; Toyooka, Y.; Shimosato, D.; Yagi, R.; Takahashi, K.; Okochi, H.; Okuda, A.; Matoba, R.; Sharov, A.A.; et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 2007, 9, 625–635. [Google Scholar] [CrossRef]
- Niwa, H.; Miyazaki, J.-I.; Smith, A.G. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat. Genet. 2000, 24, 372–376. [Google Scholar] [CrossRef]
- Zhang, W.C.S. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J. Stem Cells 2014, 6, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Rizzino, A.; Wuebben, E.L. Sox2/Oct4: A delicately balanced partnership in pluripotent stem cells and embryogenesis. Biochim. Biophys. Acta 2016, 1859, 780–791. [Google Scholar] [CrossRef]
- Tapia, N.; MacCarthy, C.; Esch, D.; Marthaler, A.G.; Tiemann, U.; Araúzo-Bravo, M.J.; Jauch, R.; Cojocaru, V.; Schöler, H. Dissecting the role of distinct OCT4-SOX2 heterodimer configurations in pluripotency. Sci. Rep. 2015, 5, 13533. [Google Scholar] [CrossRef] [PubMed]
- Chew, J.-L.; Loh, Y.-H.; Zhang, W.; Chen, X.; Tam, W.-L.; Yeap, L.-S.; Li, P.; Ang, Y.-S.; Lim, B.; Robson, P.; et al. Reciprocal Transcriptional Regulation of Pou5f1 and Sox2 via the Oct4/Sox2 Complex in Embryonic Stem Cells. Mol. Cell. Biol. 2005, 25, 6031–6046. [Google Scholar] [CrossRef]
- Kopp, J.L.; Ormsbee, B.D.; Desler, M.; Rizzino, A. Small Increases in the Level of Sox2 Trigger the Differentiation of Mouse Embryonic Stem Cells. Stem Cells 2008, 26, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Maki, C.; Pacchiarotti, J.; Csontos, S.; Pham, J.; Slepko, N.; Patel, A.; Silva, F. Pluripotent marker expression and differentiation of human second trimester Mesenchymal Stem Cells. Biochem. Biophys. Res. Commun. 2007, 362, 491–497. [Google Scholar] [CrossRef]
- Fong, Y.W.; Inouye, C.; Yamaguchi, T.; Cattoglio, C.; Grubisic, I.; Tjian, R. A DNA Repair Complex Functions as an Oct4/Sox2 Coactivator in Embryonic Stem Cells. Cell 2011, 147, 120–131. [Google Scholar] [CrossRef]
- Trivanovic, D.; Kocic, J.; Mojsilovic, S.; Krstic, A.; Ilic, V.; Okic-Djordjevic, I.; Santibanez, J.; Jovcic, G.; Terzic, M.; Bugarski, D. Mesenchymal stem cells isolated from peripheral blood and umbilical cord Wharton’s jelly. Srp. Arh. Celok. Lek. 2013, 141, 178–186. [Google Scholar] [CrossRef]
- Izadpanah, R.; Trygg, C.; Patel, B.; Kriedt, C.; Dufour, J.; Gimble, J.M.; Bunnell, B.A. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J. Cell. Biochem. 2006, 99, 1285–1297. [Google Scholar] [CrossRef]
- Riekstina, U.; Cakstina, I.; Parfejevs, V.; Hoogduijn, M.; Jankovskis, G.; Muiznieks, I.; Muceniece, R.; Ancans, J. Embryonic Stem Cell Marker Expression Pattern in Human Mesenchymal Stem Cells Derived from Bone Marrow, Adipose Tissue, Heart and Dermis. Stem Cell Rev. Rep. 2009, 5, 378–386. [Google Scholar] [CrossRef]
- Yoon, D.S.; Kim, Y.H.; Jung, H.S.; Paik, S.; Lee, J.W. Importance of Sox2 in maintenance of cell proliferation and multipotency of mesenchymal stem cells in low-density culture. Cell Prolif. 2011, 44, 428–440. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wei, X.; Ling, J.; Wu, L.; Xiao, Y. Expression Pattern of Oct-4, Sox2, and c-Myc in the Primary Culture of Human Dental Pulp Derived Cells. J. Endod. 2011, 37, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Beltran, A.; Chetwynd, E.; Stuebe, A.M.; Twigger, A.-J.; Metzger, P.; Trengove, N.; Lai, C.T.; Filgueira, L.; Blancafort, P.; et al. Breastmilk Is a Novel Source of Stem Cells with Multilineage Differentiation Potential. Stem Cells 2012, 30, 2164–2174. [Google Scholar] [CrossRef]
- Twigger, A.-J.; Hepworth, A.R.; Lai, C.T.; Chetwynd, E.; Stuebe, A.M.; Blancafort, P.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Gene expression in breastmilk cells is associated with maternal and infant characteristics. Sci. Rep. 2015, 5, 12933. [Google Scholar] [CrossRef] [PubMed]
- Kamachi, Y.; Kondoh, H. Sox proteins: Regulators of cell fate specification and differentiation. Development 2013, 140, 4129–4144. [Google Scholar] [CrossRef]
- Navarro, P.; Festuccia, N.; Colby, D.; Gagliardi, A.; Mullin, N.P.; Zhang, W.; Karwacki-Neisius, V.; Osorno, R.; Kelly, D.; Robertson, M.; et al. OCT4/SOX2-independent Nanog autorepression modulates heterogeneous Nanog gene expression in mouse ES cells. EMBO J. 2012, 31, 4547–4562. [Google Scholar] [CrossRef]
- Świstowska, M.; Gil-Kulik, P.; Krzyżanowski, A.; Bielecki, T.; Czop, M.; Kwaśniewska, A.; Kocki, J. Potential Effect of SOX2 on the Cell Cycle of Wharton’s Jelly Stem Cells (WJSCs). Oxid. Med. Cell. Longev. 2019, 2019, 5084689. [Google Scholar] [CrossRef]
- Adachi, K.; Suemori, H.; Nakatsuji, N.; Kawase, N.N.A.E. The Role of SOX2 in Maintaining Pluripotency and Differentiation of Human Embryonic Stem Cells. In Stem Cells in Clinic and Research; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Liu, P.; Cai, J.; Dong, D.; Chen, Y.; Liu, X.; Wang, Y.; Zhou, Y. Effects of SOX2 on Proliferation, Migration and Adhesion of Human Dental Pulp Stem Cells. PLoS ONE 2015, 10, e0141346. [Google Scholar] [CrossRef]
- Russell, J.P.; Lim, X.; Santambrogio, A.; Yianni, V.; Kemkem, Y.; Wang, B.; Fish, M.; Haston, S.; Grabek, A.; Hallang, S.; et al. ituitary stem cells produce paracrine WNT signals to control the expansion of their descendant progenitor cells. eLife 2021, 10, e59142. [Google Scholar] [CrossRef]
- Nunes, R.D.; Zandavalli, F.M. Association between maternal and fetal factors and quality of cord blood as a source of stem cells. Rev. Bras. Hematol. Hemoter. 2015, 37, 38–42. [Google Scholar] [CrossRef]
- Abdelrazik, A.M.; El Said, M.N.; Abdelaziz, H.E.M.; Badran, H.M.; Elal, E.Y.A.A. The impact of fetal and maternal physiologic factors on umbilical cord blood quality as a source of stem cells in Egyptian population. Transfusion 2015, 55, 2882–2889. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Liao, C.; Chen, J.-S.; Xu, Z.-P.; Gu, S.-L.; Wu, S.-Q.; Lu, Y.; Xie, G.-E. Analysis of maternal and neonatal factors associated with hematopoietic reconstruction potential in umbilical cord blood units. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2010, 18, 1535–1541. (In Chinese) [Google Scholar] [PubMed]
- Chandra, T.; Afreen, S.; Kumar, A.; Singh, U.; Gupta, A. Does umbilical cord blood-derived CD34+ cell concentration depend on the weight and sex of a full-term infant? J. Pediatr. Hematol. 2012, 34, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Caughey, A.B.; Sundaram, V.; Kaimal, A.J.; Cheng, Y.W.; Gienger, A.; Little, S.E.; Lee, J.F.; Wong, L.; Shaffer, B.L.; Tran, S.H.; et al. Maternal and neonatal outcomes of elective induction of labor. Evid. Rep. Technol. Assess. 2009, 176, 1–257. [Google Scholar]
- Mousavi, S.M.; Abroun, S.; Zarrabi, M.; Ahmadipanah, M. The effect of maternal and infant factors on cord blood yield. Pediatr. Blood Cancer 2016, 64, e26381. [Google Scholar] [CrossRef]
- Bielec-Berek, B.; Jastrzębska-Stojko, Ż.; Drosdzol-Cop, A.; Jendyk, C.; Boruczkowski, D.; Ołdak, T.; Nowak-Brzezińska, A.; Stojko, R. Maternal predictors and quality of umbilical cord blood units. Cell Tissue Bank. 2018, 19, 69–75. [Google Scholar] [CrossRef]
- Mancinelli, F.; Tamburini, A.; Spagnoli, A.; Malerba, C.; Suppo, G.; Lasorella, R.; de Fabritiis, P.; Calugi, A. Optimizing Umbilical Cord Blood Collection: Impact of Obstetric Factors Versus Quality of Cord Blood Units. Transplant. Proc. 2006, 38, 1174–1176. [Google Scholar] [CrossRef]
- Santos, S.V.; Marti, L.; Ribeiro, A.A.F.; Conti, F.; Barros, S.M. A cross-sectional study of umbilical cord blood donor profiles and their influence on umbilical cord blood collection in a Brazilian hospital. Cytotherapy 2011, 13, 1120–1127. [Google Scholar] [CrossRef]
- Al-Deghaither, S.Y. Impact of maternal and neonatal factors on parameters of hematopoietic potential in umbilical cord blood. Saudi Med. J. 2015, 36, 704–712. [Google Scholar] [CrossRef]
- Rowisha, M.A.; El-Shanshory, M.R.; El-Hawary, E.E.; Ahmed, A.Y.; Altoraky, S.R.M. Impact of maternal and neonatal factors on umbilical cord CD34+ cells. Stem Cell Investig. 2020, 7, 5. [Google Scholar] [CrossRef]
- Nakagawa, R.; Watanabe, T.; Kawano, Y.; Kanai, S.; Suzuya, H.; Kaneko, M.; Watanabe, H.; Okamoto, Y.; Kuroda, Y.; Nakayama, T.; et al. Analysis of maternal and neonatal factors that influence the nucleated and CD34+ cell yield for cord blood banking. Transfusion 2004, 44, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Ballen, K.; Wilson, M.; Wuu, J.; Ceredona, A.; Hsieh, C.; Stewart, F.; Popovsky, M.; Quesenberry, P. Bigger is better: Maternal and neonatal predictors of hematopoietic potential of umbilical cord blood units. Bone Marrow Transplant. 2001, 27, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Weisdorf, D.J.; Solovey, A.; Hebbel, R.P. Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Investig. 2000, 105, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Aufderhaar, U.; Holzgreve, W.; Danzer, E.; Tichelli, A.; Troeger, C.; Surbek, D. The impact of intrapartum factors on umbilical cord blood stem cells banking. J. Perinat. Med. 2003, 31, 317–322. [Google Scholar] [CrossRef]
- Obradovic, H.; Krstic, J.; Trivanovic, D.; Mojsilovic, S.; Okic, I.; Kukolj, T.; Ilic, V.; Jaukovic, A.; Terzic, M.; Bugarski, D. Improving stemness and functional features of mesenchymal stem cells from Wharton’s jelly of a human umbilical cord by mimicking the native, low oxygen stem cell niche. Placenta 2019, 82, 25–34. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Sanz-Ros, J.; Román-Domínguez, A.; Inglés, M.; Gimeno-Mallench, L.; El Alami, M.; Viña-Almunia, J.; Gambini, J.; Viña, J.; Borrás, C. Relevance of Oxygen Concentration in Stem Cell Culture for Regenerative Medicine. Int. J. Mol. Sci. 2019, 20, 1195. [Google Scholar] [CrossRef]
- Halim, D.H.; Murty, H.; Sandra, F.; Boediono, A.; Djuwantono, T.; Setiawan, B. Stem Cell Dasar Teori Dan Aplikasi Klinis, 1st ed.; Erlangga: Jakarta, Indonesia, 2010; pp. 154–196. [Google Scholar]
- Widowati, W.; Wijaya, L.; Bachtiar, I.; Gunanegara, R.F.; Sugeng, S.U.; Irawan, Y.A.; Sumitro, S.B.; Widodo, M.A. Effect of oxygen tension on proliferation and characteristics of Wharton’s jelly-derived mesenchymal stem cells. Biomark. Genomic Med. 2014, 6, 43–48. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, Y.; Fujita, M.; Tanaka, Y.; Kojima, I.; Kanatani, Y.; Ishihara, M.; Tachibana, S. Low oxygen tension enhances proliferation and maintains stemness of adipose tissue-derived stromal cells. Bio. Res. Open Access 2013, 2, 199–205. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, L.; Chen, Q.; Wang, F.; Li, Q.; Zeng, Q.; Huang, J.; Luo, M.; Li, W.; Zheng, Y.; et al. Hypoxia with Wharton’s jelly mesenchymal stem cell coculture maintains stemness of umbilical cord blood-derived CD34+ cells. Stem Cell Res. Ther. 2018, 9, 158. [Google Scholar] [CrossRef]
- Safitri, E. Effect of low oxygen tension on transcriptional factor OCT4 and SOX2 expression in New Zealand rabbit bone marrow-derived mesenchymal stem cells. Veter. World 2020, 13, 2469–2476. [Google Scholar] [CrossRef]
- Bae, K.-M.; Dai, Y.; Vieweg, J.; Siemann, D.W. Hypoxia regulates SOX2 expression to promote prostate cancer cell invasion and sphere formation. Am. J. Cancer Res. 2016, 6, 1078–1088. [Google Scholar] [PubMed]
- Alrefaei, G.I.; Al-Karim, S.; Ayuob, N.N.; Ali, S.S. Does the maternal age affect the mesenchymal stem cell markers and gene expression in the human placenta? What is the evidence? Tissue Cell 2015, 47, 406–419. [Google Scholar] [CrossRef] [PubMed]
- Alrefaei, G.; Ayuob, N.N.; Ali, S.S.; Alkarim, S. Effects of maternal age on the expression of mesenchymal stem cell markers in the components of human umbilical cord. Folia Histochem. Cytobiol. 2015, 53, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Asumda, F.Z.; Chase, P.B. Age-related changes in rat bone-marrow mesenchymal stem cell plasticity. BMC Cell Biol. 2011, 12, 44. [Google Scholar] [CrossRef]
- Huang, S.; Feng, C.; Wu, Y.; Yang, S.; Ma, K.; Wu, X.; Fu, X. Dissimilar characteristics of umbilical cord mesenchymal stem cells from donors of different ages. Cell Tissue Bank. 2013, 14, 707–713. [Google Scholar] [CrossRef]
- Gil-Kulik, P.; Chomik, P.; Krzyżanowski, A.; Radzikowska-Büchner, E.; Maciejewski, R.; Kwaśniewska, A.; Rahnama, M.; Kocki, J. Influence of the Type of Delivery, Use of Oxytocin, and Maternal Age on POU5F1 Gene Expression in Stem Cells Derived from Wharton’s Jelly within the Umbilical Cord. Oxidative Med. Cell. Longev. 2019, 2019, 1027106. [Google Scholar] [CrossRef]
- Gil-Kulik, P.; Świstowska, M.; Kondracka, A.; Chomik, P.; Krzyżanowski, A.; Kwaśniewska, A.; Rahnama, M.; Kocki, J. Increased Expression of BIRC2, BIRC3, and BIRC5 from the IAP Family in Mesenchymal Stem Cells of the Umbilical Cord Wharton’s Jelly (WJSC) in Younger Women Giving Birth Naturally. Oxidative Med. Cell. Longev. 2020, 2020, 9084730. [Google Scholar] [CrossRef]
- Mazzoccoli, G.; Miscio, G.; Fontana, A.; Copetti, M.; Francavilla, M.; Bosi, A.; Perfetto, F.; Valoriani, A.; De Cata, A.; Santodirocco, M.; et al. Time related variations in stem cell harvesting of umbilical cord blood. Sci. Rep. 2016, 6, 21404. [Google Scholar] [CrossRef]
- Thame, M.; Osmond, C.; Bennett, F. Wzrost płodu jest bezpośrednio związany z antropometrią matki i objętością łożyska. Eur. J. Clin. Nutr. 2004, 58, 894. [Google Scholar] [CrossRef]
- Ballen, K.K.; Gluckman, E.; Broxmeyer, H.E. Umbilical cord blood transplantation: The first 25 years and beyond. Blood 2013, 122, 491–498. [Google Scholar] [CrossRef]
- Page, K.M.; Mendizabal, A.; Betz-Stablein, B.; Wease, S.; Shoulars, K.; Gentry, T.; Prasad, V.K.; Sun, J.; Carter, S.; Balber, A.E.; et al. Optimizing donor selection for public cord blood banking: Influence of maternal, infant, and collection characteristics on cord blood unit quality. Transfusion 2014, 54, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Vrijens, K.; Tsamou, M.; Madhloum, N.; Gyselaers, W.; Nawrot, T.S. Placental hypoxia-regulating network in relation to birth weight and ponderal index: The ENVIRONAGE Birth Cohort Study. J. Transl. Med. 2018, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Taha, Z.; Hassan, A.A.; Wikkeling-Scott, L.; Papandreou, D. Factors Associated with Preterm Birth and Low Birth Weight in Abu Dhabi, the United Arab Emirates. Int. J. Environ. Res. Public Health 2020, 17, 1382. [Google Scholar] [CrossRef] [PubMed]
- Kotowski, M.; Safranow, K.; Kawa, M.P.; Lewandowska, J.; Kłos, P.; Dziedziejko, V.; Paczkowska, E.; Czajka, R.; Celewicz, Z.; Rudnicki, J.; et al. Circulating hematopoietic stem cell count is a valuable predictor of prematurity complications in preterm newborns. BMC Pediatr. 2012, 12, 148. [Google Scholar] [CrossRef]
- Gil-Kulik, P.; Krzyżanowski, A.; Dudzińska, E.; Karwat, J.; Chomik, P.; Świstowska, M.; Kondracka, A.; Kwaśniewska, A.; Cioch, M.; Jojczuk, M.; et al. Potential Involvement of BIRC5 in Maintaining Pluripotency and Cell Differentiation of Human Stem Cells. Oxid. Med. Cell. Longev. 2019, 2019, 8727925. [Google Scholar] [CrossRef]
- Walecka, I.; Gil-Kulik, P.; Krzyżanowski, A.; Czop, M.; Galkowski, D.; Karwat, J.; Chomik, P.; Świstowska, M.; Kwaśniewska, A.; Bogucka-Kocka, A.; et al. Phenotypic Characterization of Adherent Cells Population CD34+ CD90+ CD105+ Derived from Wharton’s Jelly. Med. Sci. Monit. 2017, 23, 1886–1895. [Google Scholar] [CrossRef][Green Version]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2011, 25, 402–408. [Google Scholar] [CrossRef]

| Parameter | N | Mean | Median | Minimum | Maximum | SD |
|---|---|---|---|---|---|---|
| maternal age [years] | 30 | 32.3 | 32.000 | 24.000 | 46.000 | 5.098 |
| number of pregnancies | 2.000 | 2.000 | 1.000 | 8.000 | 1.414 | |
| week of pregnancy | 38.786 | 39.000 | 35.000 | 41.000 | 1.548 | |
| number of deliveries | 1.862 | 2.000 | 1.000 | 7.000 | 1.187 | |
| newborn’s weight [g] | 3383.793 | 3300.000 | 2140.000 | 4740.000 | 584.089 | |
| pH * | 7.326 | 7.341 | 7.182 | 7.434 | 0.066 | |
| pCO2 [mmHg] * | 45.268 | 43.800 | 32.600 | 66.500 | 8.098 | |
| PO2 [mmHg] * | 24.643 | 23.200 | 14.600 | 42.700 | 7.887 | |
| cHCO3 [mmol/L] * | 22.729 | 23.100 | 19.200 | 26.700 | 2.045 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Kulik, P.; Świstowska, M.; Krzyżanowski, A.; Petniak, A.; Kwaśniewska, A.; Płachno, B.J.; Galkowski, D.; Bogucka-Kocka, A.; Kocki, J. Evaluation of the Impact of Pregnancy-Associated Factors on the Quality of Wharton’s Jelly-Derived Stem Cells Using SOX2 Gene Expression as a Marker. Int. J. Mol. Sci. 2022, 23, 7630. https://doi.org/10.3390/ijms23147630
Gil-Kulik P, Świstowska M, Krzyżanowski A, Petniak A, Kwaśniewska A, Płachno BJ, Galkowski D, Bogucka-Kocka A, Kocki J. Evaluation of the Impact of Pregnancy-Associated Factors on the Quality of Wharton’s Jelly-Derived Stem Cells Using SOX2 Gene Expression as a Marker. International Journal of Molecular Sciences. 2022; 23(14):7630. https://doi.org/10.3390/ijms23147630
Chicago/Turabian StyleGil-Kulik, Paulina, Małgorzata Świstowska, Arkadiusz Krzyżanowski, Alicja Petniak, Anna Kwaśniewska, Bartosz J. Płachno, Dariusz Galkowski, Anna Bogucka-Kocka, and Janusz Kocki. 2022. "Evaluation of the Impact of Pregnancy-Associated Factors on the Quality of Wharton’s Jelly-Derived Stem Cells Using SOX2 Gene Expression as a Marker" International Journal of Molecular Sciences 23, no. 14: 7630. https://doi.org/10.3390/ijms23147630
APA StyleGil-Kulik, P., Świstowska, M., Krzyżanowski, A., Petniak, A., Kwaśniewska, A., Płachno, B. J., Galkowski, D., Bogucka-Kocka, A., & Kocki, J. (2022). Evaluation of the Impact of Pregnancy-Associated Factors on the Quality of Wharton’s Jelly-Derived Stem Cells Using SOX2 Gene Expression as a Marker. International Journal of Molecular Sciences, 23(14), 7630. https://doi.org/10.3390/ijms23147630







