Electron Attachment to 5-Fluorouracil: The Role of Hydrogen Fluoride in Dissociation Chemistry
Abstract
:1. Introduction
2. Results and Discussion
3. Methods and Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lawrence, T.S.; Davis, M.A.; Maybaum, J.; Stetson, P.L.; Ensminger, W.D. The Effect of Single versus Double-Strand Substitution on Halogenated Pyrimidine-Induced Radiosensitization and DNA Strand Breakage in Human Tumor Cells. Radiat. Res. 1990, 123, 192. [Google Scholar] [CrossRef]
- Kinsella, T.J.; Dobson, P.P.; Mitchell, J.B.; Fornace, A.J.J. Enhancement of X Ray Induced DNA Damage by Pre-Treatment with Halogenated Pyrimidine Analogs. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 733–739. [Google Scholar] [CrossRef]
- Gorfinkiel, J.D.; Ptasinska, S. Electron Scattering from Molecules and Molecular Aggregates of Biological Relevance. J. Phys. B At. Mol. Opt. Phys. 2017, 50, 182001. [Google Scholar] [CrossRef] [Green Version]
- Abouaf, R.; Dunet, H. Structures in Dissociative Electron Attachment Cross-Sections in Thymine, Uracil and Halouracils. Eur. Phys. J. D 2005, 35, 405–410. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Huels, M.A.; Illenberger, E.; Sanche, L. Formation of Negative Ions from Gas Phase Halo-Uracils by Low-Energy (0–18 eV) Electron Impact. Int. J. Mass Spectrom. 2003, 228, 703–716. [Google Scholar] [CrossRef]
- Spisz, P.; Kozak, W.; Chomicz-Mańka, L.; Makurat, S.; Falkiewicz, K.; Sikorski, A.; Czaja, A.; Rak, J.; Zdrowowicz, M. 5-(N-Trifluoromethylcarboxy) Aminouracil as a Potential DNA Radiosensitizer and Its Radiochemical Conversion into N-Uracil-5-yloxamic Acid. Int. J. Mol. Sci. 2020, 21, 6352. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Polska, K.; Rak, J.; Wagner, J.R.; Sanche, L. Fundamental Mechanisms of DNA Radiosensitization: Damage Induced by Low-Energy Electrons in Brominated Oligonucleotide Trimers. J. Phys. Chem. B 2012, 116, 9676–9682. [Google Scholar] [CrossRef]
- Pimblott, S.M.; LaVerne, J.A. Production of Low-Energy Electrons by Ionizing Radiation. Radiat. Phys. Chem. 2007, 76, 1244–1247. [Google Scholar] [CrossRef]
- Boudaïffa, B.; Cloutier, P.; Hunting, D.; Huels, M.A.; Sanche, L. Resonant Formation of DNA Strand Breaks by Low-Energy (3 to 20 eV) Electrons. Science 2000, 287, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Sanche, L. Role of Secondary Low Energy Electrons in Radiobiology and Chemoradiation Therapy of Cancer. Chem. Phys. Lett. 2009, 474, 1–6. [Google Scholar] [CrossRef]
- Dong, Y.; Liao, H.; Gao, Y.; Cloutier, P.; Zheng, Y.; Sanche, L. Early Events in Radiobiology: Isolated and Cluster DNA Damage Induced by Initial Cations and Nonionizing Secondary Electrons. J. Phys. Chem. Lett. 2021, 12, 717–723. [Google Scholar] [CrossRef]
- Zheng, Y.; Sanche, L. Clustered DNA Damages Induced by 0.5 to 30 eV Electrons. Int. J. Mol. Sci. 2019, 20, 3749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huwaidi, A.; Kumari, B.; Robert, G.; Guérin, B.; Sanche, L.; Wagner, J.R. Profiling DNA Damage Induced by the Irradiation of DNA with Gold Nanoparticles. J. Phys. Chem. Lett. 2021, 12, 9947–9954. [Google Scholar] [CrossRef]
- Kohanoff, J.; McAllister, M.; Tribello, G.A.; Gu, B. Interactions between Low Energy Electrons and DNA: A Perspective from First-Principles Simulations. J. Phys. Condens. Matter 2017, 29, 383001. [Google Scholar] [CrossRef]
- Westphal, K.; Wiczk, J.; Miloch, J.; Kciuk, G.; Bobrowski, K.; Rak, J. Irreversible Electron Attachment—A Key to DNA Damage by Solvated Electrons in Aqueous Solution. Org. Biomol. Chem. 2015, 13, 10362–10369. [Google Scholar] [CrossRef]
- Arumainayagam, C.R.; Lee, H.L.; Nelson, R.B.; Haines, D.R.; Gunawardane, R.P. Low-Energy Electron-Induced Reactions in Condensed Matter. Surf. Sci. Rep. 2010, 65, 1–44. [Google Scholar] [CrossRef]
- Chomicz, L.; Rak, J.; Storoniak, P. Electron-Induced Elimination of the Bromide Anion from Brominated Nucleobases. A Computational Study. J. Phys. Chem. B 2012, 116, 5612–5619. [Google Scholar] [CrossRef]
- Rak, J.; Chomicz, L.; Wiczk, J.; Westphal, K.; Zdrowowicz, M.; Wityk, P.; Żyndul, M.; Makurat, S.; Golon, Ł. Mechanisms of Damage to DNA Labeled with Electrophilic Nucleobases Induced by Ionizing or UV Radiation. J. Phys. Chem. B 2015, 119, 8227–8238. [Google Scholar] [CrossRef]
- Chomicz, L.; Petrovici, A.; Archbold, I.; Adhikary, A.; Kumar, A.; Sevilla, M.D.; Rak, J. An ESR and DFT Study of Hydration of the 2′-Deoxyuridine-5-yl Radical: A Possible Hydroxyl Radical Intermediate. Chem. Commun. 2014, 50, 14605–14608. [Google Scholar] [CrossRef] [Green Version]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, P.; Cao, S.; Zhao, L. The Combination of Curcumin and 5-Fluorouracil in Cancer Therapy. Arch. Pharm. Res. 2018, 41, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Scheer, A.M.; Aflatooni, K.; Gallup, G.A.; Burrow, P.D. Bond Breaking and Temporary Anion States in Uracil and Halouracils: Implications for the DNA Bases. Phys. Rev. Lett. 2004, 92, 068102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira da Silva, F.; Almeida, D.; Antunes, R.; Martins, G.; Nunes, Y.; Eden, S.; Garcia, G.; Limão-Vieira, P. Electron Transfer Processes in Potassium Collisions with 5-Fluorouracil and 5-Chlorouracil. Phys. Chem. Chem. Phys. 2011, 13, 21621. [Google Scholar] [CrossRef] [PubMed]
- Blondel, C.; Delsart, C.; Goldfarb, F. Electron Spectrometry at the \µeV Level and the Electron Affinities of Si and F. J. Phys. B At. Mol. Opt. Phys. 2001, 34, L281–L288. [Google Scholar] [CrossRef]
- Blondel, C.; Cacciani, P.; Delsart, C.; Trainham, R. High-Resolution Determination of the Electron Affinity of Fluorine and Bromine Using Crossed Ion and Laser Beams. Phys. Rev. A 1989, 40, 3698–3701. [Google Scholar] [CrossRef]
- Peláez, R.J.; Blondel, C.; Delsart, C.; Drag, C. Pulsed Photodetachment Microscopy and the Electron Affinity of Iodine. J. Phys. B At. Mol. Opt. Phys. 2009, 42, 125001. [Google Scholar] [CrossRef]
- Kopyra, J.; Keller, A.; Bald, I. On the Role of Fluoro-Substituted Nucleosides in DNA Radiosensitization for Tumor Radiation Therapy. RSC Adv. 2014, 4, 6825–6829. [Google Scholar] [CrossRef] [Green Version]
- Rackwitz, J.; Kopyra, J.; Dąbkowska, I.; Ebel, K.; Ranković, M.L.; Milosavljević, A.R.; Bald, I. Sensitizing DNA Towards Low-Energy Electrons with 2-Fluoroadenine. Angew. Chem. Int. Ed. 2016, 128, 10404–10408. [Google Scholar] [CrossRef]
- Ómarsson, B.; Bjarnason, E.H.; Haughey, S.A.; Field, T.A.; Abramov, A.; Klüpfel, P.; Jónsson, H.; Ingólfsson, O. Molecular Rearrangement Reactions in the Gas Phase Triggered by Electron Attachment. Phys. Chem. Chem. Phys. 2013, 15, 4754. [Google Scholar] [CrossRef]
- Cipriani, M.; Ingólfsson, O. HF Formation through Dissociative Electron Attachment—A Combined Experimental and Theoretical Study on Pentafluorothiophenol and 2-Fluorothiophenol. Int. J. Mol. Sci. 2022, 23, 2430. [Google Scholar] [CrossRef]
- Ómarsson, B.; Ingólfsson, O. Stabilization, Fragmentation and Rearrangement Reactions in Low-Energy Electron Interaction with Tetrafluoro-Para-Benzoquinone: A Combined Theoretical and Experimental Study. Phys. Chem. Chem. Phys. 2013, 15, 16758–16767. [Google Scholar] [CrossRef] [PubMed]
- Wetmore, S.D.; Boyd, R.J.; Eriksson, L.A. A Theoretical Study of 5-Halouracils: Electron Affinities, Ionization Potentials and Dissociation of the Related Anions. Chem. Phys. Lett. 2001, 343, 151–158. [Google Scholar] [CrossRef]
- Izadi, F.; Arthur-Baidoo, E.; Strover, L.T.; Yu, L.-J.; Coote, M.L.; Moad, G.; Denifl, S. Selective Bond Cleavage in RAFT Agents Promoted by Low-Energy Electron Attachment. Angew. Chem. Int. Ed. 2021, 60, 19128–19132. [Google Scholar] [CrossRef] [PubMed]
- Denifl, S.; Matejcik, S.; Ptasinska, S.; Gstir, B.; Probst, M.; Scheier, P.; Illenberger, E.; Mark, T.D. Electron Attachment to Chlorouracil: A Comparison between 6-ClU and 5-ClU. J. Chem. Phys. 2004, 120, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Radisic, D.; Ko, Y.J.; Nilles, J.M.; Stokes, S.T.; Sevilla, M.D.; Rak, J.; Bowen, K.H. Photoelectron Spectroscopic Studies of 5-Halouracil Anions. J. Chem. Phys. 2011, 134, 015101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denifl, S.; Ptasińska, S.; Hanel, G.; Gstir, B.; Probst, M.; Scheier, P.; Märk, T.D. Electron Attachment to Gas-Phase Uracil. J. Chem. Phys. 2004, 120, 6557–6565. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sanche, L.; Sevilla, M.D. Dehalogenation of 5-Halouracils after Low Energy Electron Attachment: A Density Functional Theory Investigation. J. Phys. Chem. A 2002, 106, 11248–11253. [Google Scholar] [CrossRef] [PubMed]
- Spisz, P.; Zdrowowicz, M.; Kozak, W.; Chomicz-Mańka, L.; Falkiewicz, K.; Makurat, S.; Sikorski, A.; Wyrzykowski, D.; Rak, J.; Arthur-Baidoo, E.; et al. Uracil-5-yl O-Sulfamate: An Illusive Radiosensitizer. Pitfalls in Modeling the Radiosensitizing Derivatives of Nucleobases. J. Phys. Chem. B 2020, 124, 5600–5613. [Google Scholar] [CrossRef] [PubMed]
- Denifl, S.; Zappa, F.; Mauracher, A.; Ferreira da Silva, F.; Bacher, A.; Echt, O.; Märk, T.D.; Bohme, D.K.; Scheier, P. Dissociative Electron Attachment to DNA Bases Near Absolute Zero Temperature: Freezing Dissociation Intermediates. ChemPhysChem 2008, 9, 1387–1389. [Google Scholar] [CrossRef]
- Da Silva, F.F.; Matias, C.; Almeida, D.; García, G.; Ingólfsson, O.; Flosadóttir, H.D.; Ómarsson, B.; Ptasinska, S.; Puschnigg, B.; Scheier, P.; et al. NCO-, a Key Fragment upon Dissociative Electron Attachment and Electron Transfer to Pyrimidine Bases: Site Selectivity for a Slow Decay Process. J. Am. Soc. Mass Spectrom. 2013, 24, 1787–1797. [Google Scholar] [CrossRef]
- Kossoski, F.; Bettega, M.H.F.; Varella, M.T.D.N. Shape Resonance Spectra of Uracil, 5-Fluorouracil, and 5-Chlorouracil. J. Chem. Phys. 2014, 140, 024317. [Google Scholar] [CrossRef] [PubMed]
- Kossoski, F.; Varella, M.T.d.N. Negative Ion States of 5-Bromouracil and 5-Iodouracil. Phys. Chem. Chem. Phys. 2015, 17, 17271–17278. [Google Scholar] [CrossRef] [PubMed]
- Ptasinska, S.; Denifl, S.; Scheier, P.; Illenberger, E.; Märk, T.D. Bond- and Site-Selective Loss of H Atoms from Nucleobases by Very-Low-Energy Electrons (<3 EV). Angew. Chem. Int. Ed. 2005, 44, 6941–6943. [Google Scholar] [CrossRef]
- Burrow, P.D.; Gallup, G.A.; Scheer, A.M.; Denifl, S.; Ptasinska, S.; Märk, T.; Scheier, P. Vibrational Feshbach Resonances in Uracil and Thymine. J. Chem. Phys. 2006, 124, 124310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kossoski, F.; Varella, M.T.D.N. How Does Methylation Suppress the Electron-Induced Decomposition of 1-Methyl-Nitroimidazoles? J. Chem. Phys. 2017, 147, 164310. [Google Scholar] [CrossRef] [PubMed]
- Kawarai, Y.; Weber, T.; Azuma, Y.; Winstead, C.; McKoy, V.; Belkacem, A.; Slaughter, D.S. Dynamics of the Dissociating Uracil Anion Following Resonant Electron Attachment. J. Phys. Chem. Lett. 2014, 5, 3854–3858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saqib, M.; Arthur-Baidoo, E.; Ončák, M.; Denifl, S. Electron Attachment Studies with the Potential Radiosensitizer 2-Nitrofuran. Int. J. Mol. Sci. 2020, 21, 8906. [Google Scholar] [CrossRef]
- Meißner, R.; Kočišek, J.; Feketeová, L.; Fedor, J.; Fárník, M.; Limão-Vieira, P.; Illenberger, E.; Denifl, S. Low-Energy Electrons Transform the Nimorazole Molecule into a Radiosensitiser. Nat. Commun. 2019, 10, 2388. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. I. The Atoms Boron through Neon and Hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron Affinities of the First-row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef] [Green Version]
- Purvis, G.D.; Bartlett, R.J. A Full Coupled-cluster Singles and Doubles Model: The Inclusion of Disconnected Triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Simons, J. Analysis of Stabilization and Extrapolation Methods for Determining Energies and Lifetimes of Metastable Electronic States. J. Phys. Chem. A 2021, 125, 7735–7749. [Google Scholar] [CrossRef]
- Arthur-Baidoo, E.; Falkiewicz, K.; Chomicz-Mańka, L.; Czaja, A.; Demkowicz, S.; Biernacki, K.; Kozak, W.; Rak, J.; Denifl, S. Electron-Induced Decomposition of Uracil-5-yl O-(N,N-Dimethylsulfamate): Role of Methylation in Molecular Stability. Int. J. Mol. Sci. 2021, 22, 2344. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-Electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarti, K.; Laporta, V.; Tennyson, J. Calculated Cross Sections for Low Energy Electron Collision with OH. Plasma Sources Sci. Technol. 2019, 28, 85013. [Google Scholar] [CrossRef]
- Laporta, V.; Schneider, I.F.; Tennyson, J. Dissociative Electron Attachment Cross Sections for Ro-Vibrationally Excited NO Molecule and N− Anion Formation. Plasma Sources Sci. Technol. 2020, 29, 10LT01. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016; p. 29. [Google Scholar]
- Arthur-Baidoo, E.; Ameixa, J.; Ziegler, P.; Ferreira da Silva, F.; Ončák, M.; Denifl, S. Reactions in Tirapazamine Induced by the Attachment of Low-Energy Electrons: Dissociation Versus Roaming of OH. Angew. Chem. Int. Ed. 2020, 59, 17177–17181. [Google Scholar] [CrossRef]
- Arthur-Baidoo, E.; Ameixa, J.; Ončák, M.; Denifl, S. Ring-Selective Fragmentation in the Tirapazamine Molecule upon Low-Energy Electron Attachment. Int. J. Mol. Sci. 2021, 22, 3159. [Google Scholar] [CrossRef]
- Rivera, E.; Schuler, R.H. Intermediates in the Reduction of 5-Halouracils by eaq-. J. Phys. Chem. 1983, 87, 3966–3971. [Google Scholar] [CrossRef]
- Neta, P. Electron Spin Resonance Study of Radicals Produced in Irradiated Aqueous Solutions of 5-Halouracils. J. Phys. Chem. 1972, 76, 2399–2402. [Google Scholar] [CrossRef] [PubMed]
- Zdrowowicz, M.; Chomicz-Mańka, L.; Butowska, K.; Spisz, P.; Falkiewicz, K.; Czaja, A.; Rak, J. DNA Damage Radiosensitizers Geared Towards Hydrated Electrons. In Practical Aspects of Computational Chemistry V.; Leszczynski, J., Shukla, M.K., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 125–169. ISBN 978-3-030-83244-5. [Google Scholar]
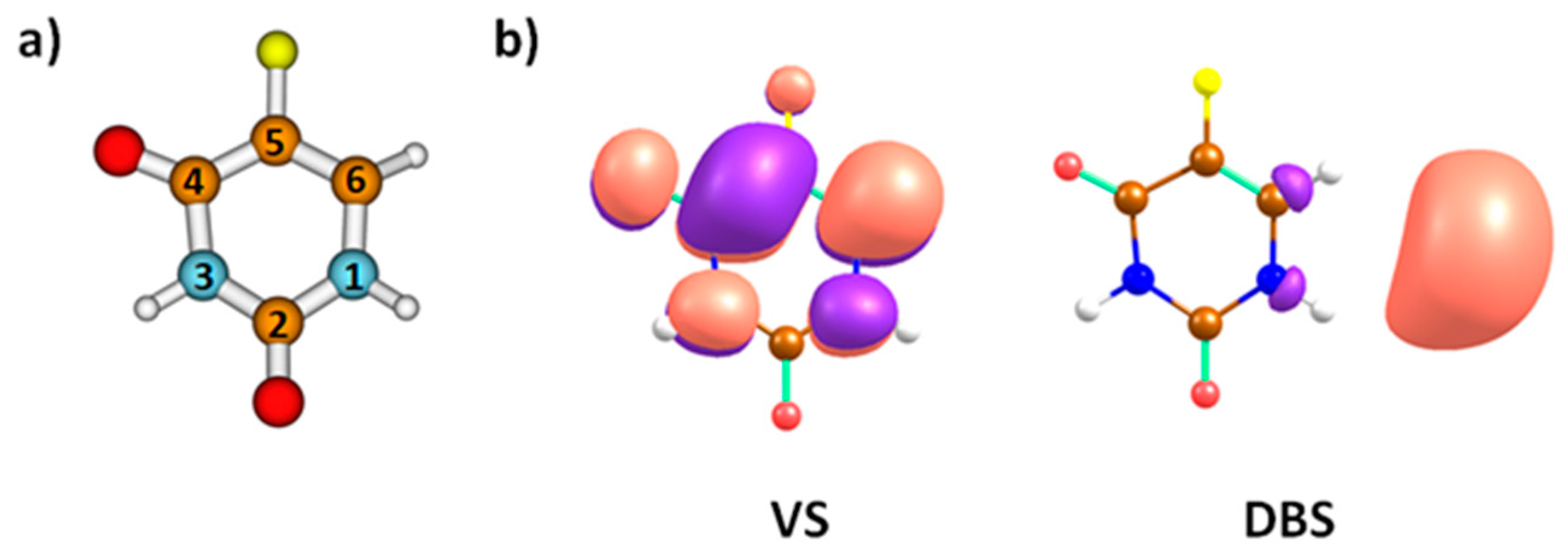

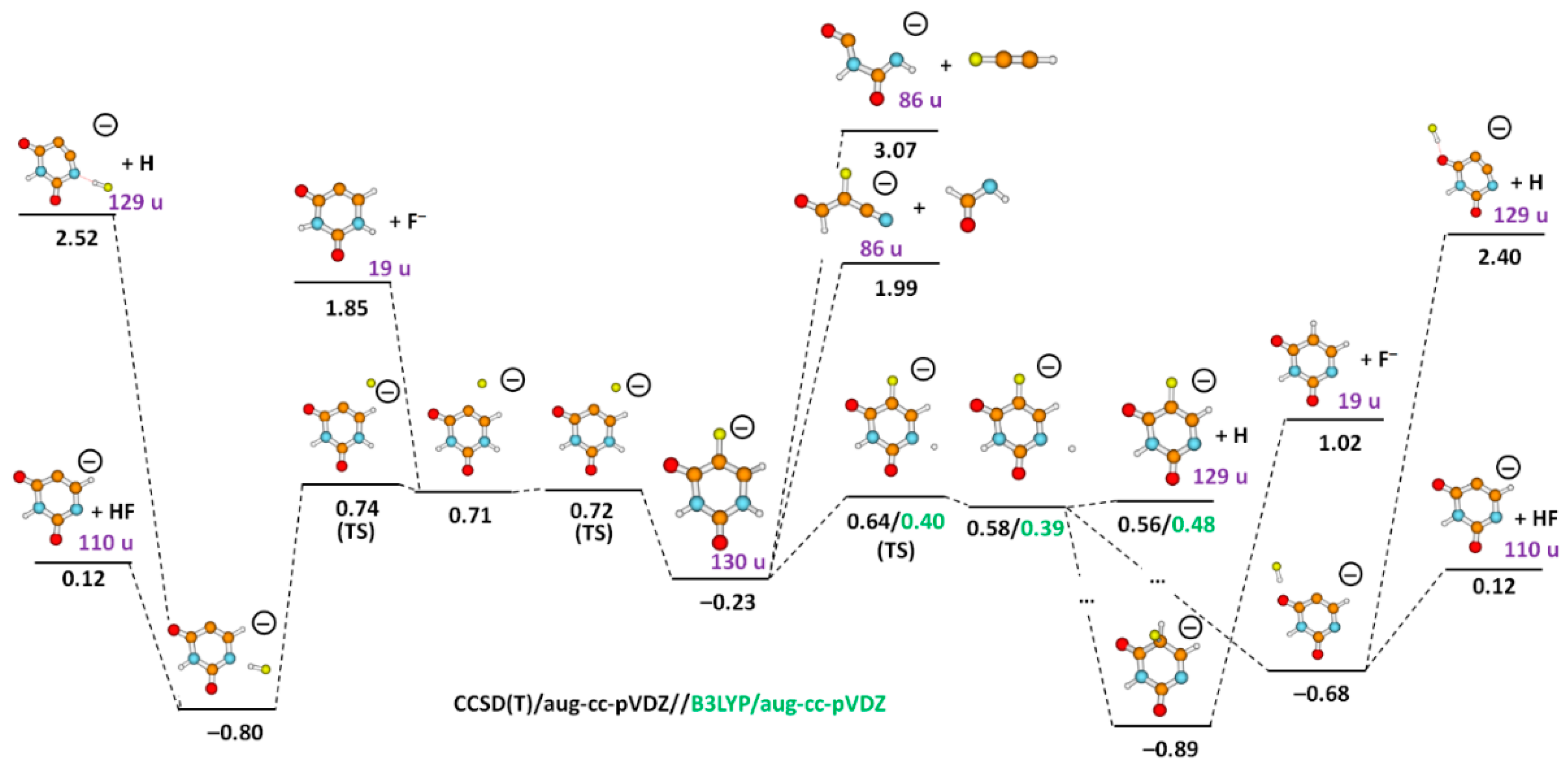
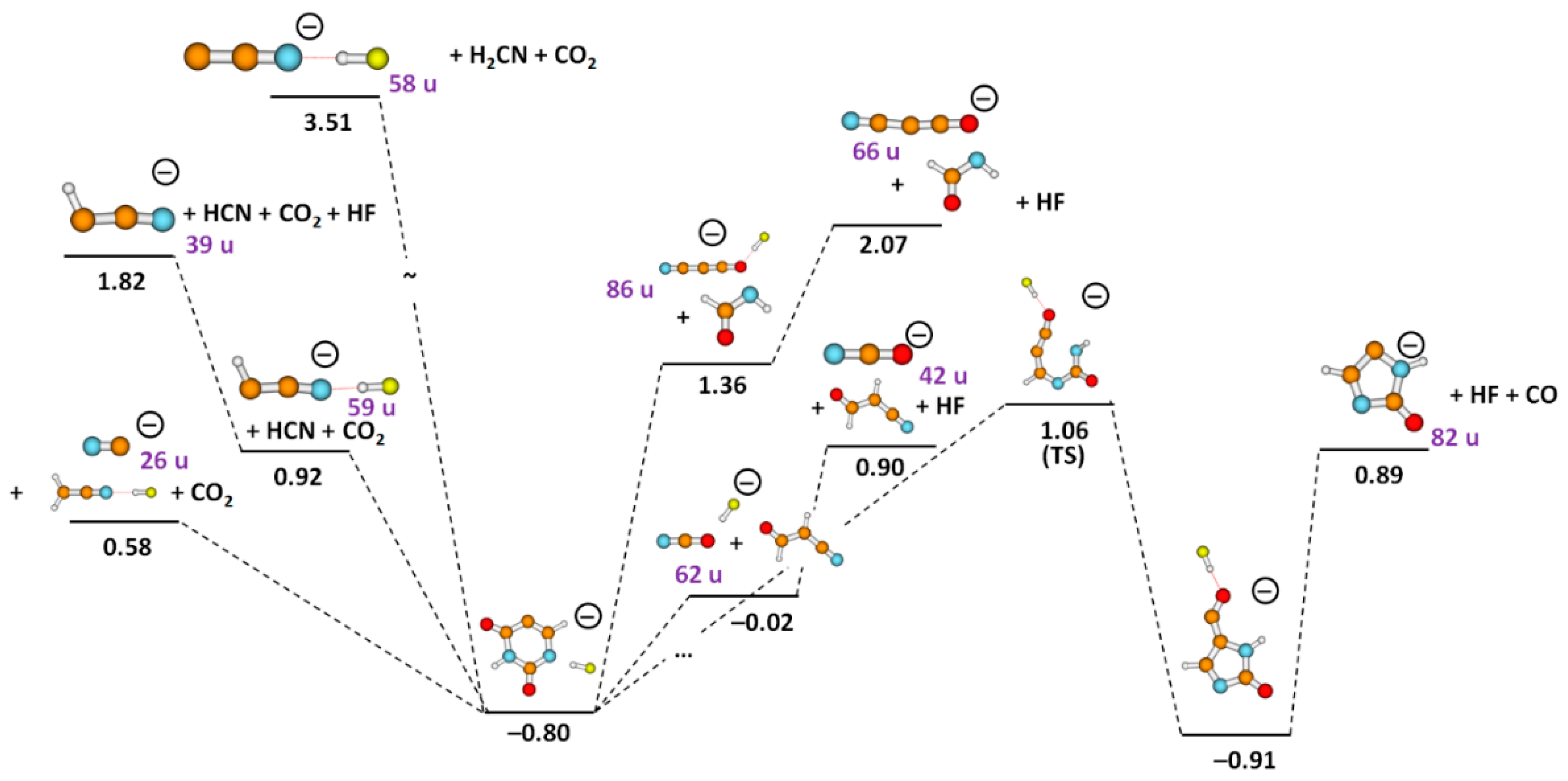
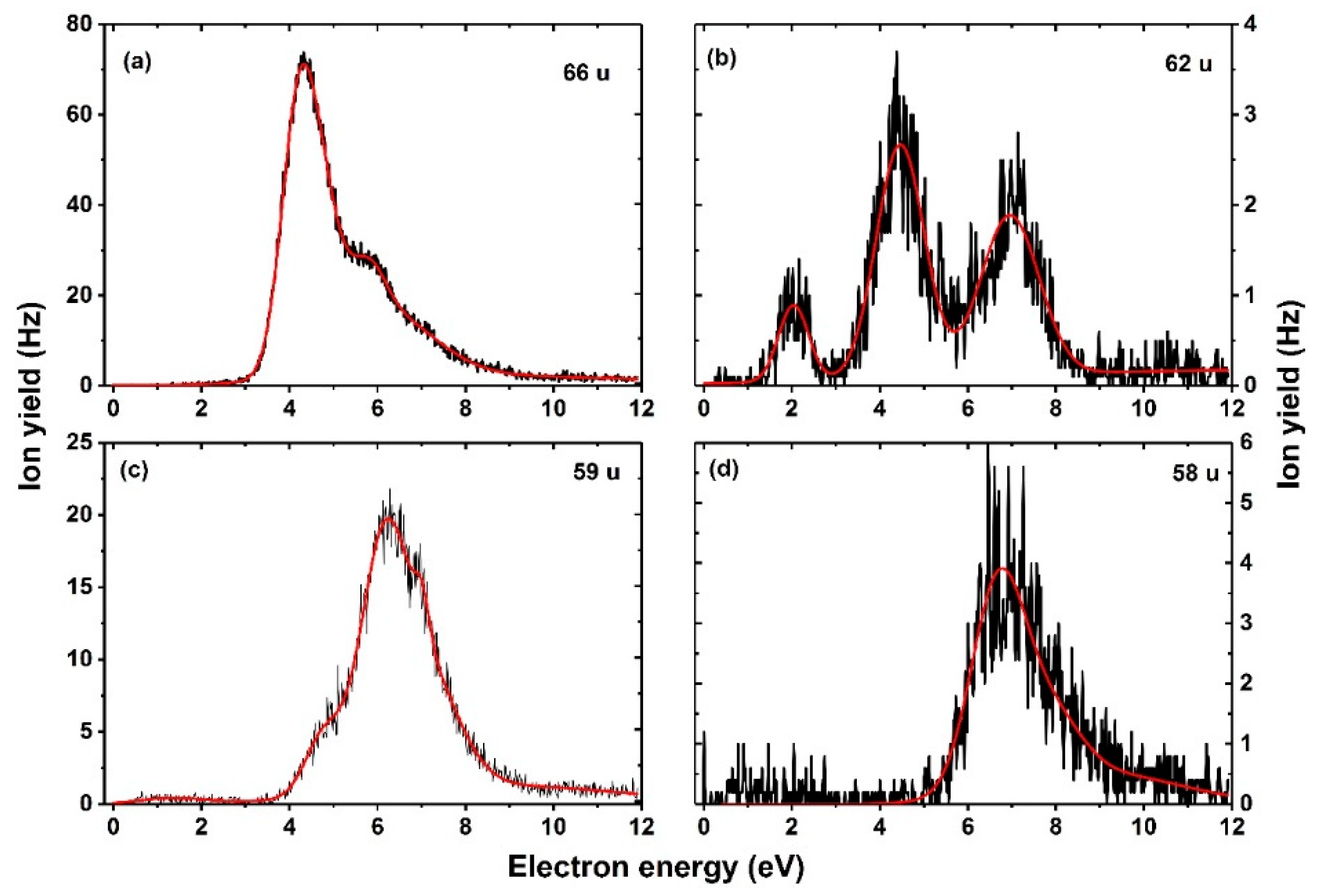
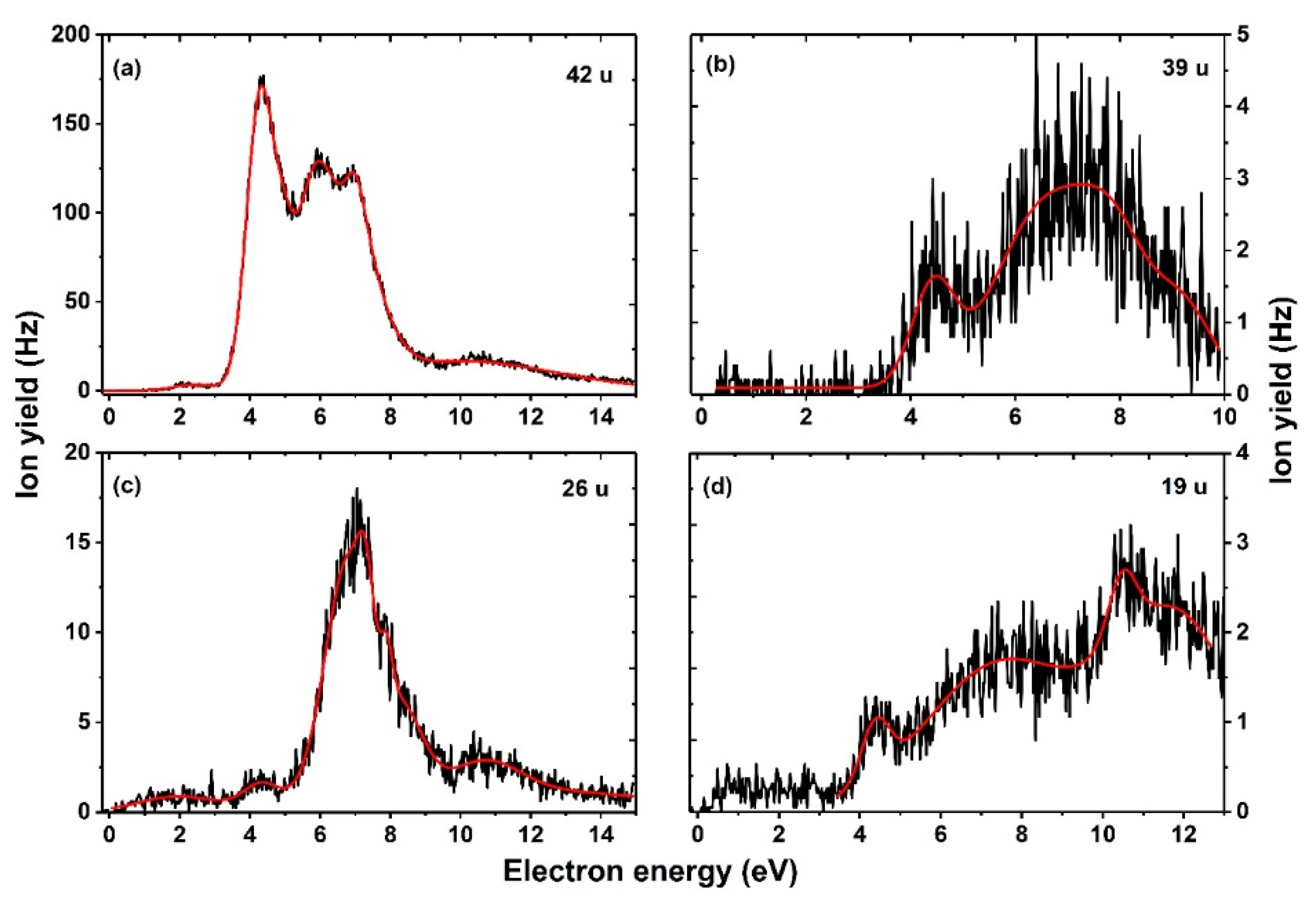
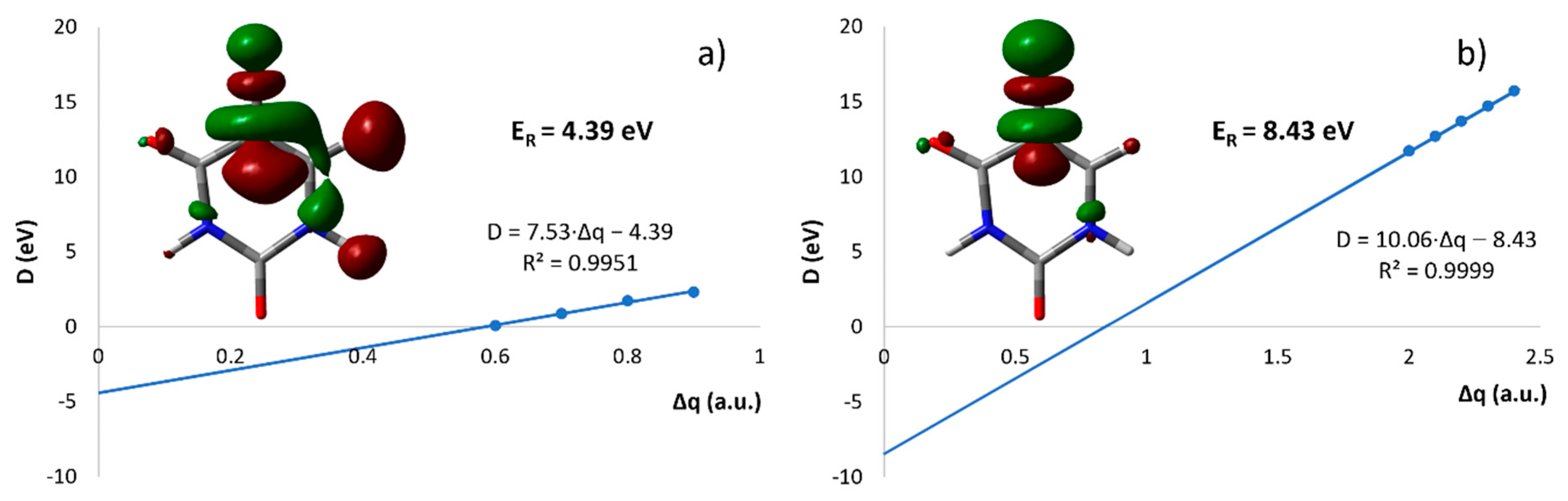
| Mass (u) | Anion | Resonance Maxima (eV) | Threshold (eV) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1. | 2. | 3. | 4. | 5. | 6. | 7. | Exp. | Theory | ||
| 129 | C4FH2N2O2− | 0.6 | 0.7 | 1.0 | 1.4 | 1.6 | 2.1 | 3.7 | 0.4 | 0.56 |
| 110 | C4H2N2O2− | 0.2 | 0.6 | 1.7 | 2.1 | – | – | – | ~0 | 0.12 |
| 86 | HC3FNO−/NC3O.HF−/H2C2N2O2− | 4.1 | 5.9 | 7.2 | – | – | – | – | 3.2 | 1.99/1.36/3.07 |
| 82 | H2C3N2O− | 2.1 | 3.9 | – | – | – | – | – | 1.3 | 0.89 |
| 66 | NC3O− | 4.2 | 4.7 | 5.8 | 6.1 | 8.7 | – | – | 3.2 | 2.07 |
| 62 | NCO−.HF | 2.0 | 4.5 | 7.1 | – | – | – | – | 1.2 | −0.02 |
| 59 | HC2N−.HF | 4.8 | 6.1 | 7.0 | 8.7 | – | – | – | 3.5 | 0.92 |
| 58 | C2N−.HF | 6.8 | 7.2 | 7.9 | 8.3 | – | – | – | 5.2 | 3.51 |
| 42 | NCO− | 2.3 | 4.3 | 5.8 | 7.0 | 7.9 | 10.3 | – | 0.9 | 0.90 |
| 39 | HC2N− | 4.4 | 6.6 | 7.9 | 9.2 | – | – | – | 3.4 | 1.82 |
| 26 | CN− | 4.3 | 6.8 | 7.2 | 7.9 | 8.3 | 10.7 | – | 3.2 | 0.58 |
| 19 | F− | 4.4 | 7.4 | 10.6 | 11.8 | – | – | – | 3.6 | 1.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arthur-Baidoo, E.; Schöpfer, G.; Ončák, M.; Chomicz-Mańka, L.; Rak, J.; Denifl, S. Electron Attachment to 5-Fluorouracil: The Role of Hydrogen Fluoride in Dissociation Chemistry. Int. J. Mol. Sci. 2022, 23, 8325. https://doi.org/10.3390/ijms23158325
Arthur-Baidoo E, Schöpfer G, Ončák M, Chomicz-Mańka L, Rak J, Denifl S. Electron Attachment to 5-Fluorouracil: The Role of Hydrogen Fluoride in Dissociation Chemistry. International Journal of Molecular Sciences. 2022; 23(15):8325. https://doi.org/10.3390/ijms23158325
Chicago/Turabian StyleArthur-Baidoo, Eugene, Gabriel Schöpfer, Milan Ončák, Lidia Chomicz-Mańka, Janusz Rak, and Stephan Denifl. 2022. "Electron Attachment to 5-Fluorouracil: The Role of Hydrogen Fluoride in Dissociation Chemistry" International Journal of Molecular Sciences 23, no. 15: 8325. https://doi.org/10.3390/ijms23158325
APA StyleArthur-Baidoo, E., Schöpfer, G., Ončák, M., Chomicz-Mańka, L., Rak, J., & Denifl, S. (2022). Electron Attachment to 5-Fluorouracil: The Role of Hydrogen Fluoride in Dissociation Chemistry. International Journal of Molecular Sciences, 23(15), 8325. https://doi.org/10.3390/ijms23158325








