Functionalised Anodised Aluminium Oxide as a Biocidal Agent
Abstract
1. Introduction
2. Materials and Methods
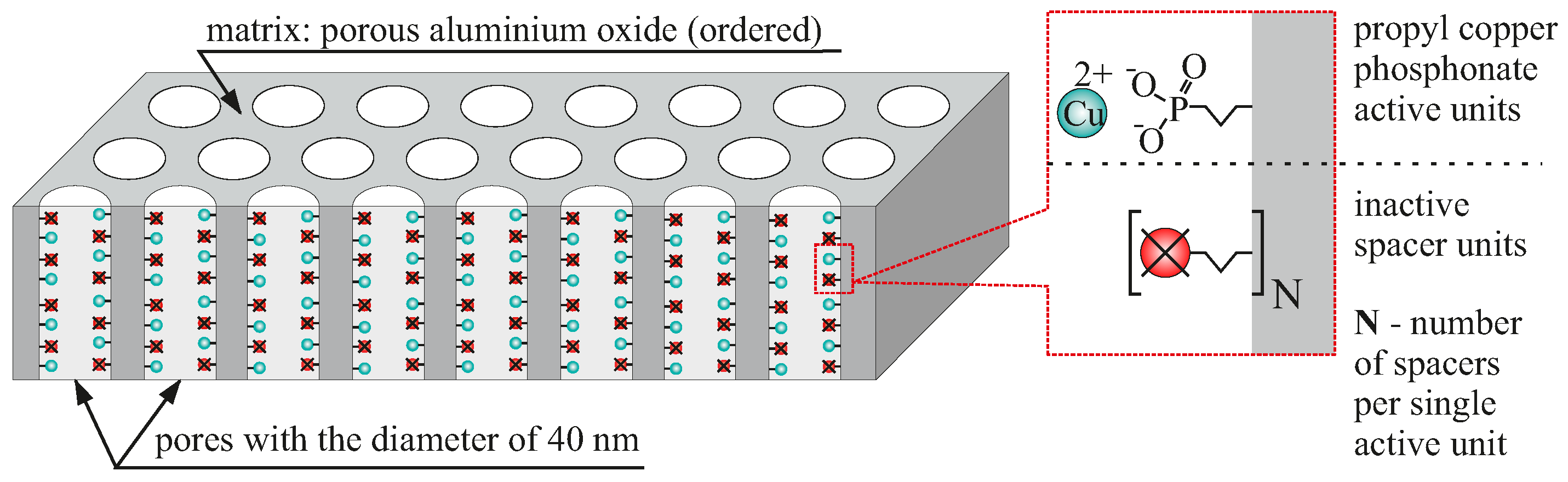
- AAO Cu 4: anodised aluminium oxide with functional copper phosphonate units distributed with the highest possible density (no spacer units between functionalities),
- AAO Cu 3: anodised aluminium oxide with single spacer between functional copper phosphonate units (half the density of AAO Cu 4),
- AAO Cu 2: anodised aluminium oxide with six spacers between functional copper phosphonate units (almost seven times less copper than AAO Cu 4),
- AAO Cu 1: anodised aluminium oxide with twelve spacers between functional copper phosphonate units (almost thirteen times less copper than AAO Cu 4).
2.1. The Preparation of AAO Membranes
2.2. The Functionalisation
2.3. Characterisation Methods
3. Results and Discussion
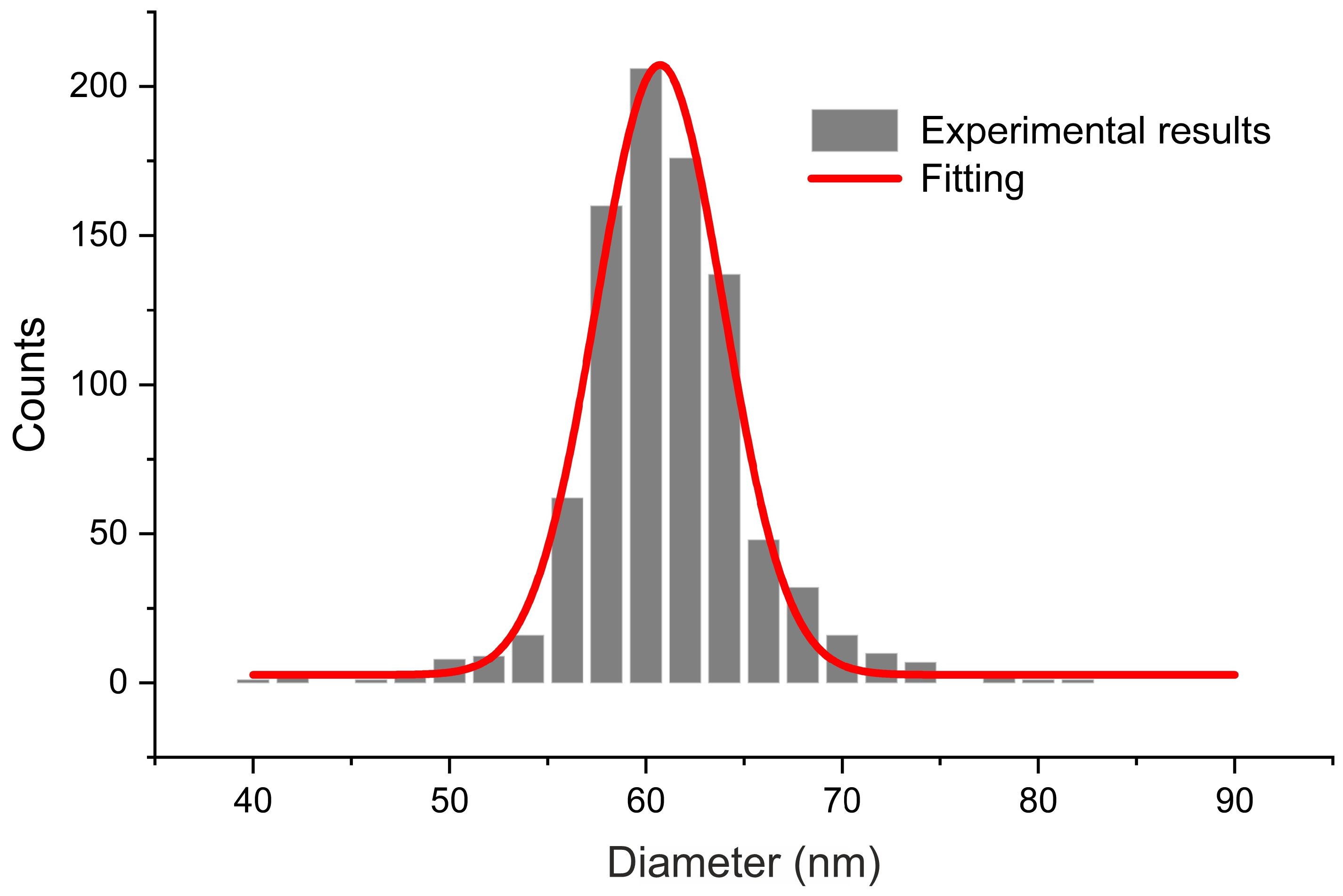
3.1. Morphology
3.2. Differential Pulse Anodic Stripping Voltammetry
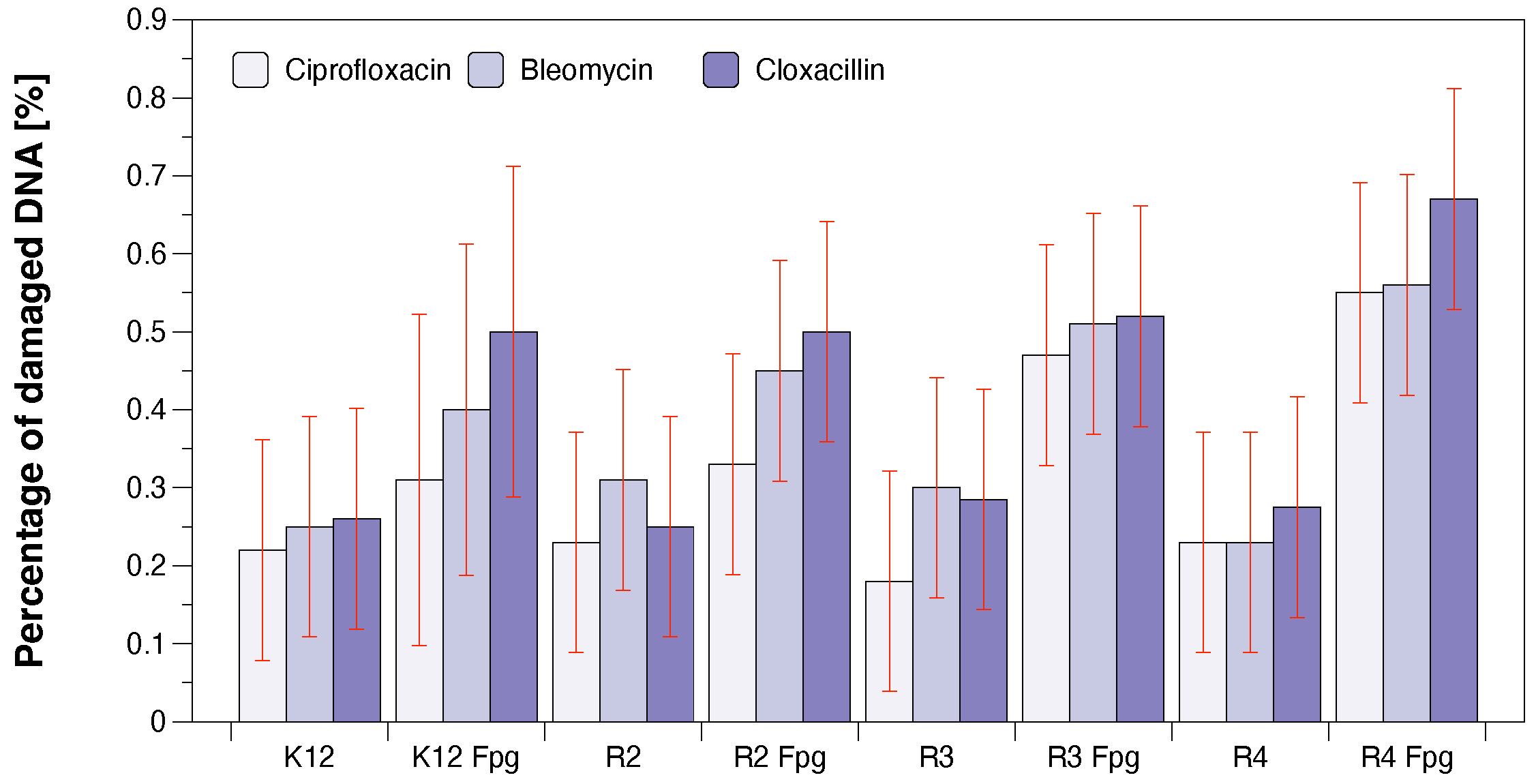
3.3. Antibacterial Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAO | anodic aluminium oxide |
| AO | aluminium oxide |
| Fpg | formamidopyrimidine-DNA glycosylase |
| MIC | minimal inhibitory concentration |
| MBC | minimum bactericidal concentration |
| LPS | lipopolysaccharides |
| DPASV | differential pulse anodic stripping voltammetry |
References
- Ghaemi, F.; Amiri, A.; Bajuri, M.Y.; Yuhana, N.Y.; Ferrara, M. Role of different types of nanomaterials against diagnosis, prevention and therapy of COVID-19. Sustain. Cities Soc. 2021, 72, 103046. [Google Scholar] [CrossRef] [PubMed]
- Quek, J.; Uroro, E.; Goswami, N.; Vasilev, K. Design principles for bacteria-responsive antimicrobial nanomaterials. Mater. Today Chem. 2022, 23, 100606. [Google Scholar] [CrossRef]
- Truong, V.K.; Kobaisi, M.A.; Vasilev, K.; Cozzolino, D.; Chapman, J. Current perspectives for engineering antimicrobial nanostructured materials. Curr. Opin. Biomed. Eng. 2022, 23, 100399. [Google Scholar] [CrossRef]
- Hu, Z.T.; Chen, Y.; Fei, Y.F.; Loo, S.L.; Chen, G.; Hu, M.; Song, Y.; Zhao, J.; Zhang, Y.; Wang, J. An overview of nanomaterial-based novel disinfection technologies for harmful microorganisms: Mechanism, synthesis, devices and application. Sci. Total Environ. 2022, 837, 155720. [Google Scholar] [CrossRef] [PubMed]
- Feger, G.; Angelov, B.; Angelova, A. Prediction of amphiphilic cell-penetrating peptide building blocks from protein-derived amino acid sequences for engineering of drug delivery nanoassemblies. J. Phys. Chem. B 2020, 124, 4069–4078. [Google Scholar] [CrossRef]
- Ogunsona, E.O.; Muthuraj, R.; Ojogbo, E.; Valerio, O.; Mekonnen, T.H. Engineered nanomaterials for antimicrobial applications: A review. Appl. Mater. Today 2020, 18, 100473. [Google Scholar] [CrossRef]
- Ye, L.; Cao, Z.; Liu, X.; Cui, Z.; Li, Z.; Liang, Y.; Zhu, S.; Wu, S. Noble metal-based nanomaterials as antibacterial agents. J. Alloy. Compd. 2022, 904, 164091. [Google Scholar] [CrossRef]
- Sharmin, S.; Rahaman, M.M.; Sarkar, C.; Atolani, O.; Islam, M.T.; Adeyemi, O.S. Nanoparticles as antimicrobial and antiviral agents: A literature-based perspective study. Heliyon 2021, 7, e06456. [Google Scholar] [CrossRef]
- Lei, C.; Deng, J. Hydrogen Peroxide Sensor Based on Coimmobilized Methylene Green and Horseradish Peroxidase in the Same Montmorillonite-Modified Bovine Serum Albumin—Glutaraldehyde Matrix on a Glassy Carbon Electrode Surface. Anal. Chem. 1996, 68, 3344–3349. [Google Scholar] [CrossRef]
- Hazarika, A.; Yadav, M.; Yadav, D.K.; Yadav, H.S. An overview of the role of nanoparticles in sustainable agriculture. Biocatal. Agric. Biotechnol. 2022, 43, 102399. [Google Scholar] [CrossRef]
- Vaid, P.; Raizada, P.; Saini, A.K.; Saini, R.V. Biogenic silver, gold and copper nanoparticles—A sustainable green chemistry approach for cancer therapy. Sustain. Chem. Pharm. 2020, 16, 100247. [Google Scholar] [CrossRef]
- Reddy, G.B.; Mangatayaru, K.G.; Reddy, D.M.; Krishna, S.B.N.; Golla, N. Biosynthesis and characterization methods of copper nanoparticles and their applications in the agricultural sector. In Copper Nanostructures: Next-Generation of Agrochemicals for Sustainable Agroecosystems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 45–80. [Google Scholar] [CrossRef]
- Khezerlou, A.; Alizadeh-Sani, M.; Azizi-Lalabadi, M.; Ehsani, A. Nanoparticles and their antimicrobial properties against pathogens including bacteria, fungi, parasites and viruses. Microb. Pathog. 2018, 123, 505–526. [Google Scholar] [CrossRef]
- Jiang, X.; Bastakoti, B.P.; Weng, W.; Higuchi, T.; Oveisi, H.; Suzuki, N.; Chen, W.J.; Huang, Y.T.; Yamauchi, Y. Preparation of Ordered Mesoporous Alumina-Doped Titania Films with High Thermal Stability and Their Application to High-Speed Passive-Matrix Electrochromic Displays. Chem. A Eur. J. 2013, 19, 10958–10964. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Madej, A.; Paprocki, D.; Szymczak, M.; Ostaszewski, R. Coumarin Derivatives as New Toxic Compounds to Selected K12, R1–R4 E. coli Strains. Materials 2020, 13, 2499. [Google Scholar] [CrossRef]
- Laskowska, M.; Pastukh, O.; Fedorchuk, A.; Schabikowski, M.; Kowalczyk, P.; Zalasiński, M.; Laskowski, Ł. Nanostructured Silica with Anchoring Units: The 2D Solid Solvent for Molecules and Metal Ions. Int. J. Mol. Sci. 2020, 21, 8137. [Google Scholar] [CrossRef]
- Blattner, F.R.; Plunkett, G., III; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The complete genome sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Madej, A.; Szymczak, M.; Ostaszewski, R. α-Amidoamids as New Replacements of Antibiotics—Research on the Chosen K12, R2–R4 E. coli Strains. Materials 2020, 13, 5169. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Trzepizur, D.; Szymczak, M.; Skiba, G.; Kramkowski, K.; Ostaszewski, R. 1,2-Diarylethanols—A New Class of Compounds That Are Toxic to E. coli K12, R2–R4 Strains. Materials 2021, 14, 1025. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Szymczak, M.; Maciejewska, M.; Laskowski, Ł.; Laskowska, M.; Ostaszewski, R.; Skiba, G.; Franiak-Pietryga, I. All that glitters is not silver—A new look at microbiological and medical applications of silver nanoparticles. Int. J. Mol. Sci. 2021, 22, 854. [Google Scholar] [CrossRef]
- Koszelewski, D.; Ostaszewski, R.; Śmigielski, P.; Hrunyk, A.; Kramkowski, K.; Laskowski, Ł.; Laskowska, M.; Lizut, R.; Szymczak, M.; Michalski, J.; et al. Pyridine Derivatives—A New Class of Compounds That Are Toxic to E. coli K12, R2–R4 Strains. Materials 2021, 14, 5401. [Google Scholar] [CrossRef]
- Kowalczyk, P.; Wilk, M.; Parul, P.; Szymczak, M.; Kramkowski, K.; Raj, S.; Skiba, G.; Sulejczak, D.; Kleczkowska, P.; Ostaszewski, R. The Synthesis and Evaluation of Aminocoumarin Peptidomimetics as Cytotoxic Agents on Model Bacterial E. coli Strains. Materials 2021, 14, 5725. [Google Scholar] [CrossRef]
- Samsonowicz-Górski, J.; Kowalczyk, P.; Koszelewski, D.; Brodzka, A.; Szymczak, M.; Kramkowski, K.; Ostaszewski, R. The Synthesis and Evaluation of Amidoximes as Cytotoxic Agents on Model Bacterial E. coli Strains. Materials 2021, 14, 7577. [Google Scholar] [CrossRef]
- Pobłocki, K.; Jacewicz, D.; Walczak, J.; Gawdzik, B.; Kramkowski, K.; Drzeżdżon, J.; Kowalczyk, P. Preparation of Allyl Alcohol Oligomers Using Dipicolinate Oxovanadium (IV) Coordination Compound. Materials 2022, 15, 695. [Google Scholar] [CrossRef] [PubMed]
- Gawdzik, B.; Kowalczyk, P.; Koszelewski, D.; Brodzka, A.; Masternak, J.; Kramkowski, K.; Wypych, A.; Ostaszewski, R. The Evaluation of DHPMs as Biotoxic Agents on Pathogen Bacterial Membranes. Membranes 2022, 12, 238. [Google Scholar] [CrossRef]
- Amouzadeh Tabrizi, M.; Ferre-Borrull, J.; Marsal, L.F. Advances in optical biosensors and sensors using nanoporous anodic alumina. Sensors 2020, 20, 5068. [Google Scholar] [CrossRef]
- Sanz, R.; Hernández-Vélez, M.; Pirota, K.R.; Baldonedo, J.L.; Vázquez, M. Fabrication and magnetic functionalization of cylindrical porous anodic alumina. Small 2007, 3, 434–437. [Google Scholar] [CrossRef]
- Thormann, A.; Teuscher, N.; Pfannmöller, M.; Rothe, U.; Heilmann, A. Nanoporous aluminum oxide membranes for filtration and biofunctionalization. Small 2007, 3, 1032–1040. [Google Scholar] [CrossRef]
- Zaraska, L.; Sulka, G.D.; Jaskuła, M. Anodic alumina membranes with defined pore diameters and thicknesses obtained by adjusting the anodizing duration and pore opening/widening time. J. Solid State Electrochem. 2011, 15, 2427–2436. [Google Scholar] [CrossRef]
- Dobosz, I. Influence of the anodization conditions and chemical treatment on the formation of alumina membranes with defined pore diameters. J. Porous Mater. 2021, 28, 1011–1022. [Google Scholar] [CrossRef]
- Popat, K.C.; Mor, G.; Grimes, C.A.; Desai, T.A. Surface modification of nanoporous alumina surfaces with poly (ethylene glycol). Langmuir 2004, 20, 8035–8041. [Google Scholar] [CrossRef]
- Choi, J.; Sauer, G.; Nielsch, K.; Wehrspohn, R.B.; Gösele, U. Hexagonally arranged monodisperse silver nanowires with adjustable diameter and high aspect ratio. Chem. Mater. 2003, 15, 776–779. [Google Scholar] [CrossRef]
- O’sullivan, J.; Wood, G. The morphology and mechanism of formation of porous anodic films on aluminium. Proc. R. Soc. London A Math. Phys. Sci. 1970, 317, 511–543. [Google Scholar]
- Sulka, G.; Stroobants, S.; Moshchalkov, V.; Borghs, G.; Celis, J.P. Synthesis of well-ordered nanopores by anodizing aluminum foils in sulfuric acid. J. Electrochem. Soc. 2002, 149, D97. [Google Scholar] [CrossRef]
- Masuda, H.; Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science 1995, 268, 1466–1468. [Google Scholar] [CrossRef] [PubMed]
- Alkire, R.C.; Gogotsi, Y.; Simon, P. Nanostructured Materials in Electrochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Briggs, E.P.; Walpole, A.R.; Wilshaw, P.R.; Karlsson, M.; Pålsgård, E. Formation of highly adherent nano-porous alumina on Ti-based substrates: A novel bone implant coating. J. Mater. Sci. Mater. Med. 2004, 15, 1021–1029. [Google Scholar] [CrossRef]
- Hulbert, S. The use of alumina and zirconia in surgical implants. Adv. Ser. Ceram. 1993, 1, 25–40. [Google Scholar]
- Losic, D.; Santos, A. Nanoporous Alumina. Fabrication, Structure, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Porta-i Batalla, M.; Xifré-Pérez, E.; Eckstein, C.; Ferré-Borrull, J.; Marsal, L.F. 3D nanoporous anodic alumina structures for sustained drug release. Nanomaterials 2017, 7, 227. [Google Scholar] [CrossRef]
- Saji, V.S.; Kumeria, T.; Gulati, K.; Prideaux, M.; Rahman, S.; Alsawat, M.; Santos, A.; Atkins, G.J.; Losic, D. Localized drug delivery of selenium (Se) using nanoporous anodic aluminium oxide for bone implants. J. Mater. Chem. B 2015, 3, 7090–7098. [Google Scholar] [CrossRef][Green Version]
- Aw, M.S.; Kurian, M.; Losic, D. Non-eroding drug-releasing implants with ordered nanoporous and nanotubular structures: Concepts for controlling drug release. Biomater. Sci. 2014, 2, 10–34. [Google Scholar]
- Kwak, D.H.; Yoo, J.B.; Kim, D.J. Drug release behavior from nanoporous anodic aluminum oxide. J. Nanosci. Nanotechnol. 2010, 10, 345–348. [Google Scholar] [CrossRef]
- Laskowska, M.; Oyama, M.; Kityk, I.; Marszalek, M.; Dulski, M.; Laskowski, L. Surface functionalization by silver-containing molecules with controlled distribution of functionalities. Appl. Surf. Sci. 2019, 481, 433–436. [Google Scholar] [CrossRef]
- Laskowska, M.; Pastukh, O.; Kuźma, D.; Laskowski, Ł. How to Control the Distribution of Anchored, Mn12–Stearate, Single-Molecule Magnets. Nanomaterials 2019, 9, 1730. [Google Scholar] [CrossRef]
- Dobosz, I.; Uhlemann, M.; Gumowska, W. Al2O3-Me layers obtained by the electrochemical method. Metall. Foundry Eng. 2013, 39, 15–23. [Google Scholar] [CrossRef]
- Nečas, D.; Klapetek, P. Gwyddion: An open-source software for SPM data analysis. Open Phys. 2012, 10, 181–188. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Fedorchuk, A.; Walcarius, A.; Laskowska, M.; Vila, N.; Kowalczyk, P.; Cpałka, K.; Laskowski, Ł. Synthesis of Vertically Aligned Porous Silica Thin Films Functionalized by Silver Ions. Int. J. Mol. Sci. 2021, 22, 7505. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, Ł.; Laskowska, M.; Dulski, M.; Zubko, M.; Jelonkiewicz, J.; Perzanowski, M.; Vilà, N.; Walcarius, A. Multi-step functionalization procedure for fabrication of vertically aligned mesoporous silica thin films with metal-containing molecules localized at the pores bottom. Microporous Mesoporous Mater. 2019, 274, 356–362. [Google Scholar] [CrossRef]
- Available online: https://www.jmp.com/en_gb/home.html (accessed on 3 June 2022).
- Kowalczyk, P.; Gawdzik, B.; Trzepizur, D.; Szymczak, M.; Skiba, G.; Raj, S.; Kramkowski, K.; Lizut, R.; Ostaszewski, R. δ-Lactones—A New Class of Compounds That Are Toxic to E. coli K12 and R2–R4 Strains. Materials 2021, 14, 2956. [Google Scholar] [CrossRef] [PubMed]


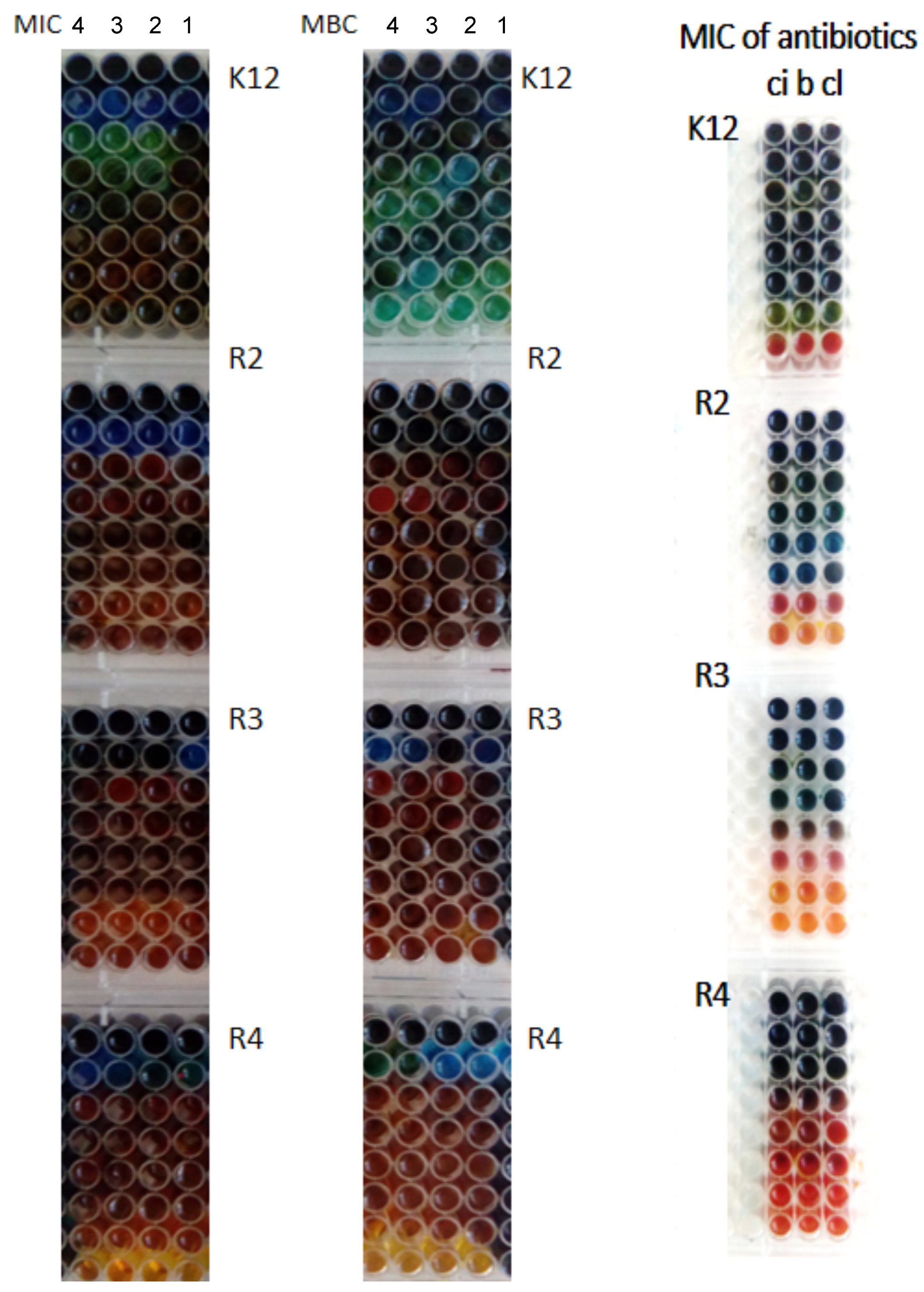


| Strain | AAO Cu 4 | AAO Cu 3 | AAO Cu 2 | AAO Cu 1 | Type of Test |
|---|---|---|---|---|---|
| K12 | * | * | * | ** | MIC |
| R2 | * | * | * | ** | MIC |
| R3 | * | * | * | ** | MIC |
| R4 | * | * | * | ** | MIC |
| K12 | * | * | ** | * | MBC |
| R2 | ** | * | ** | * | MBC |
| R3 | ** | * | ** | * | MBC |
| R4 | ** | * | ** | * | MBC |
| K12 | * | * | * | * | MBC/MIC |
| R2 | * | * | * | * | MBC/MIC |
| R3 | * | * | * | * | MBC/MIC |
| R4 | * | * | * | * | MBC/MIC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schabikowski, M.; Laskowska, M.; Kowalczyk, P.; Fedorchuk, A.; Szőri-Dorogházi, E.; Németh, Z.; Kuźma, D.; Gawdzik, B.; Wypych, A.; Kramkowski, K.; et al. Functionalised Anodised Aluminium Oxide as a Biocidal Agent. Int. J. Mol. Sci. 2022, 23, 8327. https://doi.org/10.3390/ijms23158327
Schabikowski M, Laskowska M, Kowalczyk P, Fedorchuk A, Szőri-Dorogházi E, Németh Z, Kuźma D, Gawdzik B, Wypych A, Kramkowski K, et al. Functionalised Anodised Aluminium Oxide as a Biocidal Agent. International Journal of Molecular Sciences. 2022; 23(15):8327. https://doi.org/10.3390/ijms23158327
Chicago/Turabian StyleSchabikowski, Mateusz, Magdalena Laskowska, Paweł Kowalczyk, Andrii Fedorchuk, Emma Szőri-Dorogházi, Zoltán Németh, Dominika Kuźma, Barbara Gawdzik, Aleksandra Wypych, Karol Kramkowski, and et al. 2022. "Functionalised Anodised Aluminium Oxide as a Biocidal Agent" International Journal of Molecular Sciences 23, no. 15: 8327. https://doi.org/10.3390/ijms23158327
APA StyleSchabikowski, M., Laskowska, M., Kowalczyk, P., Fedorchuk, A., Szőri-Dorogházi, E., Németh, Z., Kuźma, D., Gawdzik, B., Wypych, A., Kramkowski, K., & Laskowski, Ł. (2022). Functionalised Anodised Aluminium Oxide as a Biocidal Agent. International Journal of Molecular Sciences, 23(15), 8327. https://doi.org/10.3390/ijms23158327







