Retinal Toxicity Induced by Chemical Agents
Abstract
1. Introduction
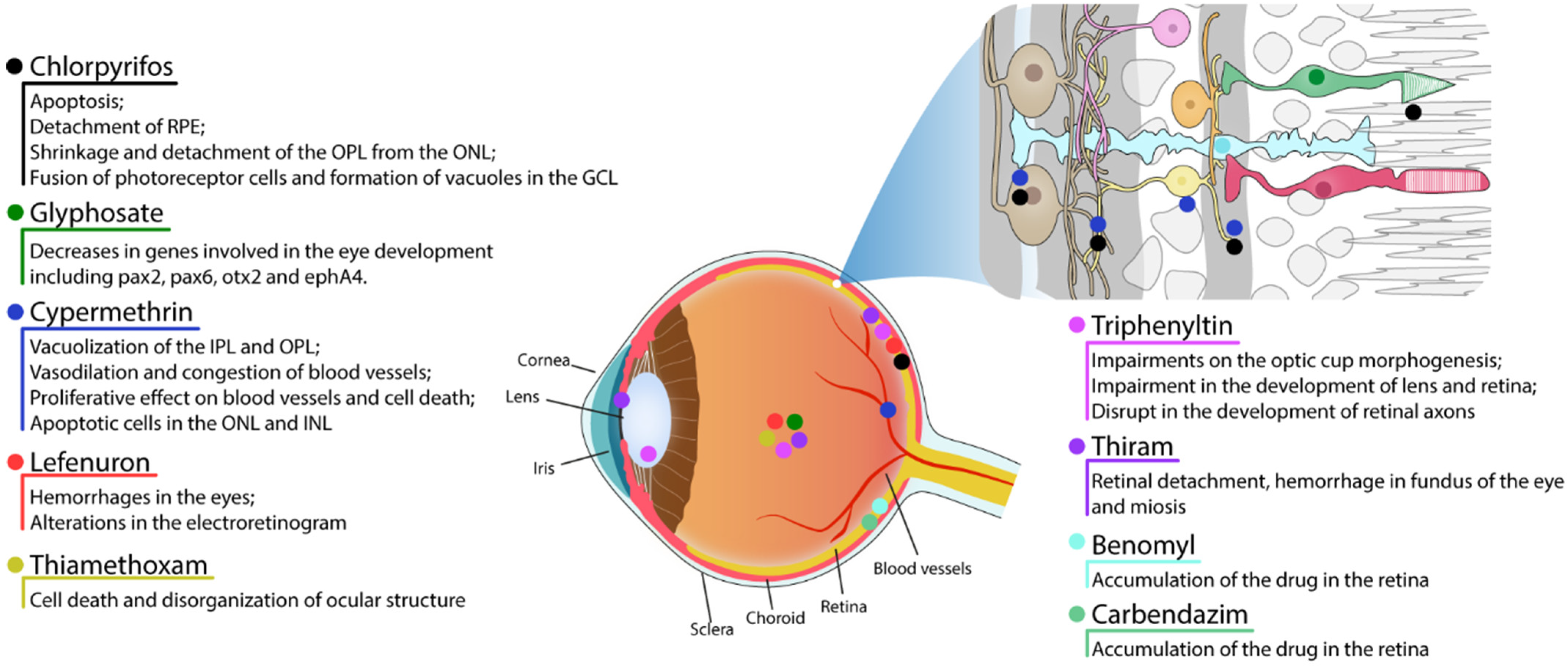
2. Pesticides
2.1. Organophosphates
2.1.1. Chlorpyrifos
2.1.2. Glyphosate
2.2. Pyrethroid
Cypermethrin
2.3. Benzoylurea
Lefenuron
2.4. Neonicotinoid
Thiamethoxam
2.5. Organotin
Triphenyltin
2.6. Organosulfur
Thiram
2.7. Benzimidazoles
Benomyl and Carbendazim
3. Natural Products
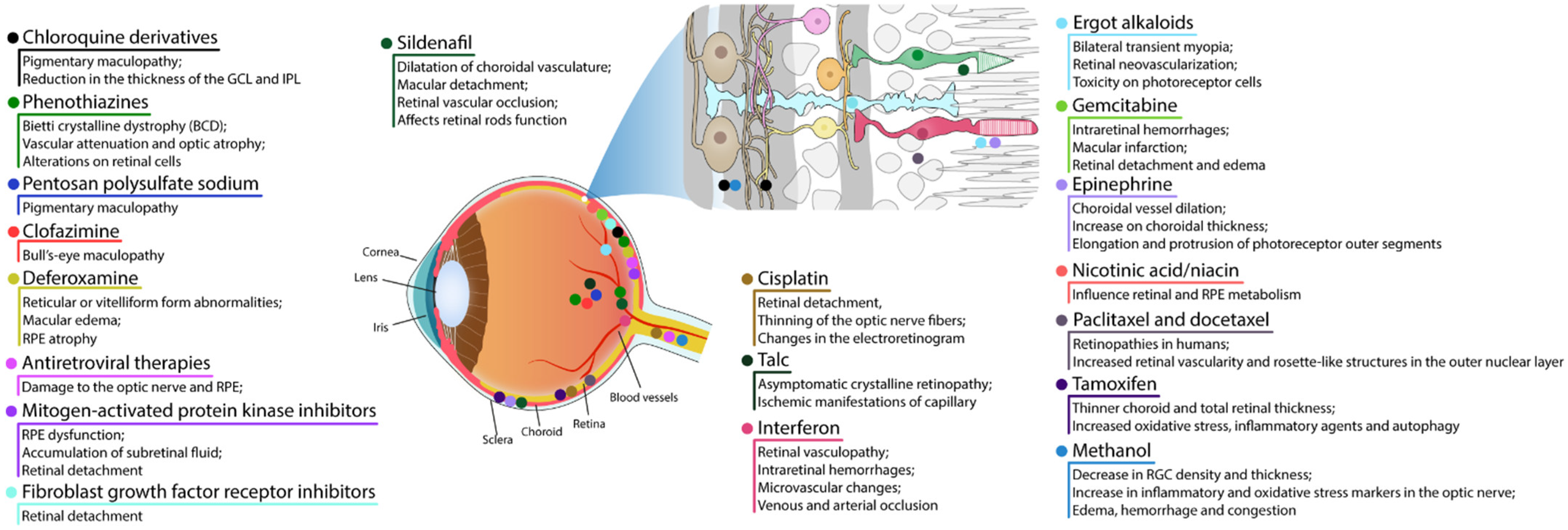
4. Drugs and Medicine
4.1. RPE and Photoreceptor Complex
4.1.1. Chloroquine Derivatives
4.1.2. Phenothiazines
4.1.3. Pentosan Polysulfate Sodium
4.1.4. Clofazimine
4.1.5. Deferoxamine
4.1.6. Antiretroviral Therapies
4.1.7. Mitogen-Activated Protein Kinase Inhibitors
4.1.8. Fibroblast Growth Factor Receptor Inhibitors
4.1.9. Sildenafil
4.1.10. Cisplatin
4.2. Retinal Vascular Damage
4.2.1. Talc
4.2.2. Interferon
4.2.3. Ergot Alkaloids
4.2.4. Gemcitabine
4.3. Cystoid Macular Edema
4.3.1. Epinephrine
4.3.2. Nicotinic Acid/Niacin
4.3.3. Paclitaxel and Docetaxel
4.4. Crystalline Retinopathy
Tamoxifen
4.5. Damage to Ganglion Cell Layer or Optic Nerve
Methanol
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World Report on Vision; Licence: CC BY-NC-SA 3.0 IGO; WHO: Geneva, Switzerland, 2019; p. 180. [Google Scholar]
- Wässle, H. Parallel processing in the mammalian retina. Nat. Rev. Neurosci. 2004, 5, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Hoon, M.; Okawa, H.; Della Santina, L.; Wong, R.O. Functional architecture of the retina: Development and disease. Prog. Retin. Eye Res. 2014, 42, 44–84. [Google Scholar] [CrossRef] [PubMed]
- De Campos, V.S.; Calaza, K.C.; Adesse, D. Implications of TORCH Diseases in Retinal Development-Special Focus on Congenital Toxoplasmosis. Front. Cell Infect. Microbiol. 2020, 10, 585727. [Google Scholar] [CrossRef] [PubMed]
- Thoreson, W.B.; Dacey, D.M. Diverse Cell Types, Circuits, and Mechanisms for Color Vision in the Vertebrate Retina. Physiol. Rev. 2019, 99, 1527–1573. [Google Scholar] [CrossRef]
- Fain, G.; Sampath, A.P. Rod and cone interactions in the retina. F1000Research 2018, 7, 657. [Google Scholar] [CrossRef]
- Coughlin, B.A.; Feenstra, D.J.; Mohr, S. Müller cells and diabetic retinopathy. Vis. Res. 2017, 139, 93–100. [Google Scholar] [CrossRef]
- Newman, E.A. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos. Trans. R. Soc. B 2015, 370, 1672. [Google Scholar] [CrossRef]
- Reichenbach, A.; Bringmann, A. Glia of the human retina. Glia 2020, 68, 768–796. [Google Scholar] [CrossRef]
- Carpi-Santos, R.; de Melo Reis, R.A.; Gomes, F.C.A.; Calaza, K.C. Contribution of Müller Cells in the Diabetic Retinopathy Development: Focus on Oxidative Stress and Inflammation. Antioxidants 2022, 11, 617. [Google Scholar] [CrossRef]
- Subirada, P.V.; Paz, M.C.; Ridano, M.E.; Lorenc, V.E.; Vaglienti, M.V.; Barcelona, P.F.; Luna, J.D.; Sánchez, M.C. A journey into the retina: Müller glia commanding survival and death. Eur. J. Neurosci. 2018, 47, 1429–1443. [Google Scholar] [CrossRef]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia-neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Santos, A.M.; Calvente, R.; Tassi, M.; Carrasco, M.C.; Martín-Oliva, D.; Marín-Teva, J.L.; Navascués, J.; Cuadros, M.A. Embryonic and postnatal development of microglial cells in the mouse retina. J. Comp. Neurol. 2008, 506, 224–239. [Google Scholar] [CrossRef]
- Li, F.; Jiang, D.; Samuel, M.A. Microglia in the developing retina. Neural. Dev. 2019, 14, 12. [Google Scholar] [CrossRef]
- Kinuthia, U.M.; Wolf, A.; Langmann, T. Microglia and Inflammatory Responses in Diabetic Retinopathy. Front. Immunol. 2020, 11, 564077. [Google Scholar] [CrossRef]
- Yu, C.; Roubeix, C.; Sennlaub, F.; Saban, D.R. Microglia versus Monocytes: Distinct Roles in Degenerative Diseases of the Retina. Trends Neurosci. 2020, 43, 433–449. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Y.; Nathans, J. The Norrin/Frizzled4 signaling pathway in retinal vascular development and disease. Trends Mol. Med. 2010, 16, 417–425. [Google Scholar] [CrossRef]
- George, S.M.; Lu, F.; Rao, M.; Leach, L.L.; Gross, J.M. The retinal pigment epithelium: Development, injury responses, and regenerative potential in mammalian and non-mammalian systems. Prog. Retin. Eye Res. 2021, 85, 100969. [Google Scholar] [CrossRef]
- EPA. Reregistration Eligibility Decision (RED) Triphenyltin Hydroxide (TPTH); United States Environmental Protection Agency: Washington, DC, USA, 1999. [Google Scholar]
- Brasil Ministério do Meio Ambiente IBAMA. Relatórios de Comercialização de Agrotóxicos; Brasil Ministério do Meio Ambiente IBAMA: São Paulo, Brazil, 2022. [Google Scholar]
- Panis, C.; Kawassaki, A.C.B.; Crestani, A.P.J.; Pascotto, C.R.; Bortoloti, D.S.; Vicentini, G.E.; Lucio, L.C.; Ferreira, M.O.; Prates, R.T.C.; Vieira, V.K.; et al. Evidence on Human Exposure to Pesticides and the Occurrence of Health Hazards in the Brazilian Population: A Systematic Review. Front. Public Health 2021, 9, 787438. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Amirabadizadeh, A.; Samarghandian, S.; Mehrpour, O. Impact of chlorpyrifos on blood glucose concentration in an animal model: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2020, 27, 2474–2481. [Google Scholar] [CrossRef]
- Montgomery, M.P.; Postel, E.; Umbach, D.M.; Richards, M.; Watson, M.; Blair, A.; Chen, H.; Sandler, D.P.; Schmidt, S.; Kamel, F. Pesticide Use and Age-Related Macular Degeneration in the Agricultural Health Study. Environ. Health Perspect 2017, 125, 77013. [Google Scholar] [CrossRef] [PubMed]
- Kamel, F.; Boyes, W.K.; Gladen, B.C.; Rowland, A.S.; Alavanja, M.C.; Blair, A.; Sandler, D.P. Retinal degeneration in licensed pesticide applicators. Am. J. Ind. Med. 2000, 37, 618–628. [Google Scholar] [CrossRef]
- Padilla, S.; Marshall, R.S.; Hunter, D.L.; Oxendine, S.; Moser, V.C.; Southerland, S.B.; Mailman, R.B. Neurochemical effects of chronic dietary and repeated high-level acute exposure to chlorpyrifos in rats. Toxicol. Sci. 2005, 88, 161–171. [Google Scholar] [CrossRef]
- Geller, A.M.; Sutton, L.D.; Marshall, R.S.; Hunter, D.L.; Madden, V.; Peiffer, R.L. Repeated spike exposure to the insecticide chlorpyrifos interferes with the recovery of visual sensitivity in rats. Doc. Ophthalmol. 2005, 110, 79–90. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Z.; Ju, B.; Wang, Y.; Wang, J.; Bai, D. Apoptotic effect of organophosphorus insecticide chlorpyrifos on mouse retina in vivo via oxidative stress and protection of combination of vitamins C and E. Exp. Toxicol. Pathol. 2008, 59, 415–423. [Google Scholar] [CrossRef]
- Sastry, B.V.; Kambam, S.R.; Singh, G.; Franks, J.J. Nature of cholinesterase in the rat retina. J. Ocul. Pharmacol. 1994, 10, 195–201. [Google Scholar] [CrossRef]
- Calaza Kda, C.; Gardino, P.F. Neurochemical phenotype and birthdating of specific cell populations in the chick retina. An. Acad. Bras. Cienc. 2010, 82, 595–608. [Google Scholar] [CrossRef][Green Version]
- Pereira-Figueiredo, D.; Nascimento, A.A.; Cunha-Rodrigues, M.C.; Brito, R.; Calaza, K.C. Caffeine and Its Neuroprotective Role in Ischemic Events: A Mechanism Dependent on Adenosine Receptors. Cell Mol. Neurobiol 2021, 42, 1693–1725. [Google Scholar] [CrossRef]
- Jasna, J.M.; Anandbabu, K.; Bharathi, S.R.; Angayarkanni, N. Paraoxonase enzyme protects retinal pigment epithelium from chlorpyrifos insult. PLoS ONE 2014, 9, e101380. [Google Scholar] [CrossRef]
- Hernandez, C.M.; Beck, W.D.; Naughton, S.X.; Poddar, I.; Adam, B.L.; Yanasak, N.; Middleton, C.; Terry, A.V., Jr. Repeated exposure to chlorpyrifos leads to prolonged impairments of axonal transport in the living rodent brain. Neurotoxicology 2015, 47, 17–26. [Google Scholar] [CrossRef]
- Gearhart, D.A.; Sickles, D.W.; Buccafusco, J.J.; Prendergast, M.A.; Terry, A.V., Jr. Chlorpyrifos, chlorpyrifos-oxon, and diisopropylfluorophosphate inhibit kinesin-dependent microtubule motility. Toxicol. Appl. Pharmacol. 2007, 218, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, H.; Lockridge, O. Nanoimages show disruption of tubulin polymerization by chlorpyrifos oxon: Implications for neurotoxicity. Toxicol. Appl. Pharmacol. 2009, 240, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, M.A.; Self, R.L.; Smith, K.J.; Ghayoumi, L.; Mullins, M.M.; Butler, T.R.; Buccafusco, J.J.; Gearhart, D.A.; Terry, A.V., Jr. Microtubule-associated targets in chlorpyrifos oxon hippocampal neurotoxicity. Neuroscience 2007, 146, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Duysen, E.G.; Hansen, H.; Shlyakhtenko, L.; Schopfer, L.M.; Lockridge, O. Mice treated with chlorpyrifos or chlorpyrifos oxon have organophosphorylated tubulin in the brain and disrupted microtubule structures, suggesting a role for tubulin in neurotoxicity associated with exposure to organophosphorus agents. Toxicol. Sci. 2010, 115, 183–193. [Google Scholar] [CrossRef]
- Marigoudar, S.R.; Mohan, D.; Nagarjuna, A.; Karthikeyan, P. Biomarker and histopathological responses of Lates calcarifer on exposure to sub lethal concentrations of chlorpyrifos. Ecotoxicol. Environ. Saf. 2018, 148, 327–335. [Google Scholar] [CrossRef]
- Marigoudar, S.R.; Nagarjuna, A.; Karthikeyan, P.; Mohan, D.; Sharma, K.V. Comparative toxicity of chlorpyrifos: Sublethal effects on enzyme activities and histopathology of Mugil cephalus and Chanos chanos. Chemosphere 2018, 211, 89–101. [Google Scholar] [CrossRef]
- Schönbrunn, E.; Eschenburg, S.; Shuttleworth, W.A.; Schloss, J.V.; Amrhein, N.; Evans, J.N.; Kabsch, W. Interaction of the herbicide glyphosate with its target enzyme 5-enolpyruvylshikimate 3-phosphate synthase in atomic detail. Proc. Natl. Acad. Sci. USA 2001, 98, 1376–1380. [Google Scholar] [CrossRef]
- Roy, N.M.; Carneiro, B.; Ochs, J. Glyphosate induces neurotoxicity in zebrafish. Environ. Toxicol. Pharmacol. 2016, 42, 45–54. [Google Scholar] [CrossRef]
- Huang, F.; Chen, Z.; Chen, H.; Lu, W.; Xie, S.; Meng, Q.H.; Wu, Y.; Xia, D. Cypermethrin Promotes Lung Cancer Metastasis via Modulation of Macrophage Polarization by Targeting MicroRNA-155/Bcl6. Toxicol. Sci. 2018, 163, 454–465. [Google Scholar] [CrossRef]
- Madu, E.P. Teratogenic and embryotoxic effects of orally administered cypermethrin in pregnant albino rats. J. Toxicol. Environ. Health Sci. 2015, 7, 60–67. [Google Scholar] [CrossRef][Green Version]
- Mohey Issa, N.; Al-Gholam, M.A. The effect of N-acetylcysteine on the sensory retina of male albino rats exposed prenatally to cypermethrin. Folia Morphol. 2021, 80, 140–148. [Google Scholar] [CrossRef]
- Paravani, E.V.; Simoniello, M.F.; Poletta, G.L.; Zolessi, F.R.; Casco, V.H. Cypermethrin: Oxidative stress and genotoxicity in retinal cells of the adult zebrafish. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2018, 826, 25–32. [Google Scholar] [CrossRef]
- Rafaela Leão Soares, P.; Lucas Corrêa de Andrade, A.; Pinheiro Santos, T.; Caroline Barros Lucas da Silva, S.; Freitas da Silva, J.; Rodrigues Dos Santos, A.; Hugo Lima da Silva Souza, E.; Magliano da Cunha, F.; Wanderley Teixeira, V.; Sales Cadena, M.R.; et al. Acute and chronic toxicity of the benzoylurea pesticide, lufenuron, in the fish, Colossoma macropomum. Chemosphere 2016, 161, 412–421. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Wang, X. Exploring the multilevel hazards of thiamethoxam using Drosophila melanogaster. J. Hazard. Mater. 2020, 384, 121419. [Google Scholar] [CrossRef]
- Antes, F.G.; Krupp, E.; Flores, E.M.; Dressler, V.L.; Feldmann, J. Speciation and degradation of triphenyltin in typical paddy fields and its uptake into rice plants. Environ. Sci Technol. 2011, 45, 10524–10530. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Z.; Wei, Q.; Zhen, H.; Zhao, Y.; Peng, H.; Wan, Y.; Giesy, J.P.; Li, L.; Zhang, B. Malformations of the endangered Chinese sturgeon, Acipenser sinensis, and its causal agent. Proc. Natl. Acad. Sci. USA 2009, 106, 9339–9344. [Google Scholar] [CrossRef]
- Nishikawa, J.; Mamiya, S.; Kanayama, T.; Nishikawa, T.; Shiraishi, F.; Horiguchi, T. Involvement of the retinoid X receptor in the development of imposex caused by organotins in gastropods. Environ. Sci. Technol. 2004, 38, 6271–6276. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, J.; Zhen, H.; Wu, X.; Huang, C. Reproductive inhibition and transgenerational toxicity of triphenyltin on medaka (Oiyzias latipes) at environmentally relevant levels. Environ. Sci. Technol. 2008, 42, 8133–8139. [Google Scholar] [CrossRef]
- Fent, K.; Meier, W. Effects of triphenyltin on fish early life stages. Arch. Environ. Contam. Toxicol. 1994, 27, 224–231. [Google Scholar] [CrossRef]
- Xiao, Y.; Jiang, J.; Hu, W.; Zhao, Y.; Hu, J. Toxicity of triphenyltin on the development of retinal axons in zebrafish at low dose. Aquat. Toxicol. 2017, 189, 9–15. [Google Scholar] [CrossRef]
- Li, P.; Li, Z.H. Toxicity evaluation of triphenyltin in zebrafish larvae by embryonic malformation, retinal development, and GH/IGF axis. Fish Physiol. Biochem. 2020, 46, 2101–2107. [Google Scholar] [CrossRef]
- Short, R.D., Jr.; Russel, J.Q.; Minor, J.L.; Lee, C.C. Developmental toxicity of ferric dimethyldithiocarbamate and bis(dimethylthiocarbamoyl) disulfide in rats and mice. Toxicol. Appl. Pharmacol. 1976, 35, 83–94. [Google Scholar] [CrossRef]
- Hodgson, J.R.; Lee, C.C. Cytotoxicity studies on dithiocarbamate fungicides. Toxicol. Appl. Pharmacol. 1977, 40, 19–22. [Google Scholar] [CrossRef]
- Maita, K.; Tsuda, S.; Shirasu, Y. Chronic toxicity studies with thiram in Wistar rats and beagle dogs. Fundam. Appl. Toxicol. 1991, 16, 667–686. [Google Scholar] [CrossRef]
- Hellman, B.; Laryea, D. Inhibitory action of benzimidazole fungicides on the in vivo incorporation of [3H]thymidine in various organs of the mouse. Food Chem. Toxicol. 1990, 28, 701–706. [Google Scholar] [CrossRef]
- Yamashita, H.; Hoenerhoff, M.J.; Peddada, S.D.; Sills, R.C.; Pandiri, A.R. Chemical Exacerbation of Light-induced Retinal Degeneration in F344/N Rats in National Toxicology Program Rodent Bioassays. Toxicol. Pathol. 2016, 44, 892–903. [Google Scholar] [CrossRef]
- Yamashita, H.; Hoenerhoff, M.J.; Shockley, K.R.; Peddada, S.D.; Gerrish, K.E.; Sutton, D.; Cummings, C.A.; Wang, Y.; Julie, F.F.; Behl, M.; et al. Reduced Disc Shedding and Phagocytosis of Photoreceptor Outer Segment Contributes to Kava Kava Extract-induced Retinal Degeneration in F344/N Rats. Toxicol. Pathol. 2018, 46, 564–573. [Google Scholar] [CrossRef]
- Dong, Q.; Hu, N.; Yue, H.; Wang, H. Inhibitory Activity and Mechanism Investigation of Hypericin as a Novel α-Glucosidase Inhibitor. Molecules 2021, 26, 4566. [Google Scholar] [CrossRef]
- Harris, M.S.; Sakamoto, T.; Kimura, H.; He, S.; Spee, C.; Gopalakrishna, R.; Gundimeda, U.; Yoo, J.S.; Hinton, D.R.; Ryan, S.J. Hypericin inhibits cell growth and induces apoptosis in retinal pigment epithelial cells: Possible involvement of protein kinase C. Curr. Eye Res. 1996, 15, 255–262. [Google Scholar] [CrossRef]
- Wielgus, A.R.; Chignell, C.F.; Miller, D.S.; Van Houten, B.; Meyer, J.; Hu, D.N.; Roberts, J.E. Phototoxicity in human retinal pigment epithelial cells promoted by hypericin, a component of St. John’s wort. Photochem. Photobiol. 2007, 83, 706–713. [Google Scholar] [CrossRef][Green Version]
- Devi Daimary, U.; Girisa, S.; Parama, D.; Verma, E.; Kumar, A.; Kunnumakkara, A.B. Embelin: A novel XIAP inhibitor for the prevention and treatment of chronic diseases. J. Biochem. Mol. Toxicol. 2022, 36, e22950. [Google Scholar] [CrossRef] [PubMed]
- Kifle, Z.D.; Belayneh, Y.M. Antidiabetic and Anti-hyperlipidemic Effects of the Crude Hydromethanol Extract of Hagenia abyssinica (Rosaceae) Leaves in Streptozotocin-Induced Diabetic Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 4085–4094. [Google Scholar] [CrossRef] [PubMed]
- Kifle, Z.D.; Atnafie, S.A.; Yimer Tadesse, T.; Belachew, T.F.; Kidanu, B.B. Methanolic Crude Extract of Hagenia abyssinica Possesses Significant Antidiarrheal Effect: Evidence for In Vivo Antidiarrheal Activity. Evid. Based Complement. Alternat. Med. 2021, 2021, 9944629. [Google Scholar] [CrossRef] [PubMed]
- Low, G.; Rogers, L.J.; Brumley, S.P.; Ehrlich, D. Visual deficits and retinotoxicity caused by the naturally occurring anthelmintics, Embelia ribes and Hagenia abyssinica. Toxicol. Appl. Pharmacol. 1985, 81, 220–230. [Google Scholar] [CrossRef]
- Mandal, M.N.; Patlolla, J.M.; Zheng, L.; Agbaga, M.P.; Tran, J.T.; Wicker, L.; Kasus-Jacobi, A.; Elliott, M.H.; Rao, C.V.; Anderson, R.E. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free Radic. Biol. Med. 2009, 46, 672–679. [Google Scholar] [CrossRef]
- Vasireddy, V.; Chavali, V.R.; Joseph, V.T.; Kadam, R.; Lin, J.H.; Jamison, J.A.; Kompella, U.B.; Reddy, G.B.; Ayyagari, R. Rescue of photoreceptor degeneration by curcumin in transgenic rats with P23H rhodopsin mutation. PLoS ONE 2011, 6, e21193. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.; Guo, H.; Kern, T.S.; Huang, K.; Zheng, L. Curcumin inhibits neuronal and vascular degeneration in retina after ischemia and reperfusion injury. PLoS ONE 2011, 6, e23194. [Google Scholar] [CrossRef]
- Xie, T.; Chen, X.; Chen, W.; Huang, S.; Peng, X.; Tian, L.; Wu, X.; Huang, Y. Curcumin is a Potential Adjuvant to Alleviates Diabetic Retinal Injury via Reducing Oxidative Stress and Maintaining Nrf2 Pathway Homeostasis. Front. Pharmacol. 2021, 12, 796565. [Google Scholar] [CrossRef]
- López-Malo, D.; Villarón-Casares, C.A.; Alarcón-Jiménez, J.; Miranda, M.; Díaz-Llopis, M.; Romero, F.J.; Villar, V.M. Curcumin as a Therapeutic Option in Retinal Diseases. Antioxidants 2020, 9, 48. [Google Scholar] [CrossRef]
- Chandrasekaran, P.R.; Madanagopalan, V.G. Role of Curcumin in Retinal Diseases-A review. Graefes. Arch. Clin. Exp. Ophthalmol. 2022, 260, 1457–1473. [Google Scholar] [CrossRef]
- Lu, H.F.; Lai, K.C.; Hsu, S.C.; Lin, H.J.; Yang, M.D.; Chen, Y.L.; Fan, M.J.; Yang, J.S.; Cheng, P.Y.; Kuo, C.L.; et al. Curcumin induces apoptosis through FAS and FADD, in caspase-3-dependent and -independent pathways in the N18 mouse-rat hybrid retina ganglion cells. Oncol. Rep. 2009, 22, 97–104. [Google Scholar] [CrossRef]
- Lin, H.L.; Yang, J.S.; Yang, J.H.; Fan, S.S.; Chang, W.C.; Li, Y.C.; Chung, J.G. The role of Ca2+ on the DADS-induced apoptosis in mouse-rat hybrid retina ganglion cells (N18). Neurochem. Res. 2006, 31, 383–393. [Google Scholar] [CrossRef]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef]
- Zhang, H.W.; Zhang, H.; Grant, S.J.; Wan, X.; Li, G. Single herbal medicine for diabetic retinopathy. Cochrane Database Syst. Rev. 2018, 12, Cd007939. [Google Scholar] [CrossRef]
- Yusuf, I.H.; Sharma, S.; Luqmani, R.; Downes, S.M. Hydroxychloroquine retinopathy. Eye 2017, 31, 828–845. [Google Scholar] [CrossRef]
- Godinho, G.; Madeira, C.; Falcão, M.; Penas, S.; Dinah-Bragança, T.; Brandão, E.; Carneiro, Â.; Santos-Silva, R.; Falcão-Reis, F.; Beato, J. Longitudinal Retinal Changes Induced by Hydroxychloroquine in Eyes without Retinal Toxicity. Ophthalmic. Res. 2021, 64, 290–296. [Google Scholar] [CrossRef]
- Chen, T.Y.; Lien, W.C.; Cheng, H.L.; Kuan, T.S.; Sheu, S.Y.; Wang, C.Y. Chloroquine inhibits human retina pigmented epithelial cell growth and microtubule nucleation by downregulating p150(glued). J. Cell Physiol. 2019, 234, 10445–10457. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Z.; Liu, X.; Liu, Q.; Wu, M.; Yao, X.; Liu, Y.; Cui, C.; Li, H.; Song, C.; et al. Cytotoxicity Evaluation of Chloroquine and Hydroxychloroquine in Multiple Cell Lines and Tissues by Dynamic Imaging System and Physiologically Based Pharmacokinetic Model. Front. Pharmacol. 2020, 11, 574720. [Google Scholar] [CrossRef]
- Nguyen Hoang, A.T.; Lee, H.; Lee, S.J. Casein kinase I inhibitor D4476 influences autophagy and apoptosis in chloroquine-induced adult retinal pigment epithelial-19 cells. Exp. Eye Res. 2022, 218, 109004. [Google Scholar] [CrossRef]
- Mondal, K.; Porter, H.; Cole, J., 2nd; Pandya, H.K.; Basu, S.K.; Khanam, S.; Chiu, C.Y.; Shah, V.; Stephenson, D.J.; Chalfant, C.E.; et al. Hydroxychloroquine Causes Early Inner Retinal Toxicity and Affects Autophagosome-Lysosomal Pathway and Sphingolipid Metabolism in the Retina. Mol. Neurobiol. 2022, 59, 3873–3887. [Google Scholar] [CrossRef]
- Corradetti, G.; Violanti, S.; Au, A.; Sarraf, D. Wide field retinal imaging and the detection of drug associated retinal toxicity. Int. J. Retin. Vitr. 2019, 5, 26. [Google Scholar] [CrossRef]
- Richa, S.; Yazbek, J.C. Ocular adverse effects of common psychotropic agents: A review. CNS Drugs 2010, 24, 501–526. [Google Scholar] [CrossRef]
- Dorgau, B.; Georgiou, M.; Chaudhary, A.; Moya-Molina, M.; Collin, J.; Queen, R.; Hilgen, G.; Davey, T.; Hewitt, P.; Schmitt, M.; et al. Human Retinal Organoids Provide a Suitable Tool for Toxicological Investigations: A Comprehensive Validation Using Drugs and Compounds Affecting the Retina. Stem. Cells Transl. Med. 2022, 11, 159–177. [Google Scholar] [CrossRef]
- Wang, D.; Au, A.; Gunnemann, F.; Hilely, A.; Scharf, J.; Tran, K.; Sun, M.; Kim, J.H.; Sarraf, D. Pentosan-associated maculopathy: Prevalence, screening guidelines, and spectrum of findings based on prospective multimodal analysis. Can. J. Ophthalmol. 2020, 55, 116–125. [Google Scholar] [CrossRef]
- Hanif, A.M.; Armenti, S.T.; Taylor, S.C.; Shah, R.A.; Igelman, A.D.; Jayasundera, K.T.; Pennesi, M.E.; Khurana, R.N.; Foote, J.E.; O’Keefe, G.A.; et al. Phenotypic Spectrum of Pentosan Polysulfate Sodium-Associated Maculopathy: A Multicenter Study. JAMA Ophthalmol. 2019, 137, 1275–1282. [Google Scholar] [CrossRef]
- Leung, E.H.; Sharma, S.; Levie-Sprick, A.; Lee, G.D.; Cho, H.; Mukkamala, K. Pentosan Polysulfate Sodium-Associated Pigmentary Retinopathy: Risk Factors and Fundus Findings. Clin. Ophthalmol. 2021, 15, 4809–4816. [Google Scholar] [CrossRef]
- Van Bergen, T.; Etienne, I.; Jia, J.; Li, J.P.; Vlodavsky, I.; Stitt, A.; Vermassen, E.; Feyen, J.H.M. Heparanase Deficiency Is Associated with Disruption, Detachment, and Folding of the Retinal Pigment Epithelium. Curr. Eye Res. 2021, 46, 1166–1170. [Google Scholar] [CrossRef]
- Khan, M.J.; Papakostas, T.; Kovacs, K.; Gupta, M.P. Drug-induced maculopathy. Curr. Opin. Ophthalmol. 2020, 31, 563–571. [Google Scholar] [CrossRef]
- Viola, F.; Barteselli, G.; Dell’Arti, L.; Vezzola, D.; Mapelli, C.; Villani, E.; Ratiglia, R. Multimodal imaging in deferoxamine retinopathy. Retina 2014, 34, 1428–1438. [Google Scholar] [CrossRef]
- Sakamoto, K.; Suzuki, T.; Takahashi, K.; Koguchi, T.; Hirayama, T.; Mori, A.; Nakahara, T.; Nagasawa, H.; Ishii, K. Iron-chelating agents attenuate NMDA-Induced neuronal injury via reduction of oxidative stress in the rat retina. Exp. Eye Res. 2018, 171, 30–36. [Google Scholar] [CrossRef]
- Totsuka, K.; Ueta, T.; Uchida, T.; Roggia, M.F.; Nakagawa, S.; Vavvas, D.G.; Honjo, M.; Aihara, M. Oxidative stress induces ferroptotic cell death in retinal pigment epithelial cells. Exp. Eye Res. 2019, 181, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, W.; Shah, A.; Yu, M.; Liu, Y.; He, L.; Dang, J.; Yang, L.; Yan, M.; Ying, Y.; et al. Sodium iodate induces ferroptosis in human retinal pigment epithelium ARPE-19 cells. Cell Death Dis. 2021, 12, 230. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, J.; Wang, Y.; Liu, Z.; Wu, Y. Ferroptosis drives photoreceptor degeneration in mice with defects in all-trans-retinal clearance. J. Biol. Chem. 2021, 296, 100187. [Google Scholar] [CrossRef] [PubMed]
- Abalem, M.F.; Carricondo, P.C.; Rao, R.C. Bullseye Retinopathy from Antiretroviral Therapy. Ophthalmology 2017, 124, 1539. [Google Scholar] [CrossRef]
- Hu, X.; Calton, M.A.; Tang, S.; Vollrath, D. Depletion of Mitochondrial DNA in Differentiated Retinal Pigment Epithelial Cells. Sci. Rep. 2019, 9, 15355. [Google Scholar] [CrossRef]
- Urner-Bloch, U.; Urner, M.; Jaberg-Bentele, N.; Frauchiger, A.L.; Dummer, R.; Goldinger, S.M. MEK inhibitor-associated retinopathy (MEKAR) in metastatic melanoma: Long-term ophthalmic effects. Eur. J. Cancer 2016, 65, 130–138. [Google Scholar] [CrossRef]
- Van Dijk, E.H.C.; Duits, D.E.M.; Versluis, M.; Luyten, G.P.M.; Bergen, A.A.B.; Kapiteijn, E.W.; de Lange, M.J.; Boon, C.J.F.; van der Velden, P.A. Loss of MAPK Pathway Activation in Post-Mitotic Retinal Cells as Mechanism in MEK Inhibition-Related Retinopathy in Cancer Patients. Medicine 2016, 95, e3457. [Google Scholar] [CrossRef]
- Cebulla, C.M.; Kim, B.; George, V.; Heisler-Taylor, T.; Hamadmad, S.; Reese, A.Y.; Kothari, S.S.; Kusibati, R.; Wilson, H.; Abdel-Rahman, M.H. Oral Selumetinib Does Not Negatively Impact Photoreceptor Survival in Murine Experimental Retinal Detachment. Investig. Ophthalmol. Vis. Sci. 2019, 60, 349–357. [Google Scholar] [CrossRef]
- Pellino, A.; Loupakis, F.; Cadamuro, M.; Dadduzio, V.; Fassan, M.; Guido, M.; Cillo, U.; Indraccolo, S.; Fabris, L. Precision medicine in cholangiocarcinoma. Transl. Gastroenterol. Hepatol. 2018, 3, 40. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Alekseev, O.; Ojuok, E.; Cousins, S. Multifocal serous retinopathy with pemigatinib therapy for metastatic colon adenocarcinoma. Int. J. Retin. Vitr. 2021, 7, 34. [Google Scholar] [CrossRef]
- Etminan, M.; Sodhi, M.; Mikelberg, F.S.; Maberley, D. Risk of Ocular Adverse Events Associated with Use of Phosphodiesterase 5 Inhibitors in Men in the US. JAMA Ophthalmol. 2022, 140, 480–484. [Google Scholar] [CrossRef]
- Zahavi, A.; Weiss, S.; Vieyra, M.; Nicholson, J.D.; Muhsinoglu, O.; Barinfeld, O.; Zadok, D.; Goldenberg-Cohen, N. Ocular Effects of Sildenafil in Naïve Mice and a Mouse Model of Optic Nerve Crush. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1987–1995. [Google Scholar] [CrossRef]
- Berkowitz, B.A.; Podolsky, R.H.; Lins Childers, K.; Saadane, A.; Kern, T.S.; Roberts, R.; Olds, H.; Joy, J.; Richards, C.; Rosales, T.; et al. Sildenafil-evoked photoreceptor oxidative stress in vivo is unrelated to impaired visual performance in mice. PLoS ONE 2021, 16, e0245161. [Google Scholar] [CrossRef]
- Umeya, N.; Yoshizawa, Y.; Fukuda, K.; Ikeda, K.; Kamada, M.; Miyawaki, I. Availability of multistep light stimulus method for evaluation of visual dysfunctions. J. Pharmacol. Toxicol. Methods 2019, 96, 27–33. [Google Scholar] [CrossRef]
- Haugnes, H.S.; Bosl, G.J.; Boer, H.; Gietema, J.A.; Brydøy, M.; Oldenburg, J.; Dahl, A.A.; Bremnes, R.M.; Fosså, S.D. Long-term and late effects of germ cell testicular cancer treatment and implications for follow-up. J. Clin. Oncol. 2012, 30, 3752–3763. [Google Scholar] [CrossRef]
- Alkan, A.; Talaz, S. Cilioretinal artery occlusion associated with cisplatin. J. Oncol. Pharm. Pract. 2019, 25, 969–971. [Google Scholar] [CrossRef]
- Dulz, S.; Asselborn, N.H.; Dieckmann, K.P.; Matthies, C.; Wagner, W.; Weidmann, J.; Seidel, C.; Oing, C.; Berger, L.A.; Alsdorf, W.; et al. Retinal toxicity after cisplatin-based chemotherapy in patients with germ cell cancer. J. Cancer Res. Clin. Oncol. 2017, 143, 1319–1325. [Google Scholar] [CrossRef]
- Langevin, S.; Chang, J.S.; Chang, S. Serous retinopathy associated with cisplatin treatment. Retin. Case. Brief Rep. 2019, 13, 211–214. [Google Scholar] [CrossRef]
- Khadka, S.; Byanju, R.; Poon, S. Chemotherapy-Induced Central Retinal Artery Occlusion in Gestational Trophoblastic Neoplasia: Case Report. Int. Med. Case. Rep. J. 2020, 13, 431–435. [Google Scholar] [CrossRef]
- Fındık, H.; Tumkaya, L.; Yılmaz, A.; Gökhan Aslan, M.; Okutucu, M.; Akyildiz, K.; Mercantepe, T. The protective effects of astaxanthin against cisplatin-induced retinal toxicity. Cutan. Ocul. Toxicol. 2019, 38, 59–65. [Google Scholar] [CrossRef]
- Taşlı, N.G.; Uçak, T.; Karakurt, Y.; Keskin Çimen, F.; Özbek Bilgin, A.; Kurt, N.; Süleyman, H. The effects of rutin on cisplatin induced oxidative retinal and optic nerve injury: An experimental study. Cutan. Ocul. Toxicol. 2018, 37, 252–257. [Google Scholar] [CrossRef]
- Karakurt, Y.; Uçak, T.; Tasli, N.; Ahiskali, I.; Şipal, S.; Kurt, N.; Süleyman, H. The effects of lutein on cisplatin-induced retinal injury: An experimental study. Cutan. Ocul. Toxicol. 2018, 37, 374–379. [Google Scholar] [CrossRef]
- Sunar, M.; Yazici, G.N.; Mammadov, R.; Kurt, N.; Arslan, Y.K.; Süleyman, H. Coenzyme Q10 effect on cisplatin-induced oxidative retinal injury in rats. Cutan. Ocul. Toxicol. 2021, 40, 312–318. [Google Scholar] [CrossRef]
- Ibrahim, A.E.; Shafaa, M.W.; Khedr, M.H.; Rashed, R.F. Comparative study between lutein and its liposomal form on cisplatin-induced retinal injury in rabbits. Cutan. Ocul. Toxicol. 2019, 38, 279–285. [Google Scholar] [CrossRef]
- Chen, T.Y.; Huang, B.M.; Tang, T.K.; Chao, Y.Y.; Xiao, X.Y.; Lee, P.R.; Yang, L.Y.; Wang, C.Y. Genotoxic stress-activated DNA-PK-p53 cascade and autophagy cooperatively induce ciliogenesis to maintain the DNA damage response. Cell Death Differ. 2021, 28, 1865–1879. [Google Scholar] [CrossRef]
- Wu, C.M.; Su, F.H.; Muo, C.H.; Huang, J.C.; Wu, M.M.; Yeh, C.C. Analysis of Different Types of Interferon-Associated Retinopathy in Patients with Chronic Hepatitis C Virus Infection Treated with Pegylated Interferon Plus Ribavirin. Viruses 2021, 13, 475. [Google Scholar] [CrossRef]
- Roche, S.L.; Ruiz-Lopez, A.M.; Moloney, J.N.; Byrne, A.M.; Cotter, T.G. Microglial-induced Müller cell gliosis is attenuated by progesterone in a mouse model of retinitis pigmentosa. Glia 2018, 66, 295–310. [Google Scholar] [CrossRef]
- Kutty, R.K.; Samuel, W.; Duncan, T.; Postnikova, O.; Jaworski, C.; Nagineni, C.N.; Redmond, T.M. Proinflammatory cytokine interferon-γ increases the expression of BANCR, a long non-coding RNA, in retinal pigment epithelial cells. Cytokine 2018, 104, 147–150. [Google Scholar] [CrossRef]
- Wei, T.T.; Zhang, M.Y.; Zheng, X.H.; Xie, T.H.; Wang, W.; Zou, J.; Li, Y.; Li, H.Y.; Cai, J.; Wang, X.; et al. Interferon-γ induces retinal pigment epithelial cell Ferroptosis by a JAK1-2/STAT1/SLC7A11 signaling pathway in Age-related Macular Degeneration. FEBS J. 2022, 289, 1968–1983. [Google Scholar] [CrossRef]
- Eshaq, R.S.; Harris, N.R. The role of tumor necrosis factor-α and interferon-γ in the hyperglycemia-induced ubiquitination and loss of platelet endothelial cell adhesion molecule-1 in rat retinal endothelial cells. Microcirculation 2021, 28, e12717. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, M.; Someya, H.; Inada, M.; Nishio, Y.; Takayama, K.; Harimoto, K.; Karasawa, Y.; Ito, M.; Takeuchi, M. Retinal changes in mice spontaneously developing diabetes by Th17-cell deviation. Exp. Eye Res. 2020, 198, 108155. [Google Scholar] [CrossRef] [PubMed]
- Jung, I.; Jung, D.; Zha, Z.; Jeong, J.; Noh, S.; Shin, J.; Park, J.K.; Kim, K.S.; Jeong, Y.; Hur, J.; et al. Interferon-γ inhibits retinal neovascularization in a mouse model of ischemic retinopathy. Cytokine 2021, 143, 155542. [Google Scholar] [CrossRef]
- Silberstein, S.D.; McCrory, D.C. Ergotamine and dihydroergotamine: History, pharmacology, and efficacy. Headache 2003, 43, 144–166. [Google Scholar] [CrossRef] [PubMed]
- Arana, L.A.; Bach, M.B.; Vedana, G.; Volkmann, M.A.; Arana, J. Cefalium-induced bilateral transient myopia, retinal folds, and focal choroidal delay. Retin. Case. Brief Rep. 2018, 12, 118–121. [Google Scholar] [CrossRef]
- Leinonen, H.; Choi, E.H.; Gardella, A.; Kefalov, V.J.; Palczewski, K. A Mixture of U.S. Food and Drug Administration-Approved Monoaminergic Drugs Protects the Retina from Light Damage in Diverse Models of Night Blindness. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1442–1453. [Google Scholar] [CrossRef]
- Kasica, N.; Święch, A.; Saładziak, K.; Mackiewicz, J.; Osęka, M. The Inhibitory Effect of Selected D(2) Dopaminergic Receptor Agonists on VEGF-Dependent Neovascularization in Zebrafish Larvae: Potential New Therapy in Ophthalmic Diseases. Cells 2022, 11, 1202. [Google Scholar] [CrossRef]
- Hu, Q.D.; Xu, L.L.; Gong, Y.; Wu, G.H.; Wang, Y.W.; Wu, S.J.; Zhang, Z.; Mao, W.; Zhou, Y.S.; Li, Q.B.; et al. Lysergic acid diethylamide causes photoreceptor cell damage through inducing inflammatory response and oxidative stress. Cutan. Ocul. Toxicol. 2018, 37, 233–239. [Google Scholar] [CrossRef]
- Chen, K.; He, X.; Li, C.; Ou, Y.; Li, Y.; Lai, J.; Lv, M.; Li, X.; Ran, P.; Li, Y. Lysergic acid diethylamide causes mouse retinal damage by up-regulating p-JAK1/p-STAT1. Cutan. Ocul. Toxicol. 2020, 39, 106–110. [Google Scholar] [CrossRef]
- Jhaj, G.; Jhaj, R.; Shrier, E.M. Gemcitabine-Induced Retinopathy. Retina 2017, 37, e130–e131. [Google Scholar] [CrossRef]
- Behera, U.C.; Modi, R.R.; Sheth, J.; Singh, A. Bilateral macular infarction after gemcitabine and carboplatin chemotherapy. Int. Ophthalmol. 2018, 38, 2195–2198. [Google Scholar] [CrossRef]
- Loscalzo, F.; Balbarrey, M.; Grigera, J.D. Gemcitabine-Associated Retinopathy with Bilateral Exudative Retinal Detachment and Elschnig’s Spots. Ophthalmic. Surg. Lasers Imaging Retin. 2022, 53, 222–226. [Google Scholar] [CrossRef]
- Martins, J.R.; Reichhart, N.; Kociok, N.; Stindl, J.; Foeckler, R.; Lachmann, P.; Todorov, V.; Castrop, H.; Strauß, O. Systemic ß adrenergic stimulation/sympathetic nerve system stimulation influences intraocular RAS through cAMP in the RPE. Exp. Eye Res. 2019, 189, 107828. [Google Scholar] [CrossRef]
- Skarphedinsdottir, S.B.; Eysteinsson, T.; Árnason, S.S. Mechanisms of Ion Transport Across the Mouse Retinal Pigment Epithelium Measured In Vitro. Investig. Ophthalmol. Vis. Sci. 2020, 61, 31. [Google Scholar] [CrossRef]
- Cheong, K.X.; Barathi, V.A.; Teo, K.Y.C.; Chakravarthy, U.; Tun, S.B.B.; Busoy, J.M.; Ho, C.E.H.; Agrawal, R.; Takahashi, K.; Cheung, C.M.G. Choroidal and Retinal Changes After Systemic Adrenaline and Photodynamic Therapy in Non-Human Primates. Investig. Ophthalmol. Vis. Sci. 2021, 62, 25. [Google Scholar] [CrossRef]
- Hazim, R.A.; Volland, S.; Yen, A.; Burgess, B.L.; Williams, D.S. Rapid differentiation of the human RPE cell line, ARPE-19, induced by nicotinamide. Exp. Eye Res. 2019, 179, 18–24. [Google Scholar] [CrossRef]
- Kaya, M.; Atas, F.; Gulsum Guc, Z.; Oztop, I.; Durak, I.; Saatci, A.O. A cross-sectional optical coherence tomography study in patients on taxane-based therapy and a case report with the literature review. Cutan. Ocul. Toxicol. 2020, 39, 287–293. [Google Scholar] [CrossRef]
- Nghiem-Buffet, S.; Cohen, S.Y.; Giocanti-Auregan, A. Docetaxel Retinopathy: A Case Report. Case. Rep. Ophthalmol. 2017, 8, 21–25. [Google Scholar] [CrossRef]
- Torrado, L.A.; Fivgas, G.D. Unilateral cystoid macular edema and bilateral subfoveal hyperreflectivity following docetaxel chemotherapy: A case report. Am. J. Ophthalmol. Case. Rep. 2020, 20, 100995. [Google Scholar] [CrossRef]
- Tapia Quijada, H.E.; Quijada Fumero, E.; Mesa Lugo, F.I.; Serrano García, M.; Betancor Caro, N. Nepafenac for cystoid macular oedema secondary to paclitaxel. Arch. Soc. Esp. Oftalmol. 2021, 96, 434–437. [Google Scholar] [CrossRef]
- Malcolm, J.; Lune Wong, C.O.; Ching, J.; Saidkasimova, S. Paclitaxel may be a risk factor for retinal phototoxicity. Am. J. Ophthalmol. Case. Rep. 2022, 25, 101292. [Google Scholar] [CrossRef]
- Lin, P.K.; Salvador, J.; Xie, J.; Aguera, K.N.; Koller, G.M.; Kemp, S.S.; Griffin, C.T.; Davis, G.E. Selective and Marked Blockade of Endothelial Sprouting Behavior Using Paclitaxel and Related Pharmacologic Agents. Am. J. Pathol. 2021, 191, 2245–2264. [Google Scholar] [CrossRef]
- Cinici, E.; Dilekmen, N.; Kutlu, Z.; Dincer, B.; Cinici, O.; Balta, H.; Calık, I. Carvone protects against paclitaxel-induced retinal and optic nerve cytotoxicity: A histopathological study. Cutan. Ocul. Toxicol. 2019, 38, 290–293. [Google Scholar] [CrossRef]
- Kim, H.A.; Lee, S.; Eah, K.S.; Yoon, Y.H. Prevalence and Risk Factors of Tamoxifen Retinopathy. Ophthalmology 2020, 127, 555–557. [Google Scholar] [CrossRef]
- Doshi, R.R.; Fortun, J.A.; Kim, B.T.; Dubovy, S.R.; Rosenfeld, P.J. Pseudocystic foveal cavitation in tamoxifen retinopathy. Am. J. Ophthalmol. 2014, 157, 1291–1298. [Google Scholar] [CrossRef]
- Hwang, N.; Chung, S.W. Sulfasalazine attenuates tamoxifen-induced toxicity in human retinal pigment epithelial cells. BMB Rep. 2020, 53, 284–289. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Zhang, Y.; Ma, W.; Gonzalez, S.R.; Fan, J.; Kretschmer, F.; Badea, T.C.; Qian, H.H.; Wong, W.T. Tamoxifen Provides Structural and Functional Rescue in Murine Models of Photoreceptor Degeneration. J. Neurosci. 2017, 37, 3294–3310. [Google Scholar] [CrossRef]
- Liberski, S.; Kaluzny, B.J.; Kocięcki, J. Methanol-induced optic neuropathy: A still-present problem. Arch. Toxicol. 2022, 96, 431–451. [Google Scholar] [CrossRef]
- Klein, K.A.; Warren, A.K.; Baumal, C.R.; Hedges, T.R., 3rd. Optical coherence tomography findings in methanol toxicity. Int. J. Retin. Vitr. 2017, 3, 36. [Google Scholar] [CrossRef]
- Fu, J.; Jiao, J.; Weng, K.; Yu, D.; Li, R. Zebrafish methanol exposure causes patterning defects and suppressive cell proliferation in retina. Am. J. Transl. Res. 2017, 9, 2975–2983. [Google Scholar]
- Ghanbari, A.; Ghareghani, M.; Zibara, K.; Delaviz, H.; Ebadi, E.; Jahantab, M.H. Light-Emitting Diode (LED) therapy improves occipital cortex damage by decreasing apoptosis and increasing BDNF-expressing cells in methanol-induced toxicity in rats. Biomed. Pharmacother. 2017, 89, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Laksmita, Y.A.; Sidik, M.; Siregar, N.C.; Nusanti, S. Neuroprotective Effects of Citicoline on Methanol-Intoxicated Retina Model in Rats. J. Ocul. Pharmacol. Ther. 2021, 37, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Taşlı, N.G.; Çimen, F.K.; Karakurt, Y.; Uçak, T.; Mammadov, R.; Süleyman, B.; Kurt, N.; Süleyman, H. Protective effects of Rutin against methanol induced acute toxic optic neuropathy: An experimental study. Int. J. Ophthalmol. 2018, 11, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Ahiskali, I.; Pinar, C.L.; Kiki, M.; Cankaya, M.; Kunak, C.S.; Altuner, D. Effect of taxifolin on methanol-induced oxidative and inflammatory optic nerve damage in rats. Cutan. Ocul. Toxicol. 2019, 38, 384–389. [Google Scholar] [CrossRef]
- Nielsen, M.W.; Alegria, S.; Börjeson, L.; Etzkowitz, H.; Falk-Krzesinski, H.J.; Joshi, A.; Leahey, E.; Smith-Doerr, L.; Woolley, A.W.; Schiebinger, L. Opinion: Gender diversity leads to better science. Proc. Natl. Acad. Sci. USA 2017, 114, 1740–1742. [Google Scholar] [CrossRef]

| Chemical Category | Pesticide | Action |
|---|---|---|
| Organophosphates | Chlorpyrifos | Inhibit AChE |
| Glyphosate | Inhibition of the enzyme 5-enolpyruvylshikimate-3-phosphate synthase | |
| Pyrethroid | Cypermethrin | Data not available |
| Benzoylurea | Lefenuron | Data not available |
| Neonicotinoid | Thiamethoxam | Data not available |
| Organotin | Triphenyltin | Agonist of retinoid X receptors |
| Organosulfur | Thiram | Data not available |
| Benzimidazoles | Benomyl | Data not available |
| Carbendazim | Data not available 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza Monteiro de Araújo, D.; Brito, R.; Pereira-Figueiredo, D.; dos Santos-Rodrigues, A.; De Logu, F.; Nassini, R.; Zin, A.; Calaza, K.C. Retinal Toxicity Induced by Chemical Agents. Int. J. Mol. Sci. 2022, 23, 8182. https://doi.org/10.3390/ijms23158182
Souza Monteiro de Araújo D, Brito R, Pereira-Figueiredo D, dos Santos-Rodrigues A, De Logu F, Nassini R, Zin A, Calaza KC. Retinal Toxicity Induced by Chemical Agents. International Journal of Molecular Sciences. 2022; 23(15):8182. https://doi.org/10.3390/ijms23158182
Chicago/Turabian StyleSouza Monteiro de Araújo, Daniel, Rafael Brito, Danniel Pereira-Figueiredo, Alexandre dos Santos-Rodrigues, Francesco De Logu, Romina Nassini, Andrea Zin, and Karin C. Calaza. 2022. "Retinal Toxicity Induced by Chemical Agents" International Journal of Molecular Sciences 23, no. 15: 8182. https://doi.org/10.3390/ijms23158182
APA StyleSouza Monteiro de Araújo, D., Brito, R., Pereira-Figueiredo, D., dos Santos-Rodrigues, A., De Logu, F., Nassini, R., Zin, A., & Calaza, K. C. (2022). Retinal Toxicity Induced by Chemical Agents. International Journal of Molecular Sciences, 23(15), 8182. https://doi.org/10.3390/ijms23158182






