Actions of Esomeprazole on the Maternal Vasculature in Lean and Obese Pregnant Mice with Impaired Nitric Oxide Synthesis: A Model of Preeclampsia
Abstract
1. Introduction
2. Results
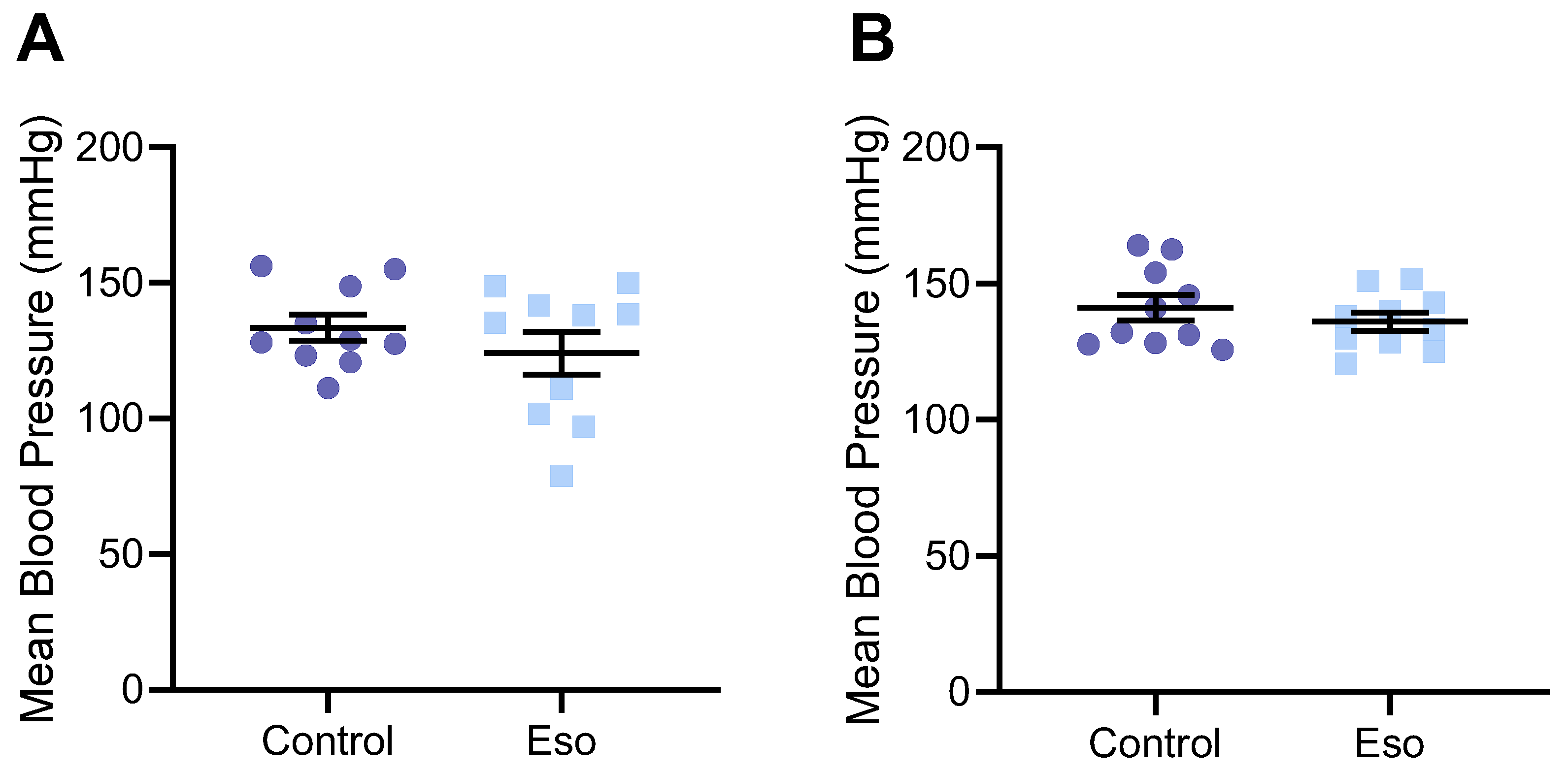
2.1. Esomeprazole Does Not Alter Blood Pressure in Lean Mice
2.2. Esomeprazole Treatment of Lean L-NAME Mice Does Not Alter Vascular Reactivity
2.3. Esomeprazole Treatment of Lean Mice Receiving L-NAME Does Not Alter Circulating Constrictor, Anti-Angiogenic, or Inflammatory Factors
2.4. Esomeprazole Treatment Did Not Improve Fetal or Placental Growth in Lean Mice Receiving L-NAME
2.5. Esomeprazole Treatment Did Not Alter Expression of Genes Involved in Kidney Function and Did Not Improve Histopathological Changes in the Kidney or Liver
2.6. Esomeprazole Did Not Significantly Alter Blood Pressure of Obese Mice Administered L-NAME
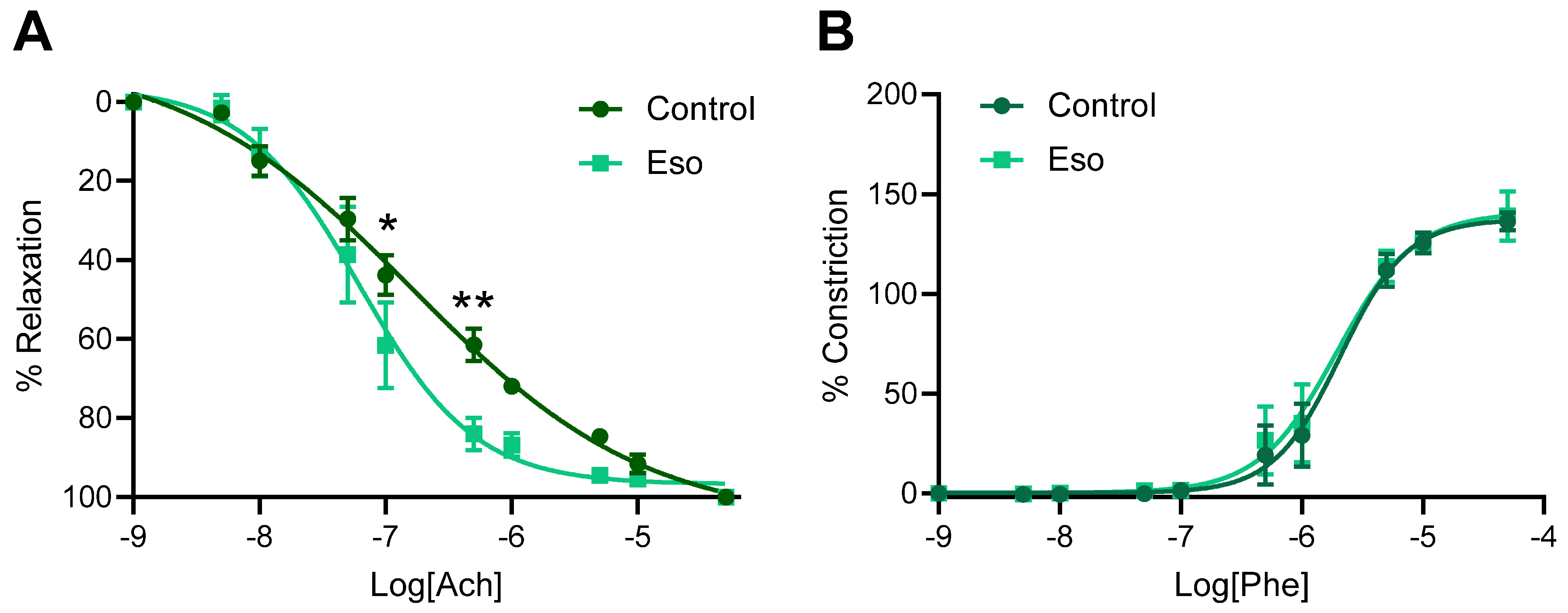
2.7. Vasodilation to Acetylcholine Is Enhanced in Mesenteric Arteries Collected from Obese Mice Receiving L-NAME and Treated with Esomeprazole
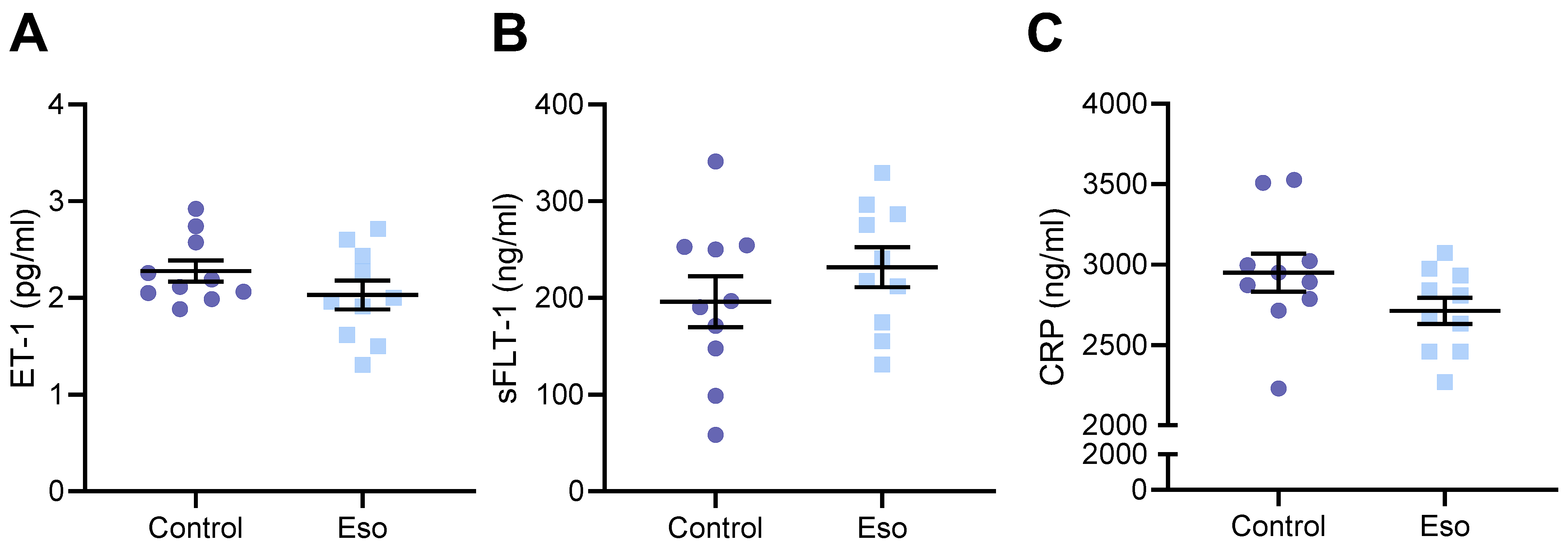
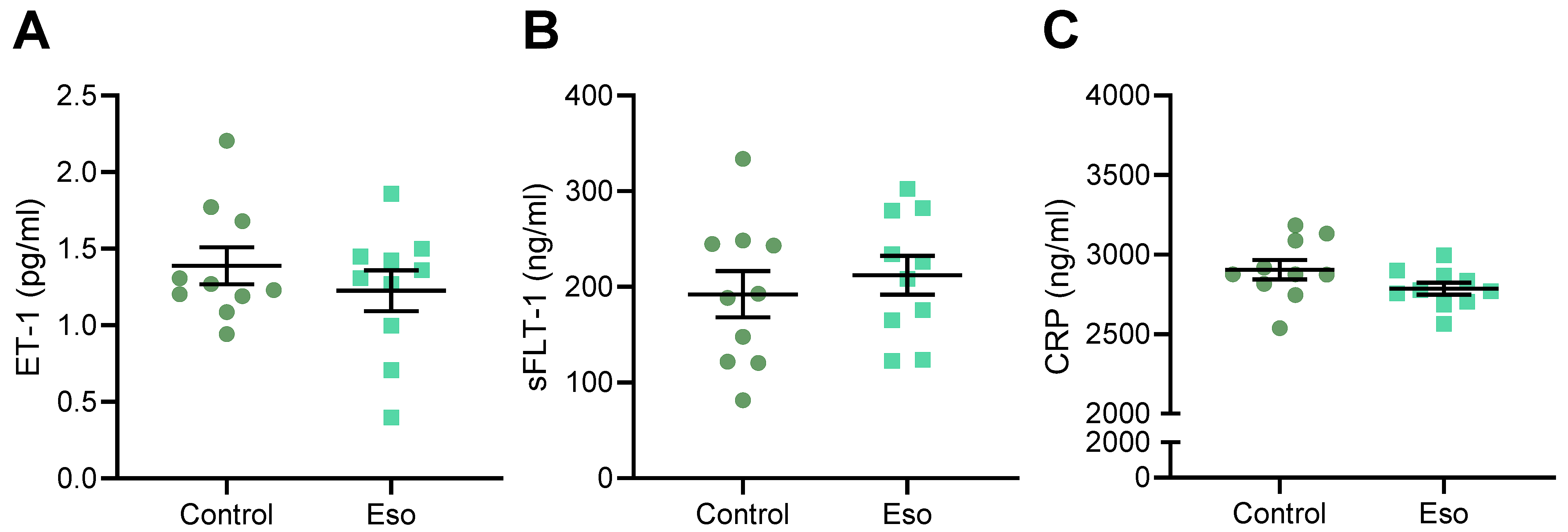
2.8. Esomeprazole Did Not Alter Levels of Circulating ET-1, sFlt-1 or CRP in Obese Mice Administered L-NAME
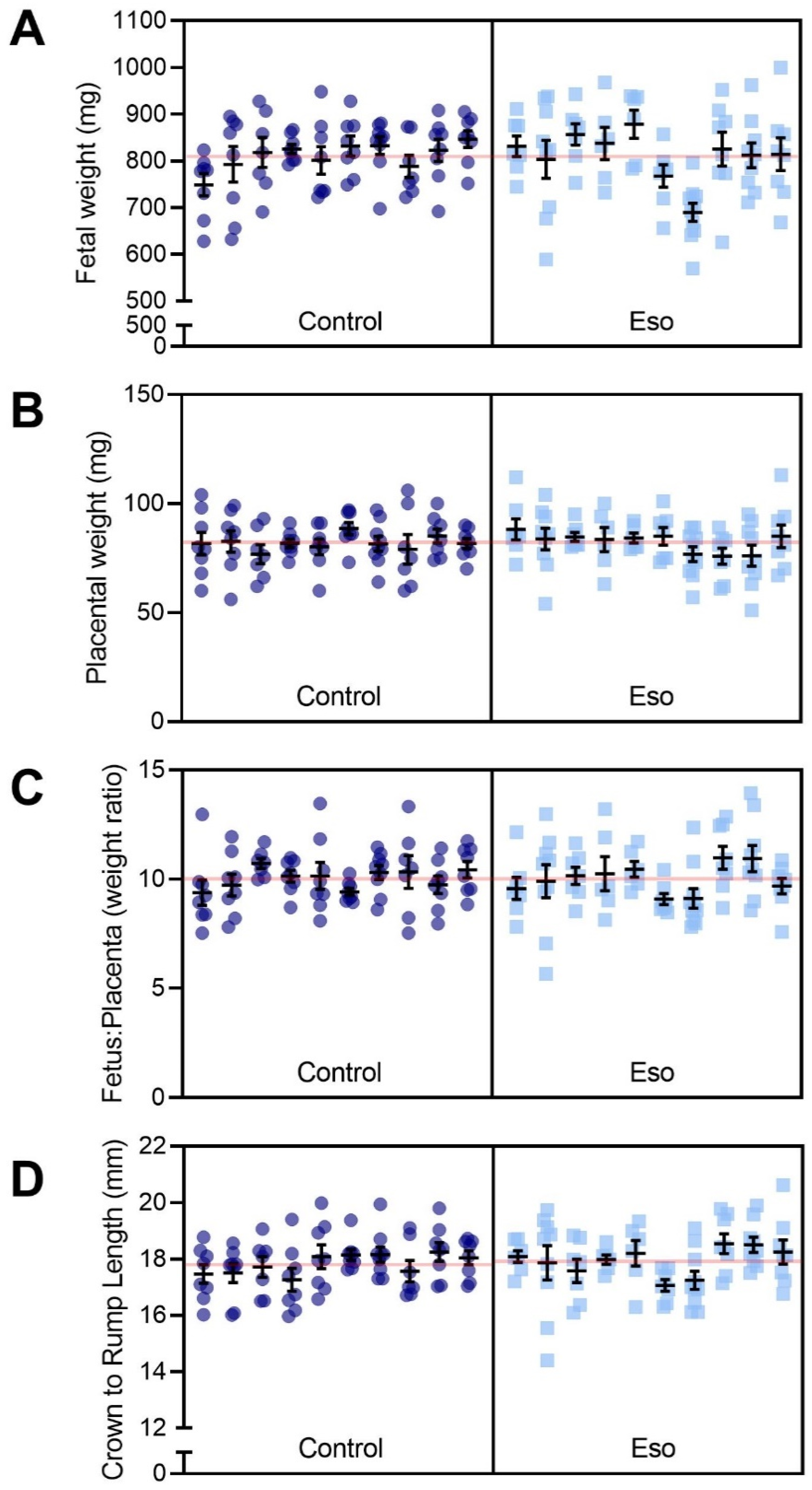
2.9. Esomeprazole Did Not Improve Fetal Size but Reduced Placental Weight in Obese Mice Receiving L-NAME
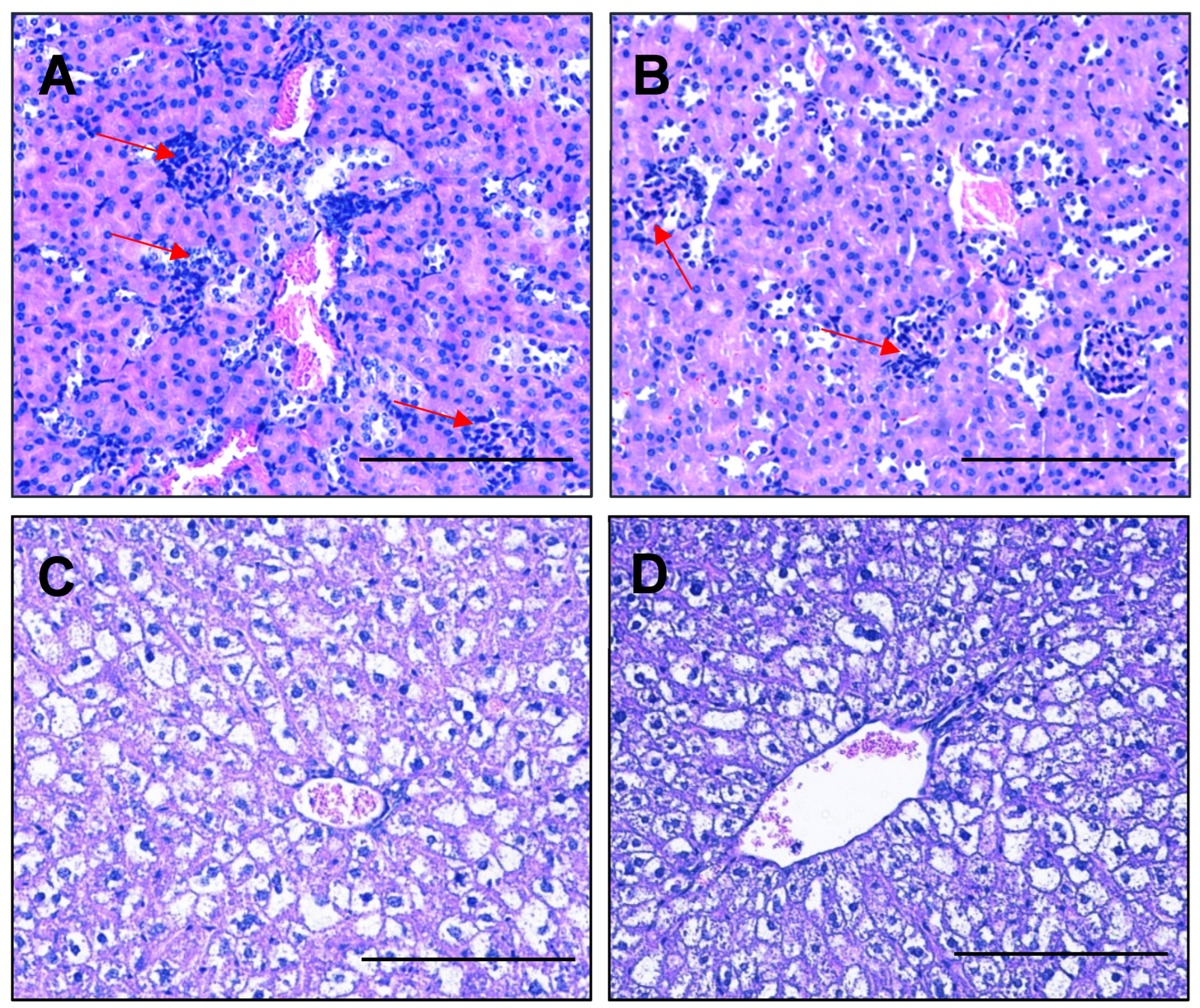
2.10. Esomeprazole Does Not Alter Expression of Genes Involved in Kidney Function, nor Histopathology of the Kidney or Liver in Obese Mice Administered L-NAME
3. Discussion
4. Materials and Methods
4.1. Animal Studies
4.2. L-NAME Mouse Model of Preeclampsia and Esomeprazole Treatment
4.3. Tissue Collection
4.4. Maternal Kidney and Liver Histology
4.5. Vascular Reactivity Studies
4.6. Enzyme Linked Immunosorbent Assay (ELISA)
4.7. Quantitative Polymerase Chain Reaction (qPCR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abalos, E.; Cuesta, C.; Grosso, A.L.; Chou, D.; Say, L. Global and regional estimates of preeclampsia and eclampsia: A systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chappell, L.C.; Cluver, C.A.; Kingdom, J.; Tong, S. Pre-eclampsia. Lancet 2021, 398, 341–354. [Google Scholar] [CrossRef]
- Wright, E.; Audette, M.C.; Ye, X.Y.; Keating, S.; Hoffman, B.; Lye, S.J.; Shah, P.S.; Kingdom, J.C. Maternal Vascular Malperfusion and Adverse Perinatal Outcomes in Low-Risk Nulliparous Women. Obstet. Gynecol. 2017, 130, 1112–1120. [Google Scholar] [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.P.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ. Res. 2019, 124, 1094–1112. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef] [PubMed]
- Saigal, S.; Doyle, L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef]
- Matok, I.; Levy, A.; Wiznitzer, A.; Uziel, E.; Koren, G.; Gorodischer, R. The safety of fetal exposure to proton-pump inhibitors during pregnancy. Dig. Dis. Sci. 2012, 57, 699–705. [Google Scholar] [CrossRef]
- Pasternak, B.; Hviid, A. Use of Proton-Pump Inhibitors in Early Pregnancy and the Risk of Birth Defects. N. Engl. J. Med. 2010, 363, 2114–2123. [Google Scholar] [CrossRef]
- Onda, K.; Tong, S.; Beard, S.; Binder, N.; Muto, M.; Senadheera, S.N.; Parry, L.; Dilworth, M.; Renshall, L.; Brownfoot, F.; et al. Proton Pump Inhibitors Decrease Soluble fms-Like Tyrosine Kinase-1 and Soluble Endoglin Secretion, Decrease Hypertension, and Rescue Endothelial Dysfunction. Hypertension 2017, 69, 457–468. [Google Scholar] [CrossRef]
- Sutton, E.F.; Gemmel, M.; Powers, R.W. Nitric oxide signaling in pregnancy and preeclampsia. Nitric Oxide 2020, 95, 55–62. [Google Scholar] [CrossRef]
- Levine, R.J.; Maynard, S.E.; Qian, C.; Lim, K.H.; England, L.J.; Yu, K.F.; Schisterman, E.F.; Thadhani, R.; Sachs, B.P.; Epstein, F.H.; et al. Circulating angiogenic factors and the risk of preeclampsia. N. Engl. J. Med. 2004, 350, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.D.; Zsengellér, Z.K.; Khankin, E.V.; Lo, A.S.; Rajakumar, A.; DuPont, J.J.; McCurley, A.; Moss, M.E.; Zhang, D.; Clark, C.D.; et al. Soluble fms-like tyrosine kinase 1 promotes angiotensin II sensitivity in preeclampsia. J. Clin. Investig. 2016, 126, 2561–2574. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, L.M.; Ness, R.B.; Markovic, N.; Roberts, J.M. The risk of preeclampsia rises with increasing prepregnancy body mass index. Ann. Epidemiol. 2005, 15, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Callaway, L.K.; Prins, J.B.; Chang, A.M.; McIntyre, H.D. The prevalence and impact of overweight and obesity in an Australian obstetric population. Med. J. Aust. 2006, 184, 56–59. [Google Scholar] [CrossRef]
- WHO. Obesity: Preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and maternal obesity: Epidemiology and health consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef]
- Olson, K.N.; Redman, L.M.; Sones, J.L. Obesity “complements” preeclampsia. Physiol. Genom. 2019, 51, 73–76. [Google Scholar] [CrossRef]
- Virdis, A. Endothelial Dysfunction in Obesity: Role of Inflammation. High. Blood Press. Cardiovasc. Prev. 2016, 23, 83–85. [Google Scholar] [CrossRef]
- Ramsay, J.E.; Ferrell, W.R.; Crawford, L.; Wallace, A.M.; Greer, I.A.; Sattar, N. Maternal Obesity Is Associated with Dysregulation of Metabolic, Vascular, and Inflammatory Pathways. J. Clin. Endocrinol. Metab. 2002, 87, 4231–4237. [Google Scholar] [CrossRef]
- Higashi, Y.; Sasaki, S.; Nakagawa, K.; Matsuura, H.; Chayama, K.; Oshima, T. Effect of obesity on endothelium-dependent, nitric oxide-mediated vasodilation in normotensive individuals and patients with essential hypertension. Am. J. Hypertens 2001, 14, 1038–1045. [Google Scholar] [CrossRef]
- Sansbury, B.E.; Hill, B.G. Regulation of obesity and insulin resistance by nitric oxide. Free Radic. Biol. Med. 2014, 73, 383–399. [Google Scholar] [CrossRef]
- de Alwis, N.; Binder, N.K.; Beard, S.; Mangwiro, Y.T.; Kadife, E.; Cuffe, J.S.; Keenan, E.; Fato, B.R.; Kaitu’u-Lino, T.U.J.; Brownfoot, F.C.; et al. The L-NAME mouse model of preeclampsia and impact to long-term maternal cardiovascular health. Life Sci. Alliance 2022. accepted. [Google Scholar]
- Maynard, S.E.; Min, J.Y.; Merchan, J.; Lim, K.H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Kumru, S.; Godekmerdan, A.; Kutlu, S.; Ozcan, Z. Correlation of maternal serum high-sensitive C-reactive protein levels with biochemical and clinical parameters in preeclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2006, 124, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Young, O.M.; Twedt, R.; Catov, J.M. Pre-pregnancy maternal obesity and the risk of preterm preeclampsia in the American primigravida. Obesity 2016, 24, 1226–1229. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef]
- Choi, J.W.; Im, M.W.; Pai, S.H. Nitric oxide production increases during normal pregnancy and decreases in preeclampsia. Ann. Clin. Lab. Sci. 2002, 32, 257–263. [Google Scholar]
- Seligman, S.P.; Buyon, J.P.; Clancy, R.M.; Young, B.K.; Abramson, S.B. The role of nitric oxide in the pathogenesis of preeclampsia. Am. J. Obstet. Gynecol. 1994, 171, 944–948. [Google Scholar] [CrossRef]
- Davidge, S.T.; Stranko, C.P.; Roberts, J.M. Urine but not plasma nitric oxide metabolites are decreased in women with preeclampsia. Am. J. Obstet. Gynecol. 1996, 174, 1008–1013. [Google Scholar] [CrossRef]
- Schiessl, B.; Strasburger, C.; Bidlingmaier, M.; Mylonas, I.; Jeschke, U.; Kainer, F.; Friese, K. Plasma- and urine concentrations of nitrite/nitrate and cyclic Guanosinemonophosphate in intrauterine growth restricted and preeclamptic pregnancies. Arch. Gynecol. Obstet. 2006, 274, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Hodžić, J.; Izetbegović, S.; Muračević, B.; Iriškić, R.; Štimjanin Jović, H. Nitric oxide biosynthesis during normal pregnancy and pregnancy complicated by preeclampsia. Med. Glas. 2017, 14, 211–217. [Google Scholar] [CrossRef]
- Silver, R.K.; Kupferminc, M.J.; Russell, T.L.; Adler, L.; Mullen, T.A.; Caplan, M.S. Evaluation of nitric oxide as a mediator of severe preeclampsia. Am. J. Obstet. Gynecol. 1996, 175, 1013–1017. [Google Scholar] [CrossRef]
- Smárason, A.K.; Allman, K.G.; Young, D.; Redman, C.W. Elevated levels of serum nitrate, a stable end product of nitric oxide, in women with pre-eclampsia. Br. J. Obstet. Gynaecol. 1997, 104, 538–543. [Google Scholar] [CrossRef]
- Nobunaga, T.; Tokugawa, Y.; Hashimoto, K.; Kimura, T.; Matsuzaki, N.; Nitta, Y.; Fujita, T.; Kidoguchi, K.I.; Azuma, C.; Saji, F. Plasma nitric oxide levels in pregnant patients with preeclampsia and essential hypertension. Gynecol. Obstet. Investig. 1996, 41, 189–193. [Google Scholar] [CrossRef]
- Pathak, N.; Sawhney, H.; Vasishta, K.; Majumdar, S. Estimation of oxidative products of nitric oxide (nitrates, nitrites) in preeclampsia. Aust. N. Z. J. Obstet. Gynaecol. 1999, 39, 484–487. [Google Scholar] [CrossRef]
- Pfeiffer, S.; Leopold, E.; Schmidt, K.; Brunner, F.; Mayer, B. Inhibition of nitric oxide synthesis by NG-nitro-L-arginine methyl ester (L-NAME): Requirement for bioactivation to the free acid, NG-nitro-L-arginine. Br. J. Pharmacol. 1996, 118, 1433–1440. [Google Scholar] [CrossRef]
- Rajapakse, N.W.; Karim, F.; Straznicky, N.E.; Fernandez, S.; Evans, R.G.; Head, G.A.; Kaye, D.M. Augmented endothelial-specific L-arginine transport prevents obesity-induced hypertension. Acta Physiol. 2014, 212, 39–48. [Google Scholar] [CrossRef]
- Kellogg, D.L., Jr.; Zhao, J.L.; Coey, U.; Green, J.V. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J. Appl. Physiol. 2005, 98, 629–632. [Google Scholar] [CrossRef]
- Kellogg, D.L., Jr.; Zhao, J.L.; Friel, C.; Roman, L.J. Nitric oxide concentration increases in the cutaneous interstitial space during heat stress in humans. J. Appl. Physiol. 2003, 94, 1971–1977. [Google Scholar] [CrossRef]
- Weldon, S.M.; Winquist, R.J.; Madwed, J.B. Differential effects of L-NAME on blood pressure and heart rate responses to acetylcholine and bradykinin in cynomolgus primates. J. Pharmacol. Exp. Ther. 1995, 272, 126–133. [Google Scholar] [PubMed]
- Dabisch, P.A.; Liles, J.T.; Baber, S.R.; Golwala, N.H.; Murthy, S.N.; Kadowitz, P.J. Analysis of L-NAME-dependent and -resistant responses to acetylcholine in the rat. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H688–H698. [Google Scholar] [CrossRef] [PubMed]
- Küng, C.F.; Moreau, P.; Takase, H.; Lüscher, T.F. L-NAME hypertension alters endothelial and smooth muscle function in rat aorta. Prevention by trandolapril and verapamil. Hypertens 1995, 26, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Janke, J.; Gorzelniak, K.; Böhnke, J.; Ghose, N.; Lindschau, C.; Luft, F.C.; Sharma, A.M. Regulation of the nitric oxide system in human adipose tissue. J. Lipid Res. 2004, 45, 1640–1648. [Google Scholar] [CrossRef] [PubMed]
- Engeli, S.; Boschmann, M.; Adams, F.; Franke, G.; Gorzelniak, K.; Janke, J.; Luft, F.C.; Jordan, J. Dissociation between adipose nitric oxide synthase expression and tissue metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 2706–2711. [Google Scholar] [CrossRef] [PubMed]
- Soliman, A.T.; Eldabbagh, M.; Saleem, W.; Zahredin, K.; Shatla, E.; Adel, A. Placental weight: Relation to maternal weight and growth parameters of full-term babies at birth and during childhood. J. Trop. Pediatr. 2013, 59, 358–364. [Google Scholar] [CrossRef][Green Version]
- Dubova, E.A.; Pavlov, K.A.; Borovkova, E.I.; Bayramova, M.A.; Makarov, I.O.; Shchegolev, A.I. Vascular endothelial growth factor and its receptors in the placenta of pregnant women with obesity. Bull. Exp. Biol. Med. 2011, 151, 253–258. [Google Scholar] [CrossRef]
- Moran, M.C.; Mulcahy, C.; Zombori, G.; Ryan, J.; Downey, P.; McAuliffe, F.M. Placental volume, vasculature and calcification in pregnancies complicated by pre-eclampsia and intra-uterine growth restriction. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 195, 12–17. [Google Scholar] [CrossRef]
- Cluver, C.A.; Hannan, N.J.; van Papendorp, E.; Hiscock, R.; Beard, S.; Mol, B.W.; Theron, G.B.; Hall, D.R.; Decloedt, E.H.; Stander, M.; et al. Esomeprazole to treat women with preterm preeclampsia: A randomized placebo controlled trial. Am. J. Obstet. Gynecol. 2018, 219, 388.e1–388.e17. [Google Scholar] [CrossRef]
- Atwa, K.; Ibrahim, Z.; Elshaer, M.; Taha, O.; Aboelroose, A.A. Role of Esomeprazole in Early Preeclampsia: A Randomized Controlled Trial. Austin J. Obs. Gynecol. 2021, 8, 1189. [Google Scholar]
- Amaral, J.H.; Montenegro, M.F.; Pinheiro, L.C.; Ferreira, G.C.; Barroso, R.P.; Costa-Filho, A.J.; Tanus-Santos, J.E. TEMPOL enhances the antihypertensive effects of sodium nitrite by mechanisms facilitating nitrite-derived gastric nitric oxide formation. Free Radic. Biol. Med. 2013, 65, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.C.; Montenegro, M.F.; Amaral, J.H.; Ferreira, G.C.; Oliveira, A.M.; Tanus-Santos, J.E. Increase in gastric pH reduces hypotensive effect of oral sodium nitrite in rats. Free Radic. Biol. Med. 2012, 53, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Sertorio, J.T. Proton pump inhibitors and nitric oxide mechanisms in type 2 diabetes. Diabetologia 2013, 56, 2763–2764. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Ohtake, K.; Uchida, H. NO-Rich Diet for Lifestyle-Related Diseases. Nutrients 2015, 7, 4911–4937. [Google Scholar] [CrossRef]
- Deeney, S.; Powers, K.N.; Crombleholme, T.M. A comparison of sexing methods in fetal mice. Lab. Anim. 2016, 45, 380–384. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Alwis, N.; Binder, N.K.; Mangwiro, Y.T.M.; Beard, S.; Pritchard, N.; Kadife, E.; Fato, B.R.; Keenan, E.; Brownfoot, F.C.; Kaitu’u-Lino, T.J.; et al. Actions of Esomeprazole on the Maternal Vasculature in Lean and Obese Pregnant Mice with Impaired Nitric Oxide Synthesis: A Model of Preeclampsia. Int. J. Mol. Sci. 2022, 23, 8185. https://doi.org/10.3390/ijms23158185
de Alwis N, Binder NK, Mangwiro YTM, Beard S, Pritchard N, Kadife E, Fato BR, Keenan E, Brownfoot FC, Kaitu’u-Lino TJ, et al. Actions of Esomeprazole on the Maternal Vasculature in Lean and Obese Pregnant Mice with Impaired Nitric Oxide Synthesis: A Model of Preeclampsia. International Journal of Molecular Sciences. 2022; 23(15):8185. https://doi.org/10.3390/ijms23158185
Chicago/Turabian Stylede Alwis, Natasha, Natalie K. Binder, Yeukai T. M. Mangwiro, Sally Beard, Natasha Pritchard, Elif Kadife, Bianca R. Fato, Emerson Keenan, Fiona C. Brownfoot, Tu’uhevaha J. Kaitu’u-Lino, and et al. 2022. "Actions of Esomeprazole on the Maternal Vasculature in Lean and Obese Pregnant Mice with Impaired Nitric Oxide Synthesis: A Model of Preeclampsia" International Journal of Molecular Sciences 23, no. 15: 8185. https://doi.org/10.3390/ijms23158185
APA Stylede Alwis, N., Binder, N. K., Mangwiro, Y. T. M., Beard, S., Pritchard, N., Kadife, E., Fato, B. R., Keenan, E., Brownfoot, F. C., Kaitu’u-Lino, T. J., & Hannan, N. J. (2022). Actions of Esomeprazole on the Maternal Vasculature in Lean and Obese Pregnant Mice with Impaired Nitric Oxide Synthesis: A Model of Preeclampsia. International Journal of Molecular Sciences, 23(15), 8185. https://doi.org/10.3390/ijms23158185






