Multiparametric Classification of Non-Muscle Invasive Papillary Urothelial Neoplasms: Combining Morphological, Phenotypical, and Molecular Features for Improved Risk Stratification
Abstract
:1. Introduction
2. Results
2.1. Description of the Cohort
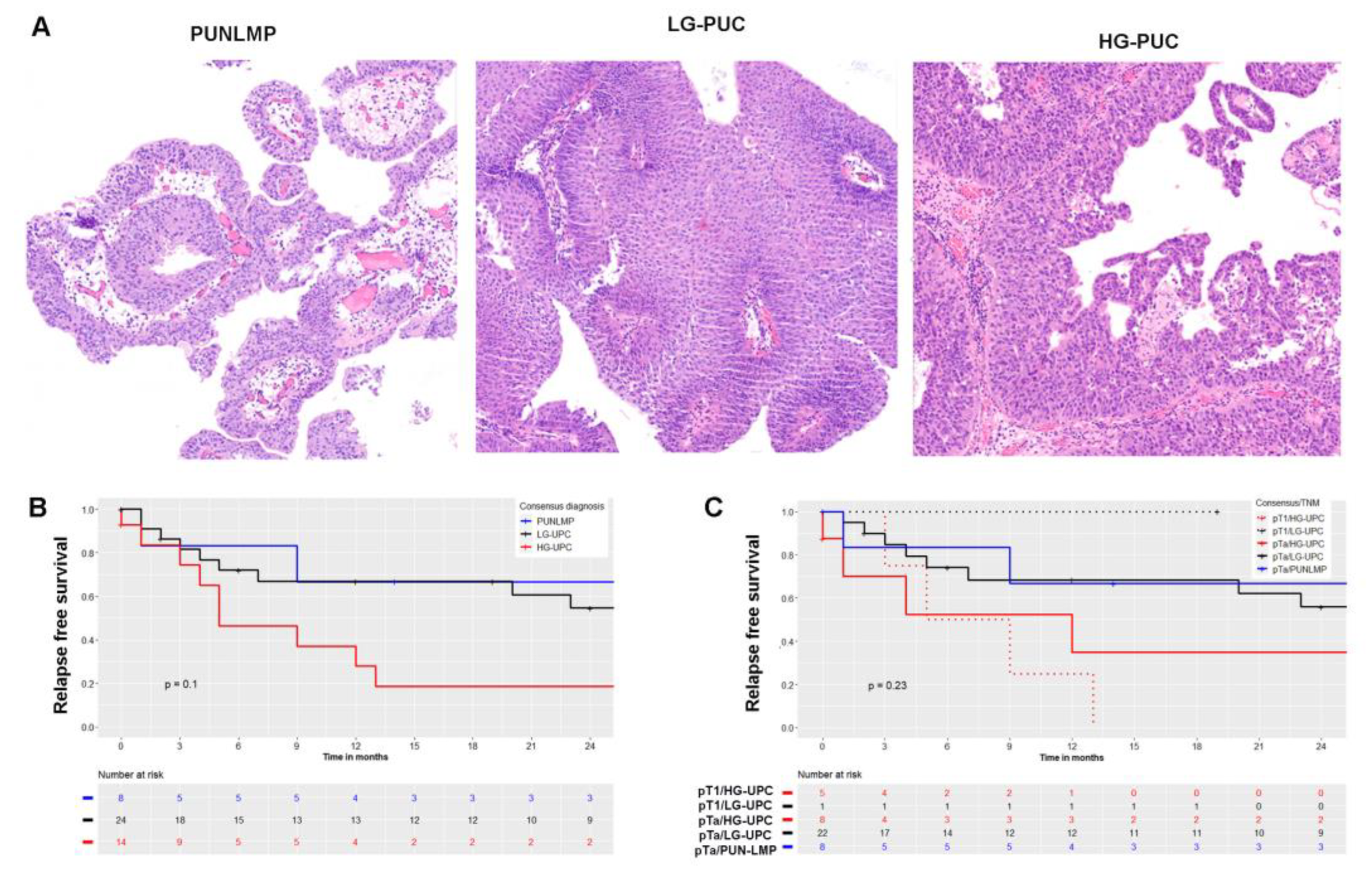
2.2. Histological Classification
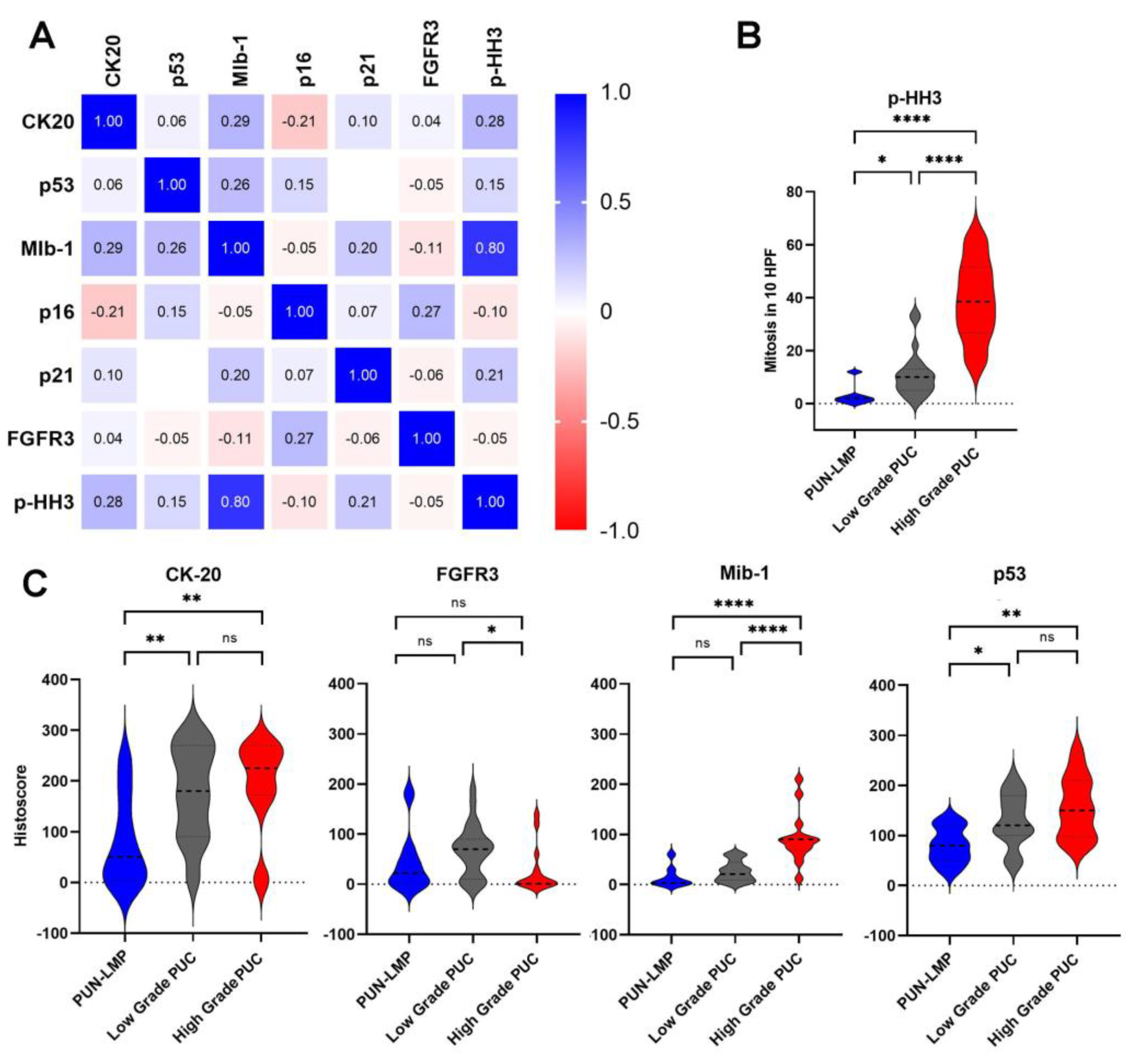
2.3. Immunohistochemical Characteristics and Correlation with Tumor Grade
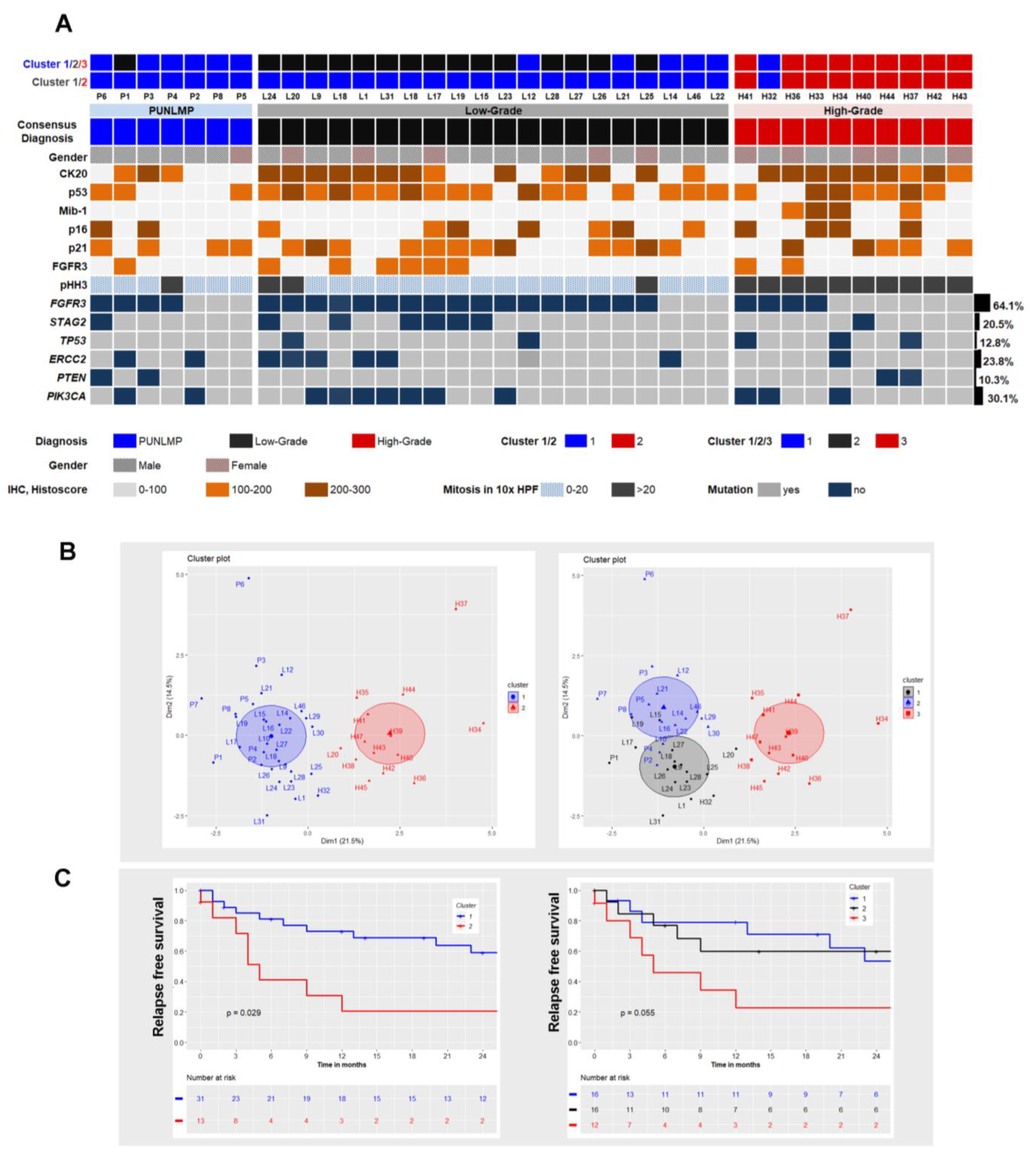
2.4. Mutational Landscape
2.5. Cluster Analysis Integrating Morphology, Immunophenotype and Mutational Status
3. Discussion
4. Materials and Methods
4.1. Tumor Samples
4.2. Histological Classification According to the 2016 WHO Classification
4.3. Clinical Data
4.4. Immunohistochemistry
4.5. DNA Extraction
4.6. Targeted Next Generation Sequencing
4.7. Cluster Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richters, A.; Aben, K.K.H.; Kiemeney, L. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woldu, S.L.; Bagrodia, A.; Lotan, Y. Guideline of guidelines: Non-muscle-invasive bladder cancer. BJU Int. 2017, 119, 371–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prout, G.R., Jr.; Barton, B.A.; Griffin, P.P.; Friedell, G.H. Treated history of noninvasive grade 1 transitional cell carcinoma. The National Bladder Cancer Group. J. Urol. 1992, 148, 1413–1419. [Google Scholar] [CrossRef]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylvester, R.J.; van der Meijden, A.P.; Oosterlinck, W.; Witjes, J.A.; Bouffioux, C.; Denis, L.; Newling, D.W.; Kurth, K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: A combined analysis of 2596 patients from seven EORTC trials. Eur. Urol. 2006, 49, 465–466, discussion 467–475. [Google Scholar] [CrossRef]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder cancer. Nat. Rev. Dis. Primers 2017, 3, 17022. [Google Scholar] [CrossRef]
- Epstein, J.I. The new World Health Organization/International Society of Urological Pathology (WHO/ISUP) classification for TA, T1 bladder tumors: Is it an improvement? Crit. Rev. Oncol. Hematol. 2003, 47, 83–89. [Google Scholar] [CrossRef]
- Compérat, E.M.; Burger, M.; Gontero, P.; Mostafid, A.H.; Palou, J.; Rouprêt, M.; van Rhijn, B.W.G.; Shariat, S.F.; Sylvester, R.J.; Zigeuner, R.; et al. Grading of Urothelial Carcinoma and The New “World Health Organisation Classification of Tumours of the Urinary System and Male Genital Organs 2016”. Eur. Urol. Focus 2019, 5, 457–466. [Google Scholar] [CrossRef]
- Hedegaard, J.; Lamy, P.; Nordentoft, I.; Algaba, F.; Høyer, S.; Ulhøi, B.P.; Vang, S.; Reinert, T.; Hermann, G.G.; Mogensen, K.; et al. Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell 2016, 30, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Sjödahl, G.; Lauss, M.; Lövgren, K.; Chebil, G.; Gudjonsson, S.; Veerla, S.; Patschan, O.; Aine, M.; Fernö, M.; Ringnér, M.; et al. A Molecular Taxonomy for Urothelial Carcinoma. Clin. Cancer Res. 2012, 18, 3377. [Google Scholar] [CrossRef] [Green Version]
- Damrauer, J.S.; Hoadley, K.A.; Chism, D.D.; Fan, C.; Tiganelli, C.J.; Wobker, S.E.; Yeh, J.J.; Milowsky, M.I.; Iyer, G.; Parker, J.S.; et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proc. Natl. Acad. Sci. USA 2014, 111, 3110–3115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, M.C.; Chang, S.S.; Dalbagni, G.; Pruthi, R.S.; Seigne, J.D.; Skinner, E.C.; Wolf, J.S., Jr.; Schellhammer, P.F. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J. Urol. 2007, 178, 2314–2330. [Google Scholar] [CrossRef] [PubMed]
- van den Bosch, S.; Alfred Witjes, J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: A systematic review. Eur. Urol. 2011, 60, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.D.; Palou Redorta, J.; Robert, G.; Hutson, T.E.; Cesari, R.; Hariharan, S.; Rodriguez Faba, O.; Briganti, A.; Steinberg, G.D. Non-muscle-invasive bladder cancer: An overview of potential new treatment options. Urol. Oncol. 2021, 39, 642–663. [Google Scholar] [CrossRef] [PubMed]
- Mollica, V.; Rizzo, A.; Montironi, R.; Cheng, L.; Giunchi, F.; Schiavina, R.; Santoni, M.; Fiorentino, M.; Lopez-Beltran, A.; Brunocilla, E.; et al. Current Strategies and Novel Therapeutic Approaches for Metastatic Urothelial Carcinoma. Cancers (Basel) 2020, 12, 1449. [Google Scholar] [CrossRef]
- Rizzo, A.; Mollica, V.; Massari, F. Expression of Programmed Cell Death Ligand 1 as a Predictive Biomarker in Metastatic Urothelial Carcinoma Patients Treated with First-line Immune Checkpoint Inhibitors Versus Chemotherapy: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2022, 8, 152–159. [Google Scholar] [CrossRef]
- Zhang, X.K.; Wang, Y.Y.; Chen, J.W.; Qin, T. Bladder papillary urothelial neoplasm of low malignant potential in Chinese: A clinical and pathological analysis. Int. J. Clin. Exp. Pathol. 2015, 8, 5549–5555. [Google Scholar]
- Kvikstad, V.; Mangrud, O.M.; Gudlaugsson, E.; Dalen, I.; Espeland, H.; Baak, J.P.A.; Janssen, E.A.M. Prognostic value and reproducibility of different microscopic characteristics in the WHO grading systems for pTa and pT1 urinary bladder urothelial carcinomas. Diagn. Pathol. 2019, 14, 90. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.D.; Cheng, L. Reappraisal of the papillary urothelial neoplasm of low malignant potential (PUNLMP). Histopathology 2020, 77, 525–535. [Google Scholar] [CrossRef]
- Jaworski, D.; Szylberg, L.; Gzil, A.; Stawinski, P.; Kasperska, A.; Marszalek, A. Diagnostic difficulties in cases of papillary urothelial neoplasm of low malignant potential, urothelial proliferation of uncertain malignant potential, urothelial dysplasia and urothelial papilloma: A review of current literature. Ann. Diagn. Pathol. 2019, 40, 182–188. [Google Scholar] [CrossRef]
- van Rhijn, B.W.; Burger, M.; Lotan, Y.; Solsona, E.; Stief, C.G.; Sylvester, R.J.; Witjes, J.A.; Zlotta, A.R. Recurrence and progression of disease in non-muscle-invasive bladder cancer: From epidemiology to treatment strategy. Eur. Urol. 2009, 56, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Lashen, A.; Toss, M.; Mihai, R.; Rakha, E. Assessment of mitotic activity in breast cancer: Revisited in the digital pathology era. J. Clin. Pathol. 2022, 75, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Quintero, A.; Alvarez-Kindelan, J.; Luque, R.J.; Gonzalez-Campora, R.; Requena, M.J.; Montironi, R.; Lopez-Beltran, A. Ki-67 MIB1 labelling index and the prognosis of primary TaT1 urothelial cell carcinoma of the bladder. J. Clin. Pathol. 2006, 59, 83–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallofre, C.; Castillo, M.; Morente, V.; Sole, M. Immunohistochemical expression of CK20, p53, and Ki-67 as objective markers of urothelial dysplasia. Mod. Pathol. 2003, 16, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.; Rashid, S.; Gashir, M.B.; Taha, N.M.; Al Bozom, I. CK20 and CK5/6 Immunohistochemical Staining of Urothelial Neoplasms: A Perspective. Adv. Urol. 2020, 2020, 4920236. [Google Scholar] [CrossRef]
- Arias-Stella, J.A., 3rd; Shah, A.B.; Gupta, N.S.; Williamson, S.R. CK20 and p53 Immunohistochemical Staining Patterns in Urinary Bladder Specimens With Equivocal Atypia. Arch. Pathol. Lab. Med. 2018, 142, 64–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaveri, E.; Brewer, J.L.; Roydasgupta, R.; Fridlyand, J.; DeVries, S.; Koppie, T.; Pejavar, S.; Mehta, K.; Carroll, P.; Simko, J.P.; et al. Bladder cancer stage and outcome by array-based comparative genomic hybridization. Clin. Cancer Res. 2005, 11, 7012–7022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billerey, C.; Chopin, D.; Aubriot-Lorton, M.H.; Ricol, D.; Gil Diez de Medina, S.; Van Rhijn, B.; Bralet, M.P.; Lefrere-Belda, M.A.; Lahaye, J.B.; Abbou, C.C.; et al. Frequent FGFR3 mutations in papillary non-invasive bladder (pTa) tumors. Am. J. Pathol. 2001, 158, 1955–1959. [Google Scholar] [CrossRef] [Green Version]
- di Martino, E.; L’Hote, C.G.; Kennedy, W.; Tomlinson, D.C.; Knowles, M.A. Mutant fibroblast growth factor receptor 3 induces intracellular signaling and cellular transformation in a cell type- and mutation-specific manner. Oncogene 2009, 28, 4306–4316. [Google Scholar] [CrossRef] [Green Version]
- Knowles, M.A.; Platt, F.M.; Ross, R.L.; Hurst, C.D. Phosphatidylinositol 3-kinase (PI3K) pathway activation in bladder cancer. Cancer Metastasis Rev. 2009, 28, 305–316. [Google Scholar] [CrossRef] [Green Version]
- Lindgren, D.; Frigyesi, A.; Gudjonsson, S.; Sjodahl, G.; Hallden, C.; Chebil, G.; Veerla, S.; Ryden, T.; Mansson, W.; Liedberg, F.; et al. Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome. Cancer Res. 2010, 70, 3463–3472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platt, F.M.; Hurst, C.D.; Taylor, C.F.; Gregory, W.M.; Harnden, P.; Knowles, M.A. Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin. Cancer Res. 2009, 15, 6008–6017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeks, J.J.; Carneiro, B.A.; Pai, S.G.; Oberlin, D.T.; Rademaker, A.; Fedorchak, K.; Balasubramanian, S.; Elvin, J.; Beaubier, N.; Giles, F.J. Genomic characterization of high-risk non-muscle invasive bladder cancer. Oncotarget 2016, 7, 75176–75184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solomon, D.A.; Kim, J.S.; Bondaruk, J.; Shariat, S.F.; Wang, Z.F.; Elkahloun, A.G.; Ozawa, T.; Gerard, J.; Zhuang, D.; Zhang, S.; et al. Frequent truncating mutations of STAG2 in bladder cancer. Nat. Genet. 2013, 45, 1428–1430. [Google Scholar] [CrossRef]
- Shariat, S.F.; Tokunaga, H.; Zhou, J.; Kim, J.; Ayala, G.E.; Benedict, W.F.; Lerner, S.P. p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J. Clin. Oncol. 2004, 22, 1014–1024. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reynies, A.; Allory, Y.; Sjodahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Spruck, C.H., 3rd; Ohneseit, P.F.; Gonzalez-Zulueta, M.; Esrig, D.; Miyao, N.; Tsai, Y.C.; Lerner, S.P.; Schmutte, C.; Yang, A.S.; Cote, R.; et al. Two molecular pathways to transitional cell carcinoma of the bladder. Cancer Res. 1994, 54, 784–788. [Google Scholar]
- Casey, R.G.; Catto, J.W.; Cheng, L.; Cookson, M.S.; Herr, H.; Shariat, S.; Witjes, J.A.; Black, P.C. Diagnosis and management of urothelial carcinoma in situ of the lower urinary tract: A systematic review. Eur. Urol. 2015, 67, 876–888. [Google Scholar] [CrossRef]
- Liu, D.; Plimack, E.R.; Hoffman-Censits, J.; Garraway, L.A.; Bellmunt, J.; Van Allen, E.; Rosenberg, J.E. Clinical Validation of Chemotherapy Response Biomarker ERCC2 in Muscle-Invasive Urothelial Bladder Carcinoma. JAMA Oncol. 2016, 2, 1094–1096. [Google Scholar] [CrossRef] [Green Version]
- Van Allen, E.M.; Mouw, K.W.; Kim, P.; Iyer, G.; Wagle, N.; Al-Ahmadie, H.; Zhu, C.; Ostrovnaya, I.; Kryukov, G.V.; O’Connor, K.W.; et al. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014, 4, 1140–1153. [Google Scholar] [CrossRef] [Green Version]
- Puzio-Kuter, A.M.; Castillo-Martin, M.; Kinkade, C.W.; Wang, X.; Shen, T.H.; Matos, T.; Shen, M.M.; Cordon-Cardo, C.; Abate-Shen, C. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009, 23, 675–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babjuk, M.; Burger, M.; Comperat, E.M.; Gontero, P.; Mostafid, A.H.; Palou, J.; van Rhijn, B.W.G.; Roupret, M.; Shariat, S.F.; Sylvester, R.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (TaT1 and Carcinoma In Situ)—2019 Update. Eur. Urol. 2019, 76, 639–657. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.S.; Boorjian, S.A.; Chou, R.; Clark, P.E.; Daneshmand, S.; Konety, B.R.; Pruthi, R.; Quale, D.Z.; Ritch, C.R.; Seigne, J.D.; et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J. Urol. 2016, 196, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, J.J.; Langerak, A.W.; Brüggemann, M.; Evans, P.A.; Hummel, M.; Lavender, F.L.; Delabesse, E.; Davi, F.; Schuuring, E.; García-Sanz, R.; et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: Report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia 2003, 17, 2257–2317. [Google Scholar] [CrossRef] [Green Version]
- Abdulhafedh, A. Incorporating K-means, Hierarchical Clustering and PCA in Customer Segmentation. J. City Dev. 2021, 3, 12–30. [Google Scholar] [CrossRef]
- Kassambara, A. Practical Guide to Cluster Analysis in R; STHDA: Shanghai, China, 2017. [Google Scholar]
| n = 45 (%) | |
|---|---|
| Median age (range) | 75 years (50–95) |
| Gender | n (%) |
| Male | 33 (73.3) |
| Female | 12 (26.6) |
| M:F Ratio | 2.75:1 |
| Tumor Stage, TNM | n (%) |
| pTa | 39 (86.6) |
| pTa and pT1 | 6 (13.3) |
| Localization of tumor | |
| 2 or more locations | 19 (42.2) |
| Lateral walls | 10 (22.2) |
| Posterior wall | 6 (13.3) |
| Trigonum | 3 (6.6) |
| Neck/Apex | 3 (6.6) |
| Dome of the bladder | 2 (4.4) |
| Anterior wall | 2 (4.4) |
| Detrusor muscle in the histological slides | 30 (66.7) |
| Immediate therapy after diagnosis * (n = 44) | n (%) |
| No therapy | 19 (43.2) |
| Immediate intravesical instillation of MMC | 12 (27.3) |
| Re-TUR-BT | 13 (29.5) |
| Recurrence in two-year follow-up % * | 48.4% |
| Further therapy during the clinical course | |
| No further therapy | 12 (27.3) |
| Further BCG or MMC instillations | 10 (22.7) |
| TUR-B | 31 (70.5) |
| Cystectomy and TUR-B: | 2 (4.5) |
| Cystectomy, TUR-B and BCG therapy | 2 (4.5) |
| Grade | ODR | Diagnosis P 1 | Diagnosis P 2 | Diagnosis P 3 | Consensus Diagnosis | Interobserver Variability (%) | Fleiss Kappa |
|---|---|---|---|---|---|---|---|
| PUN-LMP | 18 (40%) | 16 (35.6%) | 8 (17.8%) | 6 (13.3%) | 8 (17.8%) | 57% | 0.43 ** |
| LG-PUC | 14 (31.1%) | 15 (33.3%) | 22 (48.9%) | 27 (60%) | 23 (51.1%) | 67% | 0.41 ** |
| HG-PUC | 13 (28.9%) | 14 (31.1%) | 15 (33.3%) | 12 (26.7%) | 14 (31.1%) | 87% | 0.81 ** |
| CK20 | Mib-1 | p-HH3 * | FGFR3 | p16 | p21 | p53 | |
|---|---|---|---|---|---|---|---|
| PUN-LMP | 60 (6–180) | 3 (3–30) | 2 (1–12) | 9 (1–40) | 10 (15–270) | 150 (60–170) | 90 (50–130) |
| LG-PUC | 202 (110–270) | 25 (9–45) | 10 (5–15) | 70 (10–82.5) | 85 (17.5–180) | 110 (57.5–160) | 127.5 (100–182.5) |
| HG-PUC | 225 (172–270) | 90 (57–97.5) | 38.5 (25.3–51.5) | 26.3 (10–82.5) | 25 (3–225) | 97.5 (6–191.3) | 142.5 (97.5–210) |
| p-value | 0.027 | <0.001 | <0.001 | 0.032 | 0.065 | <0.001 | 0.066 |
| Marker | Cluster 1 | Cluster 2 | p-Value |
|---|---|---|---|
| Immunohistochemistry | median (p25–p75) | median (p25–p75) | Wilcoxon rank sum test |
| CK20 | 160 (80–240) | 225 (172.5–270.0) | 0.150 |
| p53 | 110 (80–160) | 158.7 (107.5–212.5) | 0.013 |
| MIB-1 | 15 (3–33.9) | 90 (68.5–97.5) | <0.001 |
| FGFR3 | 60 (1–80) | 3 (1.0–65.0) | 0.101 |
| p-HH3 * | 8 (3–12) | 38.5 (25.7–51.5) | <0.001 |
| Mutational status | n (%) | n (%) | χ2 test |
| FGFR3 | 18 (40.0%) | 3 (6.0%) | 0.028 |
| STAG2 | 8 (17.2%) | 1 (2.2% | 0.236 |
| TP53 | 2 (4.4%) | 5 (11.1%) | 0.023 |
| ERCC2 | 7 (15.5%) | 4 (8.8%) | 0.717 |
| PIK3CA | 10 (22.2%) | 3 (6.6%) | 0.724 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montes-Mojarro, I.A.; Hassas, S.; Staehle, S.; Sander, P.; Harland, N.; Serna-Higuita, L.M.; Bonzheim, I.; Bösmüller, H.; Stenzl, A.; Fend, F. Multiparametric Classification of Non-Muscle Invasive Papillary Urothelial Neoplasms: Combining Morphological, Phenotypical, and Molecular Features for Improved Risk Stratification. Int. J. Mol. Sci. 2022, 23, 8133. https://doi.org/10.3390/ijms23158133
Montes-Mojarro IA, Hassas S, Staehle S, Sander P, Harland N, Serna-Higuita LM, Bonzheim I, Bösmüller H, Stenzl A, Fend F. Multiparametric Classification of Non-Muscle Invasive Papillary Urothelial Neoplasms: Combining Morphological, Phenotypical, and Molecular Features for Improved Risk Stratification. International Journal of Molecular Sciences. 2022; 23(15):8133. https://doi.org/10.3390/ijms23158133
Chicago/Turabian StyleMontes-Mojarro, Ivonne A., Saki Hassas, Sina Staehle, Philip Sander, Niklas Harland, Lina Maria Serna-Higuita, Irina Bonzheim, Hans Bösmüller, Arnulf Stenzl, and Falko Fend. 2022. "Multiparametric Classification of Non-Muscle Invasive Papillary Urothelial Neoplasms: Combining Morphological, Phenotypical, and Molecular Features for Improved Risk Stratification" International Journal of Molecular Sciences 23, no. 15: 8133. https://doi.org/10.3390/ijms23158133
APA StyleMontes-Mojarro, I. A., Hassas, S., Staehle, S., Sander, P., Harland, N., Serna-Higuita, L. M., Bonzheim, I., Bösmüller, H., Stenzl, A., & Fend, F. (2022). Multiparametric Classification of Non-Muscle Invasive Papillary Urothelial Neoplasms: Combining Morphological, Phenotypical, and Molecular Features for Improved Risk Stratification. International Journal of Molecular Sciences, 23(15), 8133. https://doi.org/10.3390/ijms23158133










