1. Introduction
The CatSper channel is located in the principal piece of the sperm tail and is a unique cation channel whose activity is necessary for sperm hyperactivation and motility [
1]. Four
Catsper (cation channel of sperm) genes encoding different pore-forming α units have been cloned and characterized as essential genes for hyperactivation and male fertility [
2,
3]. These genes display exclusive expression in male germ cells and form a molecular structure similar to voltage-dependent K
+ channels; however, CatSper is a calcium-permeable channel [
1,
4]. In addition, the activity of CatSper channels also relies on the presence of accessory subunits (β,δ, ε, and ζ Catsper), which interact with EFCAB9, a pH sensor protein that triggers sperm hyperactivation [
5,
6,
7,
8]. During the sperm capacitation process, CatSper activity increases due to intracellular alkalization. Likewise, CatSper activation produces an intraflagellar Ca
2+ influx leading to sperm hyperactivation [
9].
The mouse
Catsper1 gene is the best-characterized member of the family regarding its sequence and activity. It shows >50% identity with rat and human DNA sequences and proteins [
1]. In addition, the
Catsper 2, 3, and 4 genes share characteristics with
Catsper1, such as α pore-forming subunits and have an exclusive expression in the testis [
2,
3]. The
Catsper1 knock-out abrogates channel formation and severely affects mouse sperm fertilization capability [
1]; similarly, patients harboring mutations in the
CATSPER1 gene show infertility [
10]. Therefore,
Catsper1 may be considered a prominent target for the design of male contraceptive strategies due to its exclusive expression in sperm cells, preventing possible side effects in other cell types.
The Sox family comprises transcription factors containing the HMG (High Mobility Group) box. Interestingly,
Sox9 expression has been associated with differentiation of the testis [
11,
12]. Sox proteins contain transactivation domains and nuclear localization signals. For nuclear transport, Sox proteins have binding domains for CaM and importins [
13,
14]. In addition, the murine
Catsper1 promoter has elements for functional interaction with Sox, Creb, Crem, and ER transcription factors [
15]. Indeed, positive Sox regulation in vitro on the
Catsper1 promoter has been suggested [
16]. The
Catsper1 promoter has three Sox sites (A, B, and C), and the mutation of the Sox-B site results in the downregulation of basal transcription in vitro. On the contrary, the presence of Sox9 or Sox5 increases the
Catsper1 promoter activity. Both transcription factors have a synergistic effect, revealing a central role for Sox in the
Catsper1 gene transcription [
16]. The
Catsper1 promoter has not been evaluated in the testicular environment [
17,
18,
19,
20,
21,
22], where Sox factors could affect its activity as they do in vitro.
Likewise, several Sox proteins depend on Calmodulin (CaM) to be transported into the nucleus. CaM is a Ca
2+-binding protein and a ubiquitous regulator of numerous cell processes, including nuclear transport. Calmidazolium (CMZ) (1-[bis(p-chlorophenyl) methyl]-3-[2,4-dichloro-3-(2,4-dichlorobenzyloxy) phenethyl] imidazolinium chloride), is a potent CaM inhibitor. Interestingly, it has been suggested that CMZ may affect the nuclear transport of Sox proteins containing the CaM-binding domain (Sox9 and Sry) in male germ cells [
13,
14]. Inhibition of Sox nuclear transport could affect the expression of genes during spermatogenesis and eventually affect the fertility of the male gametes.
Here, we investigated the activity of the Catsper1 promoter and derived mutants in the Sox binding sites by in vivo transfection of the murine testis. We also studied the effects of the inhibition of Sox protein nuclear transport by CaM on the Catsper1 gene transcription, sperm motility, and fertilization capacity of sperm. This approach could positively impact human reproduction and economically important species through implementing male contraceptive strategies.
We propose one contraceptive strategy at the molecular level to downregulate Catsper1, which is known to be the principal protein of the calcium channel.
3. Discussion
Previous in vitro analysis of the
Catsper1 promoter allowed identifying transcriptional regulators and intrinsic elements that mediate transcriptional activation. These observations require validation in vivo using spermatogenic cells, where natural expression of
Catsper1 occurs. The generation of transgenic animals might help to solve this issue; however, the in vivo transient transfection of plasmids could also be helpful to evaluate the
Catsper1 promoter, as has been reported for other promoters of testis-specific genes in mice [
21,
22]. Deletions of the
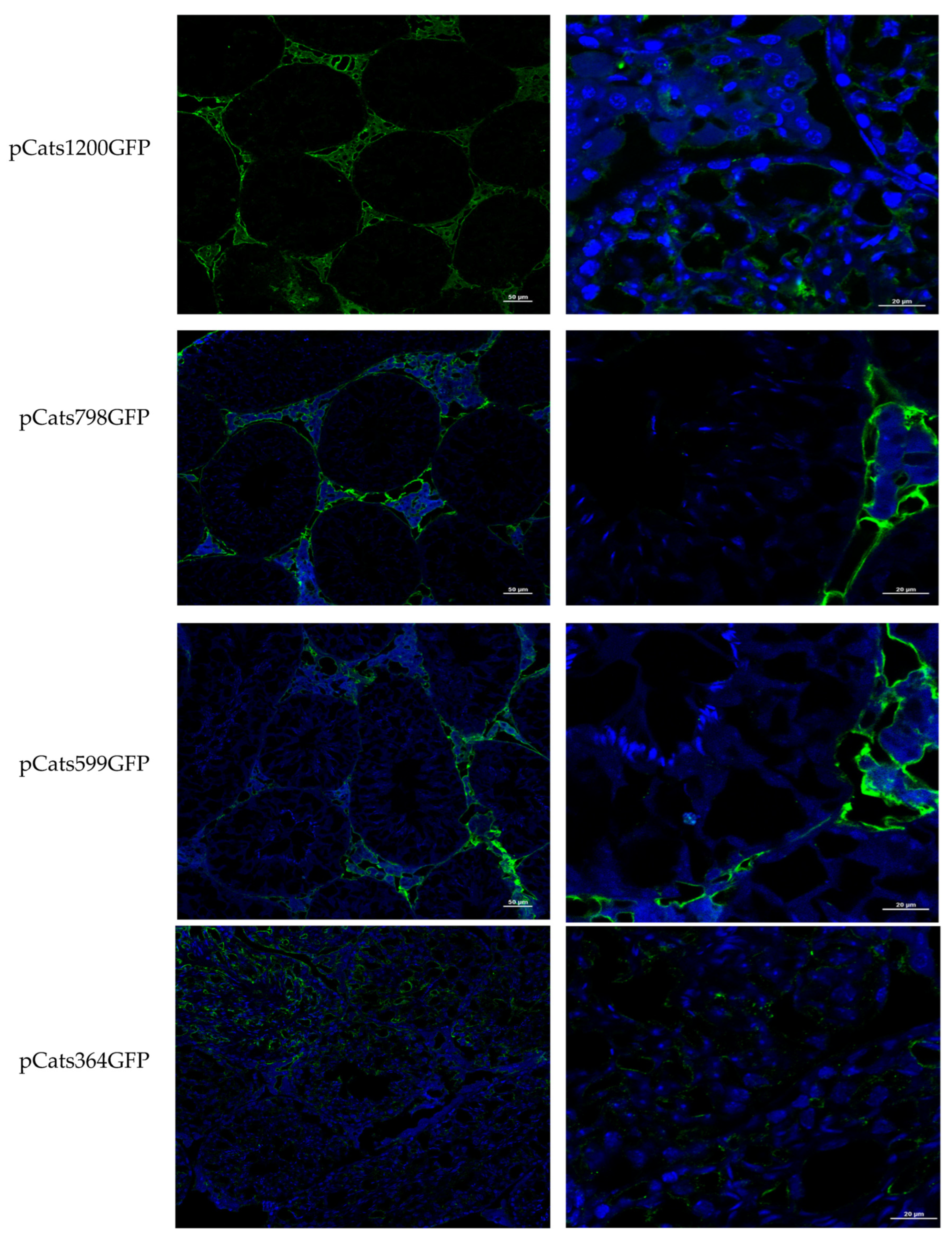
Catsper1 promoter region and the effect of the mutations introduced in the three Sox binding sites were evaluated in murine testis. Both the direct observation of GFP in seminiferous tubules and GFP immunodetection in histological testis sections demonstrated the functionality of the
Catsper1 promoter in vivo (
Figure 1b and
Figure 2b). However, to determine its localization in seminiferous tubules, GFP expression was immunodetected. Only the pCats1215 GFP and pCats364GFP exhibit GFP expression in the cytoplasm of germ cells and behave as germ cell-specific promoters. However, GFP expression from other plasmids was observed also in somatic cells, which indicates that these constructions lack elements for the tissue-specific expression of
Catsper1 (
Figure 3).
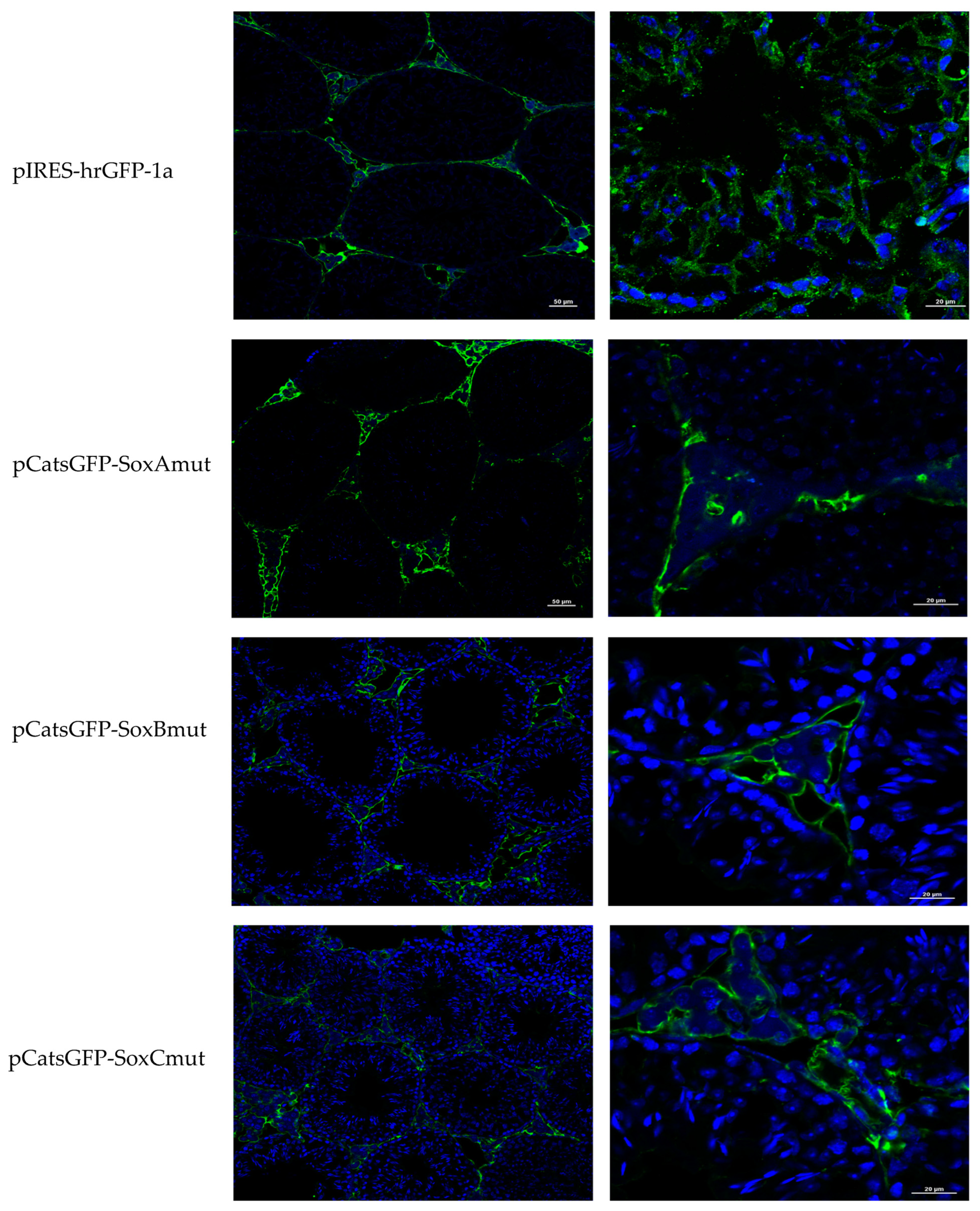
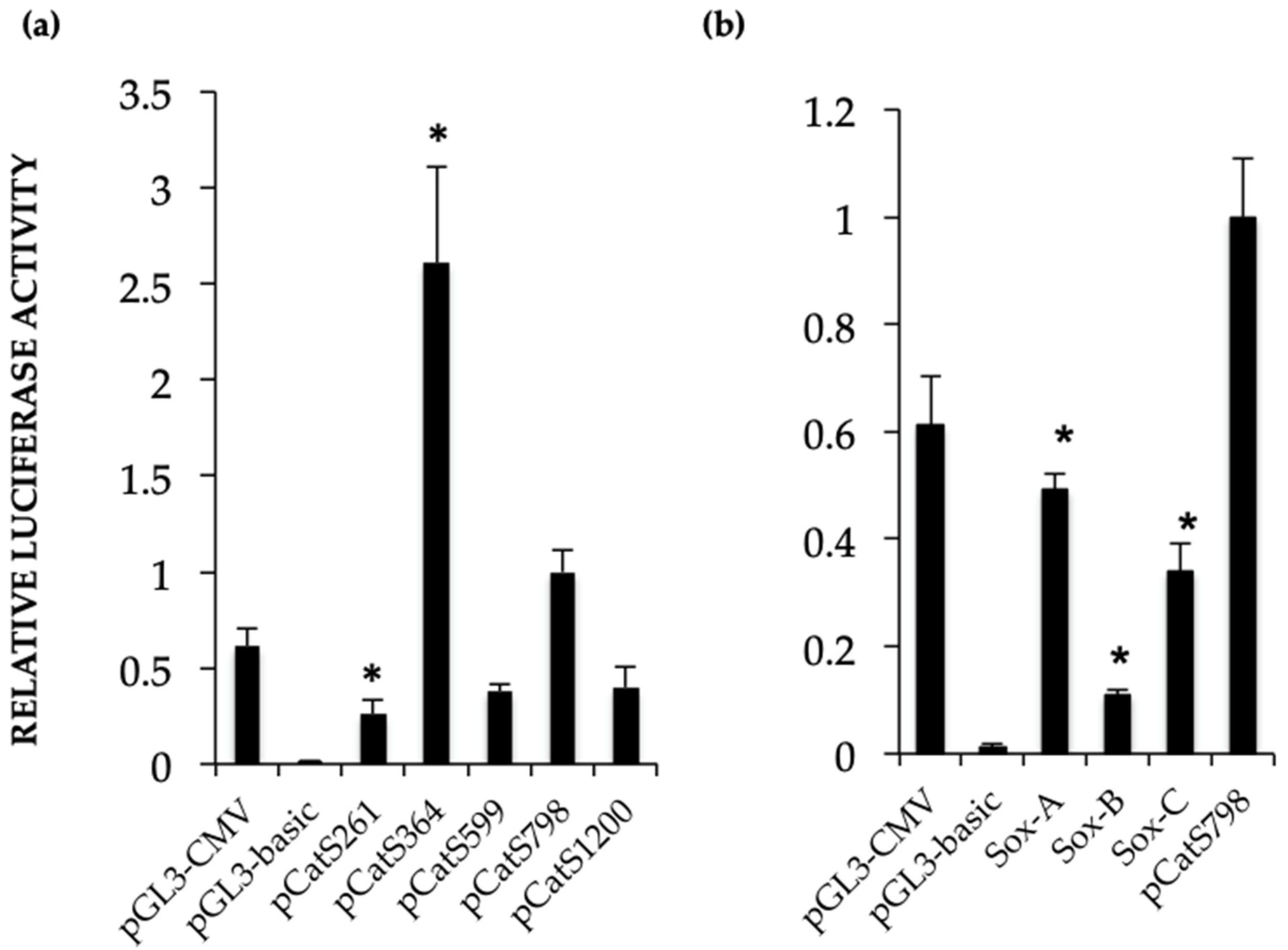
Catsper1 transcription is dependent on Sox sites of the promoter since mutation of any of three sites decreased transcriptional activity in vivo, implying the participation of Sox family proteins in its regulation (
Figure 2 and
Figure 4). Several Sox factors, which act as transactivators, contain domains for dimerization and importin or calmodulin-mediated nuclear transport. Sox factors 3, 5, 6, and 17 have been detected in spermatocytes and spermatids in the testis, while Sox factors 3, 4, 12, 13, and 18 locate in spermatogonia. Finally, Sox factors 4, 8, 9, and 12 are present in Sertoli cells of the adult mouse [
23]. The inhibition of Sox nuclear transport by CMZ and the decay of
Catsper1 mRNA indicate that these factors could interact with the Sox sites in the
Catsper1 promoter and transactivate its expression in vivo.
Thus, the characterization of the
Catsper1 promoter has allowed us to understand the role of Sox factors in its regulation. In particular, here, we show that the inhibition of Sox nuclear transport prevents
Catsper1 promoter transcriptional activity and gene expression. The decrease in the expression of
Catsper1 precludes the formation of the Catsper ion channel and produces a progressive reduction in motility after five weeks of treatment with CMZ (
Table 2) and a reduction in its fertilizing capacity, resulting in fewer litters and pups (
Table 3). This infertility effect might be reversed after stopping the CMZ treatment, but only a small recovery of fertility rate was observed (
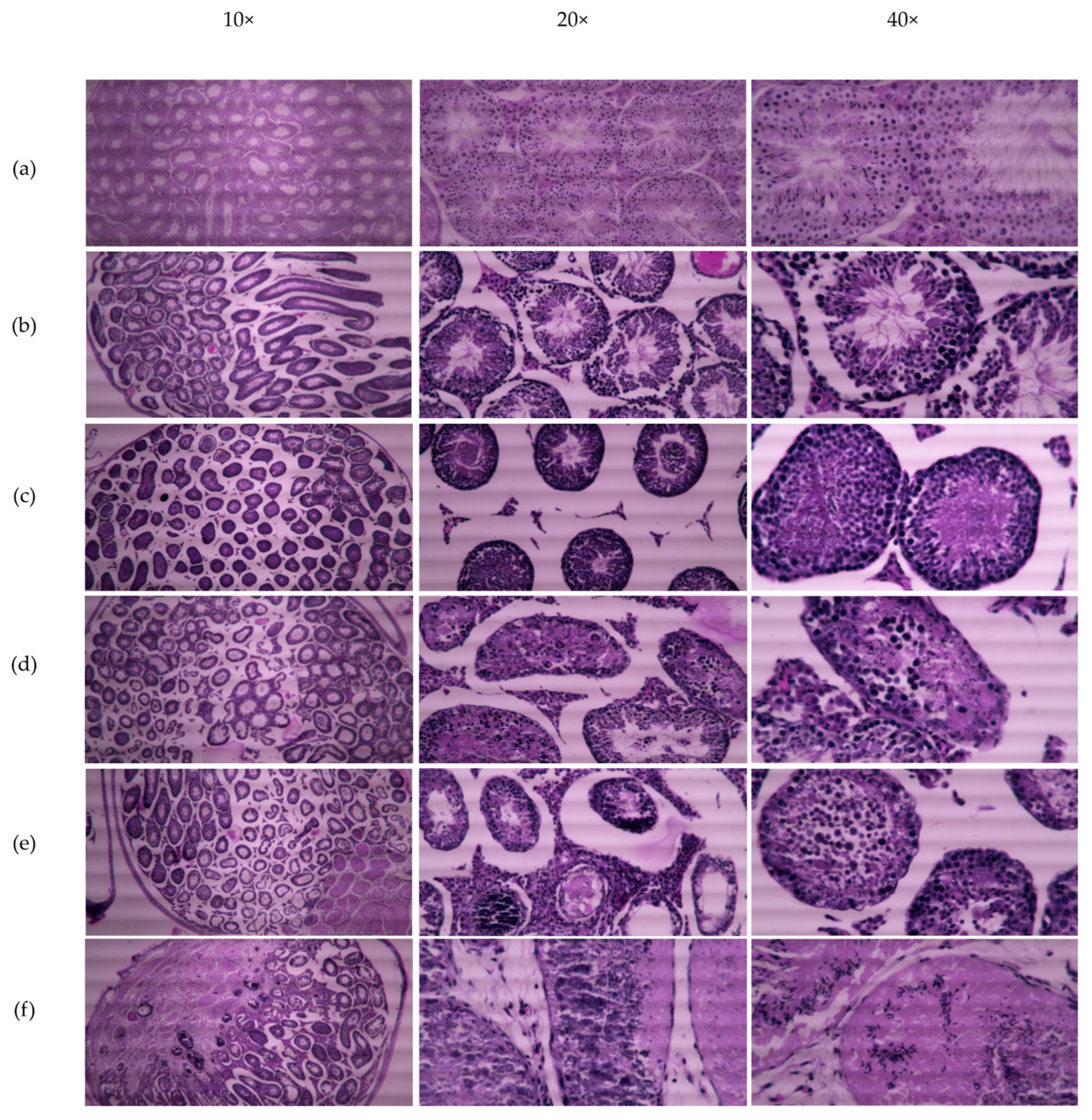
Table 3). This irreversibility in male fertility is due to cellular damage to seminiferous epithelium after 4 and 5 weeks of CMZ treatment, however, some functional tubules still produce sperm (
Figure 7).
Unexpectedly, in the group treated with dimethylsulfoxide-DMSO, a decrease in the offspring to 30.5% was observed, but this should have no effect as it had protective properties. CMZ must be dissolved in DMSO for its administration, however, our results indicate that DMSO may impair male reproductive performance. It has been reported in the literature that DMSO acts as a free-radical scavenger against the effects of radiation and helps preserve some male fertility [
24]. Possible causes of impaired male fertility could be the number of injections in testicles and cell damage, since DMSO with DNA used as a transfection agent causes marked histological changes in seminiferous tubules [
18].
CMZ can interact with different biomolecules and provoke different effects. For instance, drug administration at micromolar concentrations in osteosarcoma cell cultures has been shown to result in an influx of extracellular calcium and cell proliferation inhibition [
25]. In Madin Darby canine kidney (MDCK) cells, CMZ treatment produces extracellular calcium influx and increases the release of calcium from intracellular stores dependent on phospholipases C and A2 activity [
26]. Another calmodulin inhibitor, W7, prevents the ATPase activity of the sarcoplasmic reticulum and stimulates the mobilization of intracellular calcium in human umbilical vascular endothelial cells (HUVECs). The drug also produces changes in the expression of the E-Selectin and ICAM-1 gene [
27]. Therefore, the effects of CMZ on calcium dynamics after intratesticular administration cannot be ruled out. In addition, the presence of CMZ in vitro affects sperm capacitation, inhibits tyrosine phosphorylation in proteins, and affects sperm motility but not viability [
28]. Even though these experiments were performed in mature sperm in vitro, CMZ in the female tract might lead to similar results during capacitation and eventually could be beneficial in contraception. Furthermore, CMZ (>2 µM) stimulates steroidogenesis in primary cultures of rodent Leydig cells. The increase in testosterone and pregnenolone is independent of cAMP, Ca
2+, and the translation of new proteins. This side effect of CMZ treatment in the testis might also affect spermatogenesis [
29]. The increase in the synthesis of steroids by Leydig cells in the testis might affect the expression of
Catsper1 and all four
Catsper genes.
The inhibition of calmodulin by CMZ prevents the nuclear transport of Sox 9 and SRY, proteins that contain calmodulin-binding domains [
13,
14]. CMZ might alter the expression of diverse genes under Sox control during spermatogenesis and affect the fertility of male germ cells. Since various Sox proteins (Sox 9, 17, and 30) have well-conserved nuclear localization signals (NLS), the inhibition of calmodulin would prevent their transport to the nucleus and affect
Catsper1 gene transcription among other genes. Indeed, it has been reported that Sox 30 is essential for fertility. Interestingly, the Sox30-null mouse has decreased
Catsper1 expression, despite other transcriptional factors that might regulate its expression [
30]. Therefore, the
Catsper1 promoter activity may require calmodulin-dependent nuclear transport of Sox factors. Consistent with this, our data indicate that mutations in the Sox sites in the
Catsper1 promoter prevent its interaction with Sox factors and affect the activity of the
Catsper1 promoter in vivo (
Figure 2b and
Figure 4).
Inhibition of Sox nuclear transport disrupts
Catsper1 gene expression, and in consequence, the formation of the essential pore-forming α subunit. In this scenario, functional CatSper channels may not be formed, given that other Catsper subunits cannot replace the Catsper1 α subunit, affecting sperm hyperactivation and male fertility [
1]. For this reason, Catsper1 has been considered a relevant target for different contraceptive strategies.
There are very few methods for men regarding male fertility regulation to share equally the burden and benefits of family planning. Among these contraceptive strategies, antibodies directed to the transmembranal domains and the pore region of the Catsper1 channel have been tested in vitro, which produce a significant inhibitory effect on sperm motility and fertility [
31,
32]. In contrast, a DNA vaccine against Catsper1 provoked a reduced contraceptive effect in male mice [
33].
Likewise, the transcriptional control of
Catsper1 could be a viable alternative for male contraception. Some transcription modulators developed for the PPAR transcription factors that regulate the transcription of various metabolic genes related to dyslipidemia, and type 2 diabetes mellitus [
34], have shown promising results.
Moreover, the intratesticular injection of CMZ limited its effects on the testis, and it prevents side effects in other organs. However, the intratesticular injection could not be an optimal route of administration for humans. Therefore, the study of different administration methods and the use of analogous molecules with specific effects will be necessary to reveal the real contraceptive potential of this strategy.
4. Materials and Methods
4.1. Animals
For in vivo experiments, Balb/C 8- to 10-week-old mice, sexually mature and suitable for fertilization, were used (n = 129 male; 62 female). The animals were maintained in the Animal Facility of the National Medical Center SXXI, IMSS. They were fed a commercial diet (5859, HARLAN) with 9% crude protein (PC) (standard diet) and drinking water ad libitum. Mice received the veterinary care and attention specified in strict accordance with the Mexican Guidelines for Animal Use and Experimentation. The National Scientific Research Committee IMSS approved all experimental procedures and the euthanasia method designed to cause minimal pain and distress, license number R-2014-785-002. Based on the reported in vivo transfection experiments, three animals were the minimum necessary to evaluate each experimental condition and perform the statistical analysis that compares experimental conditions and controls. A maximum of 35 days was the duration of the experiments for male fertility.
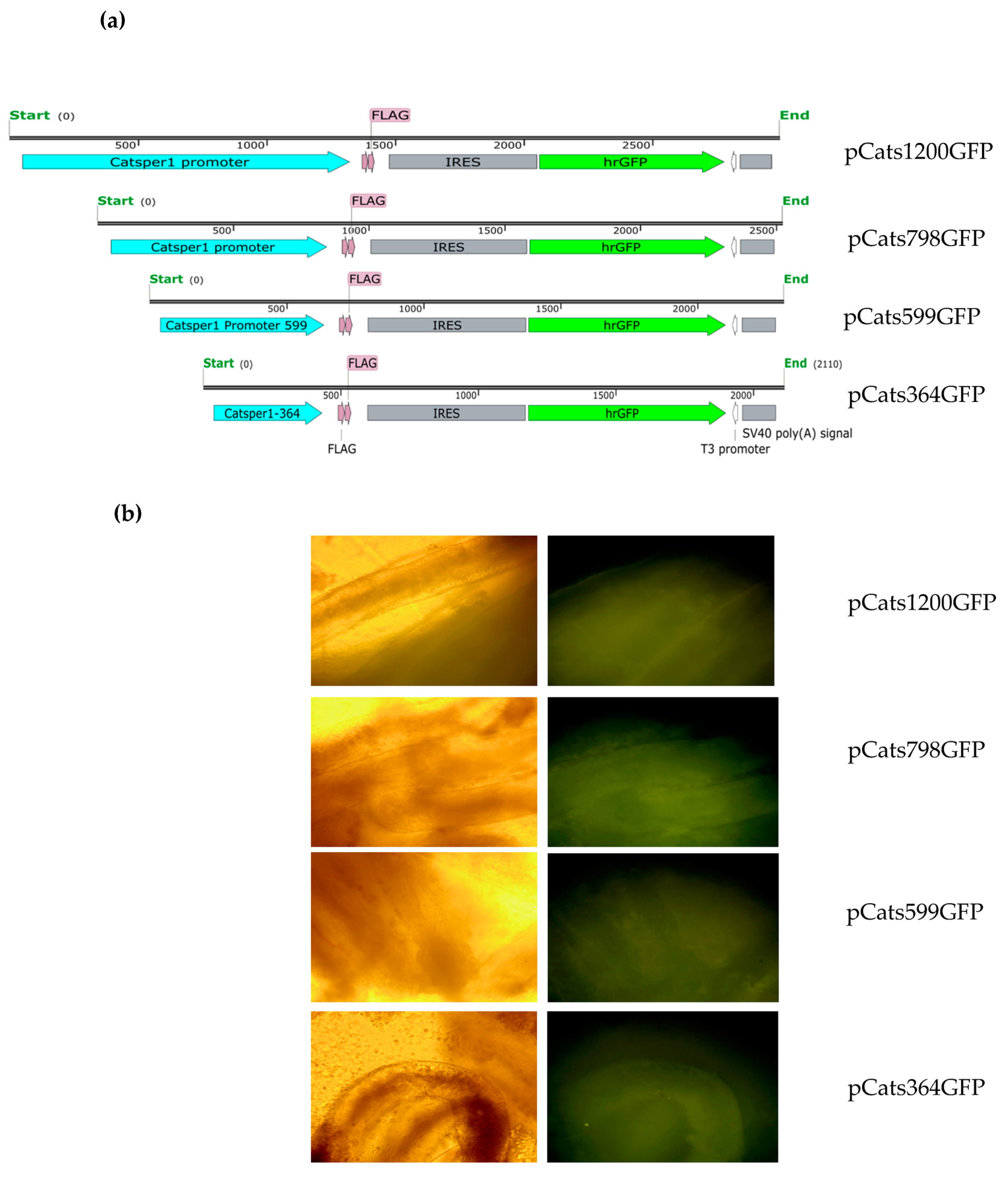
4.2. Constructs
For in vivo testis transfection in this study, constructs containing the
Catsper1 promoter upstream of the
Photinus luciferase reporter (pCatS series) were used [
16]. A new series of pCats plasmids harboring deletions and mutations of Sox sites in the
Catsper1 promoter was linked to the humanized
Renilla green fluorescent protein (hrGFP). The pCRTOPO-798Cats was used as the source to substitute CMV promoter by the
Catsper1 promoter region into
NsiI-
BamHI sites of the pIRES-hrGFP-1a vector to evaluate transcriptional activity in vivo. The resulting construct, pCatS798GFP, allowed observation of the
Catsper1 promoter activity in tissues and was the basic construction for different deletions and mutations.
The plasmid pCatS798GFP was used as a template to generate the Sox site mutations by PCR mutagenesis (Quick-Change site-directed mutagenesis, Stratagene, La Jolla, CA, USA). The plasmid pCats798GFP was the initial template to shorten the region to 599 and 364 bp lengths by PCR mutagenesis, a modification to the directed mutagenesis protocol, which consists of performing the PCR assays in two stages. The first stage includes two separate reactions that contain one of the oligonucleotides (forward or reverse) in a five-cycle amplification. The second stage consists of mixing both reactions to continue PCR amplification for 16 cycles [
35]. The fidelity of the cloned
Catsper1 promoter regions and site-directed mutation in the new constructs was confirmed by digestion with specific restriction enzymes and automatic sequencing. All plasmids were transformed and amplified in
E.coli DH5α, and the plasmid purification was performed with the Maxiprep Plasmid kit (Qiagen, Hilden, Germany).
4.3. Catsper1 Promoter Transfection in Adult Mouse Testis
Adult Balb/C mice were anesthetized intramuscularly (IM) with a mixture of xylazine hydrochloride 1 mg/kg and ketamine 10 mg/kg. In addition, preoperative local analgesia was subcutaneously injected into the testis (4 mg/kg of Rimadyl, Pfizer Ltd., New York, NY, USA). For each mouse, the right testis was the experimental organ, while the left testis remained intact and was used as a negative control. The scrotal region was shaved to expose the testis and the epididymis through the skin. A solution containing 25 μg of the experimental plasmid and 3 μL of in vivo TurboFect (Thermo Scientific, Waltham, MA, USA) reagent was mixed in 5% glucose with trypan blue total volume of 20 μL. The transfection mix for each plasmid was injected with an insulin syringe and a 32G ultra-fine needle into the testis. The trypan blue allows visualizing the DNA solution spreading from the injection site to the surface of the testis. For the post-treatment recovery, a single dose of 100 μL of crystalline sodium benzylpenicillin 1,000,000 U/2 mL and 2.5 μL of dipyrone (Dipirone 50-Virbac, 0.5 mL/5 kg) was injected IM in each mouse.
4.4. Catsper1 Promoter Activity by GFP Detection
The plasmid pCatsGFP798 and the three mutated Sox site constructs were transfected into mice testis. The animals were kept under close observation for three days to allow for GFP accumulation and then sacrificed by cervical dislocation to cause a painless death, and the testes were surgically removed. The seminiferous tubules were exposed to a Dulbecco’s Modified Eagle Medium (DMEM) and immediately observed in an inverted Olympus epifluorescence microscope. Subsequently, the images were captured using photographic equipment integrated into the microscope.
4.5. Immunofluorescence
The constructs pCatsGFP with deletions or mutations were transfected into the testis following the same procedure. The testes were removed, cryoprotected with isopentane (Sigma Aldrich, Burlington, MA, USA), frozen on dry ice, and stored at −80 °C before immunofluorescence (IF) analysis. All testes were mounted with tissue-tek (Sakura Finetek, Nagano, Japan) in a cryostat microtome GM 1510S (Leica Microsystems, Wetzlar, Germany) and cut into 10 µm sections. Sections were placed onto poly-L-lysine-coated slides and fixed with 4% paraformaldehyde in 0.12 M PBS [pH 7.4] for 24 h, and then washed with 0.12 M PBS and 0.025% Tritón X100 for 5 min. Sections were incubated for 30 min at room temperature in a blocking solution containing 10% bovine serum albumin Fraction V (Roche, Basilea, Suiza) in 0.12 M PBS and subsequently exposed to anti-GFP (Stratagene, San Diego, CA, USA), anti-Catsper1 (H-300), and anti-Sox5 (H-19) antibodies (Santa Cruz Biotechnology, CA, USA), diluted in PBS (1:100) for 24 h at 4 °C. Sections were then rewashed with 0.12 M PBS and 0.1% Tween20 for 10 min, and immediately incubated with anti-rabbit IgG Alexa 486 for GFP and Sox5 (Santa Cruz Biotechnologies, Santa Cruz, CA, USA) antibodies, and anti-rabbit IgG fluorescein for Catsper1 and diluted 1:400 in PBS, for two h at room temperature. Last, the sections were washed with 0.12 M PBS and 0.1% Tween20 for 10 min before adding 100 μL of 300 nM DAPI diluted in 0.12 M PBS for 5 min. Samples were rewashed (0.12 M PBS), dyed, and gently mounted onto coverslips with Vectashield (Vector Labs, Newark, CA, USA) mounting reagent. Images were acquired on a Nikon Ti Eclipse inverted confocal microscope equipped with an A1 imaging system. Imaging was performed using either a 20× (dry, NA 0.8) or 60× (oil immersion, NA 1.4) objective lens. Dyes were excited in a sequential mode using the built-in laser lines: 403 nm (DAPI), 488 nm (Alexa 486) Corresponding fluorescences were read in the following ranges: 425–475 nm (DAPI), 500–550 nm (Alexa 486) with the manufacturer-provided filter sets. Images were acquired and analyzed using software NIS Elements v.5.50.
4.6. Analysis of Catsper1 Promoter Activity In Vivo
The different deletions of the Catsper1 promoter and the Sox mutated sites were evaluated by transfecting mice testes with pCatS plasmids containing the Luciferase reporter gene. After three days, the testes were collected and prepared for the luciferase assay. Briefly, the seminiferous tubules were cut into small pieces and suspended in 3 mL of phosphate saline buffer (PBS), mixed by continuous pipetting for 2 min at room temperature to obtain the germ cells. The supernatant was recovered and centrifuged at 2000 rpm to obtain the cell pellet. Cells were suspended in 800 µL of lysis buffer included in the dual luciferase assay kit (Promega, Madison, WI, USA). The luciferase assays were performed using 200 µL of the cell lysate, 200 µL buffer with the substrate (LAR-II) for Photinus luciferase, and 200 µL of STOP-GLO for Renilla luciferase activity were added later. The chemiluminescence activity was measured in a luminometer (Junior LB 95O9, Berthold Technologies, Bad Wildbad, Germany) as relative luminescence units (RLU).
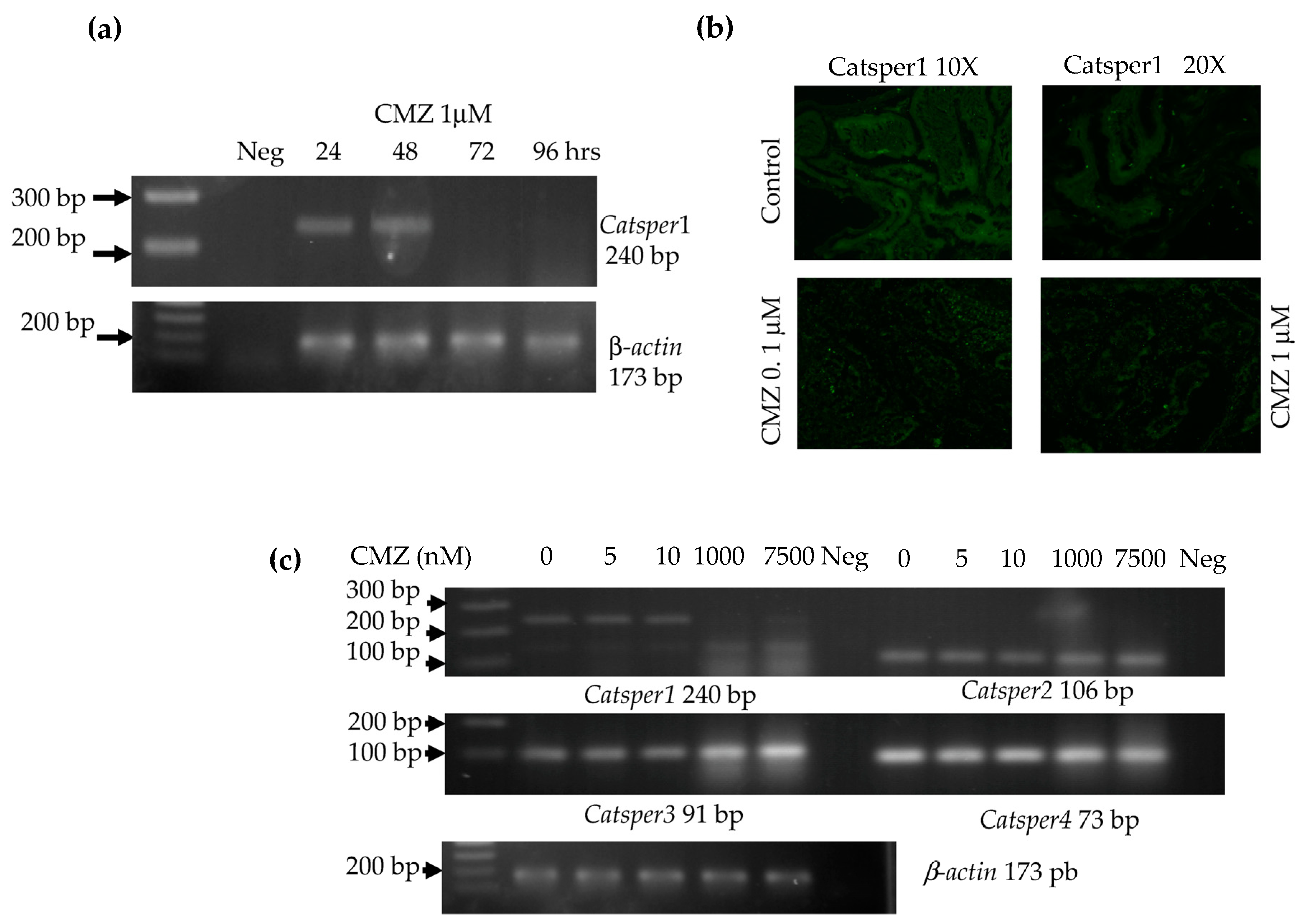
4.7. Intra-Testicular Injection of Calmidazolium
The inhibitor of calmodulin, calmidazolium 1 μM (CMZ) was dissolved in 5 mM DMSO as a vehicle and tested by intratesticular injection in male mice. First, a time course was made with 1 μM of CMZ intratesticular injected in mice. Testes were recollected after 24, 48, 72 up to 96 h for total RNA extraction, followed by RT-PCR for Catsper1 expression and immunofluorescence of Catsper1. Second, a test of different concentrations of 5, 10, 1000, and 7500 nM CMZ in a final volume of 10 µL for each testis was assayed in mice. RNA total was extracted from testes and RT-PCR of four Catsper genes was made. Further, to test male fertility, mice were intratesticular injected once a week with 1 μM CMZ or DMSO 5 mM doses for five weeks. Mice were sacrificed by cervical dislocation to cause minimal pain and distress after each treatment to obtain the testes and epididymis.
4.8. CMZ Treatment and Fertility Evaluation
Male fertility was assessed in mice intratesticular injected with 1 μM CMZ per testis or 5mM DMSO as the vehicle. Control groups of untreated mice and DMSO-treated mice were used to evaluate fertilization capability (n = 3). Briefly, three separate experiments were performed in groups of six, mice received intratesticular injections of CMZ (1 μM) or DMSO in each testis every week for five weeks. Each mouse received five CMZ intratesticular injections in each testis, spanning the complete spermatogenic cycle. Each male mouse was caged with two adult female mice and the presence of a vaginal plug was observed to verify the mate. Male mice were removed after mating and sacrificed by cervical dislocation performed correctly to cause as painless a death as possible, to obtain the testes and epididymis. Testes were analyzed by standard histological hematoxylin-eosin staining and sperm from the epididymis were evaluated for their motility with microscopy. Eight mice treated with CMZ for five weeks were kept for a second mating after eight weeks without treatment (Recuperates). The mating females were observed to count the number of pregnant females per group, the litter size, and to determine the fertility and reproduction rate in the condition tested.
4.9. RT-PCR Analysis for Catsper Genes
The Catsper expression was evaluated by RT-PCR 72 h after CMZ treatment. Testis RNA extraction was performed with 1ml Trizol. RNA was separated with 0.2 mL of chloroform by centrifugation at 12,000× g for 10 min. The aqueous phase was recovered, and RNA samples were precipitated with 0.5 mL isopropyl alcohol. The RNA pellet was recovered by centrifugation at 12,000× g for 15 min and then washed with 70% ethanol-DEPC water, air-dried at room temperature, and redissolved in water. Final RNA concentration was estimated by spectrophotometry (nanodrop 2000, Thermofisher, Waltham, MA, USA). The first-strand synthesis reaction was performed in a total volume of 20 µL with 2 µg of RNA, random primers, dNTPs mix, and adequate buffer conditions for the multiScribe Reverse Transcriptase (High-capacity cDNA reverse transcription kit, Applied Biosystems, Waltham, MA, USA). Pairs of oligonucleotides yielding a Tm of 60 °C were used to amplify the four Catsper genes with products of 90–240 bp: Catsper1F 5′ CTGAGCTAGAGATCCGAGGTG 3′, Catsper1R 5′ AGACTTTGAGAATCCGGAACAGGC 3′, Catsper2F 5′ TTTGTGCTGATGGTTGAAATAGA 3′, Catsper2R 5′ TGAAGCTAAGCAAGATGAACCA 3′, Catsper3F 5′ GCTTTCTTTACTCTCTTCAGTTTGG 3′, Catsper3R 5′ CCCGGCTCACAGTAAACTTC 3′, Catsper4F 5′ AGATCCGAGAGGAACTCAACAT 3′, and Catsper4R 5′ ACATTTTGCTGCTCCTGGTT 3′.
RT-PCR was performed in a 20 µL reaction with 1 µL cDNA, 10 pM of each primer forward and reverse, and Mix GoTaq 2X (Promega, Madison, WI, USA). Conditions for Catsper gene amplification by PCR were: 94 °C denaturalization for 1 min, 30 cycles of denaturalization 94 °C 30 s, alignment 60 °C 30 s, extension 72 °C 30 s, and final extension of 72 °C 7 min. A 173 bp product of β-actin gene was used as an endogenous control for sample normalization with oligonucleotides β-actinF 5′CACCATGTACCCAGGCATTG 3′ and β-actinR 5′ CCTGCTTGCTGATCCACATC 3′. All PCR products were analyzed by 2% agarose gel electrophoresis.
4.10. Sperm Motility Determination
Sperm were obtained from the epididymis and divided into sections in 2 mL of Human Tubal Fluid (HTF) medium supplemented with 10% SFB, progesterone, and calcium and kept in incubation at 37 °C for 20 min. Cell count and viability estimation were carried out in a Neubauer chamber with 10 µL of the sperm suspension and trypan blue to stain non-viable cells. Sperm motility was evaluated under light microscopy, and three types of movement were observed: progressive, non-progressive, and no motility [
36]. It is worth mentioning that an indicator of murine sperm hyperactivity is the rapid progressive linear movement, which in the control group can reach 36–40% and, together with the type B movement, approaches 55–50% [
36,
37]. Motility was evaluated during the first 60 min after obtaining the sample, starting with an aliquot of 10 µL in the Neubauer chamber. Two hundred sperm cells were evaluated for motility in each separate experiment using the 40× objective in different quadrants chosen randomly, and cells were classified in A, B, and C categories, as mentioned above.
4.11. Experimental Design
To analyze the transcriptional activity of the Catsper1 promoter with different deletions or mutations in the Sox sites, each plasmid with GFP or Luciferase reporter genes was intratesticularly injected in mice (n = 3). Transcriptional activity was observed by GFP fluorescence in vivo from seminiferous tubules under a fluorescence microscope. On the other hand, similar constructs of the Catsper1 promoter coupled to the Photinus luciferase gene luciferase, activities were measured in protein extracts from the plasmid-transfected testis. The localization of GFP in testicular tissue was immunodetected with a specific antibody to determine the cell types with the activity of the Catsper1 promoter.
To determine the effect of the calmodulin inhibitor, 1 µM CMZ dose was analyzed over a time course of 24, 48, 72, and 96 h for the Catsper1 expression by RT-PCR (n = 3 each time) and immunofluorescence of Catsper1 in testis sections were observed. Likewise, sperm from epididymis was evaluated for motility in this time course for up to 72 hrs. Different doses of CMZ were injected intratesticular and 96 h after the expression of four Catsper genes was evaluated (n = 3).
For the evaluation of fertility, a control group without treatment (n = 3), one with the DMSO vehicle (n = 3), and 1 μM CMZ of mice (n = 33) treated for 5 weeks underwent intratesticular injections with CMZ 1 μM every week, of which three mice were sacrificed weekly for evaluation of motility and histological analysis of the testis (n = 15). Mice from a control group (n = 3), a group with a DMSO vehicle (n = 3), and a group of mice (n = 18) treated with CMZ 1 μM up to 5 weeks, were crossed with two mature and virgin females in a 1:2 male, female ratio (n = 36). The seminal plug in the females was verified before removing the males, and the number of pregnant females and the number of pups given birth by each female were observed.
4.12. Statistic Analysis
The differences between mice testes transfected with experimental plasmids were estimated by paired t-student tests after determining normal distribution through the Kolmogorov–Smirnov test. Comparison of sperm motility among control, treated and recuperated groups were analyzed with a one-way ANOVA statistical test. T-student tests were used to compare the mean fertility rate and litter size between the control and treated groups. All statistical tests were two-tailed, and p values < 0.05 were considered statistically significant.