Treatment of Mouse Sperm with a Non-Catalytic Mutant of PLA2G10 Reveals That PLA2G10 Improves In Vitro Fertilization through Both Its Enzymatic Activity and as Ligand of PLA2R1
Abstract
1. Introduction
2. Results
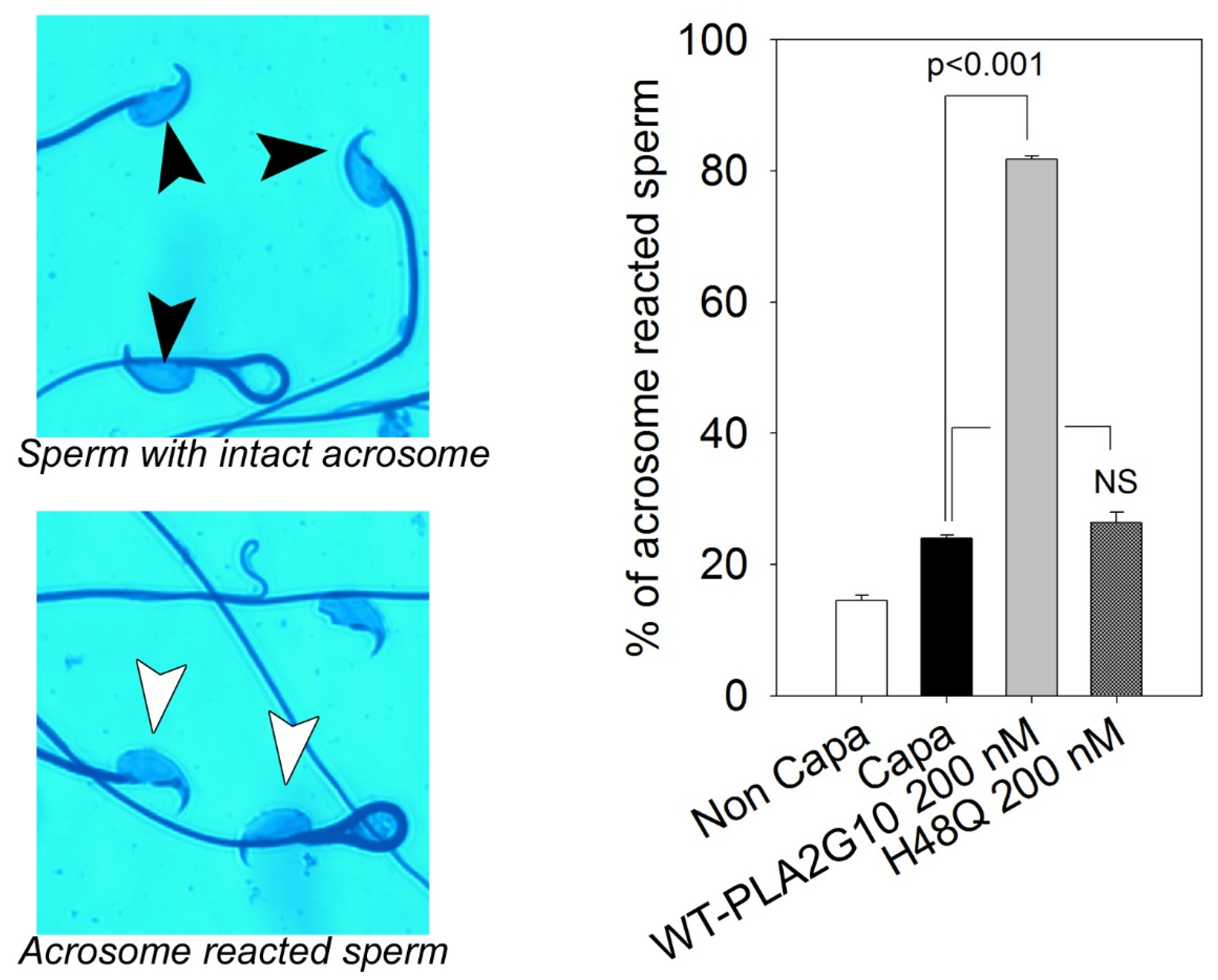
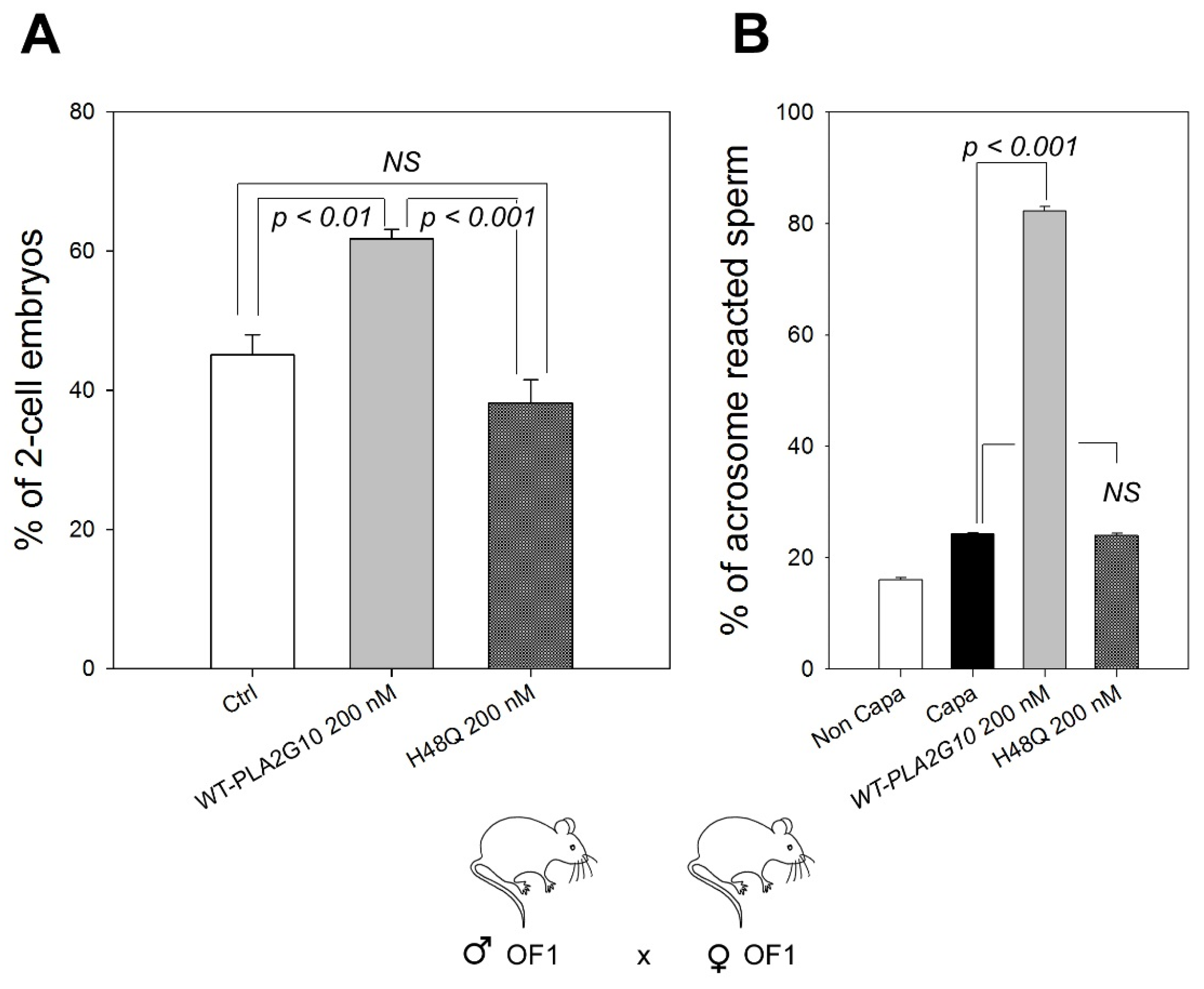
2.1. H48Q-PLA2G10 Has No Effect on the Acrosome Reaction
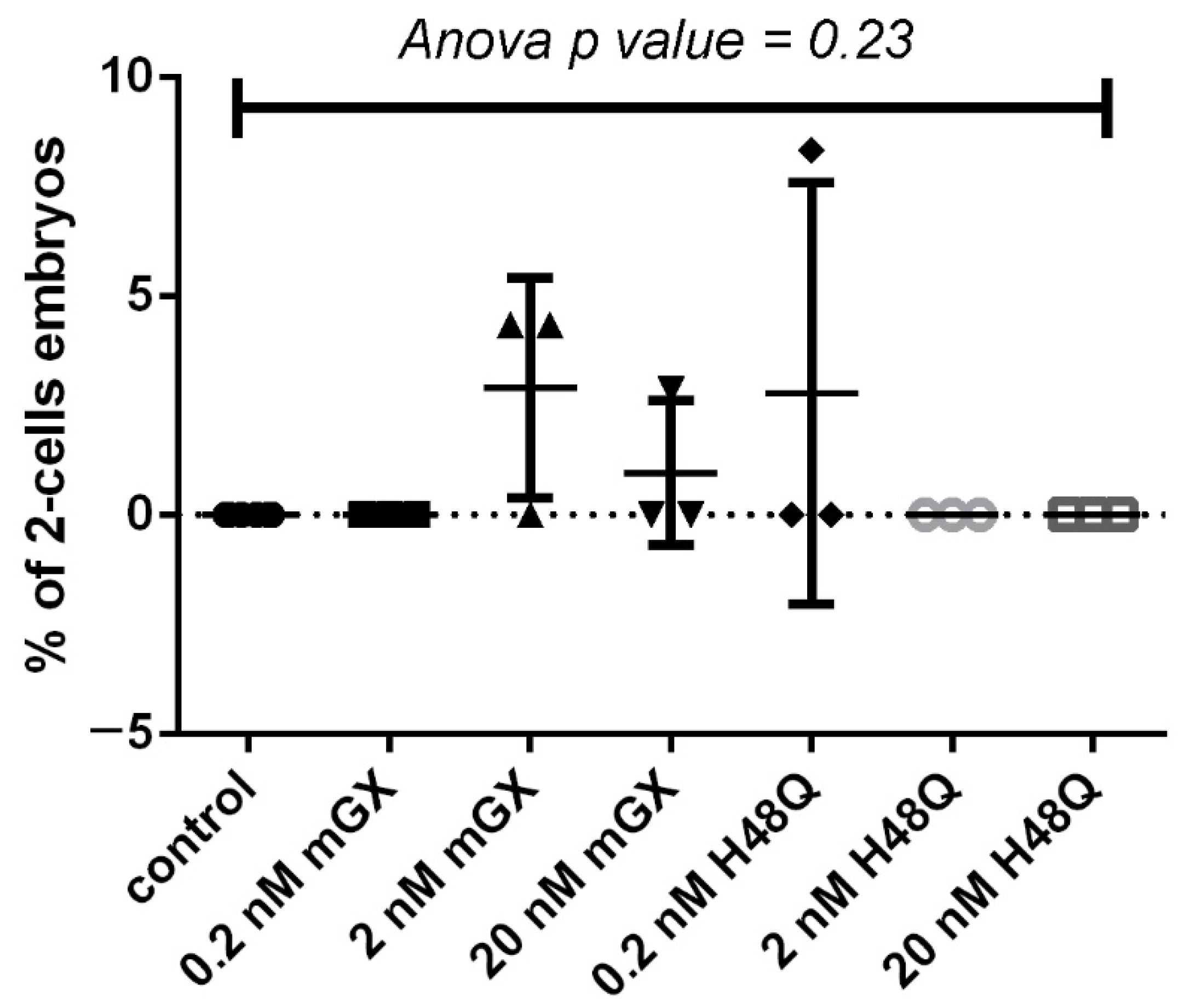
2.2. H48Q-PLA2G10 Has No Effect on Parthenogenesis
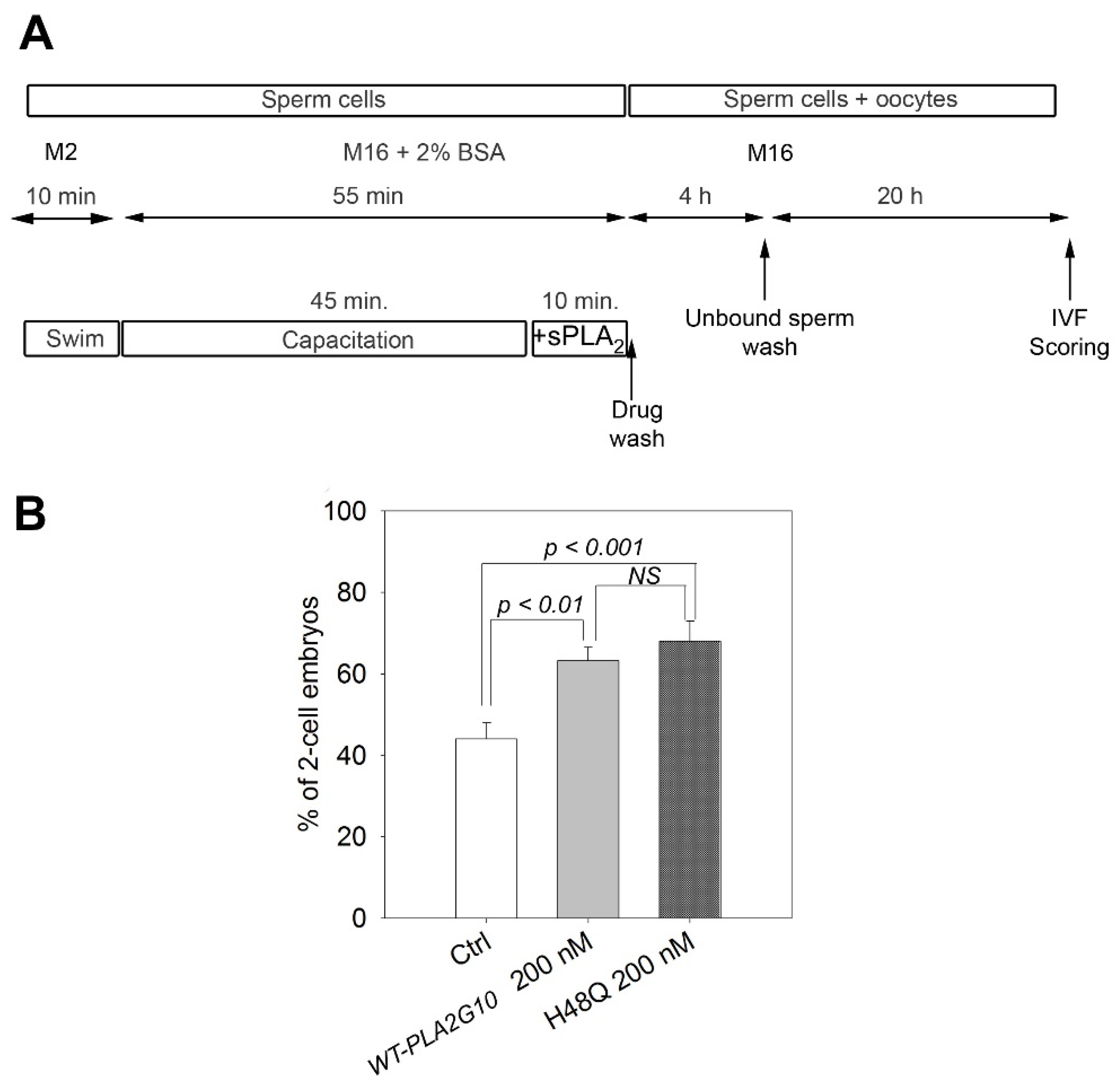
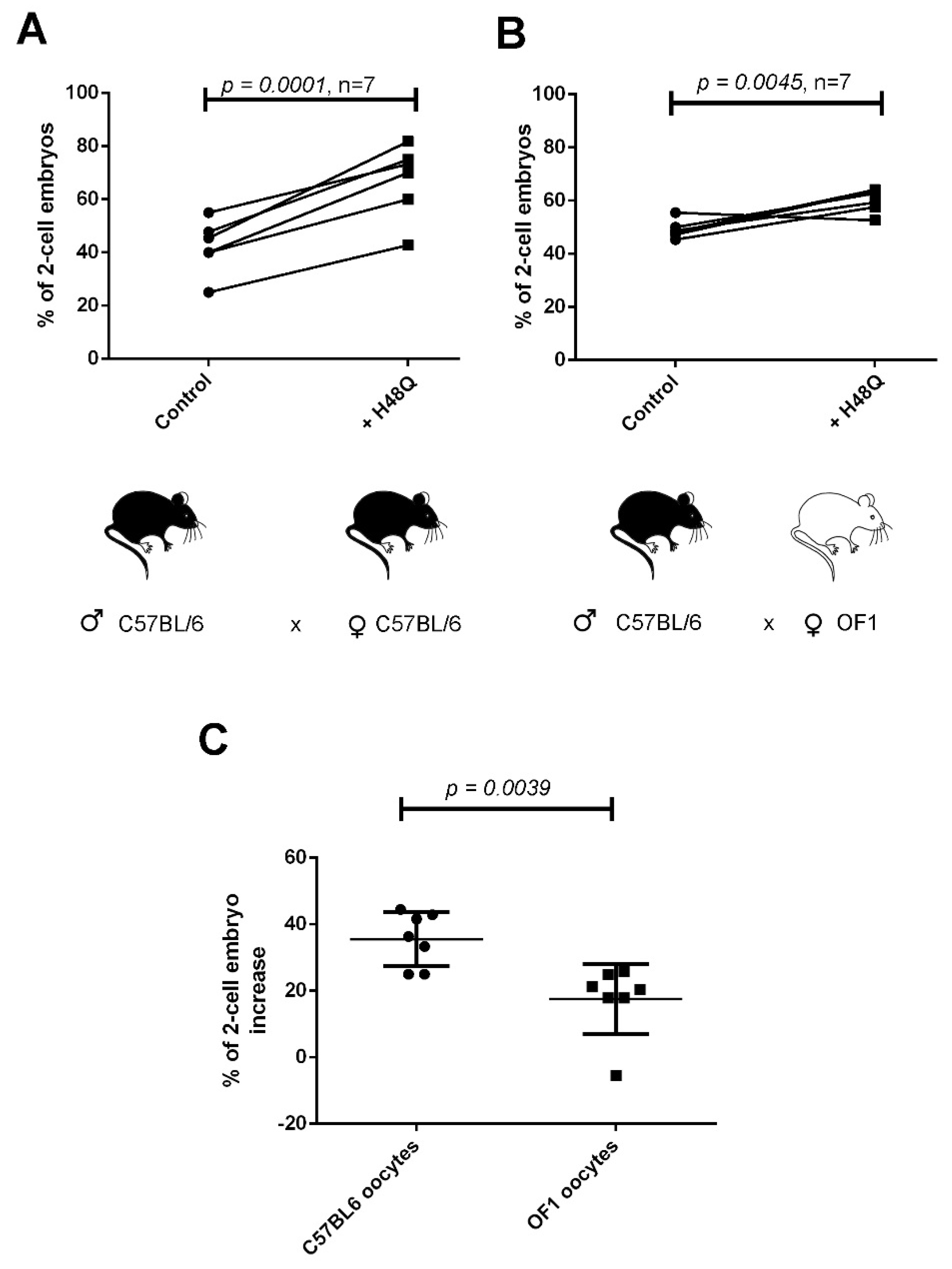
2.3. H48Q-PLA2G10 Improves IVF Yield
2.4. “sPLA2-Dependent Improvement of Fertilization” Induced by H48Q-PLA2G10 Is Strain Dependent
2.5. Expression of PLA2R1 in Gametes and Role in IVF
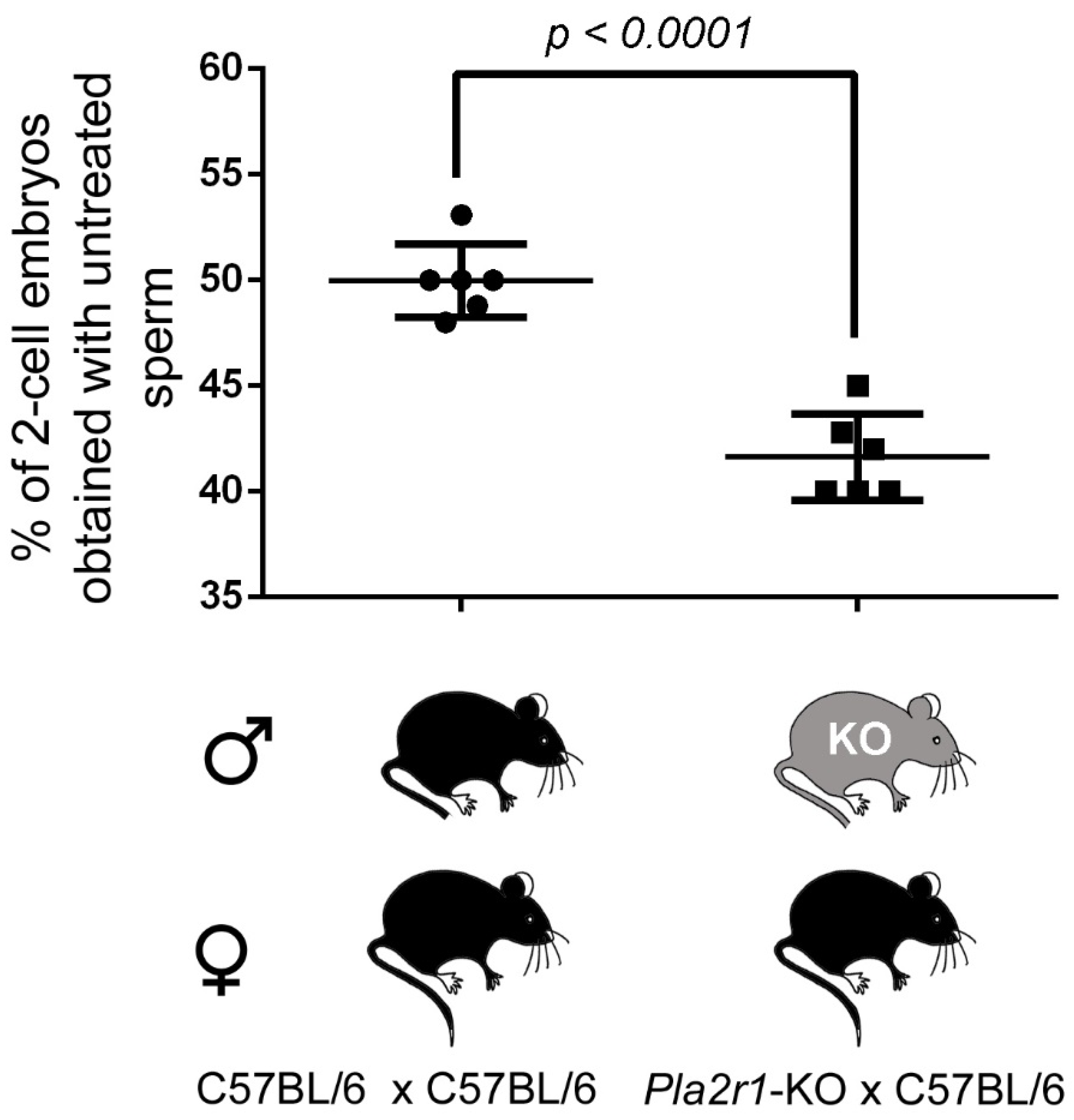
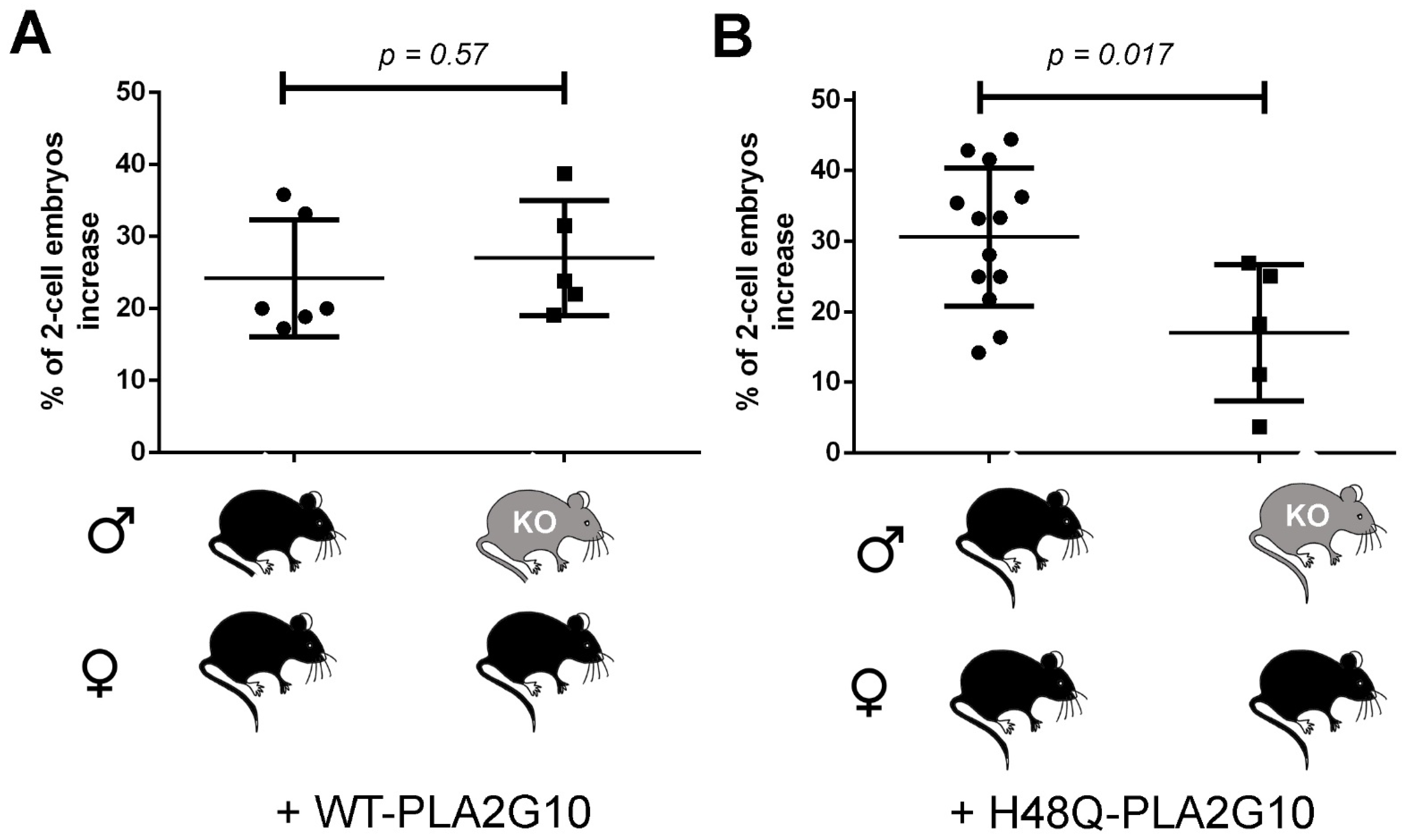
2.6. H48Q-PLA2G10-Induced Improvement of Fertilization Is Altered with Gametes from Pla2r1 -Deficient Mice
3. Discussion
3.1. Coexistence of Two Modes of Action
3.2. PLA2R1 of Both Sperm and MII Oocyte Is Involved in the H48Q-PLA2G10-Dependent Improvement of Fertilization
3.3. The Positive Effect of H48Q-PLA2G10 on Embryo Production Suggests That PLA2R1 Activates an Intracellular Pathway
3.4. Importance of the Physiological Mechanisms for the Optimization of Fertilization
4. Material and Methods
4.1. Animals
4.2. Chemical Compounds
4.3. Production of Recombinant sPLA2s
4.4. Capacitation
4.5. AR Assay
4.6. In Vitro Fertilization (IVF)
4.7. RT-PCR: Assessment of Pla2r1 Expression
4.8. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed]
- Lambeau, G.; Gelb, M.H. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 2008, 77, 495–520. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.G.; Ghomashchi, F.; Le Calvez, C.; Bollinger, J.; Bezzine, S.; Rouault, M.; Sadilek, M.; Nguyen, E.; Lazdunski, M.; Lambeau, G.; et al. Interfacial kinetic and binding properties of the complete set of human and mouse groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 2002, 277, 48535–48549. [Google Scholar] [CrossRef] [PubMed]
- Valentin, E.; Ghomashchi, F.; Gelb, M.H.; Lazdunski, M.; Lambeau, G. On the diversity of secreted phospholipases A(2). Cloning, tissue distribution, and functional expression of two novel mouse group II enzymes. J. Biol. Chem. 1999, 274, 31195–31202. [Google Scholar] [CrossRef]
- Escoffier, J.; Jemel, I.; Tanemoto, A.; Taketomi, Y.; Payre, C.; Coatrieux, C.; Sato, H.; Yamamoto, K.; Masuda, S.; Pernet-Gallay, K.; et al. Group X phospholipase A2 is released during sperm acrosome reaction and controls fertility outcome in mice. J. Clin. Investig. 2010, 120, 1415–1428. [Google Scholar] [CrossRef]
- Murase, R.; Sato, H.; Yamamoto, K.; Ushida, A.; Nishito, Y.; Ikeda, K.; Kobayashi, T.; Yamamoto, T.; Taketomi, Y.; Murakami, M. Group X Secreted Phospholipase A2 Releases ω3 Polyunsaturated Fatty Acids, Suppresses Colitis, and Promotes Sperm Fertility. J. Biol. Chem. 2016, 291, 6895–6911. [Google Scholar] [CrossRef]
- Darios, F.; Connell, E.; Davletov, B. Phospholipases and fatty acid signalling in exocytosis. J. Physiol. 2007, 585 Pt 3, 699–704. [Google Scholar] [CrossRef]
- Abi Nahed, R.; Martinez, G.; Escoffier, J.; Yassine, S.; Karaouzene, T.; Hograindleur, J.P.; Turk, J.; Kokotos, G.; Ray, P.F.; Bottari, S.; et al. Progesterone-induced Acrosome Exocytosis Requires Sequential Involvement of Calcium-independent Phospholipase A2beta (iPLA2beta) and Group X Secreted Phospholipase A2 (sPLA2). J. Biol. Chem. 2016, 291, 3076–3089. [Google Scholar] [CrossRef]
- Abi Nahed, R.; Escoffier, J.; Revel, C.; Jeammet, L.; Payre, C.; Ray, P.F.; Hennebicq, S.; Lambeau, G.; Arnoult, C. The effect of group X secreted phospholipase A2 on fertilization outcome is specific and not mimicked by other secreted phospholipases A2 or progesterone. Biochimie. 2014, 99, 88–95. [Google Scholar] [CrossRef]
- Arnoult, C.; Escoffier, J.; Munch, L.; Pierre, V.; Hennebicq, S.; Lambeau, G.; Ray, P. Do phospholipases, key enzymes in sperm physiology, represent therapeutic challenges? Med. Sci. 2012, 28, 512–518. [Google Scholar]
- Martinez, G.; Hograindleur, J.P.; Jeammet, L.; Le Blevec, E.; Coutton, C.; Mermillod, P.; Lambeau, G.; Schmitt, E.; Ray, P.F.; Arnoult, C. Enzymatic activity of mouse group X-sPLA2 improves in vitro production of preimplantation bovine embryos. Theriogenology 2019, 131, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Lambeau, G. Emerging roles of secreted phospholipase A(2) enzymes: An update. Biochimie 2013, 95, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.D.; Yanagimachi, R. Evidence suggesting the importance of fatty acids and the fatty acid moieties of sperm membrane phospholipids in the acrosome reaction of guinea pig spermatozoa. J. Exp. Zool. 1984, 229, 485–489. [Google Scholar] [CrossRef]
- Riffo, M.S.; Parraga, M. Role of phospholipase A2 in mammalian sperm-egg fusion: Development of hamster oolemma fusibility by lysophosphatidylcholine. J. Exp. Zool. 1997, 279, 81–88. [Google Scholar] [CrossRef]
- Roldan, E.R.; Shi, Q.X. Sperm phospholipases and acrosomal exocytosis. Front. Biosci. 2007, 12, 89–104. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rouault, M.; Le Calvez, C.; Boilard, E.; Surrel, F.; Singer, A.; Ghomashchi, F.; Bezzine, S.; Scarzello, S.; Bollinger, J.; Gelb, M.H.; et al. Recombinant production and properties of binding of the full set of mouse secreted phospholipases A2 to the mouse M-type receptor. Biochemistry 2007, 46, 1647–1662. [Google Scholar] [CrossRef]
- Saegusa, J.; Akakura, N.; Wu, C.Y.; Hoogland, C.; Ma, Z.; Lam, K.S.; Liu, F.T.; Takada, Y.K.; Takada, Y. Pro-inflammatory secretory phospholipase A2 type IIA binds to integrins alphavbeta3 and alpha4beta1 and induces proliferation of monocytic cells in an integrin-dependent manner. J. Biol. Chem. 2008, 283, 26107–26115. [Google Scholar] [CrossRef]
- Boilard, E.; Bourgoin, S.G.; Bernatchez, C.; Poubelle, P.E.; Surette, M.E. Interaction of low molecular weight group IIA phospholipase A2 with apoptotic human T cells: Role of heparan sulfate proteoglycans. FASEB J. 2003, 17, 1068–1080. [Google Scholar] [CrossRef]
- Murakami, M.; Kambe, T.; Shimbara, S.; Yamamoto, S.; Kuwata, H.; Kudo, I. Functional association of type IIA secretory phospholipase A(2) with the glycosylphosphatidylinositol-anchored heparan sulfate proteoglycan in the cyclooxygenase-2-mediated delayed prostanoid-biosynthetic pathway. J. Biol. Chem. 1999, 274, 29927–29936. [Google Scholar] [CrossRef]
- Fujisawa, D.; Yamazaki, Y.; Lomonte, B.; Morita, T. Catalytically inactive phospholipase A2 homologue binds to vascular endothelial growth factor receptor-2 via a C-terminal loop region. Biochem. J. 2008, 411, 515–522. [Google Scholar] [CrossRef]
- Ivanušec, A.; Šribar, J.; Križaj, I. Secreted Phospholipases A(2)—Not just Enzymes: Revisited. Int. J. Biol. Sci. 2022, 18, 873–888. [Google Scholar] [CrossRef] [PubMed]
- Yokota, Y.; Higashino, K.; Nakano, K.; Arita, H.; Hanasaki, K. Identification of group X secretory phospholipase A(2) as a natural ligand for mouse phospholipase A(2) receptor. FEBS Lett. 2000, 478, 187–191. [Google Scholar] [CrossRef]
- Surrel, F.; Jemel, I.; Boilard, E.; Bollinger, J.G.; Payre, C.; Mounier, C.M.; Talvinen, K.A.; Laine, V.J.; Nevalainen, T.J.; Gelb, M.H.; et al. Group X phospholipase A2 stimulates the proliferation of colon cancer cells by producing various lipid mediators. Mol. Pharmacol. 2009, 76, 778–790. [Google Scholar] [CrossRef]
- Janssen, M.J.; van de Wiel, W.A.; Beiboer, S.H.; van Kampen, M.D.; Verheij, H.M.; Slotboom, A.J.; Egmond, M.R. Catalytic role of the active site histidine of porcine pancreatic phospholipase A2 probed by the variants H48Q, H48N and H48K. Protein Eng. 1999, 12, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Morioka, Y.; Saiga, A.; Yokota, Y.; Suzuki, N.; Ikeda, M.; Ono, T.; Nakano, K.; Fujii, N.; Ishizaki, J.; Arita, H.; et al. Mouse group X secretory phospholipase A2 induces a potent release of arachidonic acid from spleen cells and acts as a ligand for the phospholipase A2 receptor. Arch. Biochem. Biophys. 2000, 381, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Hanasaki, K.; Yokota, Y.; Ishizaki, J.; Itoh, T.; Arita, H. Resistance to endotoxic shock in phospholipase A2 receptor-deficient mice. J. Biol. Chem. 1997, 272, 32792–32797. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.P.; Payette, P.; Mudgett, J.; Vadas, P.; Pruzanski, W.; Kwan, M.; Tang, C.; Rancourt, D.E.; Cromlish, W.A. A natural disruption of the secretory group II phospholipase A2 gene in inbred mouse strains. J. Biol. Chem. 1995, 270, 22378–22385. [Google Scholar] [CrossRef]
- Masuda, S.; Murakami, M.; Matsumoto, S.; Eguchi, N.; Urade, Y.; Lambeau, G.; Gelb, M.H.; Ishikawa, Y.; Ishii, T.; Kudo, I. Localization of various secretory phospholipase A2 enzymes in male reproductive organs. Biochim. Biophys. Acta 2004, 1686, 61–76. [Google Scholar] [CrossRef]
- Diaz, B.L.; Satake, Y.; Kikawada, E.; Balestrieri, B.; Arm, J.P. Group V secretory phospholipase A2 amplifies the induction of cyclooxygenase 2 and delayed prostaglandin D2 generation in mouse bone marrow culture-derived mast cells in a strain-dependent manner. Biochim. Biophys. Acta 2006, 1761, 1489–1497. [Google Scholar]
- Kuroda, E.; Yamashita, U. Mechanisms of enhanced macrophage-mediated prostaglandin E2 production and its suppressive role in Th1 activation in Th2-dominant BALB/c mice. J. Immunol. 2003, 170, 757–764. [Google Scholar]
- Nicolas, J.P.; Lambeau, G.; Lazdunski, M. Identification of the binding domain for secretory phospholipases A2 on their M-type 180-kDa membrane receptor. J. Biol. Chem. 1995, 270, 28869–28873. [Google Scholar] [CrossRef] [PubMed]
- Ancian, P.; Lambeau, G.; Lazdunski, M. Multifunctional activity of the extracellular domain of the M-type (180 kDa) membrane receptor for secretory phospholipases A2. Biochemistry 1995, 34, 13146–13151. [Google Scholar] [CrossRef] [PubMed]
- Higashino Ki, K.; Yokota, Y.; Ono, T.; Kamitani, S.; Arita, H.; Hanasaki, K. Identification of a soluble form phospholipase A2 receptor as a circulating endogenous inhibitor for secretory phospholipase A2. J. Biol. Chem. 2002, 277, 13583–13588. [Google Scholar] [CrossRef]
- Lambeau, G.; Lazdunski, M. Receptors for a growing family of secreted phospholipases A2. Trends Pharmacol. Sci. 1999, 20, 162–170. [Google Scholar] [CrossRef]
- Sukocheva, O.; Menschikowski, M.; Hagelgans, A.; Yarla, N.S.; Siegert, G.; Reddanna, P.; Bishayee, A. Current insights into functions of phospholipase A2 receptor in normal and cancer cells: More questions than answers. Semin. Cancer Biol. 2019, 56, 116–127. [Google Scholar] [CrossRef]
- Girard, C.A.; Seitz-Polski, B.; Dolla, G.; Augert, A.; Vindrieux, D.; Bernard, D.; Lambeau, G. Nouveaux rôles physiopathologiques pour le récepteur PLA2R1 dans le cancer et la glomérulonéphrite extramembraneuse. Med. Sci. 2014, 30, 519–525. [Google Scholar] [CrossRef][Green Version]
- Zvaritch, E.; Lambeau, G.; Lazdunski, M. Endocytic properties of the M-type 180-kDa receptor for secretory phospholipases A2. J. Biol. Chem. 1996, 271, 250–257. [Google Scholar] [CrossRef]
- Granata, F.; Petraroli, A.; Boilard, E.; Bezzine, S.; Bollinger, J.; Del Vecchio, L.; Gelb, M.H.; Lambeau, G.; Marone, G.; Triggiani, M. Activation of cytokine production by secreted phospholipase A2 in human lung macrophages expressing the M-type receptor. J. Immunol. 2005, 174, 464–474. [Google Scholar] [CrossRef]
- Park, D.W.; Kim, J.R.; Kim, S.Y.; Sonn, J.K.; Bang, O.S.; Kang, S.S.; Kim, J.H.; Baek, S.H. Akt as a mediator of secretory phospholipase A2 receptor-involved inducible nitric oxide synthase expression. J. Immunol. 2003, 170, 2093–2099. [Google Scholar] [CrossRef]
- Silliman, C.C.; Moore, E.E.; Zallen, G.; Gonzalez, R.; Johnson, J.L.; Elzi, D.J.; Meng, X.; Hanasaki, K.; Ishizaki, J.; Arita, H.; et al. Presence of the M-type sPLA(2) receptor on neutrophils and its role in elastase release and adhesion. Am. J. Physiol. Cell Physiol. 2002, 283, C1102–C1113. [Google Scholar] [CrossRef]
- Fonteh, A.N.; Atsumi, G.; LaPorte, T.; Chilton, F.H. Secretory phospholipase A2 receptor-mediated activation of cytosolic phospholipase A2 in murine bone marrow-derived mast cells. J. Immunol. 2000, 165, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Vindrieux, D.; Augert, A.; Girard, C.A.; Gitenay, D.; Lallet-Daher, H.; Wiel, C.; Le Calvé, B.; Gras, B.; Ferrand, M.; Verbeke, S.; et al. PLA2R1 mediates tumor suppression by activating JAK2. Cancer Res. 2013, 73, 6334–6345. [Google Scholar] [CrossRef]
- Griveau, A.; Devailly, G.; Eberst, L.; Navaratnam, N.; Le Calvé, B.; Ferrand, M.; Faull, P.; Augert, A.; Dante, R.; Vanacker, J.M.; et al. The PLA2R1-JAK2 pathway upregulates ERRα and its mitochondrial program to exert tumor-suppressive action. Oncogene 2016, 35, 5033–5042. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Jiang, X.; Hameed, U.; Shi, Q. Role of Lipid Metabolism and Signaling in Mammalian Oocyte Maturation, Quality, and Acquisition of Competence. Front. Cell Dev. Biol. 2021, 9, 639704. [Google Scholar] [CrossRef] [PubMed]
- Stival, C.; Puga Molina Ldel, C.; Paudel, B.; Buffone, M.G.; Visconti, P.E.; Krapf, D. Sperm Capacitation and Acrosome Reaction in Mammalian Sperm. Adv. Anat. Embryol. Cell Biol. 2016, 220, 93–106. [Google Scholar]
- Jungnickel, M.K.; Sutton, K.A.; Baker, M.A.; Cohen, M.G.; Sanderson, M.J.; Florman, H.M. The flagellar protein Enkurin is required for mouse sperm motility and for transport through the female reproductive tract. Biol. Reprod. 2018, 99, 789–797. [Google Scholar] [CrossRef]
- Miller, B.J.; Georges-Labouesse, E.; Primakoff, P.; Myles, D.G. Normal fertilization occurs with eggs lacking the integrin alpha6beta1 and is CD9-dependent. J. Cell Biol. 2000, 149, 1289–1296. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Montoya, M.C.; Oatley, M.J.; Lord, T.; Oatley, J.M.; Klein, J.; Ravichandran, R.; Tillman, H.; Kim, M.; Connelly, J.P.; et al. MAGE cancer-testis antigens protect the mammalian germline under environmental stress. Sci. Adv. 2019, 5, eaav4832. [Google Scholar] [CrossRef]
- Sutton, K.A.; Jungnickel, M.K.; Florman, H.M. A polycystin-1 controls postcopulatory reproductive selection in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 8661–8666. [Google Scholar] [CrossRef]
- Festa-Bianchet, M.; King, W.J. Effects of Litter Size and Population Dynamics on Juvenile and Maternal Survival in Columbian Ground Squirrels. J. Anim. Ecol. 1991, 60, 1077–1090. [Google Scholar] [CrossRef]
- Cardillo, M. Biological determinants of extinction risk: Why are smaller species less vulnerable? Animal Conservation 2003, 6, 63–69. [Google Scholar]
- Ghomashchi, F.; Brglez, V.; Payre, C.; Jeammet, L.; Bezzine, S.; Gelb, M.H.; Lambeau, G. Preparation of the Full Set of Recombinant Mouse- and Human-Secreted Phospholipases A2. Methods Enzymol. 2017, 583, 35–69. [Google Scholar] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abi Nahed, R.; Dhellemmes, M.; Payré, C.; Le Blévec, E.; Perrier, J.-P.; Hennebicq, S.; Escoffier, J.; Ray, P.F.; Loeuillet, C.; Lambeau, G.; et al. Treatment of Mouse Sperm with a Non-Catalytic Mutant of PLA2G10 Reveals That PLA2G10 Improves In Vitro Fertilization through Both Its Enzymatic Activity and as Ligand of PLA2R1. Int. J. Mol. Sci. 2022, 23, 8033. https://doi.org/10.3390/ijms23148033
Abi Nahed R, Dhellemmes M, Payré C, Le Blévec E, Perrier J-P, Hennebicq S, Escoffier J, Ray PF, Loeuillet C, Lambeau G, et al. Treatment of Mouse Sperm with a Non-Catalytic Mutant of PLA2G10 Reveals That PLA2G10 Improves In Vitro Fertilization through Both Its Enzymatic Activity and as Ligand of PLA2R1. International Journal of Molecular Sciences. 2022; 23(14):8033. https://doi.org/10.3390/ijms23148033
Chicago/Turabian StyleAbi Nahed, Roland, Magali Dhellemmes, Christine Payré, Emilie Le Blévec, Jean-Philippe Perrier, Sylviane Hennebicq, Jessica Escoffier, Pierre F. Ray, Corinne Loeuillet, Gérard Lambeau, and et al. 2022. "Treatment of Mouse Sperm with a Non-Catalytic Mutant of PLA2G10 Reveals That PLA2G10 Improves In Vitro Fertilization through Both Its Enzymatic Activity and as Ligand of PLA2R1" International Journal of Molecular Sciences 23, no. 14: 8033. https://doi.org/10.3390/ijms23148033
APA StyleAbi Nahed, R., Dhellemmes, M., Payré, C., Le Blévec, E., Perrier, J.-P., Hennebicq, S., Escoffier, J., Ray, P. F., Loeuillet, C., Lambeau, G., & Arnoult, C. (2022). Treatment of Mouse Sperm with a Non-Catalytic Mutant of PLA2G10 Reveals That PLA2G10 Improves In Vitro Fertilization through Both Its Enzymatic Activity and as Ligand of PLA2R1. International Journal of Molecular Sciences, 23(14), 8033. https://doi.org/10.3390/ijms23148033





