Possible Implications of Bacteriospermia on the Sperm Quality, Oxidative Characteristics, and Seminal Cytokine Network in Normozoospermic Men
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Processing
4.2. Assessment of Semen Quality
4.3. Oxidative Profile
4.4. Biochip Assay
4.5. ELISA
4.6. Bacteriological Analysis
4.7. Biodiversity Calculation
4.8. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rusz, A.; Pilatz, A.; Wagenlehner, F.; Linn, T.; Diemer, T.; Schuppe, H.C.; Lohmeyer, J.; Hossain, H.; Weidner, W. Influence of urogenital infections and inflammation on semen quality and male fertility. World J. Urol. 2012, 30, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Pergialiotis, V.; Karampetsou, N.; Perrea, D.N.; Konstantopoulos, P.; Daskalakis, G. The impact of bacteriospermia on semen parameters: A meta-analysis. J. Family Reprod. Health 2018, 12, 73–83. [Google Scholar]
- Fraczek, M.; Kurpisz, M. Mechanisms of the harmful effects of bacterial semen infection on ejaculated human spermatozoa: Potential inflammatory markers in semen. Folia Histochem. Cytobiol. 2015, 53, 201–217. [Google Scholar] [CrossRef]
- Weng, S.L.; Chiu, C.M.; Lin, F.M.; Huang, W.C.; Liang, C.; Yang, T.; Yang, T.L.; Liu, C.Y.; Wu, W.Y.; Chang, Y.A.; et al. Bacterial communities in semen from men of infertile couples: Metagenomic sequencing reveals relationships of seminal microbiota to semen quality. PLoS ONE 2014, 9, e110152. [Google Scholar] [CrossRef]
- Eini, F.; Kutenaei, M.A.; Zareei, F.; Dastjerdi, Z.S.; Shirzeyli, M.H.; Salehi, E. Effect of bacterial infection on sperm quality and DNA fragmentation in subfertile men with leukocytospermia. BMC Mol. Cell. Biol. 2021, 22, 42. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Capitani, S.; Figura, N.; Pammolli, A.; Federico, M.G.; Giannerini, V.; Collodel, G. The presence of bacteria species in semen and sperm quality. J. Assisted Reprod. Genet. 2009, 26, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.; Hryhorowicz, M.; Gill, K.; Zarzycka, M.; Gaczarzewicz, D.; Jedrzejczak, P.; Bilinska, B.; Piasecka, M.; Kurpisz, M. The effect of bacteriospermia and leukocytospermia on conventional and nonconventional semen parameters in healthy young normozoospermic males. J. Reprod. Immunol. 2016, 118, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Voroshilina, E.S.; Zornikov, D.L.; Ivanov, A.V.; Pochernikov, D.G.; Panacheva, E.A. Microbiota of semen samples with normozoospermia: Analysis of real-time PCR data. Bull. Russ. State Med. Univ. 2021, 5, 54–61. [Google Scholar] [CrossRef]
- Zhu, X.; Li, X.; Wangg, W.; Ning, K. Bacterial contamination screening and interpretation for biological laboratory environments. Med. Microecol. 2020, 5, 100021. [Google Scholar] [CrossRef]
- Zeyad, A.; Hamad, M.F.; Hammadeh, M.E. The effects of bacterial infection on human sperm nuclear protamine P1/P2 ratio and DNA integrity. Andrologia 2018, 50, e12841. [Google Scholar] [CrossRef]
- Marchiani, S.; Baccani, I.; Tamburrino, L.; Mattiuz, G.; Nicolò, S.; Bonaiuto, C.; Panico, C.; Vignozzi, L.; Antonelli, A.; Rossolini, G.M.; et al. Effects of common Gram-negative pathogens causing male genitourinary-tract infections on human sperm functions. Sci. Rep. 2021, 11, 19177. [Google Scholar] [CrossRef] [PubMed]
- Enwuru, C.A.; Iwalokun, B.; Enwuru, V.N.; Ezechi, O.; Oluwadun, A. The effect of presence of facultative bacteria species on semen and sperm quality of men seeking fertility care. Afr. J. Urol. 2016, 22, 213–222. [Google Scholar] [CrossRef][Green Version]
- Baud, D.; Pattaroni, C.; Vulliemoz, N.; Castella, V.; Marsland, B.J.; Stojanov, M. Sperm microbiota and its impact on semen parameters. Front. Microbiol. 2019, 10, 234. [Google Scholar] [CrossRef]
- Fraczek, M.; Hryhorowicz, M.; Gaczarzewicz, D.; Szumala-Kakol, A.; Kolanowski, T.J.; Beutin, L.; Kurpisz, M. Can apoptosis and necrosis coexist in ejaculated human spermatozoa during in vitro semen bacterial infection? J. Assist. Reprod. Genet. 2015, 32, 771–779. [Google Scholar] [CrossRef]
- Vilvanathan, S.; Kandasamy, B.; Jayachandran, A.L.; Sathiyanarayanan, S.; Tanjore Singaravelu, V.; Krishnamurthy, V.; Elangovan, V. Bacteriospermia and its impact on basic semen parameters among infertile men. Interdiscip. Perspect. Infect. Dis. 2016, 2016, 2614692. [Google Scholar] [CrossRef] [PubMed]
- Chaichian, S.; Tamannaie, Z.; Rohani, H.; Ahmadi, M.; Nasr, M.H.; Pazouki, A.; Mehdizadehkashi, A. Relationship between sperm parameters and intracytoplasmic sperm injection outcome. Middle East Fertil. Soc. J. 2015, 20, 251–254. [Google Scholar] [CrossRef]
- Prabha, V.; Sandhu, R.; Kaur, S.; Kaur, K.; Sarwal, A.; Mavuduru, R.S.; Singh, S.K. Mechanism of sperm immobilization by Escherichia coli. Adv. Urol. 2010, 2010, 240268. [Google Scholar] [CrossRef]
- Fraczek, M.; Szumala-Kakol, A.; Jedrzejczak, P.; Kamieniczna, M.; Kurpisz, M. Bacteria trigger oxygen radical release and sperm lipid peroxidation in in vitro model of semen inflammation. Fertil. Steril. 2007, 88, 1076–1085. [Google Scholar] [CrossRef]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, e13126. [Google Scholar] [CrossRef]
- Das, S.; Roychoudhury, S.; Dey, A.; Jha, N.K.; Kumar, D.; Roychoudhury, S.; Slama, P.; Kesari, K.K. Bacteriospermia and male infertility: Role of oxidative stress. Adv. Exp. Med. Biol. 2022, 1358, 141–163. [Google Scholar]
- Matousková, I.; Oborná, I.; Fingerová, H.; Kohnová, I.; Novotný, J.; Svobodová, M.; Brezinová, J.; Vyslouzilová, J.; Radová, L. Bacteriospermia and the production of reactive oxygen species in the semen of males from infertile couples. Klin. Mikrobiol. Infekc. Lek. 2009, 15, 192–195. [Google Scholar] [PubMed]
- Domes, T.; Lo, K.C.; Grober, E.D.; Mullen, J.B.; Mazzulli, T.; Jarvi, K. The incidence and effect of bacteriospermia and elevated seminal leukocytes on semen parameters. Fertil. Steril. 2012, 97, 1050–1055. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Pilatz, A.; Hedger, M.P.; Nicolas, N.; Bhushan, S.; Michel, V.; Tung, K.S.K.; Schuppe, H.C.; Meinhardt, A. Infectious, inflammatory and ‘autoimmune’ male factor infertility: How do rodent models inform clinical practice? Hum. Reprod. Update 2018, 24, 416–441. [Google Scholar] [CrossRef] [PubMed]
- Lackner, J.E.; Herwig, R.; Schmidbauer, J.; Schatzl, G.; Kratzik, C.; Marberger, M. Correlation of leukocytospermia with clinical infection and the positive effect of antiinflammatory treatment on semen quality. Fertil. Steril. 2006, 86, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Ventimiglia, E.; Capogrosso, P.; Boeri, L.; Cazzaniga, W.; Matloob, R.; Pozzi, E.; Chierigo, F.; Abbate, C.; Viganò, P.; Montorsi, F.; et al. Leukocytospermia is not an informative predictor of positive semen culture in infertile men: Results from a validation study of available guidelines. Hum. Reprod. Open. 2020, 2020, hoaa039. [Google Scholar] [CrossRef]
- Boitrelle, F.; Robin, G.; Lefebvre, C.; Bailly, M.; Selva, J.; Courcol, R.; Lornage, J.; Albert, M. Bacteriospermia in Assisted Reproductive Techniques: Effects of bacteria on spermatozoa and seminal plasma, diagnosis and treatment. Gynecol. Obstet. Fertil. 2012, 40, 226–234. [Google Scholar] [CrossRef]
- Heidari Pebdeni, P.; Saffari, F.; Reza Mirshekari, T.; Ashourzadeh, S.; Taheri Soodejani, M.; Ahmadrajabi, R. Bacteriospermia and its association with seminal fluid parameters and infertility in infertile men, Kerman, Iran: A cross-sectional study. Int. J. Reprod. Biomed. 2022, 20, 202–212. [Google Scholar] [CrossRef]
- Dissanayake, D.M.; Amaranath, K.A.; Perera, R.R.; Wijesinghe, P.S. Antibiotics supplemented culture media can eliminate non-specific bacteria from human semen during sperm preparation for intra uterine insemination. J. Hum. Reprod. Sci. 2014, 7, 58–62. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef]
- Santos, C.S.; Silva, A.R. Current and alternative trends in antibacterial agents used in mammalian semen technology. Anim. Reprod. 2020, 17, e20190111. [Google Scholar] [CrossRef]
- Hou, D.; Zhou, X.; Zhong, X.; Settles, M.L.; Herring, J.; Wang, L.; Abdo, Z.; Forney, L.J.; Xu, C. Microbiota of the seminal fluid from healthy and infertile men. Fertil. Steril. 2013, 100, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak, P.; Fraczek, M.; Szumała-Kakol, A.; Taszarek-Hauke, G.; Pawelczyk, L.; Kurpisz, M. Consequences of semen inflammation and lipid peroxidation on fertilization capacity of spermatozoa in vitro conditions. Int. J. Androl. 2005, 28, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Al-Jebouri, M.M.; Mdish, S.A. Tracing of antibiotic-resistant bacteria isolated from semen of Iraqi males with primary infertility. Open J. Urol. 2019, 9, 19–29. [Google Scholar] [CrossRef][Green Version]
- Zhang, F.; Dai, J.; Chen, T. Role of Lactobacillus in female infertility via modulating sperm agglutination and immobilization. Front. Cell. Infect. Microbiol. 2021, 10, 620529. [Google Scholar] [CrossRef]
- Valcarce, D.G.; Genovés, S.; Riesco, M.F.; Martorell, P.; Herráez, M.P.; Ramón, D.; Robles, V. Probiotic administration improves sperm quality in asthenozoospermic human donors. Benef. Microbes. 2017, 8, 193–206. [Google Scholar] [CrossRef]
- Isaiah, I.; Nche, B.T.; Nwagu, I.G.; Nnanna, I.I. Current Studies on Bacterospermia the Leading Cause of Male Infertility: Aprotege and Potential Threat towards Man’s Extinction. Nor. Am. J. Med. Sci. 2011, 3, 562–564. [Google Scholar] [CrossRef]
- Kastrop, P.M.; de Graaf-Miltenburg, L.A.; Gutknecht, D.R.; Weima, S.M. Microbial contamination of embryo cultures in an ART laboratory: Sources and management. Hum. Reprod. 2007, 22, 2243–2248. [Google Scholar] [CrossRef]
- Machen, G.L.; Bird, E.T.; Brown, M.L.; Ingalsbe, D.A.; East, M.M.; Reyes, M.; Kuehl, T.J. Time trends for bacterial species and resistance patterns in semen in patients undergoing evaluation for male infertility. Proceedings (Bayl. Univ. Med. Cent.) 2018, 31, 165–167. [Google Scholar] [CrossRef]
- Sanocka-Maciejewska, D.; Ciupińska, M.; Kurpisz, M. Bacterial infection and semen quality. J. Reprod. Immunol. 2005, 67, 51–56. [Google Scholar] [CrossRef]
- Berjis, K.; Ghiasi, M.; Sangy, S. Study of seminal infection among an infertile male population in Qom, Iran, and its effect on sperm quality. Iran. J. Microbiol. 2018, 10, 111–116. [Google Scholar] [PubMed]
- Fraczek, M.; Piasecka, M.; Gaczarzewicz, D.; Szumala-Kakol, A.; Kazienko, A.; Lenart, S.; Laszczynska, M.; Kurpisz, M. Membrane stability and mitochondrial activity of human-ejaculated spermatozoa during in vitro experimental infection with Escherichia coli, Staphylococcus haemolyticus and Bacteroides ureolyticus. Andrologia 2012, 44, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Sánchez, R.; Soto, L.; Risopatrón, J.; Villegas, J. Effect of Escherichia coli and its soluble factors on mitochondrial membrane potential, phosphatidylserine translocation, viability, and motility of human spermatozoa. Fertil. Steril. 2010, 94, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Collodel, G.; Mazzi, L.; Campagna, M.; Iacoponi, F.; Figura, N. Resistin, interleukin-6, tumor necrosis factor-alpha, and human semen parameters in the presence of leukocytospermia, smoking habit, and varicocele. Fertil. Steril. 2014, 102, 354–360. [Google Scholar] [CrossRef]
- Fraczek, M.; Szumala-Kakol, A.; Dworacki, G.; Sanocka, D.; Kurpisz, M. In vitro reconstruction of inflammatory reaction in human semen: Effect on sperm DNA fragmentation. J. Reprod. Immunol. 2013, 100, 76–85. [Google Scholar] [CrossRef]
- Sanocka, D.; Fraczek, M.; Jedrzejczak, P.; Szumała-Kakol, A.; Kurpisz, M. Male genital tract infection: An influence of leukocytes and bacteria on semen. J. Reprod. Immunol. 2004, 62, 111–124. [Google Scholar] [CrossRef]
- Henkel, R.R. Leukocytes and oxidative stress: Dilemma for sperm function and male fertility. Asian J. Androl. 2011, 13, 43–52. [Google Scholar] [CrossRef]
- Keck, C.; Gerber-Schäfer, C.; Clad, A.; Wilhelm, C.; Breckwoldt, M. Seminal tract infections: Impact on male fertility and treatment options. Hum. Reprod. Update 1998, 4, 891–903. [Google Scholar] [CrossRef]
- Schuppe, H.C.; Pilatz, A.; Hossain, H.; Diemer, T.; Wagenlehner, F.; Weidner, W. Urogenital Infection as a Risk Factor for Male Infertility. Dtsch. Arztebl. Int. 2017, 114, 339–346. [Google Scholar] [CrossRef]
- Volz, Y.; Ebner, B.; Pfitzinger, P.; Berg, E.; Lellig, E.; Marcon, J.; Trottmann, M.; Becker, A.; Stief, C.G.; Magistro, G. Asymptomatic bacteriospermia and infertility-what is the connection? Infection 2022, 1–7. [Google Scholar] [CrossRef]
- Martínez, P.; Proverbio, F.; Camejo, M.I. Sperm lipid peroxidation and pro-inflammatory cytokines. Asian J. Androl. 2007, 9, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Perdichizzi, A.; Nicoletti, F.; La Vignera, S.; Barone, N.; D’Agata, R.; Vicari, E.; Calogero, A.E. Effects of tumour necrosis factor-alpha on human sperm motility and apoptosis. J. Clin. Immunol. 2007, 27, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Barroso, G.; Morshedi, M.; Oehninger, S. Analysis of DNA fragmentation, plasma membrane translocation of phosphatidylserine and oxidative stress in human spermatozoa. Hum. Reprod. 2000, 15, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Dziadecki, W.; Celińska, A.; Fracki, S.; Bablok, L.; Barcz, E. Interleukin 1b and interleukin 18 and their connection with leukocytospermia in human semen. Centr. Eur. J. Immunol. 2010, 35, 157–161. [Google Scholar]
- Koçak, I.; Yenisey, C.; Dündar, M.; Okyay, P.; Serter, M. Relationship between seminal plasma interleukin-6 and tumor necrosis factor alpha levels with semen parameters in fertile and infertile men. Urol. Res. 2002, 30, 263–267. [Google Scholar]
- Lu, Z.; Sethu, R.; Imlay, J.A. Endogenous superoxide is a key effector of the oxygen sensitivity of a model obligate anaerobe. Proc. Natl. Acad. Sci. USA 2018, 115, E3266–E3275. [Google Scholar] [CrossRef]
- Chakraborty, S.P.; Pramanik, P.; Roy, S. Staphylococcus aureus Infection induced oxidative imbalance in neutrophils: Possible protective role of nanoconjugated vancomycin. ISRN Pharmacol. 2012, 2012, 435214. [Google Scholar] [CrossRef]
- Duracka, M.; Lukac, N.; Kacaniova, M.; Kantor, A.; Hleba, L.; Ondruska, L.; Tvrda, E. Antibiotics Versus Natural Biomolecules: The Case of In Vitro Induced Bacteriospermia by Enterococcus Faecalis in Rabbit Semen. Molecules 2019, 24, 4329. [Google Scholar] [CrossRef]
- Collodel, G.; Baccetti, B.; Capitani, S.; Moretti, E. Necrosis in human spermatozoa. I. Ultrastructural features and FISH study in semen from patients with urogenital infections. J. Submicrosc. Cytol. Pathol. 2007, 37, 67–73. [Google Scholar]
- Edström, A.M.; Malm, J.; Frohm, B.; Martellini, J.A.; Giwercman, A.; Mörgelin, M.; Cole, A.M.; Sørensen, O.E. The major bactericidal activity of human seminal plasma is zinc-dependent and derived from fragmentation of the semenogelins. J. Immunol. 2008, 181, 3413–3421. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Radner, M. Lysozyme activities and immunoglobulin concentrations in seminal plasma and spermatozoa of different teleost species and indications on its significance for sperm function. Theriogenology 2010, 74, 246–254. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; WHO Press: Geneva, Switzerland, 2010; pp. 13–36. [Google Scholar]
- Omu, A.E. Sperm parameters: Paradigmatic index of good health and longevity. Med. Princ. Pract. 2013, 22, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, D.; Mortimer, S.T. Computer-aided sperm analysis (CASA) of sperm motility and hyperactivation. Methods Mol. Biol. 2013, 927, 77–87. [Google Scholar] [PubMed]
- Tvrdá, E.; Lovíšek, D.; Kyzek, S.; Kováčik, D.; Gálová, E. The Effect of Non-Thermal Plasma on the Structural and Functional Characteristics of Human Spermatozoa. Int. J. Mol. Sci. 2021, 22, 4979. [Google Scholar] [CrossRef]
- Shen, H.M.; Dai, J.; Chia, S.E.; Lim, A.; Ong, C.N. Detection of apoptotic alterations in sperm in subfertile patients and their correlations with sperm quality. Hum. Reprod. 2002, 17, 1266–1273. [Google Scholar] [CrossRef]
- Esteves, S.C.; Sharma, R.K.; Thomas, A.J., Jr.; Agarwal, A. Evaluation of acrosomal status and sperm viability in fresh and cryopreserved specimens by the use of fluorescent peanut agglutinin lectin in conjunction with hypo-osmotic swelling test. Int. Braz. J. Urol. 2007, 33, 364–376. [Google Scholar] [CrossRef][Green Version]
- Tvrdá, E.; López-Fernández, C.; Sánchez-Martín, P.; Gosálvez, J. Sperm DNA fragmentation in donors and normozoospermic patients attending for a first spermiogram: Static and dynamic assessment. Andrologia 2018, 50, e12986. [Google Scholar] [CrossRef]
- Sharma, R.; Iovine, C.; Agarwal, A.; Henkel, R. TUNEL assay-Standardized method for testing sperm DNA fragmentation. Andrologia. 2021, 53, e13738. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.; Sharma, R. Leukocytospermia Quantitation (ENDTZ) Test. In Andrological Evaluation of Male Infertility, 1st ed.; Agarwal, A., Gupta, S., Sharma, R., Eds.; Springer Science + Business Media: Berlin, Germany, 2016; pp. 69–72. [Google Scholar]
- Agarwal, A.; Qiu, E.; Sharma, R. Laboratory assessment of oxidative stress in semen. Arab J. Urol. 2017, 16, 77–86. [Google Scholar] [CrossRef]
- Muller, C.H.; Lee, T.K.; Montaño, M.A. Improved chemiluminescence assay for measuring antioxidant capacity of seminal plasma. Methods Mol. Biol. 2013, 927, 363–376. [Google Scholar]
- Aitken, R.J.; Wingate, J.K.; De Iuliis, G.N.; McLaughlin, E.A. Analysis of lipid peroxidation in human spermatozoa using BODIPY C11. Mol. Hum. Reprod. 2007, 13, 203–311. [Google Scholar] [CrossRef] [PubMed]
- van Johannes, P.; Fred, R. Long-term quality control of the cytokine & growth factors and cell adhesion molecule arrays at the Randox Evidence Investigator. J. Med. Biochem. 2009, 28, 300–304. [Google Scholar]
- Kačániová, M.; Terentjeva, M.; Štefániková, J.; Žiarovská, J.; Savitskaya, T.; Grinshpan, D.; Kowalczewski, P.Ł.; Vukovic, N.; Tvrdá, E. Chemical Composition and Antimicrobial Activity of Selected Essential Oils against Staphylococcus spp. Isolated from Human Semen. Antibiotics 2020, 9, 765. [Google Scholar] [CrossRef] [PubMed]
- Ďuračka, M.; Belić, L.; Tokárová, K.; Žiarovská, J.; Kačániová, M.; Lukáč, N.; Tvrdá, E. Bacterial communities in bovine ejaculates and their impact on the semen quality. Syst. Biol. Reprod. Med. 2021, 67, 438–449. [Google Scholar] [CrossRef]
| Excellent Motility (n = 43) | High Motility (n = 40) | Acceptable Motility (n = 41) | |
|---|---|---|---|
| Volume [mL] | 3.30 (2.80; 3.80) | 2.90 (2.20; 4.10) | 3.25 (1.90; 3.50) |
| Concentration [×106/mL] | 77.07 (56.90; 81.30) | 73.16 (49.90; 81.80) | 74.21 (48.70; 83.13) |
| Motility [%] | 83.00 (80.00; 87.00) | 75.00 (65.00; 76.00) *EM | 50.00 (44.25; 55.75) ***EM; ***HM |
| AV-positive cells [%] | 14.79 (13.60;18.36) | 19.15 (16.39; 22.55) | 24.62 (18.14; 29.04) **EM; *HM |
| PI-positive cells [%] | 3.79 (2.95; 4.73) | 6.23 (5.32; 10.50) | 9.61 (8.89; 10.78) **EM; *HM |
| Mitochondrial membrane potential [green/red ratio] | 0.73 (0.44; 0.78) | 0.67 (0.40; 0.72) | 0.49 (0.34; 0.74) **EM; *HM |
| Acrosome integrity [%] | 83.89 (80.58; 85.01) | 79.26 (76.44; 82.19) | 74.41 (68.31; 78.43) *EM |
| Chromatin-dispersion test [%] | 12.42 (10.85; 13.99) | 15.21 (12.85; 18.74) | 20.99 (19.42; 25.70) *EM |
| TUNEL-positive cells [%] | 14.05 (13.25; 17.00) | 18.55 (16.00; 20.76) | 23.93 (16.02; 25.88) **EM |
| Leukocyte concentration [×106/mL] | 0.31 (0.00; 0.80) | 2.45 (0.00; 8.00) **EM | 5.54 (0.40; 10.00) ***EM; **HM |
| Excellent Motility (n = 43) | High Motility (n = 40) | Acceptable Motility (n = 41) | |
|---|---|---|---|
| ROS production [RLU/s/106 sperm] | 2.84 (1.07; 4.36) | 5.99 (4.68; 7.32) *EM | 9.70 (6.62; 11.53) **EM; *HM |
| Total antioxidant capacity [eq. μmol Trolox/L] | 16.58 (9.91; 19.06) | 9.95 (7.51; 12.34) *EM | 6.40 (2.81; 8.44) **EM; *HM |
| BODIPY-positive cells [%] | 5.28 (2.32; 6.96) | 10.21 (8.20; 12.18) *EM | 15.71 (10.63; 18.72) **EM; *HM |
| Excellent Motility (n = 43) | High Motility (n = 40) | Acceptable Motility (n = 41) | |
|---|---|---|---|
| IL-1 α [pg/mL] | 4.30 (1.63; 22.87) | 5.05 (2.50; 22.48) | 10.44 (2.80; 26.89) **EM; **HM |
| IL-1 β [pg/mL] | 8.82 (6.11; 10.94) | 11.08 (8.40; 16.76) | 13.10 (8.78; 32.83) *EM |
| IL-2 [pg/mL] | 0.42 (0.00; 1.00) | 2.43 (0.00; 7.56) **EM | 4.51 (0.00; 8.29) ***EM; **HM |
| IL-4 [pg/mL] | 1.72 (1.57; 2.03) | 1.95 (1.57; 2.23) | 2.03 (1.72; 2.26) |
| IL-6 [pg/mL] | 7.45 (2.72; 12.92) | 10.76 (3.92; 15.02) | 14.27 (6.55; 26.08) **EM; *HM |
| IL-8 [pg/mL] | 715.50 (580.2; 1317) | 891.90 (594.60; 1351) | 1236.00 (699.20; 1351) *EM |
| IL-10 [pg/mL] | 11.38 (10.22; 19.23) | 15.54 (14.20; 20.07) | 21.08 (13.91; 57.47) *EM |
| VEGF [pg/mL] | 3200.00 (2168.00; 3349.00) | 3132.00 (2597.00; 3749.00) | 2957.00 (2449.00; 3075.00) |
| IFN-γ [pg/mL] | 0.47 (0.00; 0.68) | 2.86 (0.00; 3.12) **EM | 2.99 (0.00; 24.71) **EM |
| TNF-α [pg/mL] | 0.15 (0.00; 0.37) | 0.26 (0.00; 0.86) | 6.56 (0.00; 8.24) ***EM; ***HM |
| MCP-1 [pg/mL] | 1414.00 (776.60; 1596.00) | 1596.00 (1104.00; 1720.00) | 1596.00 (1260.00; 1705.00) |
| EGF [pg/mL] | 1197.00 (1039.00; 1500.00) | 1197.00 (1001.00; 1444.00) | 1200.00 (1020.00; 1450.00) |
| Excellent Motility (n = 43) | High Motility (n = 40) | Acceptable Motility (n = 41) | |
|---|---|---|---|
| Lysozyme [U/L] | 3.76 (3.45; 4.02) | 3.02 (2.55; 3.33) *EM | 1.97 (1.48; 2.19) ***EM; ***HM |
| Lactoferrin [mg/100 mL] | 11.20 (10.00; 12.80) | 12.90 (11.55; 13.93) | 13.55 (13.15; 14.53) **EM |
| PLPA2 [ng/mL] | 0.87 (0.76; 0.90) | 0.89 (0.79; 0.88) | 0.99 (0.82; 1.19) |
| Excellent Motility (n = 43) | High Motility (n = 40) | Acceptable Motility (n = 41) | |
|---|---|---|---|
| Bacterial colonies [log10 CFU/mL] | 3.84 (3.71; 3.94) | 4.19 (3.88; 4.29) | 4.34 (4.26; 4.71) ***EM; *HM |
| Coliform bacteria [log10 CFU/mL] | 0.00 (0.00; 0.00) | 1.83 (1.62; 2.28) **EM | 2.36 (2.23; 2.64) ***EM |
| Number of samples without any detected bacteria | 11/43 | 7/40 | 0/41 |
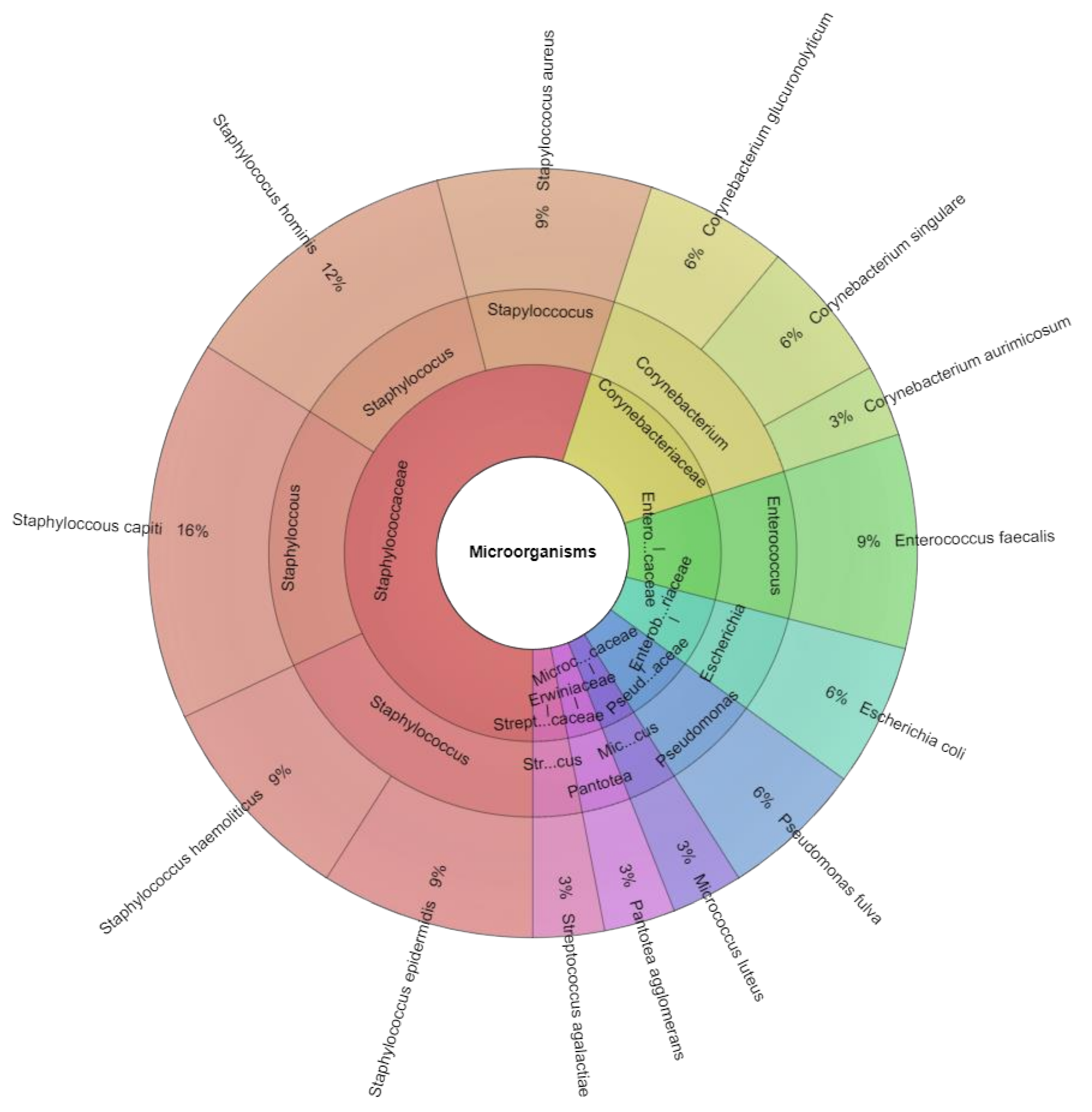
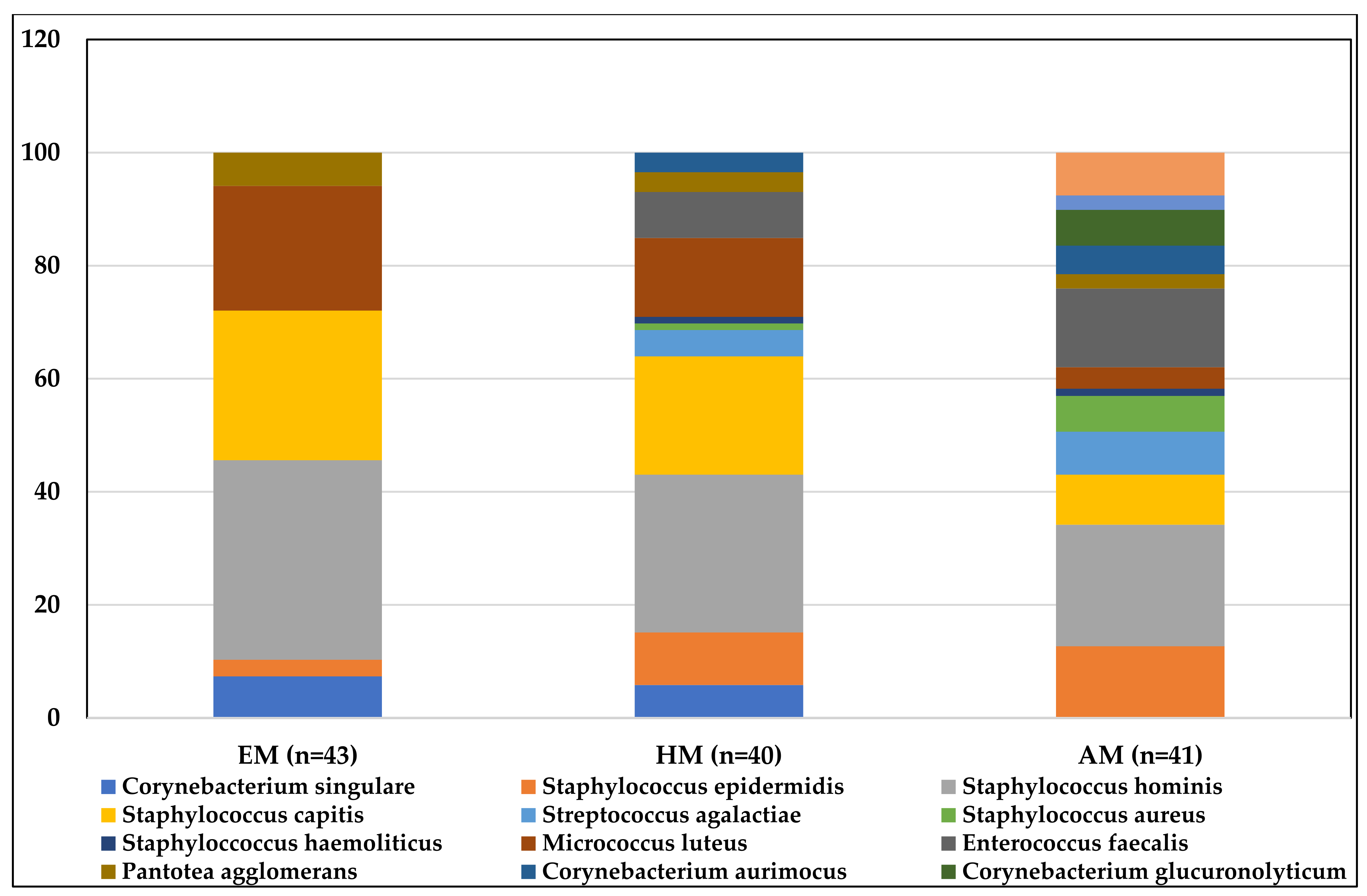
| Bacterial species (sample positivity) | Staphylococcus hominis (24/43) Staphylococcus capitis (18/43) Micrococcus luteus (15/43) Corynebacterium singulare (5/43) Pantotea agglomerans (4/43) Staphylococcus epidermidis (2/43) | Staphylococcus hominis (24/40) Staphylococcus capitis (17/40) Micrococcus luteus (12/40) Staphylococcus epidermidis (8/40) Corynebacterium singulare (5/40) Enterococcus faecalis (5/40) Streptococcus agalactiae (4/40) Corynebacterium aurimocus (3/40) Pantotea agglomerans (3/40) Staphylococcus aureus (1/40) Staphyloccoccus haemoliticus (1/40) | Staphylococcus hominis (21/41) Staphyloccoccus haemolyticus (18/41) Enterococcus faecalis (17/41) Staphylococcus epidermidis (16/41) Escherichia coli (7/41) Staphylococcus capitis (7/41) Streptococcus agalactiae (6/41) Corynebacterium glucuronolyticum (5/41) Staphylococcus aureus (5/41) Corynebacterium aurimocus (4/41) Micrococcus luteus (3/41) Pantotea agglomerans (2/41) Pseudomonas fulva (2/41) |
| Excellent Motility (n = 43) | High Motility (n = 40) | Acceptable Motility (n = 41) | |
|---|---|---|---|
| Average population size | 7.43 | 7.81 | 5.69 |
| Richness (R) | 6.00 | 11.00 | 13.00 |
| Berger Parker Index/Dominance Index | 0.46 | 0.279 | 0.229 |
| Shannon α-diversity | 1.87 | 2.15 | 3.29 |
| β-diversity | 0.34 | 0.16 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tvrdá, E.; Lovíšek, D.; Gálová, E.; Schwarzová, M.; Kováčiková, E.; Kunová, S.; Žiarovská, J.; Kačániová, M. Possible Implications of Bacteriospermia on the Sperm Quality, Oxidative Characteristics, and Seminal Cytokine Network in Normozoospermic Men. Int. J. Mol. Sci. 2022, 23, 8678. https://doi.org/10.3390/ijms23158678
Tvrdá E, Lovíšek D, Gálová E, Schwarzová M, Kováčiková E, Kunová S, Žiarovská J, Kačániová M. Possible Implications of Bacteriospermia on the Sperm Quality, Oxidative Characteristics, and Seminal Cytokine Network in Normozoospermic Men. International Journal of Molecular Sciences. 2022; 23(15):8678. https://doi.org/10.3390/ijms23158678
Chicago/Turabian StyleTvrdá, Eva, Daniel Lovíšek, Eliška Gálová, Marianna Schwarzová, Eva Kováčiková, Simona Kunová, Jana Žiarovská, and Miroslava Kačániová. 2022. "Possible Implications of Bacteriospermia on the Sperm Quality, Oxidative Characteristics, and Seminal Cytokine Network in Normozoospermic Men" International Journal of Molecular Sciences 23, no. 15: 8678. https://doi.org/10.3390/ijms23158678
APA StyleTvrdá, E., Lovíšek, D., Gálová, E., Schwarzová, M., Kováčiková, E., Kunová, S., Žiarovská, J., & Kačániová, M. (2022). Possible Implications of Bacteriospermia on the Sperm Quality, Oxidative Characteristics, and Seminal Cytokine Network in Normozoospermic Men. International Journal of Molecular Sciences, 23(15), 8678. https://doi.org/10.3390/ijms23158678








