Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review
Abstract
:1. Introduction
2. Types of Extracellular Vesicles
2.1. Exosomes
2.2. Microvesicles
2.3. Apoptotic Bodies
3. Therapeutic Value of Extracellular Vesicles
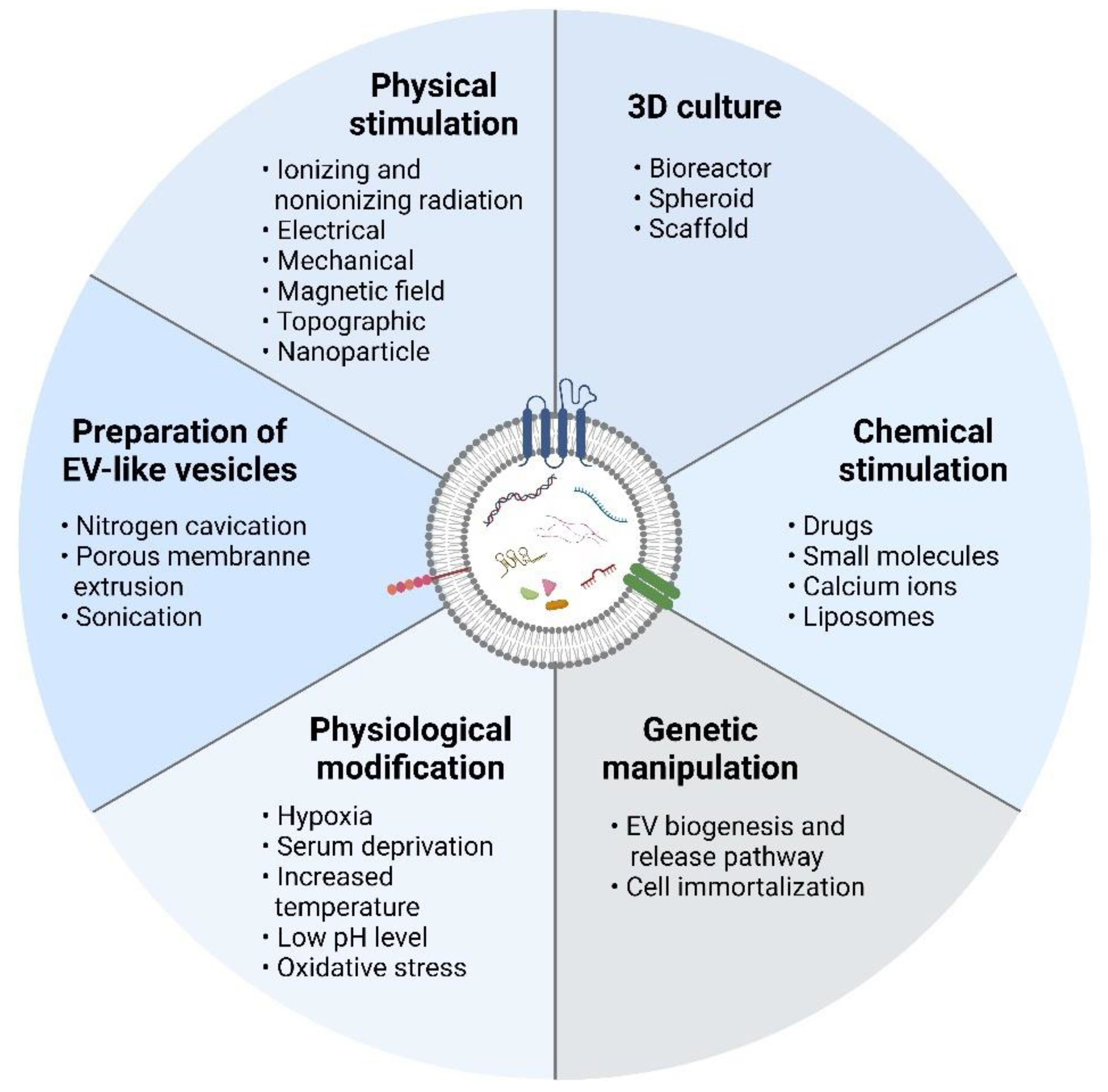
4. Strategies to Increase Production of Extracellular Vesicles
4.1. Three-Dimensional Culture
4.2. Physical Stimulation
4.3. Chemical Stimulation
4.4. Physiological Modification
4.5. Genetic Manipulation
4.6. Preparation of EV-Mimetic Nanovesicles
5. Other Factors Affecting Extracellular Vesicle Production
6. Translation of EV-Based Therapeutics
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| AChE | Acetylcholinesterase |
| ARF6 | ADP ribosylation factor 6 |
| AT | Adipose tissue |
| BM | Bone marrow |
| DNP | 2,4-dinitrophenol |
| ECM | Extracellular matrix |
| ESC | Embryonic stem cell |
| ESCRT | Endosomal sorting complex required for transport |
| EV | Extracellular vesicle |
| Fe3O4 | Magnetic iron (III) oxide |
| GMP | Good manufacturing practice |
| HDMEC | Human dermal microvascular endothelial cell |
| HEK | Human embryonic kidney |
| HIF-1a | Hypoxic-inducible factor-1a |
| HSP | Heat shock protein |
| IAA | Iodoacetate |
| MSC | Mesenchymal stem/stromal cell |
| MVB | Multivesicular body |
| NTA | Nanoparticle tracking analysis |
| PAD | Peripheral arterial disease |
| SMF | Static magnetic field |
| UC | Umbilical cord |
| UCM | Umbilical cord matrix |
References
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, S.; Fang, H.; Li, Q.; Wang, G. Extracellular Vesicles in Inflammatory Skin Disorders: From Pathophysiology to Treatment. Theranostics 2020, 10, 9937–9955. [Google Scholar] [CrossRef]
- Jin, J.; Shi, Y.; Gong, J.; Zhao, L.; Li, Y.; He, Q.; Huang, H. Exosome Secreted from Adipose-Derived Stem Cells Attenuates Diabetic Nephropathy by Promoting Autophagy Flux and Inhibiting Apoptosis in Podocyte. Stem Cell Res. Ther. 2019, 10, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-C.; Lai, L.-C. The Potential Roles of Stem Cell-Derived Extracellular Vesicles as a Therapeutic Tool. Ann. Transl. Med. 2019, 7, 693. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Chai, J.Y.; Foo, J.B.; Mohamad Yahaya, N.H.; Yang, Y.; Ng, M.H.; Law, J.X. Potential of Exosomes as Cell-Free Therapy in Articular Cartilage Regeneration: A Review. Int. J. Nanomed. 2021, 16, 6749–6781. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Hong, Z.; Xiao, C.; Li, L.; Chen, L.; Cheng, S.; Lei, T.; Zheng, H. Effects of Exosomes on Neurological Function Recovery for Ischemic Stroke in Pre-Clinical Studies: A Meta-Analysis. Front. Cell. Neurosci. 2020, 14, 593130. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Caron, C.; Mahmoud, I.; Derish, I.; Schwertani, A.; Cecere, R. Extracellular Vesicles as a Cell-Free Therapy for Cardiac Repair: A Systematic Review and Meta-Analysis of Randomized Controlled Preclinical Trials in Animal Myocardial Infarction Models. Stem Cell Rev. Rep. 2022, 18, 1143–1167. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, K.; Figueira, R.L.; Antounians, L.; Lauriti, G.; Zani, A. Systematic Review of Extracellular Vesicle-Based Treatments for Lung Injury: Are EVs a Potential Therapy for COVID-19? J. Extracell. Vesicles 2020, 9, 1795365. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Hu, S.; Huang, K.; Su, T.; Li, Z.; Vandergriff, A.; Cores, J.; Dinh, P.-U.; Allen, T.; Shen, D.; et al. Tumor Cell-Derived Exosomes Home to Their Cells of Origin and Can Be Used as Trojan Horses to Deliver Cancer Drugs. Theranostics 2020, 10, 3474–3487. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.J.; Redzic, J.S.; Graner, M.W.; Anchordoquy, T.J. Examination of the Specificity of Tumor Cell Derived Exosomes with Tumor Cells in Vitro. Biochim. Biophys. Acta 2014, 1838, 2954–2965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parolini, I.; Federici, C.; Raggi, C.; Lugini, L.; Palleschi, S.; De Milito, A.; Coscia, C.; Iessi, E.; Logozzi, M.; Molinari, A.; et al. Microenvironmental PH Is a Key Factor for Exosome Traffic in Tumor Cells. J. Biol. Chem. 2009, 284, 34211–34222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Wang, L.; Zhu, L.; Xu, Z.; Liu, Y.; Li, Z.; Zhou, J.; Luo, F. Exosomes as Drug Carriers in Anti-Cancer Therapy. Front. Cell Dev. Biol. 2022, 10, 728616. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Xie, X.; Zhang, D.; Zhou, Y.; Li, B.; Li, F.; Li, F.; Cheng, Y.; Mei, H.; Meng, H.; et al. Use of Lung-Specific Exosomes for MiRNA-126 Delivery in Non-Small Cell Lung Cancer. Nanoscale 2020, 12, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zeng, S.; Gong, Z.; Yan, Y. Exosome-Based Immunotherapy: A Promising Approach for Cancer Treatment. Mol. Cancer 2020, 19, 160. [Google Scholar] [CrossRef]
- Balaj, L.; Lessard, R.; Dai, L.; Cho, Y.-J.; Pomeroy, S.L.; Breakefield, X.O.; Skog, J. Tumour Microvesicles Contain Retrotransposon Elements and Amplified Oncogene Sequences. Nat. Commun. 2011, 2, 180. [Google Scholar] [CrossRef]
- Thakur, B.K.; Zhang, H.; Becker, A.; Matei, I.; Huang, Y.; Costa-Silva, B.; Zheng, Y.; Hoshino, A.; Brazier, H.; Xiang, J.; et al. Double-Stranded DNA in Exosomes: A Novel Biomarker in Cancer Detection. Cell Res. 2014, 24, 766–769. [Google Scholar] [CrossRef] [Green Version]
- Tortolici, F.; Vumbaca, S.; Incocciati, B.; Dayal, R.; Aquilano, K.; Giovanetti, A.; Rufini, S. Ionizing Radiation-Induced Extracellular Vesicle Release Promotes AKT-Associated Survival Response in SH-SY5Y Neuroblastoma Cells. Cells 2021, 10, 107. [Google Scholar] [CrossRef]
- Aubertin, K.; Silva, A.K.A.; Luciani, N.; Espinosa, A.; Djemat, A.; Charue, D.; Gallet, F.; Blanc-Brude, O.; Wilhelm, C. Massive Release of Extracellular Vesicles from Cancer Cells after Photodynamic Treatment or Chemotherapy. Sci. Rep. 2016, 6, 35376. [Google Scholar] [CrossRef]
- Patwardhan, S.; Mahadik, P.; Shetty, O.; Sen, S. ECM Stiffness-Tuned Exosomes Drive Breast Cancer Motility through Thrombospondin-1. Biomaterials 2021, 279, 121185. [Google Scholar] [CrossRef]
- Hedlund, M.; Nagaeva, O.; Kargl, D.; Baranov, V.; Mincheva-Nilsson, L. Thermal- and Oxidative Stress Causes Enhanced Release of NKG2D Ligand-Bearing Immunosuppressive Exosomes in Leukemia/Lymphoma T and B Cells. PLoS ONE 2011, 6, e16899. [Google Scholar] [CrossRef] [PubMed]
- Patton, M.C.; Zubair, H.; Khan, M.A.; Singh, S.; Singh, A.P. Hypoxia Alters the Release and Size Distribution of Extracellular Vesicles in Pancreatic Cancer Cells to Support Their Adaptive Survival. J. Cell. Biochem. 2020, 121, 828–839. [Google Scholar] [CrossRef] [PubMed]
- Staubach, S.; Bauer, F.N.; Tertel, T.; Börger, V.; Stambouli, O.; Salzig, D.; Giebel, B. Scaled Preparation of Extracellular Vesicles from Conditioned Media. Adv. Drug Deliv. Rev. 2021, 177, 113940. [Google Scholar] [CrossRef] [PubMed]
- Hartjes, T.A.; Mytnyk, S.; Jenster, G.W.; van Steijn, V.; van Royen, M.E. Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches. Bioengineering 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuwland, R.; Falcón-Pérez, J.M.; Théry, C.; Witwer, K.W. Rigor and Standardization of Extracellular Vesicle Research: Paving the Road towards Robustness. J. Extracell. Vesicles 2020, 10, e12037. [Google Scholar] [CrossRef]
- Welsh, J.A.; Van Der Pol, E.; Arkesteijn, G.J.A.; Bremer, M.; Brisson, A.; Coumans, F.; Dignat-George, F.; Duggan, E.; Ghiran, I.; Giebel, B.; et al. MIFlowCyt-EV: A Framework for Standardized Reporting of Extracellular Vesicle Flow Cytometry Experiments. J. Extracell. Vesicles 2020, 9, 1713526. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Chen, Z.; Xu, L.; Chang, M.; Wang, K.; Deng, C.; Gu, Y.; Zhou, S.; Shen, Y.; et al. Biogenesis and Function of Extracellular Vesicles in Pathophysiological Processes of Skeletal Muscle Atrophy. Biochem. Pharmacol. 2022, 198, 114954. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of Distinct Nanoparticles and Subsets of Extracellular Vesicles by Asymmetric-Flow Field-Flow Fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Regnault, A.; Lozier, A.; Wolfers, J.; Flament, C.; Tenza, D.; Ricciardi-Castagnoli, P.; Raposo, G.; Amigorena, S. Eradication of Established Murine Tumors Using a Novel Cell-Free Vaccine: Dendritic Cell Derived Exosomes. Nat. Med. 1998, 4, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Takada, R.; Noda, C.; Kobayashi, S.; Takada, S. Different Populations of Wnt-Containing Vesicles Are Individually Released from Polarized Epithelial Cells. Sci. Rep. 2016, 6, 35562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colombo, F.; Casella, G.; Podini, P.; Finardi, A.; Racchetti, G.; Norton, E.G.; Cocucci, E.; Furlan, R. Polarized Cells Display Asymmetric Release of Extracellular Vesicles. Traffic 2021, 22, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ye, Y.; Su, X.; He, J.; Bai, W.; He, X. MSCs-Derived Exosomes and Neuroinflammation, Neurogenesis and Therapy of Traumatic Brain Injury. Front. Cell. Neurosci. 2017, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An Emerging Focus on Lipids in Extracellular Vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- D’Souza, A.; Burch, A.; Dave, K.M.; Sreeram, A.; Reynolds, M.J.; Dobbins, D.X.; Kamte, Y.S.; Zhao, W.; Sabatelle, C.; Joy, G.M.; et al. Microvesicles Transfer Mitochondria and Increase Mitochondrial Function in Brain Endothelial Cells. J. Control. Release 2021, 338, 505–526. [Google Scholar] [CrossRef]
- Ratajczak, J.; Miekus, K.; Kucia, M.; Zhang, J.; Reca, R.; Dvorak, P.; Ratajczak, M.Z. Embryonic Stem Cell-Derived Microvesicles Reprogram Hematopoietic Progenitors: Evidence for Horizontal Transfer of MRNA and Protein Delivery. Leukemia 2006, 20, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Yuan, A.; Farber, E.L.; Rapoport, A.L.; Tejada, D.; Deniskin, R.; Akhmedov, N.B.; Farber, D.B. Transfer of MicroRNAs by Embryonic Stem Cell Microvesicles. PLoS ONE 2009, 4, e4722. [Google Scholar] [CrossRef] [Green Version]
- Ismail, N.; Wang, Y.; Dakhlallah, D.; Moldovan, L.; Agarwal, K.; Batte, K.; Shah, P.; Wisler, J.; Eubank, T.D.; Tridandapani, S.; et al. Macrophage Microvesicles Induce Macrophage Differentiation and MiR-223 Transfer. Blood 2013, 121, 984–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldenström, A.; Gennebäck, N.; Hellman, U.; Ronquist, G. Cardiomyocyte Microvesicles Contain DNA/RNA and Convey Biological Messages to Target Cells. PLoS ONE 2012, 7, e34653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ratajczak, M.Z.; Ratajczak, J. Extracellular Microvesicles/Exosomes: Discovery, Disbelief, Acceptance, and the Future? Leukemia 2020, 34, 3126–3135. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, J.; Wysoczynski, M.; Hayek, F.; Janowska-Wieczorek, A.; Ratajczak, M.Z. Membrane-Derived Microvesicles: Important and Underappreciated Mediators of Cell-to-Cell Communication. Leukemia 2006, 20, 1487–1495. [Google Scholar] [CrossRef]
- Poon, I.K.H.; Parkes, M.A.F.; Jiang, L.; Atkin-Smith, G.K.; Tixeira, R.; Gregory, C.D.; Ozkocak, D.C.; Rutter, S.F.; Caruso, S.; Santavanond, J.P.; et al. Moving beyond Size and Phosphatidylserine Exposure: Evidence for a Diversity of Apoptotic Cell-Derived Extracellular Vesicles in Vitro. J. Extracell. Vesicles 2019, 8, 1608786. [Google Scholar] [CrossRef] [Green Version]
- Battistelli, M.; Falcieri, E. Apoptotic Bodies: Particular Extracellular Vesicles Involved in Intercellular Communication. Biology 2020, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Alma, Z.; Kiril, B.; Heidi, N.; Erdenechimeg, S.; Lin, G.; Bernd, D.; Mihail, H.; Thomas, K.; Nazari, J.M.; Esther, L.; et al. Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12-Dependent Vascular Protection. Sci. Signal. 2009, 2, ra81. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [Green Version]
- Poon, I.K.H.; Lucas, C.D.; Rossi, A.G.; Ravichandran, K.S. Apoptotic Cell Clearance: Basic Biology and Therapeutic Potential. Nat. Rev. Immunol. 2014, 14, 166–180. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Sui, B.; Zhang, X.; Hu, J.; Chen, J.; Liu, J.; Wu, D.; Ye, Q.; Xiang, L.; Qiu, X.; et al. Apoptotic Vesicles Restore Liver Macrophage Homeostasis to Counteract Type 2 Diabetes. J. Extracell. Vesicles 2021, 10, e12109. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological Properties of Extracellular Vesicles and Their Physiological Functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, O.G.; van Balkom, B.W.M.; Schiffelers, R.M.; Bouten, C.V.C.; Verhaar, M.C. Extracellular Vesicles: Potential Roles in Regenerative Medicine. Front. Immunol. 2014, 5, 608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alqurashi, H.; Ortega Asencio, I.; Lambert, D.W. The Emerging Potential of Extracellular Vesicles in Cell-Free Tissue Engineering and Regenerative Medicine. Tissue Eng. 2021, 27, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, S.; Elçin, A.E.; Koca, A.; Elçin, Y.M. Therapeutic Applications of Stem Cells and Extracellular Vesicles in Emergency Care: Futuristic Perspectives. Stem Cell Rev. Rep. 2021, 17, 390–410. [Google Scholar] [CrossRef]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X.; et al. Extracellular Vesicle-Based Drug Delivery Systems for Cancer Treatment. Theranostics 2019, 9, 8001–8017. [Google Scholar] [CrossRef]
- Lara, P.; Chan, A.B.; Cruz, L.J.; Quest, A.F.G.; Kogan, M.J. Exploiting the Natural Properties of Extracellular Vesicles in Targeted Delivery towards Specific Cells and Tissues. Pharmaceutics 2020, 12, 1022. [Google Scholar] [CrossRef]
- Fujimoto, S.; Fujita, Y.; Kadota, T.; Araya, J.; Kuwano, K. Intercellular Communication by Vascular Endothelial Cell-Derived Extracellular Vesicles and Their MicroRNAs in Respiratory Diseases. Front. Mol. Biosci. 2020, 7, 619697. [Google Scholar] [CrossRef]
- Mensà, E.; Guescini, M.; Giuliani, A.; Bacalini, M.G.; Ramini, D.; Corleone, G.; Ferracin, M.; Fulgenzi, G.; Graciotti, L.; Prattichizzo, F.; et al. Small Extracellular Vesicles Deliver MiR-21 and MiR-217 as pro-Senescence Effectors to Endothelial Cells: Journal of Extracellular Vesicles. J Extracell Vesicles 2020, 9, 1725285. [Google Scholar] [CrossRef]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular Vesicles in Cancer—Implications for Future Improvements in Cancer Care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef]
- Hsu, Y.-L.; Hung, J.-Y.; Chang, W.-A.; Lin, Y.-S.; Pan, Y.-C.; Tsai, P.-H.; Wu, C.-Y.; Kuo, P.-L. Hypoxic Lung Cancer-Secreted Exosomal MiR-23a Increased Angiogenesis and Vascular Permeability by Targeting Prolyl Hydroxylase and Tight Junction Protein ZO-1. Oncogene 2017, 36, 4929–4942. [Google Scholar] [CrossRef]
- Webber, J.P.; Spary, L.K.; Sanders, A.J.; Chowdhury, R.; Jiang, W.G.; Steadman, R.; Wymant, J.; Jones, A.T.; Kynaston, H.; Mason, M.D.; et al. Differentiation of Tumour-Promoting Stromal Myofibroblasts by Cancer Exosomes. Oncogene 2015, 34, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Antonyak, M.A.; Li, B.; Boroughs, L.K.; Johnson, J.L.; Druso, J.E.; Bryant, K.L.; Holowka, D.A.; Cerione, R.A. Cancer Cell-Derived Microvesicles Induce Transformation by Transferring Tissue Transglutaminase and Fibronectin to Recipient Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 4852–4857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd Elmageed, Z.Y.; Yang, Y.; Thomas, R.; Ranjan, M.; Mondal, D.; Moroz, K.; Fang, Z.; Rezk, B.M.; Moparty, K.; Sikka, S.C.; et al. Neoplastic Reprogramming of Patient-Derived Adipose Stem Cells by Prostate Cancer Cell-Associated Exosomes. Stem Cells Dayt. Ohio 2014, 32, 983–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, B.H.; Ketova, T.; Hoshino, D.; Zijlstra, A.; Weaver, A.M. Directional Cell Movement through Tissues Is Controlled by Exosome Secretion. Nat. Commun. 2015, 6, 7164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urabe, F.; Kosaka, N.; Ito, K.; Kimura, T.; Egawa, S.; Ochiya, T. Extracellular Vesicles as Biomarkers and Therapeutic Targets for Cancer. Am. J. Physiol.-Cell Physiol. 2020, 318, C29–C39. [Google Scholar] [CrossRef]
- Chen, Y.; Yuan, H.; Ou, Z.; Ou, J. Microparticles (Exosomes) and Atherosclerosis. Curr. Atheroscler. Rep. 2020, 22, 23. [Google Scholar] [CrossRef]
- Femminò, S.; Penna, C.; Margarita, S.; Comità, S.; Brizzi, M.F.; Pagliaro, P. Extracellular Vesicles and Cardiovascular System: Biomarkers and Cardioprotective Effectors. Vascul. Pharmacol. 2020, 135, 106790. [Google Scholar] [CrossRef]
- Huang, C.; Neupane, Y.R.; Lim, X.C.; Shekhani, R.; Czarny, B.; Wacker, M.G.; Pastorin, G.; Wang, J.W. Extracellular Vesicles in Cardiovascular Disease. Adv. Clin. Chem. 2021, 103, 47–95. [Google Scholar] [CrossRef]
- Ayala-Mar, S.; Rodríguez-Morales, B.; Chacón-Ponce, P.; González-Valdez, J. Potential Applications and Functional Roles of Exosomes in Cardiometabolic Disease. Pharmaceutics 2021, 13, 2056. [Google Scholar] [CrossRef]
- Martin, T.S.; Trindade, D.; Vaz, M.; Campelo, I.; Almeida, M.; Trigo, G.; da Cruz E Silva, O.A.B.; Henriques, A.G. Diagnostic and Therapeutic Potential of Exosomes in Alzheimer’s Disease. J. Neurochem. 2021, 156, 162–181. [Google Scholar] [CrossRef]
- Rastogi, S.; Sharma, V.; Bharti, P.S.; Rani, K.; Modi, G.P.; Nikolajeff, F.; Kumar, S. The Evolving Landscape of Exosomes in Neurodegenerative Diseases: Exosomes Characteristics and a Promising Role in Early Diagnosis. Int. J. Mol. Sci. 2021, 22, 440. [Google Scholar] [CrossRef] [PubMed]
- Marostica, G.; Gelibter, S.; Gironi, M.; Nigro, A.; Furlan, R. Extracellular Vesicles in Neuroinflammation. Front. Cell Dev. Biol. 2021, 8, 623039. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, R.; Shetty, A.K. Extracellular Vesicles for the Diagnosis and Treatment of Parkinson’s Disease. Aging Dis. 2021, 12, 1438–1450. [Google Scholar] [CrossRef]
- Thietart, S.; Rautou, P.E. Extracellular Vesicles as Biomarkers in Liver Diseases: A Clinician’s Point of View. J. Hepatol. 2020, 73, 1507–1525. [Google Scholar] [CrossRef]
- Balaphas, A.; Meyer, J.; Sadoul, R.; Morel, P.; Gonelle-Gispert, C.; Bühler, L.H. Extracellular Vesicles: Future Diagnostic and Therapeutic Tools for Liver Disease and Regeneration. Liver Int. 2019, 39, 1801–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhou, X.; Zhang, H.; Yao, Q.; Liu, Y.; Dong, Z. Extracellular Vesicles in Diagnosis and Therapy of Kidney Diseases. Am. J. Physiol.—Ren. Physiol. 2016, 311, F844–F851. [Google Scholar] [CrossRef] [Green Version]
- Carnino, J.M.; Hao Kwok, Z.; Jin, Y. Extracellular Vesicles: A Novel Opportunity for Precision Medicine in Respiratory Diseases. Front. Med. 2021, 8, 1127. [Google Scholar] [CrossRef]
- Kim, H.; Lee, J.W.; Han, G.; Kim, K.; Yang, Y.; Kim, S.H. Extracellular Vesicles as Potential Theranostic Platforms for Skin Diseases and Aging. Pharmaceutics 2021, 13, 760. [Google Scholar] [CrossRef]
- Gołębiewska, J.E.; Wardowska, A.; Pietrowska, M.; Wojakowska, A.; Dębska-Ślizień, A. Small Extracellular Vesicles in Transplant Rejection. Cells 2021, 10, 2989. [Google Scholar] [CrossRef]
- Kamal, N.N.S.N.M.; Shahidan, W.N.S. Salivary Exosomes: From Waste to Promising Periodontitis Treatment. Front. Physiol. 2022, 12, 798682. [Google Scholar] [CrossRef]
- Han, P.; Bartold, P.; Ivanovski, S. The Emerging Role of Small Extracellular Vesicles in Saliva and Gingival Crevicular Fluid as Diagnostics for Periodontitis. J. Periodontal Res. 2021, 57, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Cohn, W.; Zhu, C.; Campagna, J.; Bilousova, T.; Spilman, P.; Teter, B.; Li, F.; Guo, R.; Elashoff, D.; Cole, G.; et al. Integrated Multiomics Analysis of Salivary Exosomes to Identify Biomarkers Associated with Changes in Mood States and Fatigue. Int. J. Mol. Sci. 2022, 23, 5257. [Google Scholar] [CrossRef] [PubMed]
- Möller, A.; Lobb, R.J. The Evolving Translational Potential of Small Extracellular Vesicles in Cancer. Nat. Rev. Cancer 2020, 20, 697–709. [Google Scholar] [CrossRef]
- Mathew, M.; Zade, M.; Mezghani, N.; Patel, R.; Wang, Y.; Momen-Heravi, F. Extracellular Vesicles as Biomarkers in Cancer Immunotherapy. Cancers 2020, 12, 2825. [Google Scholar] [CrossRef] [PubMed]
- Basu, B.; Ghosh, M.K. Extracellular Vesicles in Glioma: From Diagnosis to Therapy. BioEssays 2019, 41, 1800245. [Google Scholar] [CrossRef] [PubMed]
- De Jong, O.G.; Kooijmans, S.A.A.; Murphy, D.E.; Jiang, L.; Evers, M.J.W.; Sluijter, J.P.G.; Vader, P.; Schiffelers, R.M. Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res. 2019, 52, 1761–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular Vesicles for Drug Delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular Vesicles as Drug Delivery Systems: Why and How? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Kim, J.; Mitragotri, S. Targeting Strategies for Tissue-Specific Drug Delivery. Cell 2020, 181, 151–167. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes Facilitate Therapeutic Targeting of Oncogenic KRAS in Pancreatic Cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Kwok, Z.H.; Wang, C.; Jin, Y. Extracellular Vesicle Transportation and Uptake by Recipient Cells: A Critical Process to Regulate Human Diseases. Processes 2021, 9, 273. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.; Jayasinghe, M.K.; Pham, T.T.; Yang, Y.; Wei, L.; Usman, W.M.; Chen, H.; Pirisinu, M.; Gong, J.; Kim, S.; et al. Covalent Conjugation of Extracellular Vesicles with Peptides and Nanobodies for Targeted Therapeutic Delivery. J. Extracell. Vesicles 2021, 10, e12057. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, C.; Long, L.; Ren, Y.; Zhang, S.; Chang, X.; Qian, X.; Jia, H.; Zhao, J.; Sun, J.; et al. Blood Exosomes Endowed with Magnetic and Targeting Properties for Cancer Therapy. ACS Nano 2016, 10, 3323–3333. [Google Scholar] [CrossRef]
- Mohammadzadeh, R.; Ghazvini, K.; Farsiani, H.; Soleimanpour, S. Mycobacterium Tuberculosis Extracellular Vesicles: Exploitation for Vaccine Technology and Diagnostic Methods. Crit. Rev. Microbiol. 2021, 47, 13–33. [Google Scholar] [CrossRef]
- Thakur, A.; Parra, D.C.; Motallebnejad, P.; Brocchi, M.; Chen, H.J. Exosomes: Small Vesicles with Big Roles in Cancer, Vaccine Development, and Therapeutics. Bioact. Mater. 2022, 10, 281–294. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Mehanny, M.; Lehr, C.M.; Fuhrmann, G. Extracellular Vesicles as Antigen Carriers for Novel Vaccination Avenues. Adv. Drug Deliv. Rev. 2021, 173, 164–180. [Google Scholar] [CrossRef]
- Santos, P.; Almeida, F. Exosome-Based Vaccines: History, Current State, and Clinical Trials. Front. Immunol. 2021, 12, 711565. [Google Scholar] [CrossRef]
- Tsai, S.J.; Atai, N.A.; Cacciottolo, M.; Nice, J.; Salehi, A.; Guo, C.; Sedgwick, A.; Kanagavelu, S.; Gould, S.J. Exosome-mediated mRNA delivery in vivo is safe and can be used to induce SARS-CoV-2 immunity. J. Biol. Chem. 2021, 297, 101266. [Google Scholar] [CrossRef]
- Nanjundappa, R.H.; Wang, R.; Xie, Y.; Umeshappa, C.S.; Chibbar, R.; Wei, Y.; Liu, Q.; Xiang, J. GP120-Specific Exosome-Targeted T Cell-Based Vaccine Capable of Stimulating DC- and CD4+ T-Independent CTL Responses. Vaccine 2011, 29, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Giacobino, C.; Canta, M.; Fornaguera, C.; Borrós, S.; Cauda, V. Extracellular Vesicles and Their Current Role in Cancer Immunotherapy. Cancers 2021, 13, 2280. [Google Scholar] [CrossRef] [PubMed]
- Sabanovic, B.; Piva, F.; Cecati, M.; Giulietti, M. Promising Extracellular Vesicle-Based Vaccines against Viruses, Including SARS-CoV-2. Biology 2021, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, N.; Yoshioka, Y.; Fujita, Y.; Ochiya, T. Versatile Roles of Extracellular Vesicles in Cancer. J. Clin. Investig. 2016, 126, 1163–1172. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Wu, X. Exosomes Produced from 3D Cultures of Umbilical Cord Mesenchymal Stem Cells in a Hollow-Fiber Bioreactor Show Improved Osteochondral Regeneration Activity. Cell Biol. Toxicol. 2020, 36, 165–178. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Wang, B.; Tang, T.; Lv, L.; Ding, Z.; Li, Z.; Hu, R.; Wei, Q.; Shen, A.; Fu, Y.; et al. Three-Dimensional Culture of MSCs Produces Exosomes with Improved Yield and Enhanced Therapeutic Efficacy for Cisplatin-Induced Acute Kidney Injury. Stem Cell Res. Ther. 2020, 11, 206. [Google Scholar] [CrossRef]
- de Almeida Fuzeta, M.; Bernardes, N.; Oliveira, F.D.; Costa, A.C.; Fernandes-Platzgummer, A.; Farinha, J.P.; Rodrigues, C.A.V.; Jung, S.; Tseng, R.-J.; Milligan, W.; et al. Scalable Production of Human Mesenchymal Stromal Cell-Derived Extracellular Vesicles Under Serum-/Xeno-Free Conditions in a Microcarrier-Based Bioreactor Culture System. Front. Cell Dev. Biol. 2020, 8, 553444. [Google Scholar] [CrossRef]
- Watson, D.C.; Bayik, D.; Srivatsan, A.; Bergamaschi, C.; Valentin, A.; Niu, G.; Bear, J.; Monninger, M.; Sun, M.; Morales-Kastresana, A.; et al. Efficient Production and Enhanced Tumor Delivery of Engineered Extracellular Vesicles. Biomaterials 2016, 105, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Gobin, J.; Muradia, G.; Mehic, J.; Westwood, C.; Couvrette, L.; Stalker, A.; Bigelow, S.; Luebbert, C.C.; Bissonnette, F.S.-D.; Johnston, M.J.W.; et al. Hollow-Fiber Bioreactor Production of Extracellular Vesicles from Human Bone Marrow Mesenchymal Stromal Cells Yields Nanovesicles That Mirrors the Immuno-Modulatory Antigenic Signature of the Producer Cell. Stem Cell Res. Ther. 2021, 12, 127. [Google Scholar] [CrossRef]
- Zhang, Y.; Chopp, M.; Zhang, Z.G.; Katakowski, M.; Xin, H.; Qu, C.; Ali, M.; Mahmood, A.; Xiong, Y. Systemic Administration of Cell-Free Exosomes Generated by Human Bone Marrow Derived Mesenchymal Stem Cells Cultured under 2D and 3D Conditions Improves Functional Recovery in Rats after Traumatic Brain Injury. Neurochem. Int. 2017, 111, 69–81. [Google Scholar] [CrossRef]
- Patel, D.B.; Luthers, C.R.; Lerman, M.J.; Fisher, J.P.; Jay, S.M. Enhanced Extracellular Vesicle Production and Ethanol-Mediated Vascularization Bioactivity via a 3D-Printed Scaffold-Perfusion Bioreactor System. Acta Biomater. 2019, 95, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yun, H.-W.; Park, D.Y.; Choi, B.H.; Min, B.-H. Three-Dimensional Spheroid Culture Increases Exosome Secretion from Mesenchymal Stem Cells. Tissue Eng. Regen. Med. 2018, 15, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Al-Mayah, A.; Bright, S.; Chapman, K.; Irons, S.; Luo, P.; Carter, D.; Goodwin, E.; Kadhim, M. The Non-Targeted Effects of Radiation Are Perpetuated by Exosomes. Mutat. Res. Mol. Mech. Mutagen. 2015, 772, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Wysoczynski, M.; Ratajczak, M.Z. Lung Cancer Secreted Microvesicles: Underappreciated Modulators of Microenvironment in Expanding Tumors. Int. J. Cancer 2009, 125, 1595–1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arscott, W.T.; Tandle, A.T.; Zhao, S.; Shabason, J.E.; Gordon, I.K.; Schlaff, C.D.; Zhang, G.; Tofilon, P.J.; Camphausen, K.A. Ionizing Radiation and Glioblastoma Exosomes: Implications in Tumor Biology and Cell Migration. Transl. Oncol. 2013, 6, 638–648. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.; Zhang, X.; Shi, M.; Li, F.; Wang, S.; Wang, Y.; Wang, Y.; Wei, W.; Ma, G. Tumor Exosomes Reprogrammed by Low PH Are Efficient Targeting Vehicles for Smart Drug Delivery and Personalized Therapy against Their Homologous Tumor. Adv. Sci. 2021, 8, 2002787. [Google Scholar] [CrossRef]
- Ambattu, L.A.; Ramesan, S.; Dekiwadia, C.; Hanssen, E.; Li, H.; Yeo, L.Y. High Frequency Acoustic Cell Stimulation Promotes Exosome Generation Regulated by a Calcium-Dependent Mechanism. Commun. Biol. 2020, 3, 553. [Google Scholar] [CrossRef]
- Fukuta, T.; Nishikawa, A.; Kogure, K. Low Level Electricity Increases the Secretion of Extracellular Vesicles from Cultured Cells. Biochem. Biophys. Rep. 2020, 21, 100713. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, J.; Xie, J.; Wang, Y.; Sun, J.; Liu, T.; Zhao, Y.; Zhao, X.; Wang, X.; Ma, Y.; et al. Large-Scale Generation of Functional MRNA-Encapsulating Exosomes via Cellular Nanoporation. Nat. Biomed. Eng. 2020, 4, 69–83. [Google Scholar] [CrossRef]
- Wu, D.; Chang, X.; Tian, J.; Kang, L.; Wu, Y.; Liu, J.; Wu, X.; Huang, Y.; Gao, B.; Wang, H.; et al. Bone Mesenchymal Stem Cells Stimulation by Magnetic Nanoparticles and a Static Magnetic Field: Release of Exosomal MiR-1260a Improves Osteogenesis and Angiogenesis. J. Nanobiotechnol. 2021, 19, 209. [Google Scholar] [CrossRef]
- Wang, Z.; Maruyama, K.; Sakisaka, Y.; Suzuki, S.; Tada, H.; Suto, M.; Saito, M.; Yamada, S.; Nemoto, E. Cyclic Stretch Force Induces Periodontal Ligament Cells to Secrete Exosomes That Suppress IL-1β Production through the Inhibition of the NF-ΚB Signaling Pathway in Macrophages. Front. Immunol. 2019, 10, 1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Xu, R.; Yang, Y.; Liang, C.; Yu, X.; Liu, Y.; Wang, T.; Yu, Y.; Deng, F. Micro/Nano-Textured Hierarchical Titanium Topography Promotes Exosome Biogenesis and Secretion to Improve Osseointegration. J. Nanobiotechnol. 2021, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Jeyaraj, M.; Kim, J.-H. Platinum Nanoparticles Enhance Exosome Release in Human Lung Epithelial Adenocarcinoma Cancer Cells (A549): Oxidative Stress and the Ceramide Pathway Are Key Players. Int. J. Nanomed. 2021, 16, 515–538. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, D.; Li, H. Bioglass Enhances the Production of Exosomes and Improves Their Capability of Promoting Vascularization. Bioact. Mater. 2021, 6, 823–835. [Google Scholar] [CrossRef]
- Ludwig, N.; Yerneni, S.S.; Menshikova, E.V.; Gillespie, D.G.; Jackson, E.K.; Whiteside, T.L. Simultaneous Inhibition of Glycolysis and Oxidative Phosphorylation Triggers a Multi-Fold Increase in Secretion of Exosomes: Possible Role of 2′,3′-CAMP. Sci. Rep. 2020, 10, 6948. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Bonacquisti, E.E.; Brown, A.D.; Nguyen, J. Boosting the Biogenesis and Secretion of Mesenchymal Stem Cell-Derived Exosomes. Cells 2020, 9, 660. [Google Scholar] [CrossRef] [Green Version]
- Ruan, X.; Ju, C.; Shen, Y.; Liu, Y.; Kim, I.; Yu, H.; Weintraub, N.; Wang, X.; Tang, Y. Suxiao Jiuxin Pill Promotes Exosome Secretion from Mouse Cardiac Mesenchymal Stem Cells in Vitro. Acta Pharmacol. Sin. 2018, 39, 569–578. [Google Scholar] [CrossRef]
- Obata, Y.; Kita, S.; Koyama, Y.; Fukuda, S.; Takeda, H.; Takahashi, M.; Fujishima, Y.; Nagao, H.; Masuda, S.; Tanaka, Y.; et al. Adiponectin/T-Cadherin System Enhances Exosome Biogenesis and Decreases Cellular Ceramides by Exosomal Release. JCI Insight 2018, 3, e99680. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Kita, S.; Tanaka, Y.; Fukuda, S.; Obata, Y.; Okita, T.; Nishida, H.; Takahashi, Y.; Kawachi, Y.; Tsugawa-Shimizu, Y.; et al. Adiponectin Stimulates Exosome Release to Enhance Mesenchymal Stem-Cell-Driven Therapy of Heart Failure in Mice. Mol. Ther. 2020, 28, 2203–2219. [Google Scholar] [CrossRef]
- Hannafon, B.N.; Carpenter, K.J.; Berry, W.L.; Janknecht, R.; Dooley, W.C.; Ding, W.-Q. Exosome-Mediated MicroRNA Signaling from Breast Cancer Cells Is Altered by the Anti-Angiogenesis Agent Docosahexaenoic Acid (DHA). Mol. Cancer 2015, 14, 133. [Google Scholar] [CrossRef] [Green Version]
- Savina, A.; Furlán, M.; Vidal, M.; Colombo, M.I. Exosome Release Is Regulated by a Calcium-Dependent Mechanism in K562 Cells. J. Biol. Chem. 2003, 278, 20083–20090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savina, A.; Fader, C.M.; Damiani, M.T.; Colombo, M.I. Rab11 Promotes Docking and Fusion of Multivesicular Bodies in a Calcium-Dependent Manner. Traffic 2005, 6, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Shyong, Y.-J.; Chang, K.-C.; Lin, F.-H. Calcium Phosphate Particles Stimulate Exosome Secretion from Phagocytes for the Enhancement of Drug Delivery. Colloids Surf. B Biointerfaces 2018, 171, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Messenger, S.W.; Woo, S.S.; Sun, Z.; Martin, T.F.J. A Ca2+-Stimulated Exosome Release Pathway in Cancer Cells Is Regulated by Munc13-4. J. Cell Biol. 2018, 217, 2877–2890. [Google Scholar] [CrossRef]
- Iliev, D.; Strandskog, G.; Nepal, A.; Aspar, A.; Olsen, R.; Jørgensen, J.; Wolfson, D.; Ahluwalia, B.S.; Handzhiyski, J.; Mironova, R. Stimulation of Exosome Release by Extracellular DNA Is Conserved across Multiple Cell Types. FEBS J. 2018, 285, 3114–3133. [Google Scholar] [CrossRef]
- Emam, S.E.; Ando, H.; Abu Lila, A.S.; Shimizu, T.; Ukawa, M.; Okuhira, K.; Ishima, Y.; Mahdy, M.A.; Ghazy, F.S.; Ishida, T. A Novel Strategy to Increase the Yield of Exosomes (Extracellular Vesicles) for an Expansion of Basic Research. Biol. Pharm. Bull. 2018, 41, 733–742. [Google Scholar] [CrossRef] [Green Version]
- King, H.W.; Michael, M.Z.; Gleadle, J.M. Hypoxic Enhancement of Exosome Release by Breast Cancer Cells. BMC Cancer 2012, 12, 421. [Google Scholar] [CrossRef] [Green Version]
- Dong, L.; Wang, Y.; Zheng, T.; Pu, Y.; Ma, Y.; Qi, X.; Zhang, W.; Xue, F.; Shan, Z.; Liu, J.; et al. Hypoxic HUCMSC-Derived Extracellular Vesicles Attenuate Allergic Airway Inflammation and Airway Remodeling in Chronic Asthma Mice. Stem Cell Res. Ther. 2021, 12, 4. [Google Scholar] [CrossRef]
- Rong, Y.; Zhang, J.; Jiang, D.; Ji, C.; Liu, W.; Wang, J.; Ge, X.; Tang, P.; Yu, S.; Cui, W.; et al. Hypoxic Pretreatment of Small Extracellular Vesicles Mediates Cartilage Repair in Osteoarthritis by Delivering MiR-216a-5p. Acta Biomater. 2021, 122, 325–342. [Google Scholar] [CrossRef]
- Liu, W.; Rong, Y.; Wang, J.; Zhou, Z.; Ge, X.; Ji, C.; Jiang, D.; Gong, F.; Li, L.; Chen, J.; et al. Exosome-Shuttled MiR-216a-5p from Hypoxic Preconditioned Mesenchymal Stem Cells Repair Traumatic Spinal Cord Injury by Shifting Microglial M1/M2 Polarization. J. Neuroinflamm. 2020, 17, 47. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-C.; Liu, X.-B.; Huang, S.; Bi, X.-Y.; Wang, H.-X.; Xie, L.-X.; Wang, Y.-Q.; Cao, X.-F.; Lv, J.; Xiao, F.-J.; et al. Microvesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells Stimulated by Hypoxia Promote Angiogenesis Both in Vitro and in Vivo. Stem Cells Dev. 2012, 21, 3289–3297. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Lu, K.; Zhang, N.; Zhao, Y.; Ma, Q.; Shen, J.; Lin, Y.; Xiang, P.; Tang, Y.; Hu, X.; et al. Myocardial Reparative Functions of Exosomes from Mesenchymal Stem Cells Are Enhanced by Hypoxia Treatment of the Cells via Transferring MicroRNA-210 in an NSMase2-Dependent Way. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1659–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-King, H.; García, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepúlveda, P. Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells Dayt. Ohio 2017, 35, 1747–1759. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.D.; Johansson, H.J.; Graham, C.S.; Vesterlund, M.; Pham, M.T.; Bramlett, C.S.; Montgomery, E.N.; Mellema, M.S.; Bardini, R.L.; Contreras, Z.; et al. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells Dayt. Ohio 2016, 34, 601–613. [Google Scholar] [CrossRef] [Green Version]
- Clayton, A.; Turkes, A.; Navabi, H.; Mason, M.D.; Tabi, Z. Induction of Heat Shock Proteins in B-Cell Exosomes. J. Cell Sci. 2005, 118, 3631–3638. [Google Scholar] [CrossRef] [Green Version]
- Harmati, M.; Gyukity-Sebestyen, E.; Dobra, G.; Janovak, L.; Dekany, I.; Saydam, O.; Hunyadi-Gulyas, E.; Nagy, I.; Farkas, A.; Pankotai, T.; et al. Small Extracellular Vesicles Convey the Stress-Induced Adaptive Responses of Melanoma Cells. Sci. Rep. 2019, 9, 15329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ban, J.-J.; Lee, M.; Im, W.; Kim, M. Low PH Increases the Yield of Exosome Isolation. Biochem. Biophys. Res. Commun. 2015, 461, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Laulagnier, K.; Grand, D.; Dujardin, A.; Hamdi, S.; Vincent-Schneider, H.; Lankar, D.; Salles, J.-P.; Bonnerot, C.; Perret, B.; Record, M. PLD2 Is Enriched on Exosomes and Its Activity Is Correlated to the Release of Exosomes. FEBS Lett. 2004, 572, 11–14. [Google Scholar] [CrossRef] [Green Version]
- Hinger, S.A.; Abner, J.J.; Franklin, J.L.; Jeppesen, D.K.; Coffey, R.J.; Patton, J.G. Rab13 Regulates SEV Secretion in Mutant KRAS Colorectal Cancer Cells. Sci. Rep. 2020, 10, 15804. [Google Scholar] [CrossRef]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b Control Different Steps of the Exosome Secretion Pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Böker, K.O.; Lemus-Diaz, N.; Rinaldi Ferreira, R.; Schiller, L.; Schneider, S.; Gruber, J. The Impact of the CD9 Tetraspanin on Lentivirus Infectivity and Exosome Secretion. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 634–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Xiao, X.; Chen, M.; Aldharee, H.; Chen, Y.; Long, W. Liver Kinase B1 Restoration Promotes Exosome Secretion and Motility of Lung Cancer Cells. Oncol. Rep. 2018, 39, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-Y.; Chen, C.-K.; Ho, C.-M.; Lee, S.-S.; Chang, C.-Y.; Chen, K.-J.; Jou, Y.-S. EIF3C-Enhanced Exosome Secretion Promotes Angiogenesis and Tumorigenesis of Human Hepatocellular Carcinoma. Oncotarget 2018, 9, 13193–13205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hessvik, N.P.; Øverbye, A.; Brech, A.; Torgersen, M.L.; Jakobsen, I.S.; Sandvig, K.; Llorente, A. PIKfyve Inhibition Increases Exosome Release and Induces Secretory Autophagy. Cell. Mol. Life Sci. 2016, 73, 4717–4737. [Google Scholar] [CrossRef]
- Chen, T.S.; Arslan, F.; Yin, Y.; Tan, S.S.; Lai, R.C.; Choo, A.B.H.; Padmanabhan, J.; Lee, C.N.; de Kleijn, D.P.V.; Lim, S.K. Enabling a Robust Scalable Manufacturing Process for Therapeutic Exosomes through Oncogenic Immortalization of Human ESC-Derived MSCs. J. Transl. Med. 2011, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Wang, S.; Wang, Z. High Yield, Scalable and Remotely Drug-Loaded Neutrophil-Derived Extracellular Vesicles (EVs) for Anti-Inflammation Therapy. Biomaterials 2017, 135, 62–73. [Google Scholar] [CrossRef]
- Thamphiwatana, S.; Angsantikul, P.; Escajadillo, T.; Zhang, Q.; Olson, J.; Luk, B.T.; Zhang, S.; Fang, R.H.; Gao, W.; Nizet, V.; et al. Macrophage-like Nanoparticles Concurrently Absorbing Endotoxins and Proinflammatory Cytokines for Sepsis Management. Proc. Natl. Acad. Sci. USA 2017, 114, 11488–11493. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Abhange, K.K.; Wen, Y.; Chen, Y.; Xue, F.; Wang, G.; Tong, J.; Zhu, C.; He, X.; Wan, Y. Preparation of Engineered Extracellular Vesicles Derived from Human Umbilical Cord Mesenchymal Stem Cells with Ultrasonication for Skin Rejuvenation. ACS Omega 2019, 4, 22638–22645. [Google Scholar] [CrossRef] [Green Version]
- Jang, S.C.; Kim, O.Y.; Yoon, C.M.; Choi, D.-S.; Roh, T.-Y.; Park, J.; Nilsson, J.; Lötvall, J.; Kim, Y.-K.; Gho, Y.S. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano 2013, 7, 7698–7710. [Google Scholar] [CrossRef]
- Lunavat, T.R.; Jang, S.C.; Nilsson, L.; Park, H.T.; Repiska, G.; Lässer, C.; Nilsson, J.A.; Gho, Y.S.; Lötvall, J. RNAi Delivery by Exosome-Mimetic Nanovesicles—Implications for Targeting c-Myc in Cancer. Biomaterials 2016, 102, 231–238. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, J.-Y.; Cho, R.; Shin, D.-M.; Lee, S.W.; Oh, Y.-M. Adipose Stem Cell-Derived Nanovesicles Inhibit Emphysema Primarily via an FGF2-Dependent Pathway. Exp. Mol. Med. 2017, 49, e284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.-Y.; Ji, A.-L.; Wang, Z.; Qiang, G.-H.; Qu, Z.; Wu, J.-H.; Jiang, C.-P. Exosome-Mimetic Nanovesicles from Hepatocytes Promote Hepatocyte Proliferation in Vitro and Liver Regeneration in Vivo. Sci. Rep. 2018, 8, 2471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, G.; Lee, J.; Choi, D.-S.; Kim, S.S.; Gho, Y.S. Extracellular Vesicle–Mimetic Ghost Nanovesicles for Delivering Anti-Inflammatory Drugs to Mitigate Gram-Negative Bacterial Outer Membrane Vesicle–Induced Systemic Inflammatory Response Syndrome. Adv. Healthc. Mater. 2019, 8, 1801082. [Google Scholar] [CrossRef]
- Ingato, D.; Edson, J.A.; Zakharian, M.; Kwon, Y.J. Cancer Cell-Derived, Drug-Loaded Nanovesicles Induced by Sulfhydryl-Blocking for Effective and Safe Cancer Therapy. ACS Nano 2018, 12, 9568–9577. [Google Scholar] [CrossRef]
- Patel, D.B.; Gray, K.M.; Santharam, Y.; Lamichhane, T.N.; Stroka, K.M.; Jay, S.M. Impact of Cell Culture Parameters on Production and Vascularization Bioactivity of Mesenchymal Stem Cell-Derived Extracellular Vesicles. Bioeng. Transl. Med. 2017, 2, 170–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S.; Takasugi, M.; Watanabe, S.; Kanemaki, M.T.; Obuse, C.; et al. Exosomes Maintain Cellular Homeostasis by Excreting Harmful DNA from Cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, B.D.; Paine, M.S.; Brooks, A.M.; McCubrey, J.A.; Renegar, R.H.; Wang, R.; Terrian, D.M. Senescence-Associated Exosome Release from Human Prostate Cancer Cells. Cancer Res. 2008, 68, 7864–7871. [Google Scholar] [CrossRef] [Green Version]
- Takasugi, M.; Okada, R.; Takahashi, A.; Virya Chen, D.; Watanabe, S.; Hara, E. Small Extracellular Vesicles Secreted from Senescent Cells Promote Cancer Cell Proliferation through EphA2. Nat. Commun. 2017, 8, 15729. [Google Scholar] [CrossRef]
- Hassan, M.N.F.B.; Yazid, M.D.; Yunus, M.H.M.; Chowdhury, S.R.; Lokanathan, Y.; Idrus, R.B.H.; Ng, A.M.H.; Law, J.X. Large-Scale Expansion of Human Mesenchymal Stem Cells. Stem Cells Int. 2020, 2020, 9529465. [Google Scholar] [CrossRef]
- Guo, S.; Debbi, L.; Zohar, B.; Samuel, R.; Arzi, R.S.; Fried, A.I.; Carmon, T.; Shevach, D.; Redenski, I.; Schlachet, I.; et al. Stimulating Extracellular Vesicles Production from Engineered Tissues by Mechanical Forces. Nano Lett. 2021, 21, 2497–2504. [Google Scholar] [CrossRef]
- Artuyants, A.; Chang, V.; Reshef, G.; Blenkiron, C.; Chamley, L.W.; Leung, E.; Hisey, C.L. Production of Extracellular Vesicles Using a CELLine Adherent Bioreactor Flask. In Bioreactors in Stem Cell Biology: Methods and Protocols; Turksen, K., Ed.; Springer: New York, NY, USA, 2022; pp. 183–192. ISBN 978-1-07-162018-2. [Google Scholar]
- Piffoux, M.; Nicolás-Boluda, A.; Mulens-Arias, V.; Richard, S.; Rahmi, G.; Gazeau, F.; Wilhelm, C.; Silva, A.K.A. Extracellular Vesicles for Personalized Medicine: The Input of Physically Triggered Production, Loading and Theranostic Properties. Adv. Drug Deliv. Rev. 2019, 138, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Widlak, P.; Pietrowska, M. The Influence of Ionizing Radiation on Exosome Composition, Secretion and Intercellular Communication. Protein Pept. Lett. 2016, 23, 656–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlakis, E.; Neumann, M.; Stiewe, T. Extracellular Vesicles: Messengers of P53 in Tumor-Stroma Communication and Cancer Metastasis. Int. J. Mol. Sci. 2020, 21, 9648. [Google Scholar] [CrossRef] [PubMed]
- Berzaghi, R.; Islam, A.; Hellevik, T.; Martinez-Zubiaurre, I. Secretion Rates and Protein Composition of Extracellular Vesicles Released by Cancer-Associated Fibroblasts after Radiation. J. Radiat. Res. 2021, 62, 401–413. [Google Scholar] [CrossRef]
- IISEV2020 Abstract Book, Journal of Extracellular Vesicles; Taylor & Francis Group: Abingdon, UK, 2020; Volume 9, p. 1784511. [CrossRef]
- Park, D.J.; Yun, W.S.; Kim, W.C.; Park, J.-E.; Lee, S.H.; Ha, S.; Choi, J.S.; Key, J.; Seo, Y.J. Improvement of Stem Cell-Derived Exosome Release Efficiency by Surface-Modified Nanoparticles. J. Nanobiotechnol. 2020, 18, 178. [Google Scholar] [CrossRef]
- Taylor, J.; Azimi, I.; Monteith, G.; Bebawy, M. Ca(2+) Mediates Extracellular Vesicle Biogenesis through Alternate Pathways in Malignancy. J. Extracell. Vesicles 2020, 9, 1734326. [Google Scholar] [CrossRef] [Green Version]
- Carreau, A.; Hafny-Rahbi, B.E.; Matejuk, A.; Grillon, C.; Kieda, C. Why Is the Partial Oxygen Pressure of Human Tissues a Crucial Parameter? Small Molecules and Hypoxia. J. Cell. Mol. Med. 2011, 15, 1239–1253. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Liu, S.; Li, Y.; Wang, X.; Xue, W.; Ge, G.; Luo, X. The Role of SDF-1-CXCR4/CXCR7 Axis in the Therapeutic Effects of Hypoxia-Preconditioned Mesenchymal Stem Cells for Renal Ischemia/Reperfusion Injury. PLoS ONE 2012, 7, e34608. [Google Scholar] [CrossRef]
- Kim, Y.; Jin, H.J.; Heo, J.; Ju, H.; Lee, H.-Y.; Kim, S.; Lee, S.; Lim, J.; Jeong, S.Y.; Kwon, J.; et al. Small Hypoxia-Primed Mesenchymal Stem Cells Attenuate Graft-versus-Host Disease. Leukemia 2018, 32, 2672–2684. [Google Scholar] [CrossRef]
- Rosová, I.; Dao, M.; Capoccia, B.; Link, D.; Nolta, J.A. Hypoxic Preconditioning Results in Increased Motility and Improved Therapeutic Potential of Human Mesenchymal Stem Cells. Stem Cells Dayt. Ohio 2008, 26, 2173–2182. [Google Scholar] [CrossRef] [Green Version]
- Almeria, C.; Weiss, R.; Roy, M.; Tripisciano, C.; Kasper, C.; Weber, V.; Egger, D. Hypoxia Conditioned Mesenchymal Stem Cell-Derived Extracellular Vesicles Induce Increased Vascular Tube Formation in Vitro. Front. Bioeng. Biotechnol. 2019, 7, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Endzeliņš, E.; Ābols, A.; Bušs, A.; Zandberga, E.; Palviainen, M.; Siljander, P.I.A.; Linē, A. Extracellular Vesicles Derived from Hypoxic Colorectal Cancer Cells Confer Metastatic Phenotype to Non-Metastatic Cancer Cells. Anticancer Res. 2018, 38, 5139–5147. [Google Scholar] [CrossRef] [PubMed]
- Aoi, W.; Marunaka, Y. Importance of PH Homeostasis in Metabolic Health and Diseases: Crucial Role of Membrane Proton Transport. BioMed Res. Int. 2014, 2014, e598986. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; White, K.A.; Barber, D.L. Intracellular PH Regulates Cancer and Stem Cell Behaviors: A Protein Dynamics Perspective. Front. Oncol. 2020, 10, 1401. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Minami, N.; Yamada, M.; Imai, H. Immobilized PH in Culture Reveals an Optimal Condition for Somatic Cell Reprogramming and Differentiation of Pluripotent Stem Cells. Reprod. Med. Biol. 2016, 16, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L.; Hulikova, A. The Chemistry, Physiology and Pathology of PH in Cancer. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goody, R.S.; Müller, M.P.; Wu, Y.-W. Mechanisms of Action of Rab Proteins, Key Regulators of Intracellular Vesicular Transport. Biol. Chem. 2017, 398, 565–575. [Google Scholar] [CrossRef]
- Bobrie, A.; Krumeich, S.; Reyal, F.; Recchi, C.; Moita, L.F.; Seabra, M.C.; Ostrowski, M.; Théry, C. Rab27a Supports Exosome-Dependent and -Independent Mechanisms That Modify the Tumor Microenvironment and Can Promote Tumor Progression. Cancer Res. 2012, 72, 4920–4930. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.; Morohashi, Y.; Yoshimura, S.-I.; Manrique-Hoyos, N.; Jung, S.; Lauterbach, M.A.; Bakhti, M.; Grønborg, M.; Möbius, W.; Rhee, J.; et al. Regulation of Exosome Secretion by Rab35 and Its GTPase-Activating Proteins TBC1D10A-C. J. Cell Biol. 2010, 189, 223–232. [Google Scholar] [CrossRef]
- Guerra, F.; Paiano, A.; Migoni, D.; Girolimetti, G.; Perrone, A.M.; De Iaco, P.; Fanizzi, F.P.; Gasparre, G.; Bucci, C. Modulation of RAB7A Protein Expression Determines Resistance to Cisplatin through Late Endocytic Pathway Impairment and Extracellular Vesicular Secretion. Cancers 2019, 11, 52. [Google Scholar] [CrossRef] [Green Version]
- Thakur, R.; Panda, A.; Coessens, E.; Raj, N.; Yadav, S.; Balakrishnan, S.; Zhang, Q.; Georgiev, P.; Basak, B.; Pasricha, R.; et al. Phospholipase D Activity Couples Plasma Membrane Endocytosis with Retromer Dependent Recycling. eLife 2016, 5, e18515. [Google Scholar] [CrossRef]
- Thakur, R.; Naik, A.; Panda, A.; Raghu, P. Regulation of Membrane Turnover by Phosphatidic Acid: Cellular Functions and Disease Implications. Front. Cell Dev. Biol. 2019, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Ghossoub, R.; Lembo, F.; Rubio, A.; Gaillard, C.B.; Bouchet, J.; Vitale, N.; Slavík, J.; Machala, M.; Zimmermann, P. Syntenin-ALIX Exosome Biogenesis and Budding into Multivesicular Bodies Are Controlled by ARF6 and PLD2. Nat. Commun. 2014, 5, 3477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-Regulated Shedding of Tumor Cell-Derived Plasma Membrane Microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Dubyak, G.R. P2X7 Receptors Regulate Multiple Types of Membrane Trafficking Responses and Non-Classical Secretion Pathways. Purinergic Signal. 2009, 5, 163–173. [Google Scholar] [CrossRef] [Green Version]
- Hessvik, N.P.; Llorente, A. Current Knowledge on Exosome Biogenesis and Release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kucharzewska, P.; Belting, M. Emerging Roles of Extracellular Vesicles in the Adaptive Response of Tumour Cells to Microenvironmental Stress. J. Extracell. Vesicles 2013, 2, 20304. [Google Scholar] [CrossRef]
- Yu, X.; Harris, S.L.; Levine, A.J. The Regulation of Exosome Secretion: A Novel Function of the P53 Protein. Cancer Res. 2006, 66, 4795–4801. [Google Scholar] [CrossRef] [Green Version]
- Lespagnol, A.; Duflaut, D.; Beekman, C.; Blanc, L.; Fiucci, G.; Marine, J.-C.; Vidal, M.; Amson, R.; Telerman, A. Exosome Secretion, Including the DNA Damage-Induced P53-Dependent Secretory Pathway, Is Severely Compromised in TSAP6/Steap3-Null Mice. Cell Death Differ. 2008, 15, 1723–1733. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.T.; Lai, R.C.; Padmanabhan, J.; Sim, W.K.; Hwa Choo, A.B.; Lim, S.K. Assessment of Tumorigenic Potential in Mesenchymal-Stem/Stromal-Cell-Derived Small Extracellular Vesicles (MSC-SEV). Pharmaceuticals 2021, 14, 345. [Google Scholar] [CrossRef]
- Wu, S.; Ju, G.-Q.; Du, T.; Zhu, Y.-J.; Liu, G.-H. Microvesicles Derived from Human Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cells Attenuate Bladder Tumor Cell Growth In Vitro and In Vivo. PLoS ONE 2013, 8, e61366. [Google Scholar] [CrossRef] [PubMed]
- Rodini, C.O.; Gonçalves da Silva, P.B.; Assoni, A.F.; Carvalho, V.M.; Okamoto, O.K. Mesenchymal Stem Cells Enhance Tumorigenic Properties of Human Glioblastoma through Independent Cell-Cell Communication Mechanisms. Oncotarget 2018, 9, 24766–24777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roccaro, A.M.; Sacco, A.; Maiso, P.; Azab, A.K.; Tai, Y.-T.; Reagan, M.; Azab, F.; Flores, L.M.; Campigotto, F.; Weller, E.; et al. BM Mesenchymal Stromal Cell-Derived Exosomes Facilitate Multiple Myeloma Progression. J. Clin. Investig. 2013, 123, 1542–1555. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Zhang, B.; Wu, C.; Yu, F.; Han, B.; Li, B.; Li, L. Therapeutic Roles of Mesenchymal Stem Cell-Derived Extracellular Vesicles in Cancer. J. Hematol. Oncol. 2021, 14, 136. [Google Scholar] [CrossRef]
- García-Manrique, P.; Matos, M.; Gutiérrez, G.; Pazos, C.; Blanco-López, M.C. Therapeutic Biomaterials Based on Extracellular Vesicles: Classification of Bio-Engineering and Mimetic Preparation Routes. J. Extracell. Vesicles 2018, 7, 1422676. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Tang, J.; Yao, C.; Yang, D. Recent Progress of Extracellular Vesicle Engineering. ACS Biomater. Sci. Eng. 2021, 7, 4430–4438. [Google Scholar] [CrossRef]
- Gao, J.; Chu, D.; Wang, Z. Cell Membrane-Formed Nanovesicles for Disease-Targeted Delivery. J. Control. Release 2016, 224, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Mendez, R.; Banerjee, S. Sonication-Based Basic Protocol for Liposome Synthesis. In Lipidomics: Methods and Protocols; Bhattacharya, S.K., Ed.; Springer: New York, NY, USA, 2017; pp. 255–260. ISBN 978-1-4939-6996-8. [Google Scholar]
- Choo, Y.W.; Kang, M.; Kim, H.Y.; Han, J.; Kang, S.; Lee, J.-R.; Jeong, G.-J.; Kwon, S.P.; Song, S.Y.; Go, S.; et al. M1 Macrophage-Derived Nanovesicles Potentiate the Anticancer Efficacy of Immune Checkpoint Inhibitors. ACS Nano 2018, 12, 8977–8993. [Google Scholar] [CrossRef]
- Pisano, S.; Pierini, I.; Gu, J.; Gazze, A.; Francis, L.W.; Gonzalez, D.; Conlan, R.S.; Corradetti, B. Immune (Cell) Derived Exosome Mimetics (IDEM) as a Treatment for Ovarian Cancer. Front. Cell Dev. Biol. 2020, 8, 553576. [Google Scholar] [CrossRef]
- Baumgart, T.; Hammond, A.T.; Sengupta, P.; Hess, S.T.; Holowka, D.A.; Baird, B.A.; Webb, W.W. Large-Scale Fluid/Fluid Phase Separation of Proteins and Lipids in Giant Plasma Membrane Vesicles. Proc. Natl. Acad. Sci. USA 2007, 104, 3165–3170. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Omar, O.; Vazirisani, F.; Thomsen, P.; Ekström, K. Mesenchymal Stem Cell-Derived Exosomes Have Altered MicroRNA Profiles and Induce Osteogenic Differentiation Depending on the Stage of Differentiation. PLoS ONE 2018, 13, e0193059. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.S.; Xu, Q. Neuronal Differentiation of Human Mesenchymal Stem Cells Using Exosomes Derived from Differentiating Neuronal Cells. PLoS ONE 2015, 10, e0135111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yahao, G.; Xinjia, W. The Role and Mechanism of Exosomes from Umbilical Cord Mesenchymal Stem Cells in Inducing Osteogenesis and Preventing Osteoporosis. Cell Transplant. 2021, 30, 09636897211057465. [Google Scholar] [CrossRef] [PubMed]
- Borghesan, M.; Fafián-Labora, J.; Eleftheriadou, O.; Carpintero-Fernández, P.; Paez-Ribes, M.; Vizcay-Barrena, G.; Swisa, A.; Kolodkin-Gal, D.; Ximénez-Embún, P.; Lowe, R.; et al. Small Extracellular Vesicles Are Key Regulators of Non-Cell Autonomous Intercellular Communication in Senescence via the Interferon Protein IFITM3. Cell Rep. 2019, 27, 3956–3971. [Google Scholar] [CrossRef] [Green Version]
- Weilner, S.; Schraml, E.; Wieser, M.; Messner, P.; Schneider, K.; Wassermann, K.; Micutkova, L.; Fortschegger, K.; Maier, A.B.; Westendorp, R.; et al. Secreted Microvesicular MiR-31 Inhibits Osteogenic Differentiation of Mesenchymal Stem Cells. Aging Cell 2016, 15, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Burnouf, T.; Agrahari, V.; Agrahari, V. Extracellular Vesicles as Nanomedicine: Hopes and Hurdles in Clinical Translation. Int. J. Nanomed. 2019, 14, 8847–8859. [Google Scholar] [CrossRef] [Green Version]
- Rayyan, M.; Zheutlin, A.; Byrd, J.B. Clinical Research Using Extracellular Vesicles: Insights from the International Society for Extracellular Vesicles 2018 Annual Meeting. J. Extracell. Vesicles 2018, 7, 1535744. [Google Scholar] [CrossRef] [Green Version]
- Maumus, M.; Rozier, P.; Boulestreau, J.; Jorgensen, C.; Noël, D. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front. Bioeng. Biotechnol. 2020, 8, 997. [Google Scholar] [CrossRef]
- Lener, T.; Gimona, M.; Aigner, L.; Börger, V.; Buzas, E.; Camussi, G.; Chaput, N.; Chatterjee, D.; Court, F.A.; del Portillo, H.A.; et al. Applying Extracellular Vesicles Based Therapeutics in Clinical Trials—An ISEV Position Paper. J. Extracell. Vesicles 2015, 4, 30087. [Google Scholar] [CrossRef]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The Impact of Storage on Extracellular Vesicles: A Systematic Study. J. Extracell. Vesicles 2022, 11, e12162. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nishikawa, M.; Shinotsuka, H.; Matsui, Y.; Ohara, S.; Imai, T.; Takakura, Y. Visualization and in Vivo Tracking of the Exosomes of Murine Melanoma B16-BL6 Cells in Mice after Intravenous Injection. J. Biotechnol. 2013, 165, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.P.; Mardini, O.; Ericsson, M.; Prabhakar, S.; Maguire, C.A.; Chen, J.W.; Tannous, B.A.; Breakefield, X.O. Dynamic Biodistribution of Extracellular Vesicles in Vivo Using a Multimodal Imaging Reporter. ACS Nano 2014, 8, 483–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sung, B.H.; von Lersner, A.; Guerrero, J.; Krystofiak, E.S.; Inman, D.; Pelletier, R.; Zijlstra, A.; Ponik, S.M.; Weaver, A.M. A Live Cell Reporter of Exosome Secretion and Uptake Reveals Pathfinding Behavior of Migrating Cells. Nat. Commun. 2020, 11, 2092. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Lai, R.C.; Sim, W.K.; Choo, A.B.H.; Lane, E.B.; Lim, S.K. Topical Application of Mesenchymal Stem Cell Exosomes Alleviates the Imiquimod Induced Psoriasis-like Inflammation. Int. J. Mol. Sci. 2021, 22, 720. [Google Scholar] [CrossRef]
- Royo, F.; Zuñiga-Garcia, P.; Sanchez-Mosquera, P.; Egia, A.; Perez, A.; Loizaga, A.; Arceo, R.; Lacasa, I.; Rabade, A.; Arrieta, E.; et al. Different EV Enrichment Methods Suitable for Clinical Settings Yield Different Subpopulations of Urinary Extracellular Vesicles from Human Samples. J. Extracell. Vesicles 2016, 5, 29497. [Google Scholar] [CrossRef]
- Van Deun, J.; Mestdagh, P.; Sormunen, R.; Cocquyt, V.; Vermaelen, K.; Vandesompele, J.; Bracke, M.; De Wever, O.; Hendrix, A. The Impact of Disparate Isolation Methods for Extracellular Vesicles on Downstream RNA Profiling. J. Extracell. Vesicles 2014, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Takov, K.; Yellon, D.M.; Davidson, S.M. Comparison of Small Extracellular Vesicles Isolated from Plasma by Ultracentrifugation or Size-Exclusion Chromatography: Yield, Purity and Functional Potential. J. Extracell. Vesicles 2019, 8, 1560809. [Google Scholar] [CrossRef]
- Mol, E.A.; Goumans, M.-J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher Functionality of Extracellular Vesicles Isolated Using Size-Exclusion Chromatography Compared to Ultracentrifugation. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2061–2065. [Google Scholar] [CrossRef]
- Wolf, M.; Poupardin, R.W.; Ebner-Peking, P.; Andrade, A.C.; Blöchl, C.; Obermayer, A.; Gomes, F.G.; Vari, B.; Maeding, N.; Eminger, E.; et al. A Functional Corona around Extracellular Vesicles Enhances Angiogenesis, Skin Regeneration and Immunomodulation. J. Extracell. Vesicles 2022, 11, e12207. [Google Scholar] [CrossRef]
- Wolf, M.; Poupardin, R.W.; Ebner-Peking, P.; Andrade, A.C.; Blöchl, C.; Obermayer, A.; Gomes, F.G.; Vari, B.; Eminger, E.; Binder, H.-M.; et al. A Functional Corona around Extracellular Vesicles Enhances Angiogenesis during Skin Regeneration and Signals in Immune Cells. bioRxiv 2021. [Google Scholar] [CrossRef] [Green Version]
- Tóth, E.Á.; Turiák, L.; Visnovitz, T.; Cserép, C.; Mázló, A.; Sódar, B.W.; Försönits, A.I.; Petővári, G.; Sebestyén, A.; Komlósi, Z.; et al. Formation of a Protein Corona on the Surface of Extracellular Vesicles in Blood Plasma. J. Extracell. Vesicles 2021, 10, e12140. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.C.; Yung, B.C.; Bergamaschi, C.; Chowdhury, B.; Bear, J.; Stellas, D.; Morales-Kastresana, A.; Jones, J.C.; Felber, B.K.; Chen, X.; et al. Scalable, CGMP-Compatible Purification of Extracellular Vesicles Carrying Bioactive Human Heterodimeric IL-15/Lactadherin Complexes. J. Extracell. Vesicles 2018, 7, 1442088. [Google Scholar] [CrossRef] [PubMed]
- Hisey, C.L.; Artuyants, A.; Guo, G.; Chang, V.; Reshef, G.; Middleditch, M.; Jacob, B.; Chamley, L.W.; Blenkiron, C. Investigating the Consistency of Extracellular Vesicle Production from Breast Cancer Subtypes Using CELLine Adherent Bioreactors. bioRxiv 2022. [Google Scholar] [CrossRef]

| Strategies | Type of Induction | Cells | Exposure Period or Method | EV Isolation Method | Enhancement Factor | Therapeutic Application | References |
|---|---|---|---|---|---|---|---|
| 3D culture | Hollow-fiber bioreactor (FiberCell Systems) | UC-MSCs | Cell expansion and medium conditioning | Ultracentrifugation | Bradford assay: 7.5-fold increase in small EV protein concentration | Possessed superior chondroprotective effects to those of 2D small EVs in vitro and in vivo | [105] |
| Hollow-fiber bioreactor (FiberCell System) | UC-MSCs | Cell expansion and medium conditioning | Ultracentrifugation | BCA assay: 19.4-fold increase in small EV protein concentration | Possessed superior renoprotective efficacy to that of 2D small EVs in vitro and in vivo | [106] | |
| Vertical-Wheel™ bioreactors (VWBR) | BM-MSCs, AT-MSCs, and UC-MSCs | Cell expansion and medium conditioning | Precipitation (total exosome isolation reagent) | NTA: 4.0, 4.4, and 8.8-fold increases in small EV particle concentration for BM-MSCs, AT-MSCs, and UC-MSCs, respectively | Not reported | [107] | |
| Hollow-fiber bioreactor (FiberCell System) | hetIL-15-overexpressed HEK293 cells (clone 19.7) | Cell expansion and medium conditioning | Ultracentrifugation | Bradford assay: 40-fold increase in small EV protein concentration | Bioactivity of small EV-associated hetIL-15 was maintained (hetIL-15 activates NK cells) | [108] | |
| Hollow-fiber bioreactor (FiberCell System) | BM-MSCs | Cell expansion and medium conditioning | Precipitation (total exosome isolation reagent) | NTA: 1.9 × 1010 ± 1.1 × 1010 small EV articles/mL on day 1, 8.2 × 109 ± 3.0 × 109 small EV particles/mL on day 13, and 8.1 × 109 ± 3.3 × 109 small EV particles/mL on day 25 | Possessed immunomodulatory properties | [109] | |
| Ultrafoam scaffolds (collagen type I) | MSCs | Cell expansion and medium conditioning | Precipitation (ExoQuick) | BCA assay: twofold increase in small EV protein concentration | Enhanced neurological functional recovery of traumatic brain injury model compared with 2D-culture and liposome groups | [110] | |
| 3D-printed scaffold perfusion bioreactor | Human dermal microvascular endothelial cells (hDMECs) | Cell expansion and medium conditioning | Ultracentrifugation | NTA: 100- and 10,000-fold increases in small EV particle concentration on days 1 and 3, respectively; CD63 exoELISA: 14-fold increase in CD63+ EV concentration; BCA assay: 6.7-fold increase in small EV protein concentration, but decreased protein content per EV | Enhanced vascularization bioactivity in 3D-scaffold groups (bioreactor and static) pretreated with 100 nM ethanol | [111] | |
| 3D spheroids | BM-MSCs | Cell expansion and medium conditioning | Precipitation (ExoQuick-TC) | Bradford assay: 2-fold increase in EV protein concentration for hanging-drop 3D spheroid culture; 2.4-fold increase for poly-HEMA coated-3D spheroid culture | Not reported | [112] | |
| Physical stimulation | Ionizing radiation (X-ray: 2 Gy) | MCF7 breast epithelial cancer cells | 4 h | Ultracentrifugation | TRPS: threefold increase in small EV particle concentration in the direct irradiated group; sixfold increase in bystander group | Identified that small EVs play a role in nontargeted effects of irradiation (cancer therapy) | [113] |
| Gamma irradiation (1000 cGy) or hypoxia (1% O2) | Human lung cancer cell lines (LLC and A549) | 12, 24, 36, or 48 h | Centrifugation | Flow cytometry: fourfold increase in EV particle concentration in both hypoxia and gamma irradiation treatment groups | Identified that the microenvironment caused EV change | [114] | |
| Ionizing radiation (X-ray: 4 Gy) | Human glioblastoma cell lines (LN18, U251, U87MG), glioblastoma stem-like cells (GBAM1 and GBMJ1), and astrocytes | 12 to 48 h | Ultracentrifugation | NTA: 1.23- to 2.6-fold increases in small EV particle concentration | Increased cell migration and uptake efficiency, showing that intercellular signaling reacted to therapeutic radiation | [115] | |
| Ultraviolet irradiation stress treatment (UV; 40 W), low-pH culture medium treatment (LP; pH 4.0), high-temperature treatment (HT; 40 °C), H2O2 treatment (H2O2; 250 × 10−6 m), and hypoxic environment treatment (Hyp; 100% N2) | Human gastric cancer cells (MGC803) and human liver cancer cells (HepG2) | Not reported | Ultracentrifugation | BCA assay: 1.9-fold increases in small EV protein content in UV, LP, and HT treatments; 1.7-fold increase in H2O2; 1.5-fold increase in HYP | Increased uptake efficiency | [116] | |
| Photodynamic therapy (Foscan® photosensitizer: 0.02, 0.08, 0.2, 0.5, 2 or 10 μM) and chemotherapeutic agent (doxorubicin: 0.1, 0.5, 2, 5, 10 and 50 μM) | Human prostatic cancer cells (PC-3) | 2 h exposed to light for 5 s at a wavelength of 470 nm (7.5 J/cm2) | Not reported | NTA: 15- and 6-fold increases in large EV particle concentration for PDT and doxorubicin treatment, respectively | The released large EVs may counterbalance the desired regional limitation of a treatment and represent an underestimated source of adverse effects during PDT | [19] | |
| Acoustic irradiation: surface-reflected bulk waves (SRBWs, 4 W) and electromechanical hybrid surface (order of 10 MHz) | Human glioblastoma cells (U87-MG) and adenocarcinoma human alveolar basal epithelial cells (A549) | 10 min followed by 30 min postexcitation incubation period | Column-based (PureExoⓇ Exosome Isolation Kit) | AChE activity: 1.7-fold increase in small EV AChE activity in the first 30 min, followed by a reduction | Exosome therapy: cancer vaccine and biomarker | [117] | |
| Ionizing radiation (X-ray: 0 Gy; 0.1 Gy; 1 Gy; 10 Gy) | Neuroblastoma cell lines (SH-SY5Y and SK-N-BE) | 3 h | Ultracentrifugation | Flow cytometry: 2.7-fold and 4.5-fold increases in small EV particle concentration with 0.1 Gy and 10 Gy radiation, respectively, for the SH-SY5Y cell line; 3.8-fold increase with 10 Gy radiation for the SK-N-BE cell line Spectrophotometric quantitation: 1.2-fold and 2.2-fold increases in small EV protein content with 0.1 Gy and 10 Gy radiation, respectively, for the SH-SY5Y cell line; 1.2-fold increase with 10 Gy radiation for the SK-N-BE cell line | Increased proliferation and invasiveness, showing side effects of radiation therapy | [18] | |
| Electrical stimulation (0.34 mA/cm2) | Melanoma cell line (B16F1) and murine fibroblast cell line (3T3) | 1 h | Ultracentrifugation | TRPS: 1.26- and 1.7-fold increases in EV particle concentration for B16F1 and 3T3 cells, respectively | Not reported | [118] | |
| Cellular nanoporation (CNP) | Embryonic fibroblasts (MEFs) or bone marrow-derived dendritic cells (BMDCs) | 4, 8, 12, 16, 20, and 24 h | Ultracentrifugation | DLS and NTA: 50-fold increase in EV particle concentration and >1000-fold increase in exosomal mRNA transcripts | Targeted therapy by transfer of desired peptides (through CD74) led to longer circulatory half-life, significantly inhibited glioma tumor growth in vivo, and prolonged survival | [119] | |
| Medium containing magnetic nanoparticles (Fe3O4: 50 µg/mL) and/or static magnetic field (SMF: 100 mT) | BMSCs | 7 and 14 days | Ultracentrifugation | BCA assay: 1.4-fold increase in small EV protein concentration for Fe3O4 group; 1.7-fold increase for Fe3O4 + SMF group | Enhanced osteogenesis and angiogenesis in vitro and in vivo | [120] | |
| Cyclic stretch (20% elongation at a frequency of 10 cycles/min) | Periodontal ligament cells | 24 h | Cell culture supernatant | CD63 ELISA Kit (PS Capture™ Exosome ELISA Kit): 33-fold increase in CD63+ EV concentration | Inhibited IL-1β production and pyroptosis of LPS-primed macrophage | [121] | |
| Micro-/nanotextured hierarchical titanium topography (native titanium specimens (SLM); SLM + 250 μm ZrO2 particles + 5% hydrofluoric acid (HF) (SLA); SLA + 5 M NaOH (SAH); SLA + 0.3 wt% ammonium fluoride (NH4F) + ethylene glycol (C2H6O2) solution (SAO)) | BMSCs | During cell culture | Kit (EIQ3) | AChE activity: 1.1-fold, 1.7-fold, and 1.6-fold increases in small EV AChE activity in SLA, SAH, and SAO groups, respectively, compared with SLM group | Improved osseointegration in vitro and in vivo | [122] | |
| Platinum nanoparticles (10 µM) | Human lung epithelial adenocarcinoma cancer cells (A549) | 24 h | Precipitation (ExoQuick) | BCA assay: 3.9-fold increase in small EV protein concentration; fluorescence polarization: 4.8-fold increase in small EV particle concentration; NTA: 4.1-fold increase in small EV particle concentration; EXOCET: 5.9-fold increase in small EV particle concentration | Not reported | [123] | |
| 45S5 Bioglass® | Human MSCs | 12 to 72 h or 48 h | Ultracentrifugation and ultrafiltration | AChE activity: No significant difference in small EV AChE concentration in the first 12 h; 1.3-, 1.4-, and 1.6-fold increases at 24, 48, and 72 h, respectively NTA: No significant differences in small EV particle concentration in the first 12 h; 2.4-, 1.8-, and 2.0-fold increases at 24, 48, and 72 h, respectively EXOCET kit: 2.1-fold increase in small EV particle concentration at 48 h HSFCM: 5.4-fold increase in small EV particle concentration at 48 h | Promoted vascularization of umbilical vein endothelial cells in vitro and in vivo | [124] | |
| Chemical stimulation | Sodium iodoacetate and 2,4-dinitrophenol (IAA/DNP) (in vitro: 1 or 10 µM; ex vivo: 5, 10, or 30 µM; in vivo: 0.195 or 0.975 μmol) | UMSCC47, PCI-13, Mel526, SVEC4–10 (in vitro); murine kidney tissue explant (ex vivo); mice (in vivo) | 72 h (in vitro); 48 h (ex vivo); 14 days (in vivo) | Size-exclusion chromatography | BCA assay: 3- to 16-; 1.8-, and 2.9-fold increases in small EV protein concentration in vitro, ex vivo, and in vivo, respectively | Possessed similar biological properties and functional effects on endothelial cells (SVEC4-10) | [125] |
| Fenoterol, norepinephrine, N-methyldopamine, mephenesin, and forskolin | BMSCs | 24 h | Ultracentrifugation | NTA: 1.7- to 2.3-fold increase in small EV particle concentration, which further increased when combining compounds (2.5- to 3-fold) | Possessed regenerative activities as control small EVs | [126] | |
| Suxiao Jiuxin pill, tetramethylpyrazine, or borneol | Murine cardiac MSCs | 48 h | Precipitation (polyethylene glycol 4000) | AChE activity: 3.4-fold, 2.4-fold, and 1.3-fold increases in small EV AChE activity in Suxiao Jiuxin pill, tetramethylpyrazine, and borneol treatments, respectively | Not reported | [127] | |
| Adiponectin (20 μg/mL) from serum collected from APN-knockout mice | T-cadherin-expressing murine vascular endothelial cells (F2T cells) | 36 h | Ultracentrifugation | AChE activity: 7.8-fold increase in small EV AChE activity; NTA: 2.9-fold increase in small EV particle concentration | Adiponectin-induced small EV release affected ceramide metabolism, which could be helpful for adiponectin-related organ protection therapy | [128] | |
| High-molecular-weight adiponectin (20 μg/mL; in vitro) or pioglitazone (30 mg/kg twice a day; in vivo) | Human adipose tissue-derived MSCs | 48 h (in vitro) and two weeks (in vivo) | Ultracentrifugation | Densitometry of Western blot: increased small EV production in vitro and in vivo; NTA: 3.3-fold increase in small EV particle concentration in vitro | Augmented the cardioprotective effects of MSCs in transverse aortic constriction-operated mice | [129] | |
| Docosahexaenoic acid (DHA; 100 μM) | Human breast cancer cells (MCF7 and MDA-MB-231) | 24 h | Ultracentrifugation or precipitation (ExoQuick-TC reagent) | CD63-GFP fluorescent spectrometry: 1.1-fold increase in CD63+ EV concentration in MCF7 and MDA-MB-231 cell lines | Increased RNA content in breast cancer CD63+ EVs promoted anticancer and anti-angiogenic activity | [130] | |
| Sodium ionophore (Monensin; 1, 5, 10 µM), calcium ionophore (A23187; 1 µM), or human transferrin (20 µg/mL) | Human erythroleukemia cell line (K562) | 7 h for monensin and A23187; 12 h for transferrin treatment | Ultracentrifugation | AChE activity: 20%, 71.5%, and 97.6% increases in EV AChE activity with 1, 5, and 10 µM of monensin; 1.7-fold increase with A23187; 1.4-fold with transferrin | Not reported | [131] | |
| Sodium ionophore (Monensin: 7 µM) | Rab11-transfected human erythroleukemia cell line (K562) | 7 h | Ultracentrifugation | AChE activity: 2.0-, 1.8-, 3.8-fold, and 3.7-fold increases in EV AChE activity with monensin treatment in vector, Rab11 wildtype, Rab11 Q70L (a GTPase-deficient mutant), and Rab11 S25N (aGTP-binding deficient mutant) cells, respectively | Not reported | [132] | |
| Calcium phosphate (CaP) particles (500 and 1000 μg/mL) | Macrophage-like cells (RAW264.7) and monocyte-like cells (THP-1) | 1, 2, 4, 6, 24, 48, and 72 h | Precipitation (total exosome isolation kit) | EXOCET exosome quantitation assay kit: 2-and 2.5-fold increases in small EV particle concentration at 72 h with 500 μg/mL CaP for RAW264.7 and THP-1 cell lines, respectively | Not reported | [133] | |
| Ionomycin (2.5 µM) and TGFβ-1 (5 ng/mL) | Human breast carcinoma cell line (MDA-MB-231), human lung carcinoma line (A549), and human pancreatic carcinoma line (Panc-1) | 30 min of ionomycin treatment 24 h of TGFβ-1 treatment | Density gradient ultracentrifugation | CD63+ Slot blot: 5- and 3-fold increases in CD63+ EV concentration for ionomycin and TGFβ-1 treatments, respectively | Not reported | [134] | |
| Phosphorothioate (PS) B-class CpG oligonucleotides (ODN 2006PS; 2 μM), S. salar DNA (15 μg/mL), or E. coli DNA (15 μg/mL) | Salmon head kidney leukocytes (HKLs), Atlantic salmon kidney cells (ASK cells), chinook salmon embryo cells (CHSE-214 cells), or HEK293T cells | 1 h (2006PS, S. salar or E. coli DNA) for HEK293T cells | Ultracentrifugation | Densitometry of Western blot (Alix): 10.1-, 16.7-, and 9.1-fold increases in Alix+ EV protein content for ODN 2006PS-treated HKLs, ASK cells, and CHSE-214 cells, respectively; for HEK293T cells, 3.3-, 2.1-, and 9-fold increases in Alix+ EV protein content for 2006PS, S. salar DNA, and E. coli DNA groups, respectively | Not reported | [135] | |
| Cationic bare liposomes (CL: HSPC-based or DOPE-based; 0.5 to 2 mM) or neutral bare liposomes (NL; 0.5 to 2 mM) | Murine colorectal cancer cell line (C26), murine melanoma cell line (B16BL6), human gastric cancer cell line (MKN45), and human colorectal cancer cell line (DLD-1) | 48 h | Ultracentrifugation or precipitation (ExoQuick-TCTM) | Bio-Rad DC® protein assay: 2.5-, 2.3-, 1.7-, and 1.8-fold increases in EV protein concentration with 2 mM of NL for C26, B16B16, MKN45, and DLD-1 cell lines, respectively; 3.4-, 3.4-, 3.7-, and 2.9-fold increases in EV protein concentration with 2 mM of HSPC-based CL for C26, B16B16, MKN45, and DLD-1 cell lines, respectively. DOPE-based CLs further increased EV protein concentration (up to 3.17-fold) | Liposome-stimulated EVs showed higher cellular uptake | [136] | |
| Physiological modification | Hypoxic (1% O2, 0.1% O2, or 1 mM DMOG) | Breast cancer cell lines MCF7, SKBR3, and MDAMB 231 | 24 h (1% O2) or 48 h (0.1% O2 and DMOG) | Precipitation (ExoQuickTM) | NTA: Up to 1.41-fold, 1.94-fold, and 1.45-fold increases in small EV particle concentration at 1% O2, 0.1% O2, and 1 mM DMOG, respectively | Highlighted the importance of the study of EV-mediated pathological hypoxic signaling in tumor progression | [137] |
| Hypoxic (5% O2) | Human umbilical cord MSCs | 24 h | Ultracentrifugation | NTA: twofold increase in small EV particle concentration | Better attenuated OVA-induced chronic airway inflammation and lung parenchyma fibrosis in mice | [138] | |
| Hypoxic (1% O2) | BMSCs | 48 h | Ultrafiltration and density gradient ultracentrifugation | BCA assay: 1.4-fold increase in small EV protein concentration | Better protected cartilage from degeneration and slowed down the progression of OA in vitro and in vivo | [139] | |
| Hypoxic (1% O2) | BMSCs | 48 h | Ultrafiltration and density gradient ultracentrifugation | BCA assay: 1.4-fold increase in small EV protein concentration | Promoted to a greater extent functional behavioral recovery in mice and M1 to M2 phenotype polarization in vivo and in vitro | [140] | |
| Hypoxic (1% O2) and/or serum-free stimulation | UC-MSCs | 72 h | Ultracentrifugation | Bradford assay: 5.6-, 4.3-, and 7.5-fold increases in CD29+, CD44+, CD73+, CD31−, and CD45− EVs (authors identified the EVs as microvesicles) under hypoxic conditions, serum-free stimulation, and hypoxic and serum-free conditions, respectively | CD29+, CD44+, CD73+, CD31−, and CD45− EVs promoted angiogenesis and were superior in hypoxia endothelial cells | [141] | |
| Hypoxic (0.5% O2) | Mouse MSCs | 24 h | Ultracentrifugation | NTA: 1.3-fold increase in small EV particle concentration | Superior ability in proangiogenesis and antiapoptosis in vitro and cardiac protection in vivo | [142] | |
| Lentivirus pWPI-HIF-1α-GFP transduction | Dental pulp MSCs | Lentivirus transduction | Ultracentrifugation | Densitometry of Western blot: 3.3-, 6.3-, and 1.5-fold increases in CD63, CD9, and CD81 density AChE activity; 2.1-fold increase in small EV AChE activity | Superior angiogenic ability in vitro and in vivo via enhanced expression of the Notch ligand Jagged1 | [143] | |
| Peripheral arterial disease conditions (0% serum and 1% O2) | BMSCs | 40 h | Centrifugation (higher density EVs (claimed as microvesicles)) or ultracentrifugation (lower density EVs (claimed as exosomes)) | BCA assay: 9-fold decrease in high-density EV protein concentration; 6.6-fold increase in low-density EV protein concentration | EVs contained a robust profile of angiogenic signaling proteins and induced angiogenesis | [144] | |
| Heat stress (42 °C) | Epstein–Barr virus (EBV)-immortalized human B-lymphoblastoid cell lines (B-LCL) and Jurkat cell line | 3 h | Density gradient ultracentrifugation | BCA assay: 1.25-fold increase in EV protein concentration for all cell lines | Significantly increased heat shock proteins of cells and EVs but did not trigger dendritic cell maturation | [145] | |
| Heat stress (40 °C), oxidative stress, cells were treated for 2 h with 100 μM and 50 μM H2O2 | Jurkat and Raji cells | 1 h | Density gradient ultracentrifugation | Densitometry of Western blot (CD63): 3- and 15-fold increases in CD63+ EV protein concentration after thermal and oxidative stress, respectively, for Jurkat cells; 22- and 32-fold increases after thermal and oxidative stress for Raji cells | Partly provided a mechanistic explanation of the clinically observed NK-cell dysfunction in patients suffering from leukemia/lymphoma, which could be further impaired in conditions of cellular stress | [21] | |
| Cytostatic stress (0.6 μM doxorubicin), heat stress (42 °C), oxidative stress (2.5 μg/mL light-induced Ag-TiO2) | B16F1 mouse melanoma cell | 72 h heat stress (3 × 2 h) | Ultracentrifugation | NTA: 3.6-, 2.5-, and 1.6-fold increases in small EV particle concentration after doxorubicin, heat stress, and oxidative stress, respectively | Microenvironmental conditions altered small EV cargoes and explained the importance of determining therapy-induced host response | [146] | |
| pH (pH 6.0) | Mel1 melanoma cell lines | Cell culture and medium conditioning | Density gradient ultracentrifugation | BCA assay: 3.2- and 6.3-fold increases in Lamp-2+, CD81+, and Rab 5B+ EVs on days 3 and 4, respectively | Acidic microenvironment favored EV-to-cell fusion | [12] | |
| pH (pH 4, 7, and 11) | HEK 293 | 30 min | Precipitation (ExoQuick isolation kit) | BCA assay: 5-fold increase in CD9+, CD63+, and Hsp70+ EV protein concentration at pH 4, while pH 11 gave a negative result (3.5-fold decrease) | Acidic pH could increase the stability of EVs in vitro | [147] | |
| Genetic manipulation | PLD2 cDNA electroporation | RBL-2H3 | Murine PLD2 cDNA electroporation | Ionomycin degranulation then ultracentrifugation | FACS: Twofold increase in BODIPY-ceramide labeled EV concentration | Not reported | [148] |
| Rab13 knockdown | Mutant KRAS DKO-1 colorectal cancer cells | Rab13 shRNA transfection | Ultracentrifugation | NTA: 14.3-fold decrease in small EV production when Rab13 was knocked down in mutant KRAS cells | Not reported | [149] | |
| Rab27a or Rab27b knockdown | HeLa cell line | Transfection | Ultracentrifugation | Bradford assay: 2.5- and 2.2-fold decreases in small EV protein content for Rab27a knockdown and Rab27b knockdown cells, respectively Densitometry of Western blot (HLA-DR, Hsc70, Tsg101): 19.5-, 5.8-, and 3.1-fold decreases in HLA-DR, Hsc70, and Tsg101 protein density of small EVs, respectively, for Rab27a knockdown cells; 2.5-, 2.4-, and 1.6-fold decreases in HLA-DR, Hsc70, and Tsg101 protein density, respectively, for Rab27b knockdown cells | Not reported | [150] | |
| CD9 lentiviral transduction | HEK293, HEK293FT, Raji, Jurkat, and HeLa | pLenti6.3-CD9GFP lentiviral transduction | PEG centrifugation | NTA: 2.5-fold increase in small EV particle concentration in HEK293-CD9GFP cells; more than 3-fold increase in HEK293FT-CD9GFP; 2-fold increase in Raji-CD9GFP and Jurkat-CD9GFP; no significant difference for HeLa-CD9GFP | Not reported | [151] | |
| Liver kinase B1 (LKB1) lentiviral transduction | H460 | pCDH-LKB1 lentiviral transduction | Ultracentrifugation | NTA: 12.5-fold increase in small EV particle concentration | Enhanced cell migration ability and showed the ambiguity of cancer therapy targeting LKB1 function | [152] | |
| Upregulation of EIF3C | Hepatocellular carcinoma cell lines: PLC5 and SNU-449 | NA | Precipitation (total exosome isolation reagent) | Electron microscopy and NTA: Increased small EV particle concentration | EIF3C may act as a cancer target in cancer therapy | [153] | |
| PIKfyve inhibition (apilimod or siRNA transfection) | Human prostate cancer epithelial cell line (PC-3) | Apilimod or siRNA transfection | Ultracentrifugation and OptiPrep density gradient centrifugation | NTA and BCA assay: Apilimod treatment increased small EV particle concentration 1.6-fold and small EV protein content 1.4-fold; siRNA transfection increased small EV particle concentration 1.5-fold | Not reported | [154] | |
| MYC gene overexpression | hESC-MSC (HuES9.E1 MSCs) | GFP- or MYC-containing lentivirus transduction | HPLC | Not reported | Provided an infinite supply of cells for the production of small EVs with cardioprotective activity | [155] | |
| Preparation of EV-mimetic nanovesicles | Nitrogen cavitation (400–500 psi at 0 °C) | Promyelocytic leukemia cell line (HL-60) | 20 min | Centrifugation | BCA assay: 16-fold increase in EV-mimetic nanovesicle protein concentration | Prevented sepsis-induced inflammation and increased animal survival after loading of piceatannol | [156] |
| Sonication (42 kHz and a power of 100 W) of the mixture of macrophage membranes and nanoparticle cores | Murine J774 macrophage cell line | 2 min | Macrophage membranes mixed with nanoparticle cores at the ratio of 1:1 | Not reported | Promoted proinflammatory cytokine sequestration and endotoxin neutralization in vitro and in vivo | [157] | |
| Ultrasonication (20%) | Human umbilical cord-MSCs | 1 min | Centrifugation | 18.5-fold increase in EV-mimetic nanovesicle production | Enhanced skin rejuvenation and promoted wound healing in vitro and in vivo | [158] | |
| Serial extrusion through filters with diminishing pore sizes | Human U937 monocytic cells | Not reported | OptiPrep density gradient ultracentrifugation | NTA: 100-fold increase in doxorubicin-loaded EV-mimetic nanovesicle particle concentration | Targeted delivery of chemotherapeutic drug had a similar antitumor effect to that of doxorubicin-loaded natural EVs in vitro and in vivo | [159] | |
| Serial extrusion through filters with diminishing pore sizes | Mouse embryonic fibroblasts NIH3T3 and human U937 monocytic cells | Not reported | Two-step OptiPrep density gradient ultracentrifugation | Not reported | Therapeutic vesicles (c-Myc siRNA-loaded nanovesicles) targeted diseases associated with c-Myc overexpression | [160] | |
| Serial extrusion through filters with diminishing pore sizes | Adipose-derived stem cells | Not reported | Two-step OptiPrep density gradient ultracentrifugation | NTA: 30-fold increase in EV-mimetic nanovesicle particle concentration | Possessed similar regenerative effects to those of natural EVs in vitro and enhanced regenerative ability in emphysema mouse model | [161] | |
| Serial extrusion through filters with diminishing pore sizes | Not reported | Two-step OptiPrep density gradient ultracentrifugation | BCA assay: 100-fold increase in EV-mimetic nanovesicle protein concentration | Promoted hepatocyte proliferation in vitro and liver regeneration in vivo | [162] | ||
| Alkaline solutions (sodium carbonate solution) | Human U937 monocytes | Not reported | Sonication then density gradient ultracentrifugation | Bradford assay: 200-fold increase in EV-mimetic nanovesicle protein concentration | Reduced the release of IL-8 from OMV-treated endothelial cells in vitro and mitigated the symptoms of OMV-induced SIRS in vivo by dexamethasone-loaded EV-mimetic nanovesicles | [163] | |
| Sulfhydryl-blocking agents (dithiothreitol (DTT; 2 mM) and paraformaldehyde (PFA; 25 mM)) | Mouse lymphoma cell line (EL4) | 2 h | Ultracentrifugation and centrifugal filtration | BCA assay: More than 10-fold increase in EV-mimetic nanovesicle protein concentration | Better cellular absorption and intracellular release of doxorubicin than liposomes; more effective in slowing down tumor growth than free doxorubicin and liposome-encapsulated doxorubicin | [164] | |
| Other factors | Seeding density (1 × 102 cells/cm2 or 1 × 104 cells/cm2) | BMSCs | Not reported | Ultracentrifugation and ultrafiltration | NTA: 100-, 85-, 105-, and 50-fold increases in small EV particle concentration with 1 × 102 cells/cm2 seeding density compared with 1 × 104 cells/cm2 seeding density at passages 2, 3, 4, and 5, respectively CD63 ELISA: 126-, 152-, 201-, and 126-fold increases in CD63+ EVs with 1 × 102 cells/cm2 seeding density | Not reported | [165] |
| Collection frequency (single or double collection) | BMSCs | 12 and/or 24 h collection time(s) | Ultracentrifugation and ultrafiltration | NTA: 1.6- to 2.6-fold increase in small EV particle concentration with two EV collections compared with a single collection in 24 h | Not reported | [165] | |
| Cellular senescence (presenescent and senescent cells) | Primary normal human diploid fibroblasts (TIG-3 cells) | Serial passage or ectopic expression of oncogenic Ras | Density gradient ultracentrifugation | NTA: Significant increase in small EV particle concentration in senescent cells | Revealed the role of small EV secretion in the maintenance of cellular homeostasis | [166] | |
| Cellular senescence (presenescent and senescent cells) | Human prostate cancer cells (22Rv1) and human dermal fibroblasts (NHDFs) | Replicative senescence (serial passage) or accelerated senescence (irradiation: 4 Gy) | Ultracentrifugation | Vybrant DiI labeling: 3- and 15-fold increases in Vybrant Dil-labeled EV particles in accelerated senescent and replicative senescent cells, respectively | Not reported | [167] | |
| Cellular senescence (presenescent and senescent cells) | Human diploid fibroblasts (HDFs; TIG-3 cells) | Replicative senescence (serial passage) or induced senescence (oncogenic Ras or doxorubicin induction) | Ultracentrifugation | NTA: 46.4-, 16.7-, and 6.8-fold increases in small EV particle concentration for replicative senescent cells, oncogenic Ras-induced senescent cells, and doxorubicin-induced senescent cells, respectively | Small EVs from senescent cells promoted the proliferation of human breast cancer MCF-7 cells, showing the protumorigenic effect of senescent cells | [168] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, C.Y.; Kee, L.T.; Al-Masawa, M.E.; Lee, Q.H.; Subramaniam, T.; Kok, D.; Ng, M.H.; Law, J.X. Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review. Int. J. Mol. Sci. 2022, 23, 7986. https://doi.org/10.3390/ijms23147986
Ng CY, Kee LT, Al-Masawa ME, Lee QH, Subramaniam T, Kok D, Ng MH, Law JX. Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review. International Journal of Molecular Sciences. 2022; 23(14):7986. https://doi.org/10.3390/ijms23147986
Chicago/Turabian StyleNg, Chiew Yong, Li Ting Kee, Maimonah Eissa Al-Masawa, Qian Hui Lee, Thayaalini Subramaniam, David Kok, Min Hwei Ng, and Jia Xian Law. 2022. "Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review" International Journal of Molecular Sciences 23, no. 14: 7986. https://doi.org/10.3390/ijms23147986
APA StyleNg, C. Y., Kee, L. T., Al-Masawa, M. E., Lee, Q. H., Subramaniam, T., Kok, D., Ng, M. H., & Law, J. X. (2022). Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review. International Journal of Molecular Sciences, 23(14), 7986. https://doi.org/10.3390/ijms23147986








