Effects of Different Types of Chronic Training on Bioenergetic Profile and Reactive Oxygen Species Production in LHCN-M2 Human Myoblast Cells
Abstract
:1. Introduction
2. Results
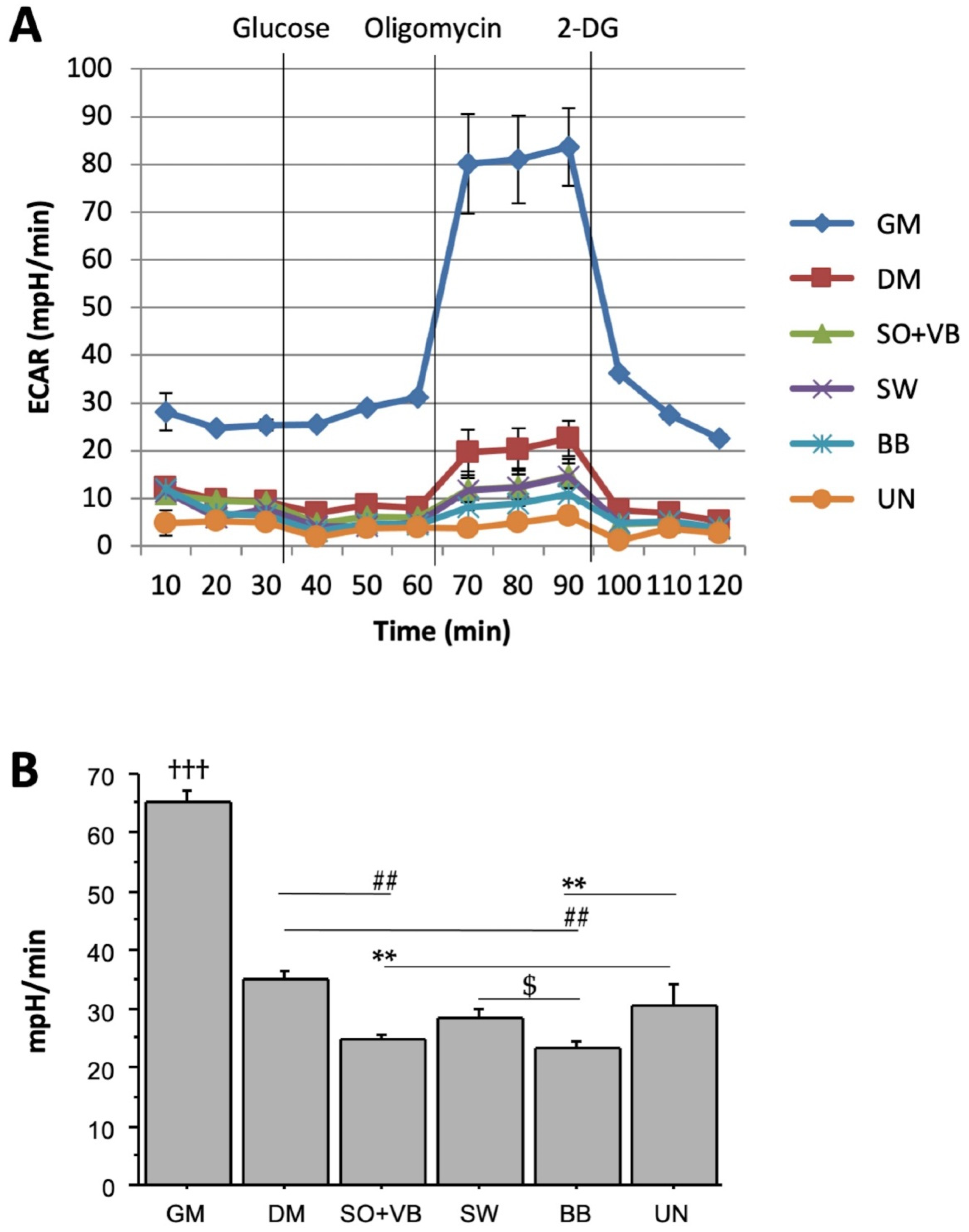
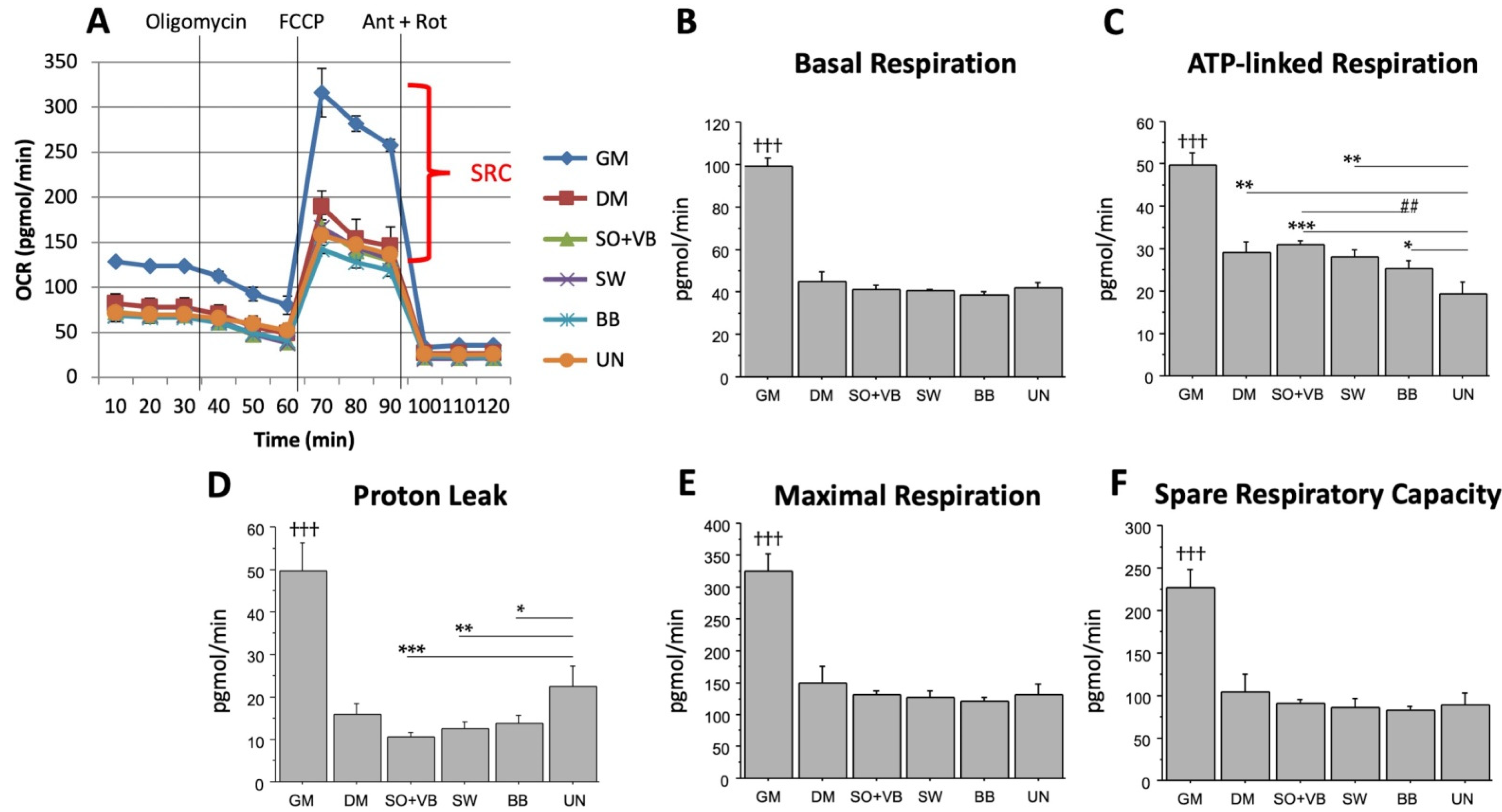
2.1. Real Time Determination of the Effects Mediated by Different-Types of Training on the Bioenergetic Profile in Human LHCN-M2 Myoblast Cells during Differentiation
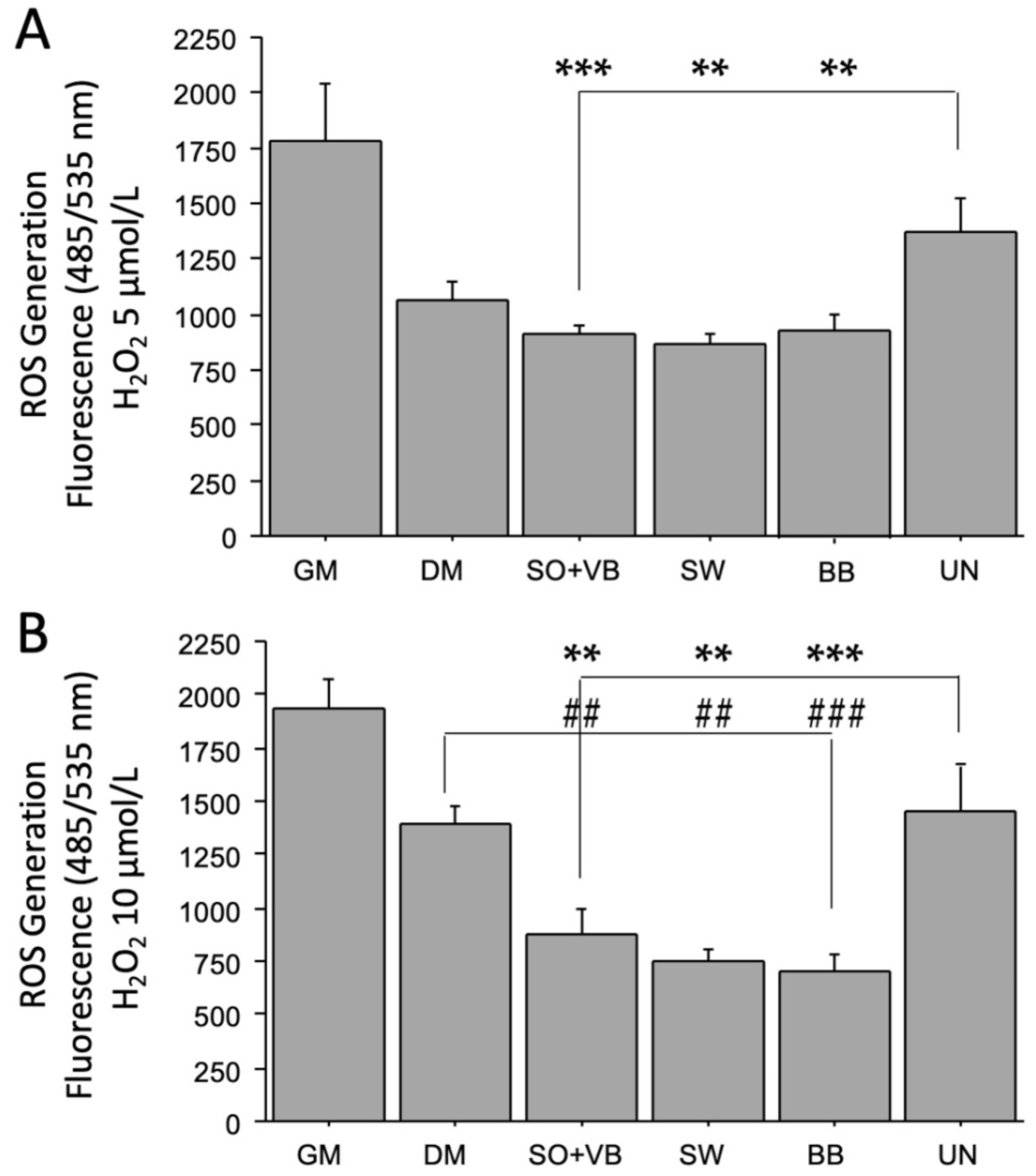
2.2. Effects of Sera from Differently Trained Volunteers on LHCN-M2 Cells and ROS Production
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Subject Recruitment
4.3. LHCN-M2 Treatment and Bioenergetics Profiling
4.4. Measurement of Intracellular ROS
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Hood, D.A.; Memme, J.M.; Oliveira, A.N.; Triolo, M. Maintenance of Skeletal Muscle Mitochondria in Health, Exercise, and Aging. Annu. Rev. Physiol. 2019, 81, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Bhaskaran, S.; Van Remmen, H. Muscle fiber type diversification during exercise and regeneration. Free Radic. Biol. Med. 2016, 98, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pessin, J.E. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 243–250. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadenas, E.; Davies, K.J. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic. Biol. Med. 2000, 29, 222–230. [Google Scholar] [CrossRef]
- Iqbal, S.; Hood, D.A. Cytoskeletal regulation of mitochondrial movements in myoblasts. Cytoskeleton 2014, 71, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Gentil, B.J.; McManus, M.J.; White, K.; St. Louis, K. Acute exercise remodels mitochondrial membrane interactions in mouse skeletal muscle. J. Appl. Physiol. 2013, 115, 1562–1571. [Google Scholar] [CrossRef]
- Holloszy, J.O. Biochemical adaptations in muscle: Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J. Biol. Chem. 1967, 242, 2278–2282. [Google Scholar] [CrossRef]
- Alfieri, A.; Martone, D.; Randers, M.B.; Labruna, G.; Mancini, A.; Nielsen, J.J.; Bangsbo, J.; Krustrup, P.; Buono, P. Effects of long-term football training on the expression profile of genes involved in muscle oxidative metabolism. Mol. Cell. Probes. 2015, 29, 43–47. [Google Scholar] [CrossRef]
- Jacobs, R.A.; Lundby, C. Mitochondria express enhanced quality as well as quantity in association with aerobic fitness across recreationally active individuals up to elite athletes. J. Appl. Physiol. 2013, 114, 344–350. [Google Scholar] [CrossRef] [Green Version]
- Holloway, G.P. Nutrition and training influences on the regulation of mitochondrial adenosine diphosphate sensitivity and bioenergetics. Sports Med. 2017, 47, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, J.; Gejl, K.D.; Hey-Mogensen, M.; Holmberg, H.-C.; Suetta, C. Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J. Physiol. 2017, 595, 2839–2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santoro, A.; Martucci, M.; Conte, M.; Capri, M.; Franceschi, C.; Salvioli, S. Inflammaging, hormesis and the rationale for anti-aging strategies. Ageing Res. Rev. 2020, 64, 101142. [Google Scholar] [CrossRef] [PubMed]
- Carter, H.N.; Chen, C.C.W.; Hood, D.A. Mitochondria, muscle health, and exercise with advancing age. Physiology 2015, 30, 208–223. [Google Scholar] [CrossRef] [Green Version]
- Mancini, A.; Vitucci, D.; Labruna, G.; Imperlini, E.; Randers, M.B.; Schmidt, J.F.; Hagman, M.; Andersen, T.R.; Russo, R.; Orrù, S.; et al. Effect of lifelong football training on the expression of muscle molecular markers involved in healthy longevity. Eur. J. Appl. Physiol. 2017, 117, 721–730. [Google Scholar] [CrossRef]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Kikusato, M.; Toyomizu, M. Crucial Role of Membrane Potential in Heat Stress-Induced Overproduction of Reactive Oxygen Species in Avian Skeletal Muscle Mitochondria. PLoS ONE 2013, 8, e64412. [Google Scholar] [CrossRef]
- Stadtman, E.R.; Levine, R.L. Protein oxidation. Ann. N. Y. Acad. Sci. 2000, 899, 191–208. [Google Scholar] [CrossRef]
- Slimen, I.B.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben, M.M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Mancini, A.; Vitucci, D.; Randers, M.B.; Schmidt, J.F.; Hagman, M.; Andersen, T.R.; Imperlini, E.; Mandola, A.; Orrù, S.; Krustrup, P.; et al. Lifelong Football Training: Effects on Autophagy and Healthy Longevity Promotion. Front. Physiol. 2019, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G.; Darley-Usmar, V.M.; Wu, M.; Jensen, P.B.; Rogers, G.W.; Ferrick, D.A. Bioenergetic Profile Experiment using C2C12 Myoblast Cells. J. Vis. Exp. 2010, 6, 2511. [Google Scholar] [CrossRef] [PubMed]
- Kase, E.T.; Nikolić, N.; Bakke, S.S.; Bogen, K.K.; Aas, V.; Thoresen, G.H.; Rustan, A.C. Remodeling of oxidative energy metabolism by galactose improves glucose handling and metabolic switching in human skeletal muscle cells. PLoS ONE 2013, 8, e59972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelmoez, A.M.; Sardón Puig, L.; Smith, J.A.B.; Gabriel, B.M.; Savikj, M.; Dollet, L.; Chibalin, A.V.; Krook, A.; Zierath, J.R.; Pillon, N.J. Comparative profiling of skeletal muscle models reveals heterogeneity of transcriptome and metabolism. Am. J. Physiol. Cell Physiol. 2020, 318, C615–C626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyatake, S.; Bilan, P.J.; Pillon, N.J.; Klip, A. Contracting C2C12 myotubes release CCL2 in an NF-kB-dependent manner to induce monocyte chemoattraction. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E160–E170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitucci, D.; Imperlini, E.; Arcone, R.; Alfieri, A.; Canciello, A.; Russomando, L.; Martone, D.; Cola, A.; Labruna, G.; Orrù, S.; et al. Serum from differently exercised subjects induces myogenic differentiation in LHCN-M2 human myoblasts. J. Sports Sci. 2018, 36, 1630–1639. [Google Scholar] [CrossRef]
- Perry, C.G.R.; Kane, D.A.; Lanza, I.R.; Neufer, P.D. Methods for assessing mitochondrial function in diabetes. Diabetes 2013, 62, 1032–1036. [Google Scholar] [CrossRef] [Green Version]
- Fukada, S.; Uezumi, A.; Ikemoto, M.; Masuda, S.; Segawa, M.; Tanimura, N. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells 2007, 25, 2448–2459. [Google Scholar] [CrossRef]
- Ryall, J.G.; Dell’orso, S.; Derfoul, A.; Juan, A.; Zare, H.; Feng, X. The NAD+-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell 2015, 16, 171–183. [Google Scholar] [CrossRef] [Green Version]
- Purohit, G.; Dhawan, J. Adult muscle stem cells: Exploring the links between systemic and cellular metabolism. Front. Cell Dev. Biol. 2019, 7, 312. [Google Scholar] [CrossRef]
- Oishi, Y.; Tsukamoto, H.; Yokokawa, T.; Hirotsu, K.; Shimazu, M.; Uchida, K.; Tomi, H.; Higashida, K.; Iwanaka, N.; Hashimoto, T. Mixed lactate and caffeine compound increases satellite cell activity and anabolic signals for muscle hypertrophy. J. Appl. Physiol. 2015, 118, 742–749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dell’orso, S.; Juan, A.H.; Ko, K.D.; Naz, F.; Perovanovic, J.; Gutierrez-Cruz, G. Single cell analysis of adult mouse skeletal muscle stem cells in homeostatic and regenerative conditions. Development 2013, 146, dev174177. [Google Scholar]
- Porter, P.; Reidy, P.T.; Bhattarai, N.; Sidossis, L.S.; Rasmussen, B.B. Resistance exercise training alters mitochondrial function in human skeletal muscle. Med. Sci. Sports Exerc. 2015, 47, 1922–1931. [Google Scholar] [CrossRef] [Green Version]
- Adhihetty, P.J.; O’Leary, M.F.N.; Chabi, B.; Wicks, K.L.; Hood, D.A. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J. Appl. Physiol. 2007, 102, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Hood, D.A. Effect of denervation-induced muscle disuse on mitochondrial protein import. Am. J. Cell Physiol. 2011, 300, C138–C145. [Google Scholar] [CrossRef] [Green Version]
- Romanello, V.; Sandri, M. Mitochondrial quality control and muscle mass maintenance. Front. Physiol. 2016, 6, 422. [Google Scholar] [CrossRef] [PubMed]
- Chabi, B.; Ljubicic, V.; Menzies, K.J.; Huang, J.H.; Saleem, A.; Hood, D.A. Mitochondrial function and apoptotic susceptibility in aging skeletal muscle. Aging Cell 2008, 7, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, V.; Tuli, H.S.; Varol, A.; Thakral, F.; Yerer, M.B.; Sak, K.; Varol, M.; Jain, A.; Khan, M.A.; Sethi, G. Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancement. Biomolecules 2019, 9, 735. [Google Scholar] [CrossRef] [Green Version]
- Brookes, P.S. Mitochondrial H+ leak and ROS generation: An odd couple. Free Radic. Biol. Med. 2005, 38, 12–23. [Google Scholar] [CrossRef]
- Harper, M.E.; Monemdjou, S.; Ramsey, J.J.; Weindruch, R. Age-related increase in mitochondrial proton leak and decrease in ATP turnover reactions in mouse hepatocytes. Am. J. Physiol. 1998, 275, E197–E206. [Google Scholar] [CrossRef]
- Sohal, R.S.; Sohal, B.H. Hydrogen peroxide release by mitochondria increases during aging. Mech. Ageing Dev. 1991, 57, 187–202. [Google Scholar] [CrossRef]
- Sohal, R.S.; Ku, H.H.; Agarwal, S.; Forster, M.J.; Lal, H. Oxidative damage, mitochondrial oxidant generation and antioxidant defenses during aging and in response to food restriction in the mouse. Mech. Ageing Dev. 1994, 74, 121–133. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Chandel, N.S. Mitochondrial reactive oxygen species regulate cellular signalling and dictate biological outcomes. Trends Biochem. Sci. 2010, 35, 505–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cadenas, S. Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta Bioenerg. 1859, 2018, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S. Generating, partitioning, targeting and functioning of superoxide in mitochondria. Biosci. Rep. 1997, 17, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, M.K.; Turner, N. Mitochondrial dysfunction and insulin resistance: An update. Endocr. Connect. 2015, 4, R1–R15. [Google Scholar] [CrossRef] [Green Version]
- Thirupathi, A.; de Souza, C.T. Multi-regulatory network of ROS: The interconnection of ROS, PGC-1 alpha, and AMPK-SIRT1 during exercise. J. Physiol. Biochem. 2017, 73, 487–494. [Google Scholar] [CrossRef]
- Garatachea, N.; Helios, P.G.; Sanchis-Gomar, F.; Santos-Lozano, A.; Fiuza-Luces, A.; Morà, M.; Emanuele, E.; Joyner, M.; Lucia, A. Exercise Attenuates the Major Hallmarks of Aging. Rejuvenation Res. 2015, 18, 57–89. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.H.; Mouly, V.; Cooper, R.N.; Mamchaoui, K.; Bigot, A.; Shay, J.W.; Wright, W.E. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: Consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell 2007, 6, 515–523. [Google Scholar] [CrossRef]
- Omodei, D.; Pucino, V.; Labruna, G.; Procaccini, C.; Galgani, M.; Perna, F.; Pirozzi, D.; De Caprio, C.; Marone, G.; Fontana, L.; et al. Immune-metabolic profiling of anorexic patients reveals an anti-oxidant and anti-inflammatory phenotype. Metabolism 2015, 64, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, L.; Nardelli, C.; D’Alessio, F.; D’Argenio, V.; Nunziato, M.; Mauriello, L.; Procaccini, C.; Maruotti, G.M.; Martinelli, P.; Matarese, G.; et al. Altered Bioenergetic Profile in Umbilical Cord and Amniotic Mesenchymal Stem Cells from Newborns of Obese Women. Stem Cells Dev. 2018, 27, 199–206. [Google Scholar] [CrossRef] [PubMed]
| Sport | Age (y) | Height (m) | Weight (kg) | BMI (kg/m2) | IGF-1 * ng/mL | Glucose mg/dL | LDH U/L | Hemoglobin (Hb) g/dL |
| 18.5–24.9 kg/m2 | 116–358 ng/mL | 60–110 mg/dL | 227–450 U/L | 13–17.5 g/dL | ||||
| SO | 22.8 ± 1.9 | 1.8 ± 0.1 | 71.2 ± 6.4 | 23.0 ± 1.6 | 230.7 ± 117.6 | 87.8 ± 11.6 | 380.4 ± 36.5 | 16.2 ± 0.9 |
| VB | 24.3 ± 1.0 | 1.8 ± 0.0 | 72.3 ± 5.0 | 23.0 ± 1.3 | 241.0 ± 92.9 | 84.0 ± 18.7 | 372.5 ± 38.1 | 15.9 ± 0.6 |
| SW | 22.7 ± 3.9 | 1.7 ± 0.0 | 69.2 ± 4.9 | 22.9 ± 1.3 | 190.2 ± 50.2 | 80.2 ± 10.5 | 400.3 ± 21.9 | 13.9 ± 1.7 |
| BB | 25.7 ± 2.4 | 1.8 ± 0 | 73.3 ± 7.2 | 23.5 ± 1.3 | 227.0 ± 39.3 | 79.5 ± 14.9 | 431.5 ± 21.7 | 15.2 ± 0.8 |
| Total Cholesterol mg/dL | HDL Cholesterol mg/dL | Triglycerides mg/dL | AST U/L | ALT U/L | GGT U/L | Sideremia µg/dL | Hematocrit (Ht) % | |
| 100–200 mg/dL | >35 mg/dL | <150 mg/dL | 1–40 U/L | 1–40 U/L | 1–50 U/L | 53–167 μg/dL | 42.0–50.0% | |
| SO | 177.3 ± 37.5 | 48.4 ± 9.6 | 127.8 ± 57.1 | 29.6 ± 10.2 | 32.0 ± 1.0 | 35.8 ± 17.5 | 125.6 ± 31.0 | 48.9 ± 2.3 |
| VB | 143.0 ± 24.5 | 49.0 ± 3.7 | 128.0 ± 81.6 | 21.0 ± 2.4 | 28.7 ± 11.2 | 33.3 ± 15.4 | 90.0 ± 22.0 | 48.8 ± 1.6 |
| SW | 166.2 ± 4.1 | 55.5 ± 12.3 | 82.5 ± 46.3 | 22.7 ± 5.9 | 23.2 ± 11.6 | 30.2 ± 19.4 | 89.5 ± 44.5 | 43.0 ± 4.1 |
| BB | 184.7 ± 25.5 | 50.7 ± 17 | 87.7 ± 20.8 | 30.2 ± 6.6 | 31.2 ± 4.0 | 13.8 ± 2.9 | 126.7 ± 58.2 | 45.2 ± 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, A.; Vitucci, D.; Labruna, G.; Orrù, S.; Buono, P. Effects of Different Types of Chronic Training on Bioenergetic Profile and Reactive Oxygen Species Production in LHCN-M2 Human Myoblast Cells. Int. J. Mol. Sci. 2022, 23, 7491. https://doi.org/10.3390/ijms23147491
Mancini A, Vitucci D, Labruna G, Orrù S, Buono P. Effects of Different Types of Chronic Training on Bioenergetic Profile and Reactive Oxygen Species Production in LHCN-M2 Human Myoblast Cells. International Journal of Molecular Sciences. 2022; 23(14):7491. https://doi.org/10.3390/ijms23147491
Chicago/Turabian StyleMancini, Annamaria, Daniela Vitucci, Giuseppe Labruna, Stefania Orrù, and Pasqualina Buono. 2022. "Effects of Different Types of Chronic Training on Bioenergetic Profile and Reactive Oxygen Species Production in LHCN-M2 Human Myoblast Cells" International Journal of Molecular Sciences 23, no. 14: 7491. https://doi.org/10.3390/ijms23147491
APA StyleMancini, A., Vitucci, D., Labruna, G., Orrù, S., & Buono, P. (2022). Effects of Different Types of Chronic Training on Bioenergetic Profile and Reactive Oxygen Species Production in LHCN-M2 Human Myoblast Cells. International Journal of Molecular Sciences, 23(14), 7491. https://doi.org/10.3390/ijms23147491








