Multipotential Role of Growth Factor Mimetic Peptides for Osteochondral Tissue Engineering
Abstract
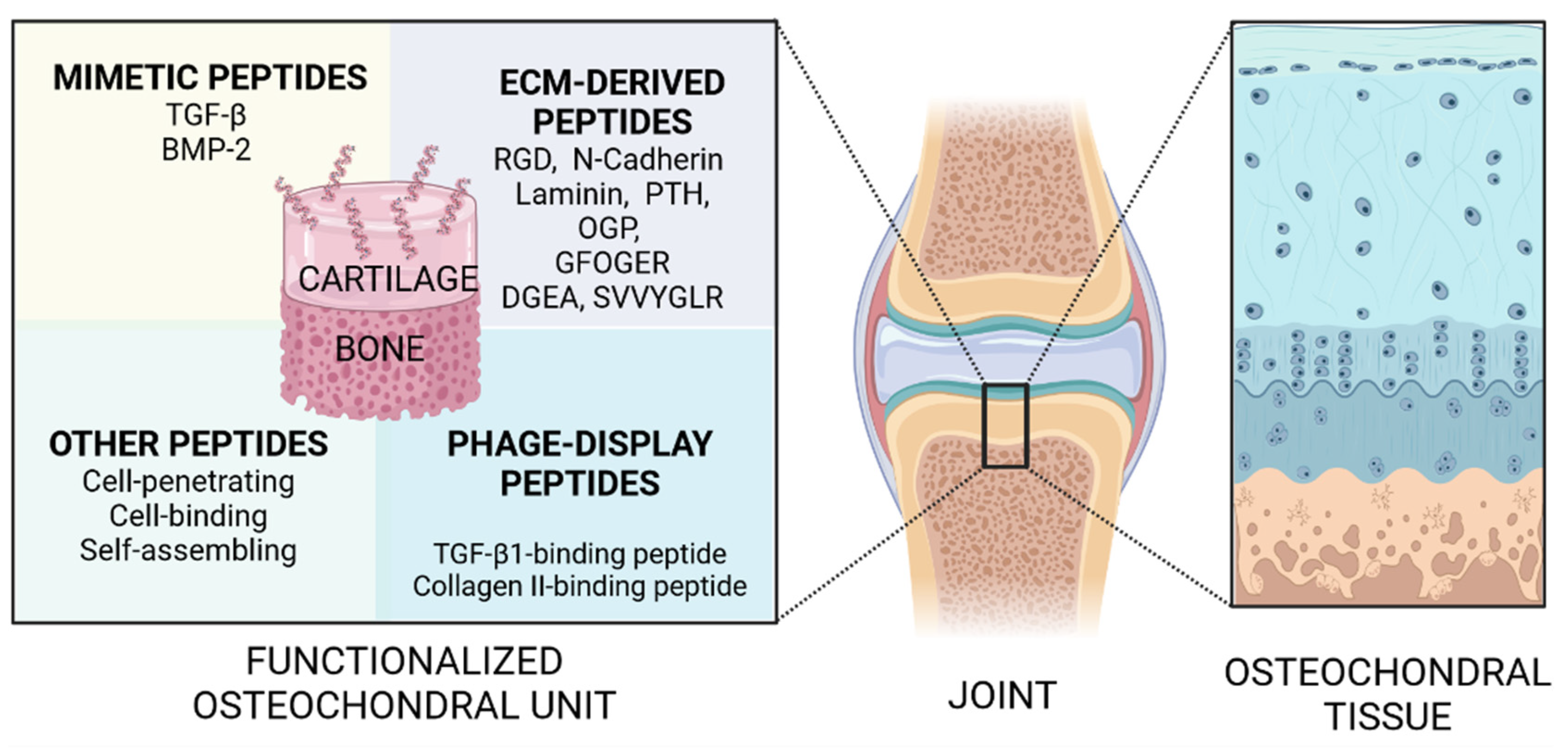
1. Introduction
2. Growth Factor-Mimetic Peptides for Osteochondral Regeneration
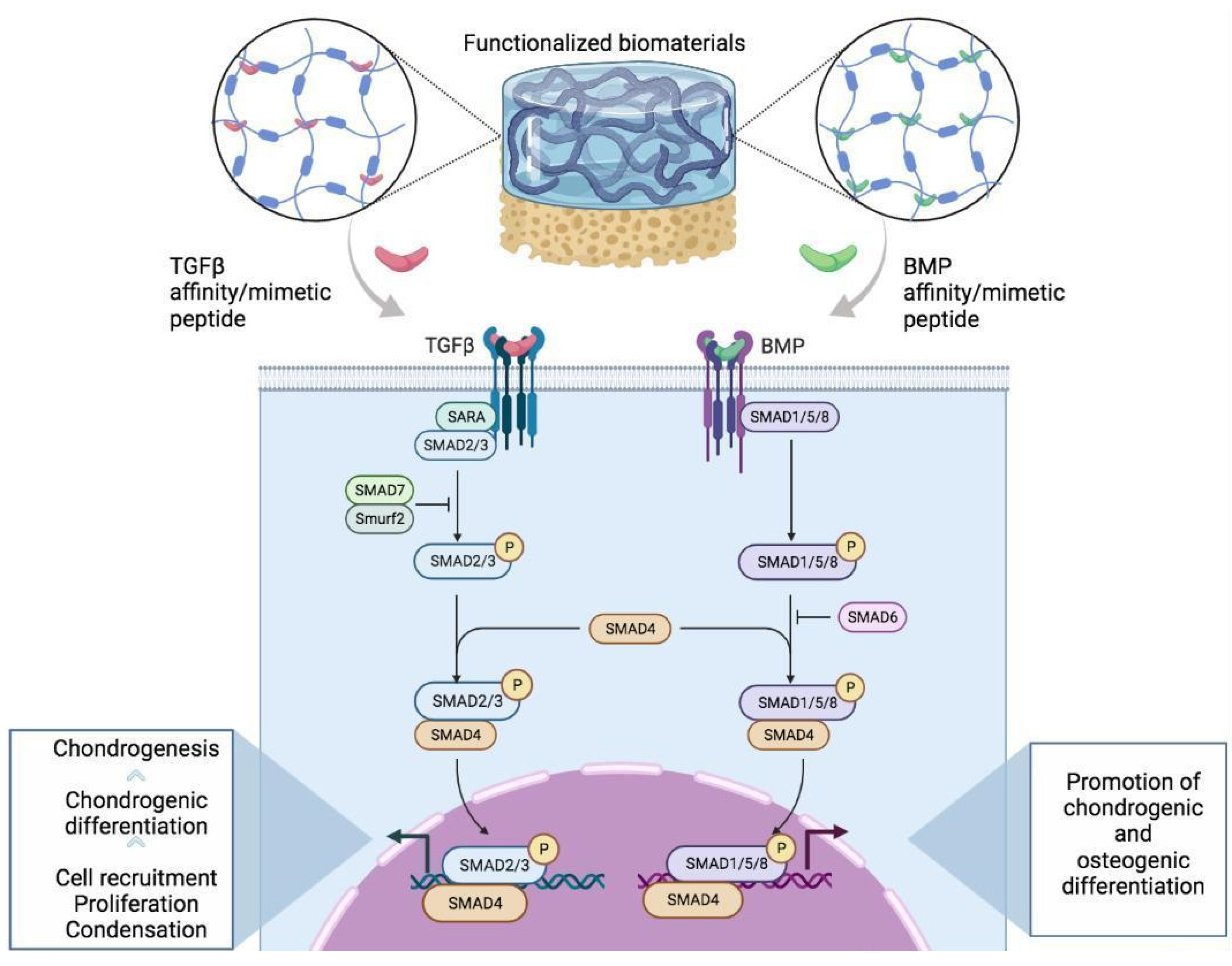
2.1. Transforming Growth Factor-β Mimetic Peptides
2.2. Bone Morphogenetic Protein 2
2.3. Cell-Penetrating Peptides
2.4. Cell-Binding Peptides
2.5. Self-Assembling Peptides
2.6. RGD Sequence Peptides
2.7. N-Cadherin Mimetic Peptides
2.8. Laminin-Derived Peptides
2.9. Parathyroid Hormone 1–34 Peptide (Teriparatide)
2.10. Osteogenic Growth Peptide
2.11. Other ECM-Derived Peptides
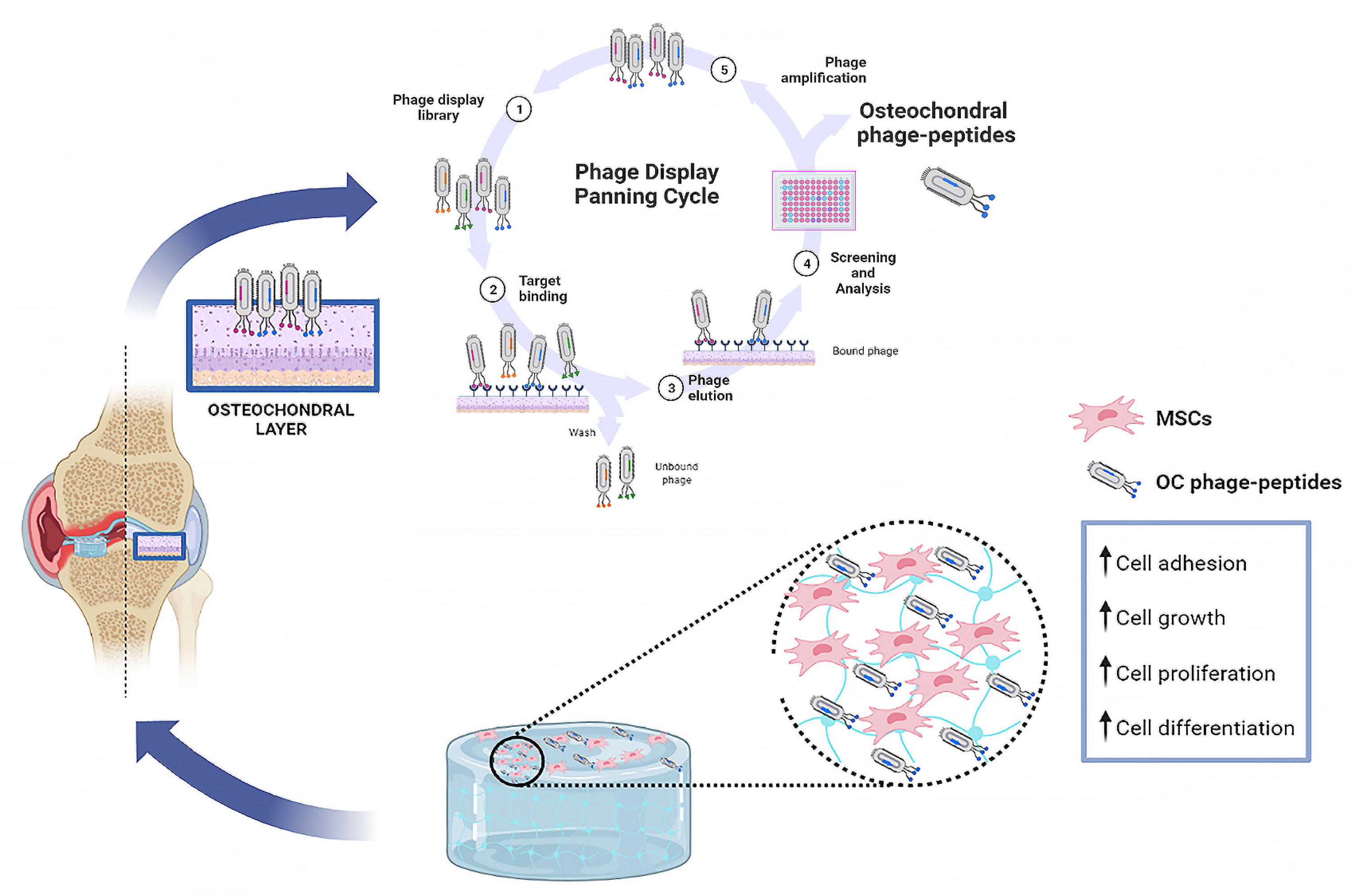
3. Phage Display Functional Peptides
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Hunzinker, E.; Rosenberg, L. Articular cartilage: Composition and structure. In Injury and Repair of the Musculoskeletal Soft Tissues; Woo, S.L.Y., Buckwalter, J.A., Eds.; American Academy of Orthopaedic Surgeons: Park Ridge, IL, USA, 1988; pp. 405–425. [Google Scholar]
- Buckwalter, J.A.; Mankin, H.J. Articular cartilage: Tissue design and chondrocyte-matrix interactions. Instr. Course Lect. 1998, 47, 477–486. [Google Scholar] [PubMed]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; Stoddart, M.; D’Amora, U.; Ambrosio, L.; Alini, M.; Musumeci, G. Mesenchymal Stem Cell-Based Cartilage Regeneration Approach and Cell Senescence: Can We Manipulate Cell Aging and Function? Tissue Eng. Part B Rev. 2017, 23, 529–539. [Google Scholar] [CrossRef]
- Liu, Y.; Shah, K.M.; Luo, J. Strategies for Articular Cartilage Repair and Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 770655. [Google Scholar] [CrossRef]
- Calabrese, G.; Petralia, S.; Fabbi, C.; Forte, S.; Franco, D.; Guglielmino, S.; Esposito, E.; Cuzzocrea, S.; Traina, F.; Conoci, S. Au, Pd and maghemite nanofunctionalized hydroxyapatite scaffolds for bone regeneration. Regen. Biomater. 2020, 7, 461–469. [Google Scholar] [CrossRef]
- Calabrese, G.; Petralia, S.; Franco, D.; Nocito, G.; Fabbi, C.; Forte, L.; Guglielmino, S.; Squarzoni, S.; Traina, F.; Conoci, S. A new Ag-nanostructured hydroxyapatite porous scaffold: Antibacterial effect and cytotoxicity study. Mater. Sci. Eng. C 2020, 118, 111394. [Google Scholar] [CrossRef]
- Franco, D.; Calabrese, G.; Petralia, S.; Neri, G.; Corsaro, C.; Forte, L.; Squarzoni, S.; Guglielmino, S.; Traina, F.; Fazio, E.; et al. Antimicrobial Effect and Cytotoxic Evaluation of Mg-Doped Hydroxyapatite Functionalized with Au-Nano Rods. Molecules 2021, 26, 1099. [Google Scholar] [CrossRef]
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar]
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Gudas, R.; Gudaitė, A.; Mickevičius, T.; Masiulis, N.; Simonaitytė, R.; Čekanauskas, E.; Skurvydas, A. Comparison of Osteochondral Autologous Transplantation, Microfracture, or Debridement Techniques in Articular Cartilage Lesions Associated with Anterior Cruciate Ligament Injury: A Prospective Study With a 3-Year Follow-up. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Keyhani, S.; Lee, E.H.; Hui, J.H.P. Evidence-Based Status of Microfracture Technique: A Systematic Review of Level I and II Studies. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Moreira-Teixeira, L.S.; Georgi, N.; Leijten, J.; Wu, L.; Karperien, M. Cartilage and Bone Development and Its Disorders. Endocr. Dev. 2011, 21, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, G.; Forte, S.; Gulino, R.; Cefalì, F.; Figallo, E.; Salvatorelli, L.; Maniscalchi, E.T.; Angelico, G.; Parenti, R.; Gulisano, M.; et al. Combination of Collagen-Based Scaffold and Bioactive Factors Induces Adipose-Derived Mesenchymal Stem Cells Chondrogenic Differentiation In vitro. Front. Physiol. 2017, 8, 50. [Google Scholar] [CrossRef]
- Calabrese, G.; Gulino, R.; Giuffrida, R.; Forte, S.; Figallo, E.; Fabbi, C.; Salvatorelli, L.; Memeo, L.; Gulisano, M.; Parenti, R. In Vivo Evaluation of Biocompatibility and Chondrogenic Potential of a Cell-Free Collagen-Based Scaffold. Front. Physiol. 2017, 8, 984. [Google Scholar] [CrossRef]
- Raucci, M.G.; D’Amora, U.; Ronca, A.; Ambrosio, L. Injectable Functional Biomaterials for Minimally Invasive Surgery. Adv. Health Mater. 2020, 9, e2000349. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Castrogiovanni, P.; Nsir, H.; Di Rosa, M.; Guglielmino, C.; Parenti, R.; Calabrese, G.; Pricoco, E.; Salvatorelli, L.; Magro, G.; et al. Engineered cartilage regeneration from adipose tissue derived-mesenchymal stem cells: A morphomolecular study on osteoblast, chondrocyte and apoptosis evaluation. Exp. Cell Res. 2017, 357, 222–235. [Google Scholar] [CrossRef]
- Szychlinska, M.A.; Calabrese, G.; Ravalli, S.; Dolcimascolo, A.; Castrogiovanni, P.; Fabbi, C.; Puglisi, C.; Lauretta, G.; Di Rosa, M.; Castorina, A.; et al. Evaluation of a Cell-Free Collagen Type I-Based Scaffold for Articular Cartilage Regeneration in an Orthotopic Rat Model. Materials 2020, 13, 2369. [Google Scholar] [CrossRef]
- Ma, P.X. Biomimetic materials for tissue engineering. Adv. Drug Deliv. Rev. 2008, 60, 184–198. [Google Scholar] [CrossRef]
- Lu, J.; Wang, X. Biomimetic Self-Assembling Peptide Hydrogels for Tissue Engineering Applications. Adv Exp Med Biol. 2018, 1064, 297–312. [Google Scholar] [CrossRef]
- Raucci, M.G.; D’Amora, U.; Ronca, A.; Demitri, C.; Ambrosio, L. Bioactivation Routes of Gelatin-Based Scaffolds to Enhance at Nanoscale Level Bone Tissue Regeneration. Front. Bioeng. Biotechnol. 2019, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.R.M.; Sidhu, S.; Dübel, S.; McCafferty, J. Beyond natural antibodies: The power of in vitro display technologies. Nat. Biotechnol. 2011, 29, 245–254. [Google Scholar] [CrossRef] [PubMed]
- De Plano, L.M.; Carnazza, S.; Franco, D.; Rizzo, M.G.; Conoci, S.; Petralia, S.; Nicoletti, A.; Zappia, M.; Campolo, M.; Esposito, E.; et al. Innovative IgG Biomarkers Based on Phage Display Microbial Amyloid Mimotope for State and Stage Diagnosis in Alzheimer’s Disease. ACS Chem. Neurosci. 2020, 11, 1013–1026. [Google Scholar] [CrossRef]
- Pande, J.; Szewczyk, M.M.; Grover, A.K. Phage display: Concept, innovations, applications and future. Biotechnol. Adv. 2010, 28, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; D’Amora, U.; Ravalli, S.; Ambrosio, L.; Di Rosa, M.; Musumeci, G. Functional Biomolecule Delivery Systems and Bioengineering in Cartilage Regeneration. Curr. Pharm. Biotechnol. 2019, 20, 32–46. [Google Scholar] [CrossRef]
- Pérez, C.M.R.; Stephanopoulos, N.; Sur, S.; Lee, S.S.; Newcomb, C.; Stupp, S.I. The Powerful Functions of Peptide-Based Bioactive Matrices for Regenerative Medicine. Ann. Biomed. Eng. 2014, 43, 501–514. [Google Scholar] [CrossRef]
- Camarero-Espinosa, S.; Cooper-White, J.J. Combinatorial presentation of cartilage-inspired peptides on nanopatterned surfaces enables directed differentiation of human mesenchymal stem cells towards distinct articular chondrogenic phenotypes. Biomaterials 2019, 210, 105–115. [Google Scholar] [CrossRef]
- Atia, A.J.; Azumah, A.D.; Deepa, B.; Dean, D. Tuning Phage for Cartilage Regeneration. In Acteriophages in Thera-Peutics; Intechopen: London, UK, 2021. [Google Scholar] [CrossRef]
- Liu, Q.; Jia, Z.; Duan, L.; Xiong, J.; Wang, D.; Ding, Y. Functional peptides for cartilage repair and re-generation. Am. J. Transl. Res. 2018, 10, 501–510. [Google Scholar]
- Timin, A.S.; Muslimov, A.R.; Zyuzin, M.V.; Peltek, O.O.; Karpov, T.E.; Sergeev, I.S.; Dotsenko, A.I.; Goncharenko, A.A.; Yolshin, N.D.; Sinelnik, A.; et al. Multifunctional Scaffolds with Improved Antimicrobial Properties and Osteogenicity Based on Piezoelectric Electrospun Fibers Decorated with Bioactive Composite Microcapsules. ACS Appl. Mater. Interfaces 2018, 10, 34849–34868. [Google Scholar] [CrossRef]
- Briquez, P.S.; Hubbell, J.A.; Martino, M.M. Extracellular Matrix-Inspired Growth Factor Delivery Systems for Skin Wound Healing. Adv. Wound Care 2015, 4, 479–489. [Google Scholar] [CrossRef]
- Sluzalska, K.D.; Sławski, J.; Sochacka, M.; Lampart, A.; Otlewski, J.; Zakrzewska, M. Intracellular partners of fibroblast growth factors 1 and 2—Implications for functions. Cytokine Growth Factor Rev. 2020, 57, 93–111. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhao, J.; Brochmann, E.J.; Wang, J.C.; Murray, S.S. Bone morphogenetic protein-2 and tumor growth: Diverse effects and possibilities for therapy. Cytokine Growth Factor Rev. 2017, 34, 73–91. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, H.S.; Pashkuleva, I. Biomimetic supramolecular designs for the controlled release of growth factors in bone regeneration. Adv. Drug Deliv. Rev. 2015, 94, 63–76. [Google Scholar] [CrossRef]
- Lawrence, D.A. Latent-TGF-β: An overview. Mol. Cell. Biochem. 2001, 219, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Li, S.; Chen, D. TGF-β signaling and the development of osteoarthritis. Bone Res. 2014, 2, 14002. [Google Scholar] [CrossRef]
- Tian-Fang, L.; Li, T.-F.; O’Keefe, R.J.; Chen, D. TGF-b signaling in chondrocytes. Front. Biosci. 2005, 10, 681–688. [Google Scholar] [CrossRef]
- Lam, H.; Li, S.; Lou, N.; Chu, J.; Bhatnagar, R. Synthetic peptides cytomodulin-1 (CM-1) and cytomodulin-2 (CM-2) promote collagen synthesis and wound healing in vitro. In Proceedings of the 26th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Francisco, CA, USA, 1–5 September 2004. [Google Scholar] [CrossRef]
- Mittal, A.; Kumar, R.; Parsad, D.; Kumar, N. Cytomodulin-functionalized porous PLGA particulate scaffolds respond better to cell migration, actin production and wound healing in rodent model. J. Tissue Eng. Regen. Med. 2012, 8, 351–363. [Google Scholar] [CrossRef]
- Renner, J.N.; Liu, J.C. Investigating the effect of peptide agonists on the chondrogenic differentiation of human mesenchymal stem cells using design of experiments. Biotechnol. Prog. 2013, 29, 1550–1557. [Google Scholar] [CrossRef]
- Zhang, Z.; Gupte, M.J.; Jin, X.; Ma, P.X. Injectable Peptide Decorated Functional Nanofibrous Hollow Microspheres to Direct Stem Cell Differentiation and Tissue Regeneration. Adv. Funct. Mater. 2014, 25, 350–360. [Google Scholar] [CrossRef]
- Shah, R.N.; Shah, N.A.; Lim, M.M.D.R.; Hsieh, C.; Nuber, G.; Stupp, S.I. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc. Natl. Acad. Sci. USA 2010, 107, 3293–3298. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, B.; Yang, J.; Heng, B.C.; Yang, Z.; Ge, Z.; Lin, J. TGF-β1 affinity peptides incorporated within a chitosan sponge scaffold can significantly enhance cartilage regeneration. J. Mater. Chem. B 2018, 6, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Gao, J.; Yu, X.; Shi, J.; Chen, J.; Yu, L.; Chen, S.; Ding, J. 3D-Printed Porous Scaffolds of Hydrogels Modified with TGF-β1 Binding Peptides to Promote In Vivo Cartilage Regeneration and Animal Gait Restoration. ACS Appl. Mater. Interfaces 2022, 14, 15982–15995. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Liu, X.; Zhang, Y.; Chen, X.; Chen, M.; Shen, H.; Feng, Y.; Liu, J.; Wang, M.; Shi, Q. A photo-crosslinked proteinogenic hydrogel enabling self-recruitment of endogenous TGF-β1 for cartilage regeneration. Smart Mater. Med. 2021, 3, 85–93. [Google Scholar] [CrossRef]
- Wang, C.; Yue, H.; Huang, W.; Lin, X.; Xie, X.; He, Z.; He, X.; Liu, S.; Bai, L.; Lu, B.; et al. Cryogenic 3D printing of heterogeneous scaffolds with gradient mechanical strengths and spatial delivery of osteogenic peptide/TGF-β1 for osteochondral tissue regeneration. Biofabrication 2020, 12, 025030. [Google Scholar] [CrossRef]
- Mahzoon, S.; Townsend, J.M.; Lam, T.N.; Sjoelund, V.; Detamore, M.S. Effects of a Bioactive SPPEPS Peptide on Chondrogenic Differentiation of Mesenchymal Stem Cells. Ann. Biomed. Eng. 2019, 47, 2308–2321. [Google Scholar] [CrossRef] [PubMed]
- Shu, B.; Zhang, M.; Xie, R.; Wang, M.; Jin, H.; Hou, W.; Tang, D.; Harris, S.E.; Mishina, Y.; O’Keefe, R.J.; et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J. Cell Sci. 2011, 124, 3428–3440. [Google Scholar] [CrossRef]
- Zhou, N.; Li, Q.; Lin, X.; Hu, N.; Liao, J.-Y.; Lin, L.-B.; Zhao, C.; Hu, Z.-M.; Liang, X.; Xu, W.; et al. BMP2 induces chondrogenic differentiation, osteogenic differentiation and endochondral ossification in stem cells. Cell Tissue Res. 2016, 366, 101–111. [Google Scholar] [CrossRef]
- Aruwajoye, O.O.; Aswath, P.B.; Kim, H.K.W. Material properties of bone in the femoral head treated with ibandronate and BMP-2 following ischemic osteonecrosis. J. Orthop. Res. 2016, 35, 1453–1460. [Google Scholar] [CrossRef][Green Version]
- Rahim, I.; Salt, S.; Heliotis, M. Successful long-term mandibular reconstruction and rehabilitation using non-vascularised autologous bone graft and recombinant human BMP-7 with subsequent endosseous implant in a patient with bisphosphonate-related osteonecrosis of the jaw. Br. J. Oral Maxillofac. Surg. 2015, 53, 870–874. [Google Scholar] [CrossRef]
- Wang, W.; Liu, L.; Dang, X.; Ma, S.; Zhang, M.; Wang, K. The effect of core decompression on local expression of BMP-2, PPAR-γ and bone regeneration in the steroid-induced femoral head osteonecrosis. BMC Musculoskelet. Disord. 2012, 13, 142. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-β family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Nohe, A.; Hassel, S.; Ehrlich, M.; Neubauer, F.; Sebald, W.; Henis, Y.I.; Knaus, P. The Mode of Bone Morphogenetic Protein (BMP) Receptor Oligomerization Determines Different BMP-2 Signaling Pathways. J. Biol. Chem. 2002, 277, 5330–5338. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhong, W.; Cao, W.; Zhang, Q.; Wu, G. Chondroinductive/chondroconductive peptides and their-functionalized biomaterials for cartilage tissue engineering. Bioact. Mater. 2021, 9, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Angelozzi, M.; Haseeb, A. SOX9 in cartilage development and disease. Curr. Opin. Cell Biol. 2019, 61, 39–47. [Google Scholar] [CrossRef]
- Bragdon, B.; Thinakaran, S.; Moseychuk, O.; King, D.; Young, K.; Litchfield, D.W.; Petersen, N.O.; Nohe, A. Casein Kinase 2 β-Subunit Is a Regulator of Bone Morphogenetic Protein 2 Signaling. Biophys. J. 2010, 99, 897–904. [Google Scholar] [CrossRef]
- Akkiraju, H.; Bonor, J.; Nohe, A. CK2.1, a novel peptide, induces articular cartilage formation in vivo. J. Orthop. Res. 2016, 35, 876–885. [Google Scholar] [CrossRef]
- Akkiraju, H.; Srinivasan, P.P.; Xu, X.; Jia, X.; Safran, C.B.K.; Nohe, A. CK2.1, a bone morphogenetic protein receptor type Ia mimetic peptide, repairs cartilage in mice with destabilized medial meniscus. Stem Cell Res. Ther. 2017, 8, 82. [Google Scholar] [CrossRef]
- Lee, J.; Perikamana, S.K.M.; Ahmad, T.; Lee, M.S.; Yang, H.S.; Kim, D.-G.; Kim, K.; Kwon, B.; Shin, H. Controlled Retention of BMP-2-Derived Peptide on Nanofibers Based on Mussel-Inspired Adhesion for Bone Formation. Tissue Eng. Part A 2017, 23, 323–334. [Google Scholar] [CrossRef]
- Saito, A.; Suzuki, Y.; Ogata, S.; Ohtsuki, C.; Tanihara, M. Activation of osteo-progenitor cells by a novel synthetic peptide derived from the bone morphogenetic protein-2 knuckle epitope. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2003, 1651, 60–67. [Google Scholar] [CrossRef]
- Zouani, O.F.; Chollet, C.; Guillotin, B.; Durrieu, M.-C. Differentiation of pre-osteoblast cells on poly(ethylene terephthalate) grafted with RGD and/or BMPs mimetic peptides. Biomaterials 2010, 31, 8245–8253. [Google Scholar] [CrossRef]
- Seol, Y.-J.; Park, Y.-J.; Lee, S.-C.; Kim, K.-H.; Lee, J.-Y.; Kim, T.-I.; Lee, Y.-M.; Ku, Y.; Rhyu, I.-C.; Han, S.-B.; et al. Enhanced osteogenic promotion around dental implants with synthetic binding motif mimicking bone morphogenetic protein (BMP)-2. J. Biomed. Mater. Res. Part A 2006, 77A, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Renner, J.; Kim, Y.; Liu, J. Bone Morphogenetic Protein-Derived Peptide Promotes Chondrogenic Differentiation of Human Mesenchymal Stem Cells. Tissue Eng. Part A 2012, 18, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zamora, P.O.; Albright, S.; Glass, J.D.; Peña, L.A. Multidomain Synthetic Peptide B2A2 Synergistically Enhances BMP-2 In Vitro. J. Bone Miner. Res. 2004, 20, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Boda, S.K.; Almoshari, Y.; Wang, H.; Wang, X.; Reinhardt, R.A.; Duan, B.; Wang, D.; Xie, J. Mineralized nanofiber segments coupled with calcium-binding BMP-2 peptides for alveolar bone regeneration. Acta Biomater. 2018, 85, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Boda, S.K.; Wang, H.; John, J.V.; Reinhardt, R.A.; Xie, J. Dual Delivery of Alendronate and E7-BMP-2 Peptide via Calcium Chelation to Mineralized Nanofiber Fragments for Alveolar Bone Regeneration. ACS Biomater. Sci. Eng. 2020, 6, 2368–2375. [Google Scholar] [CrossRef]
- Weng, L.; Boda, S.K.; Wang, H.; Teusink, M.J.; Shuler, F.D.; Xie, J. Novel 3D Hybrid Nanofiber Aerogels Coupled with BMP-2 Peptides for Cranial Bone Regeneration. Adv. Health Mater. 2018, 7, e1701415. [Google Scholar] [CrossRef]
- Toprak, Ö.; Topuz, B.; Monsef, Y.A.; Oto, Ç.; Orhan, K.; Karakeçili, A. BMP-6 carrying metal organic framework-embedded in bioresorbable electrospun fibers for enhanced bone regeneration. Mater. Sci. Eng. C 2020, 120, 111738. [Google Scholar] [CrossRef]
- Laboy-López, S.; Fernández, P.O.M.; Padilla-Zayas, J.G.; Nicolau, E. Bioactive Cellulose Acetate Electrospun Mats as Scaffolds for Bone Tissue Regeneration. Int. J. Biomater. 2022, 2022, 3255039. [Google Scholar] [CrossRef]
- Sun, H.; Dong, J.; Wang, Y.; Shen, S.; Shi, Y.; Zhang, L.; Zhao, J.; Sun, X.; Jiang, Q. Polydopamine-Coated Poly(l-lactide) Nanofibers with Controlled Release of VEGF and BMP-2 as a Regenerative Periosteum. ACS Biomater. Sci. Eng. 2021, 7, 4883–4897. [Google Scholar] [CrossRef]
- Karagiannis, E.D.; Urbanska, A.M.; Sahay, G.; Pelet, J.M.; Jhunjhunwala, S.; Langer, R.; Anderson, D.G. Rational Design of a Biomimetic Cell Penetrating Peptide Library. ACS Nano 2013, 7, 8616–8626. [Google Scholar] [CrossRef][Green Version]
- Saraf, A.; Mikos, A.G. Gene delivery strategies for cartilage tissue engineering. Adv. Drug Deliv. Rev. 2006, 58, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chu, X.; Li, W.; Pan, Q.; You, H. Chondrogenic effect of precartilaginous stem cells following NLS-TAT cell penetrating peptide-assisted transfection of eukaryotic hTGFβ3. J. Cell. Biochem. 2013, 114, 2588–2594. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Li, W.; Yuan, X.; Rakhmanov, Y.; Wang, P.; Lu, R.; Mao, Z.; Shang, X.; You, H. Chondrogenic effect of cell-based scaffold of self-assembling peptides/PLGA-PLL loading the hTGFβ3 plasmid DNA. J. Mater. Sci. Mater. Electron. 2015, 27, 19. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Song, H.; Park, S.G.; Lee, S.; Ko, J.; Park, J.; Jeong, J.; Cheon, Y.; Lee, D.R. Regulation of Differentiation Potential of Human Mesenchymal Stem Cells by Intracytoplasmic Delivery of Coactivator-Associated Arginine Methyltransferase 1 Protein Using Cell-Penetrating Peptide. Stem Cells 2012, 30, 1703–1713. [Google Scholar] [CrossRef]
- Pountos, I.; Panteli, M.; Lampropoulos, A.; Jones, E.; Calori, G.M.; Giannoudis, P.V. The role of peptides in bone healing and regeneration: A systematic review. BMC Med. 2016, 14, 103. [Google Scholar] [CrossRef]
- Bhatnagar, R.S.; Qian, J.J.; Wedrychowska, A.; Sadeghi, M.; Wu, Y.M.; Smith, N. Design of Biomimetic Habitats for Tissue Engineering with P-15, a Synthetic Peptide Analogue of Collagen. Tissue Eng. 1999, 5, 53–65. [Google Scholar] [CrossRef]
- Yang, X.B.; Bhatnagar, R.S.; Li, S.; Oreffo, R. Biomimetic Collagen Scaffolds for Human Bone Cell Growth and Differentiation. Tissue Eng. 2004, 10, 1148–1159. [Google Scholar] [CrossRef]
- Kübler, A.; Neugebauer, J.; Oh, J.-H.; Scheer, M.; Zöller, J.E. Growth and Proliferation of Human Osteoblasts on Different Bone Graft Substitutes An In Vitro Study. Implant Dent. 2004, 13, 171–179. [Google Scholar] [CrossRef]
- Trasatti, C.; Spears, R.; Gutmann, J.L.; Opperman, L.A. Increased Tgf-β1 Production by Rat Osteoblasts in the Presence of PepGen P-15 in Vitro. J. Endod. 2004, 30, 213–217. [Google Scholar] [CrossRef]
- Liu, Q.; Limthongkul, W.; Sidhu, G.; Zhang, J.; Vaccaro, A.; Shenck, R.; Hickok, N.; Shapiro, I.; Freeman, T. Covalent attachment of P15 peptide to titanium surfaces enhances cell attachment, spreading, and osteogenic gene expression. J. Orthop. Res. 2012, 30, 1626–1633. [Google Scholar] [CrossRef]
- Vordemvenne, T.; Paletta, J.R.; Hartensuer, R.; Pap, T.; Raschke, M.J.; Ochman, S. Cooperative effects in differentiation and proliferation between PDGF-BB and matrix derived synthetic peptides in human osteoblasts. BMC Musculoskelet. Disord. 2011, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Matos, S.; Guerra, F.; Krauser, J.T.; Figueiredo, H.; Marcelino, J.P.; Sanz, M. Evaluation of an anorganic bovine-derived mineral with P-15 hydrogel bone graft: Preliminary study in a rabbit cranial bone model. Clin. Oral Implant. Res. 2011, 23, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Mardas, N.; Stavropoulos, A.; Karring, T. Calvarial bone regeneration by a combination of natural anorganic bovine-derived hydroxyapatite matrix coupled with a synthetic cell-binding peptide (PepGen™): An experimental study in rats. Clin. Oral Implant. Res. 2008, 19, 1010–1015. [Google Scholar] [CrossRef] [PubMed]
- Sarahrudi, K.; Mousavi, M.; Grossschmidt, K.; Sela, N.; König, F.; Vécsei, V.; Aharinejad, S. Combination of anorganic bovine-derived hydroxyapatite with binding peptide does not enhance bone healing in a critical-size defect in a rabbit model. J. Orthop. Res. 2008, 26, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Thorwarth, M.; Schultze-Mosgau, S.; Wehrhan, F.; Srour, S.; Wiltfang, J.; Neukam, F.W.; Schlegel, K.A. Enhanced bone regeneration with a synthetic cell-binding peptide—in vivo results. Biochem. Biophys. Res. Commun. 2005, 329, 789–795. [Google Scholar] [CrossRef]
- Thorwarth, M.; Schultze-Mosgau, S.; Wehrhan, F.; Kessler, P.; Srour, S.; Wiltfang, J.; Schlegel, K.A. Bioactivation of an anorganic bone matrix by P-15 peptide for the promotion of early bone formation. Biomaterials 2005, 26, 5648–5657. [Google Scholar] [CrossRef]
- Artzi, Z.; Weinreb, M.; Tal, H.; Nemcovsky, C.E.; Rohrer, M.D.; Prasad, H.S.; Kozlovsky, A. Experimental Intrabony and Periodontal Defects Treated with Natural Mineral Combined with a Synthetic Cell-Binding Peptide in the Canine: Morphometric Evaluations. J. Periodontol. 2006, 77, 1658–1664. [Google Scholar] [CrossRef]
- Hanks, T.; Atkinson, B.L. Comparison of cell viability on anorganic bone matrix with or without P-15 cell binding peptide. Biomaterials 2004, 25, 4831–4836. [Google Scholar] [CrossRef]
- Zhang, S. Emerging biological materials through molecular self-assembly. Biotechnol. Adv. 2002, 20, 321–339. [Google Scholar] [CrossRef]
- Ryan, D.M.; Nilsson, B.L. Self-assembled amino acids and dipeptides as noncovalent hydrogels for tissue engineering. Polym. Chem. 2011, 1, 18–33. [Google Scholar] [CrossRef]
- Avinash, M.B.; Govindaraju, T. Nanoarchitectonics of biomolecular assemblies for functional applications. Nanoscale 2014, 6, 13348–13369. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Vines, J.B.; Patterson, J.L.; Chen, H.; Javed, A.; Jun, H.-W. Osteogenic differentiation of human mesenchymal stem cells synergistically enhanced by biomimetic peptide amphiphiles combined with conditioned medium. Acta Biomater. 2011, 7, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Sargeant, T.D.; Aparicio, C.; Goldberger, J.; Cui, H.; Stupp, S.I. Mineralization of peptide amphiphile nanofibers and its effect on the differentiation of human mesenchymal stem cells. Acta Biomater. 2012, 8, 2456–2465. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Choo, J.-E.; Choi, Y.-S.; Suh, J.-S.; Lee, S.-J.; Chung, C.-P.; Park, Y.-J. Osteoblastic differentiation of human bone marrow stromal cells in self-assembled BMP-2 receptor-binding peptide-amphiphiles. Biomaterials 2009, 30, 3532–3541. [Google Scholar] [CrossRef]
- Cormier, A.R.; Pang, X.; Zimmerman, M.I.; Zhou, H.-X.; Paravastu, A.K. Molecular Structure of RADA16-I Designer Self-Assembling Peptide Nanofibers. ACS Nano 2013, 7, 7562–7572. [Google Scholar] [CrossRef]
- Ikeno, M.; Hibi, H.; Kinoshita, K.; Hattori, H.; Ueda, M. Effects of self-assembling peptide hydrogel scaffold on bone regeneration with recombinant human bone morphogenetic protein-2. Int. J. Oral Maxillofac. Implant. 2013, 28, e283–e289. [Google Scholar] [CrossRef]
- Bellis, S.L. Advantages of RGD peptides for directing cell association with biomaterials. Biomaterials 2011, 32, 4205–4210. [Google Scholar] [CrossRef]
- Chen, J.; Zou, X. Self-assemble peptide biomaterials and their biomedical applications. Bioact Mater. 2019, 4, 120–131. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.-C.; Liu, Y.; Chen, Y.-R.; Deng, R.-H.; Zhang, Z.-N.; Yu, J.-K.; Yuan, F.-Z. Function and Mechanism of RGD in Bone and Cartilage Tissue Engineering. Front. Bioeng. Biotechnol. 2021, 9, 773636. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Bilem, I.; Chevallier, P.; Plawinski, L.; Sone, E.D.; Durrieu, M.C.; Laroche, G. RGD and BMP-2 mimetic peptide crosstalk enhances osteogenic commitment of human bone marrow stem cells. Acta Biomater. 2016, 36, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Frith, J.E.; Mills, R.J.; Hudson, J.E.; Cooper-White, J.J. Tailored Integrin–Extracellular Matrix Interactions to Direct Human Mesenchymal Stem Cell Differentiation. Stem Cells Dev. 2012, 21, 2442–2456. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Guvendiren, M.; Mauck, R.L.; Burdick, J.A. Hydrogels that mimic developmentally relevant matrix and N-cadherin interactions enhance MSC chondrogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 10117–10122. [Google Scholar] [CrossRef]
- Li, R.; Xu, J.; Wong, D.S.H.; Li, J.; Zhao, P.; Bian, L. Self-assembled N-cadherin mimetic peptide hydrogels promote the chondrogenesis of mesenchymal stem cells through inhibition of canonical Wnt/β-catenin signaling. Biomaterials 2017, 145, 33–43. [Google Scholar] [CrossRef]
- Guo, J.L.; Kim, Y.S.; Koons, G.L.; Lam, J.; Navara, A.M.; Barrios, S.; Xie, V.Y.; Watson, E.; Smith, B.T.; Pearce, H.A.; et al. Bilayered, peptide-biofunctionalized hydrogels for in vivo osteochondral tissue repair. Acta Biomater. 2021, 128, 120–129. [Google Scholar] [CrossRef]
- Lynch, K.; Pei, M. Age associated communication between cells and matrix: A potential impact on stem cell-based tissue regeneration strategies. Organogenesis 2014, 10, 289–298. [Google Scholar] [CrossRef]
- Martino, M.M.; Mochizuki, M.; Rothenfluh, D.A.; Rempel, S.A.; Hubbell, J.A.; Barker, T.H. Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials 2009, 30, 1089–1097. [Google Scholar] [CrossRef]
- Min, S.-K.; Kang, H.K.; Jang, D.H.; Jung, S.Y.; Kim, O.B.; Min, B.-M.; Yeo, I.-S. Titanium Surface Coating with a Laminin-Derived Functional Peptide Promotes Bone Cell Adhesion. BioMed Res. Int. 2013, 2013, 638348. [Google Scholar] [CrossRef]
- Foldager, C.B.; Toh, W.S.; Christensen, B.B.; Lind, M.; Gomoll, A.H.; Spector, M. Collagen Type IV and Laminin Expressions during Cartilage Repair and in Late Clinically Failed Repair Tissues from Human Subjects. CARTILAGE 2015, 7, 52–61. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, T.; Toh, W.S.; Pei, M. The role of laminins in cartilaginous tissues: From development to regeneration. Eur. Cells Mater. 2017, 34, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Schinke, T.; Liese, S.; Priemel, M.; Haberland, M.; Schilling, A.F.; Catala-Lehnen, P.; Blicharski, D.; Rueger, J.M.; Gagel, R.F.; Emeson, R.; et al. Decreased Bone Formation and Osteopenia in Mice Lacking α-Calcitonin Gene-Related Peptide. J. Bone Miner. Res. 2004, 19, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Fukase, M.; Baba, H.; Yamaguchi, T.; Takata, S.; Fujimi, T.; Nishikawa, M.; Nakamoto, C. New actions of parathy-roid hormone through its degradation. J. Endocrinol. Investig. 1992, 15 (Suppl 6), 121–127. [Google Scholar]
- Whitfield, J.F.; Morley, P.; Willick, G.E. Parathyroid Hormone, Its Fragments and Their Analogs for the Treatment of Osteoporosis. Treat. Endocrinol. 2002, 1, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Alkhiary, Y.; Gerstenfeld, L.C.; Krall, E.; Westmore, M.; Sato, M.; Mitlak, B.H.; Einhorn, T.A. Enhancement of Experimental Fracture-Healing by Systemic Administration of Recombinant Human Parathyroid Hormone (PTH 1-34). J. Bone Jt. Surg. 2005, 87, 731–741. [Google Scholar] [CrossRef]
- Komrakova, M.; Stuermer, E.K.; Werner, C.; Wicke, M.; Kolios, L.; Sehmisch, S.; Tezval, M.; Daub, F.; Martens, T.; Witzenhausen, P.; et al. Effect of human parathyroid hormone hPTH (1–34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone 2010, 47, 480–492. [Google Scholar] [CrossRef]
- Mognetti, B.; Marino, S.; Barberis, A.; Martin, A.-S.B.; Bala, Y.; Di Carlo, F.; Boivin, G.; Barbos, M.P. Experimental Stimulation of Bone Healing with Teriparatide: Histomorphometric and Microhardness Analysis in a Mouse Model of Closed Fracture. Calcif. Tissue Res. 2011, 89, 163–171. [Google Scholar] [CrossRef]
- Orth, P.; Cucchiarini, M.; Zurakowski, D.; Menger, M.; Kohn, D.; Madry, H. Parathyroid hormone [1–34] improves articular cartilage surface architecture and integration and subchondral bone reconstitution in osteochondral defects in vivo. Osteoarthr. Cartil. 2013, 21, 614–624. [Google Scholar] [CrossRef]
- Pigossi, S.C.; De Oliveira, G.J.P.L.; Finoti, L.S.; Nepomuceno, R.; Spolidorio, L.C.; Rossa, C.; Ribeiro, S.J.L.; Saska, S.; Scarel-Caminaga, R.M. Bacterial cellulose-hydroxyapatite composites with osteogenic growth peptide (OGP) or pentapeptide OGP on bone regeneration in critical-size calvarial defect model. J. Biomed. Mater. Res. Part A 2015, 103, 3397–3406. [Google Scholar] [CrossRef]
- Pigossi, S.C.; Medeiros, M.C.; Saska, S.; Cirelli, J.A.; Scarel-Caminaga, R.M. Role of Osteogenic Growth Peptide (OGP) and OGP(10–14) in Bone Regeneration: A Review. Int. J. Mol. Sci. 2016, 17, 1885. [Google Scholar] [CrossRef]
- Reyes, C.D.; Petrie, T.A.; Burns, K.L.; Schwartz, Z.; García, A.J. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials 2007, 28, 3228–3235. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.D.; García, A.J. α2β1integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. J. Biomed. Mater. Res. Part A 2004, 69A, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Kaneda, Y.; Akashi, Y.; Hamada, Y.; Matsumoto, T.; Saeki, M.; Thakor, D.K.; Tabata, Y.; Matsuura, N.; Yatani, H. Enhanced bone regeneration via multimodal actions of synthetic peptide SVVYGLR on osteoprogenitors and osteoclasts. Biomaterials 2009, 30, 4676–4686. [Google Scholar] [CrossRef]
- Wu, C.-H.; Liu, I.-J.; Lu, R.-M.; Wu, H.-C. Advancement and applications of peptide phage display technology in biomedical science. J. Biomed. Sci. 2016, 23, 8. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.; Carnazza, S.; De Plano, L.; Franco, D.; Nicolò, M.; Zammuto, V.; Petralia, S.; Calabrese, G.; Gugliandolo, C.; Conoci, S.; et al. Rapid detection of bacterial pathogens in blood through engineered phages-beads and integrated Real-Time PCR into MicroChip. Sensors Actuators B Chem. 2020, 329, 129227. [Google Scholar] [CrossRef]
- Zambrano-Mila, M.S.; Blacio, K.E.S.; Vispo, N.S. Peptide Phage Display: Molecular Principles and Biomedical Applications. Ther. Innov. Regul. Sci. 2020, 54, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, F.; Tanaka, Y.; Minari, Y.; Tokui, N. Designing scaffolds of peptides for phage display libraries. J. Biosci. Bioeng. 2005, 99, 448–456. [Google Scholar] [CrossRef]
- Zmolik, J.M.; Mummert, M.E. Pep-1 as a Novel Probe for the In Situ Detection of Hyaluronan. J. Histochem. Cytochem. 2005, 53, 745–751. [Google Scholar] [CrossRef]
- Metz-Estrella, D.; Jonason, J.H.; Sheu, T.-J.; Mroczek-Johnston, R.M.; Puzas, J.E. TRIP-1: A regulator of osteoblast function. J. Bone Miner. Res. 2012, 27, 1576–1584. [Google Scholar] [CrossRef]
- Yoo, S.Y.; Merzlyak, A.; Lee, S.-W. Facile growth factor immobilization platform based on engineered phage matrices. Soft Matter 2011, 7, 1660–1666. [Google Scholar] [CrossRef]
- Cabanas-Danés, J.; Nicosia, C.; Landman, E.; Karperien, M.; Huskens, J.; Jonkheijm, P. A fluorogenic monolayer to detect the co-immobilization of peptides that combine cartilage targeting and regeneration. J. Mater. Chem. B 2013, 1, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Sheu, T.-J.; Schwarz, E.M.; Martinez, D.A.; O’Keefe, R.J.; Rosier, R.N.; Zuscik, M.; Puzas, J.E. A Phage Display Technique Identifies a Novel Regulator of Cell Differentiation. J. Biol. Chem. 2003, 278, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Hsu, E.L.; Mendoza, M.; Ghodasra, J.; Nickoli, M.S.; Ashtekar, A.; Polavarapu, M.; Babu, J.; Riaz, R.M.; Nicolas, J.D.; et al. Gel Scaffolds of BMP-2-Binding Peptide Amphiphile Nanofibers for Spinal Arthrodesis. Adv. Health Mater. 2014, 4, 131–141. [Google Scholar] [CrossRef]
- Hamley, I.W. Small Bioactive Peptides for Biomaterials Design and Therapeutics. Chem. Rev. 2017, 117, 14015–14041. [Google Scholar] [CrossRef] [PubMed]
- Haggag, Y. Peptides as Drug Candidates: Limitations and Recent Development Perspectives. Biomed. J. Sci. Tech. Res. 2018, 8, 1–4. [Google Scholar] [CrossRef]
- Lee, A.C.-L.; Harris, J.L.; Khanna, K.K.; Hong, J.-H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef]
| Name | Sequence | Function | Biomaterial | Peptide Amount | In Vitro/In Vivo | Ref. |
|---|---|---|---|---|---|---|
| Cytomodulins (CMs) | AA1-AA2-AA3…AAn (AA1 = A, N, L; AA2 = V, I; AA3 = A; AAn = Q, D, E, N) | Improve collagen I expression and promote wound healing in vitro and in vivo | - | CM-1, CM2, 0.1–1 μM | Human foreskin fibroblast (HFF) cells | [39] |
| Poly(lactide-co-glycolide) (PLGA) microspheres | CM-1, 200 ng/mL | Human dermal fibroblast cells (HDFs) | [40] | |||
| Co-assembly peptide amphiphile (PA) | 5 or 10 mol% TGF-binding PA | Mesenchymal stem cells (MSCs) and rabbit model | [43] | |||
| Chitosan (CS) | CS:peptide = 10:1 w/w, 10:2 w/w, 10:3 w/w | MSCs and mouse/rabbit model | [44] | |||
| Gelatin methacryloyl (GelMA), GelMA/hydroxyapatite (HAp) (bilayered scaffold) | TGF-β1 peptide, via covalent linking, 50–400 μg/mL | MSCs and rat model | [45] | |||
| GelMA | 0.025 mM, TGF-β1-affinity peptide | Human umbilical cord mesenchymal stem cells (huMSCs) and rabbit model | [46] | |||
| β-tricalcium phosphate (TCP)/PLGA–subchondral region. Poly(D,L-lactic acid-co-trimethylene carbonate)–cartilage region (bilayered scaffold) | 20 μg of TGF-β1/collagen I (1 mL, 9 mg/mL), injected into cartilage region | Rat bone-marrow-derived mesenchymal stem cells (rBMSCs) | [47] | |||
| CK2.1 | - | Drives chondrogenesis and induces the formation of cartilage | - | 100 nM | C3H10T1/2 cells and murine model | [59] |
| Hyaluronic acid hydrogel particles (HGPS) | HGPS (10 mg) in 10 mL PBS with CK2.1 (10 mg) | [60] | ||||
| CK2.2 and CK2.3 | - | Induce osteocalcin expression and mineral deposition | - | 100 nM | [59] | |
| OP-BMP-2 | NSVNSKIPKACCVPTELSAI | Promote osteogenesis | Polyethylene terephthalate | BMPs, 10–3 M | MC3T3-E1 (pre-osteoblast-like) cells | [63] |
| pBMP-9 | RKVGKASSVPTKLSPISILYK | Titanium | BMP-2, 2 mg/0.5 mL in PBS | MC3T3-E1 cells and beagle dogs | [64] | |
| BMP-7 | RTVPKPSSAPTQLNAISTLYF | - | BMPs, 0.02 to 200 mg/mL | MSCs | [65] | |
| B2A | B2A2-K-NS | Increases cartilage proteoglycan synthesis and chondrogenesis in vitro and in vivo | - | 0–1000 ng/mL | C2C12 cells and C3H10T1/2 cells | [66] |
| NLS-TAT | PKKKRKVKGRKKRRQRRRPPQ | Promotes chondrogenesis | - | 4.10 μM | Rat precartilaginous stem cells (PSCs) | [75] |
| PepGen P-15 | GTPGPQGIAGQRGVV | Promotes osteoblastic activity in vitro and bone regeneration in vivo | - | 100 µg/mL | Human periodontal ligament fibroblast cells | [79] |
| Phytogene HAp (Algipore®), TCP (Bio-Base®), bovine HAp (low temperature (T)) (Bio-Oss®), bovine HAp (high T) (Osteograf®), and bovine Hp (high T) | PepGen P-15® | Human osteoblast cells | [81] | |||
| Grafting materials: BioOss, OsteoGraf N-300 | PepGen P-15 | Osteoblast cells | [82] | |||
| Titanium | 1 mM (dry dimethylformamide) | Preosteocyte MLO-A5 cells | [83] | |||
| - | 1000 μg/mL | Human osteoblast cells | [84] | |||
| Anorganic bovine-derived mineral bound to a P-15 (ABM/P-15): ABM/P-15 carboxymethyl cellulose (CMC)-hydrogel graft and ABM/P-15 particulate graft | 200 ng of P-15 per 1 g of ABM | Rabbit model | [85,86,87] | |||
| Algae-derived hydroxyapatite/P-15 + 25% autologous bone | P-15 was adsorbed onto the HA surface | Pig model | [88] | |||
| ABM (OsteoGrafs/N-700) and ABM/P-15 (PepGen | P-15TM, adsorbed | [89] | ||||
| ABM/P-15 | - | Dog model | [90] | |||
| ABM (OsteoGrafs/N-300) and ABM/P-15 | P-15™, adsorbed | Human foreskin fibroblast cells | [91] | |||
| Peptide amphiphiles(PA) | - | Promote osteogenic differentiation in vitro and bone regeneration in vivo | Nanofibrous PA scaffold | PA-RGDS PA-DGEA | hMSCs | [95] |
| Negatively charged PA with phosphoserine residues, negatively charged PA with serine residues, a positively charged PA with RGDS sequence, 10 mM | [96] | |||||
| OPD, 1%wt | hBMSCs | [97] | ||||
| 10 mg/mL, PuraMatrix (RADA16-I) | Rabbit model | [99] | ||||
| RGD | GRGDS, RGDS, YRGDS and c (RGDfk) | Improves cell adhesion and cartilage and bone tissue repair | Piranha activated borosilicate glass slides | 200 μL of 20 μM solution of RGD-TAMRA, BMP-2- FITC or a mixture of both peptides | hBMSCs | [104] |
| Maleimide functionalized polystyrene-block-polyethylene (PS-PEO) copolymer, spin-coated | 20 μg/mL peptide (CGRGDS, CGGGRRETAWA, CGQAASIKVAVSADR or CGGEGYGEGYIGSR) | hMSCs | [105] | |||
| N-cadherin peptide | HAVDIGGGC | Promotes both early chondrogenesis of MSCs and late cartilaginous matrix production | Methacrylated hyaluronic acid | 10 mol% of methacrylates | hMSCs and mouse model | [106] |
| Self-assembly hydrogel | Self-assembly peptide (Ac-KLDLKLDLKLDL, KLD), N-cadherin mimetic self-assembly peptide (Ac-HAVDIGGKLDLKLDLKLDL, KLD-Cad), and scrambled control peptide (Ac-AGVIDHGKLDLKLDLKLDL, KLD-Scr), 0.5% (w/v) | MSCs | [107] | |||
| Poly(glycolic acid)-poly(ethylene glycol)-poly(glycolic acid)-di(but-2-yne-1,4-dithiol) (PdBT) click conjugated with either chondrogenic “GGGHAVDI” or GGGGHKSP, mixed with poly(N-isopropylacrylamide-co-glycidyl methacrylate) (P(NIPAAm-co-GMA), (bilayered hydrogel) | - | MSCs and rabbit model | [108] | |||
| Laminin mimic peptides | IKVAV and YIGSR | Influence cartilage and bone regeneration | Maleimide functionalized PS-PEO copolymer, spin-coated | 20 μg/mL (CGRGDS, CGGGRRETAWA, CGQAASIKVAVSADR or CGGEGYGEGYIGSR) | hMSCs | [110] |
| Ln2-p3 | Enhances the expression of osteogenic markers and increases ALP activity | Titanium | 23 μg/cm2 | Human osteosarcoma (HOS) cells | [111] | |
| PTH 1–34 | H-SVSEIQLMHNLGKHLNSMERVEWLRKKLQDVHNF-OH | Stimulates osteoblast proliferation, differentiation and prevents apoptosis | - | 5 or 30 μg/kg of PTH (1–34) | Rat model | [117] |
| - | PTH (1–34) in 0.9% saline at a daily dosage of 40 μg/kg body weight (BW), subcutaneously | [118] | ||||
| - | PTH (1–34) in saline solution 200 μL, subcutaneously | Mouse model | [119] | |||
| - | 10 μg/kg BW, daily, subcutaneously | Rabbit model | [120] | |||
| OGP and OGP10–14 | YGFGG | Enhance osteoinductive potential | Bacte- rial cellulose-hydroxyapatite (BC-HA) | 10−9 mol/L, adsorption | Mouse model | [121] |
| Collagen-mimetic peptide | GFOGER | Promotes bone regeneration and osseointegration | Titanium | 20 μg/mL in Dulbecco’s PBS | Primary bone marrow stromal cells | [123] |
| DGEA | Promotes osteogenic differentiation and bone formation | Collagen Type I | 10 μg/mL | MC3T3-E1 cells | [124] | |
| SVVYGLR | Suppresses osteoclastogenesis and contributes to bone repair at the early stage | Atelocollagen sponge | 10 μg | hMSCs and rat model | [126] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rizzo, M.G.; Palermo, N.; D’Amora, U.; Oddo, S.; Guglielmino, S.P.P.; Conoci, S.; Szychlinska, M.A.; Calabrese, G. Multipotential Role of Growth Factor Mimetic Peptides for Osteochondral Tissue Engineering. Int. J. Mol. Sci. 2022, 23, 7388. https://doi.org/10.3390/ijms23137388
Rizzo MG, Palermo N, D’Amora U, Oddo S, Guglielmino SPP, Conoci S, Szychlinska MA, Calabrese G. Multipotential Role of Growth Factor Mimetic Peptides for Osteochondral Tissue Engineering. International Journal of Molecular Sciences. 2022; 23(13):7388. https://doi.org/10.3390/ijms23137388
Chicago/Turabian StyleRizzo, Maria Giovanna, Nicoletta Palermo, Ugo D’Amora, Salvatore Oddo, Salvatore Pietro Paolo Guglielmino, Sabrina Conoci, Marta Anna Szychlinska, and Giovanna Calabrese. 2022. "Multipotential Role of Growth Factor Mimetic Peptides for Osteochondral Tissue Engineering" International Journal of Molecular Sciences 23, no. 13: 7388. https://doi.org/10.3390/ijms23137388
APA StyleRizzo, M. G., Palermo, N., D’Amora, U., Oddo, S., Guglielmino, S. P. P., Conoci, S., Szychlinska, M. A., & Calabrese, G. (2022). Multipotential Role of Growth Factor Mimetic Peptides for Osteochondral Tissue Engineering. International Journal of Molecular Sciences, 23(13), 7388. https://doi.org/10.3390/ijms23137388











