Muscle Fiber Composition Changes after Selective Nerve Innervation
Abstract
:1. Introduction
2. Results
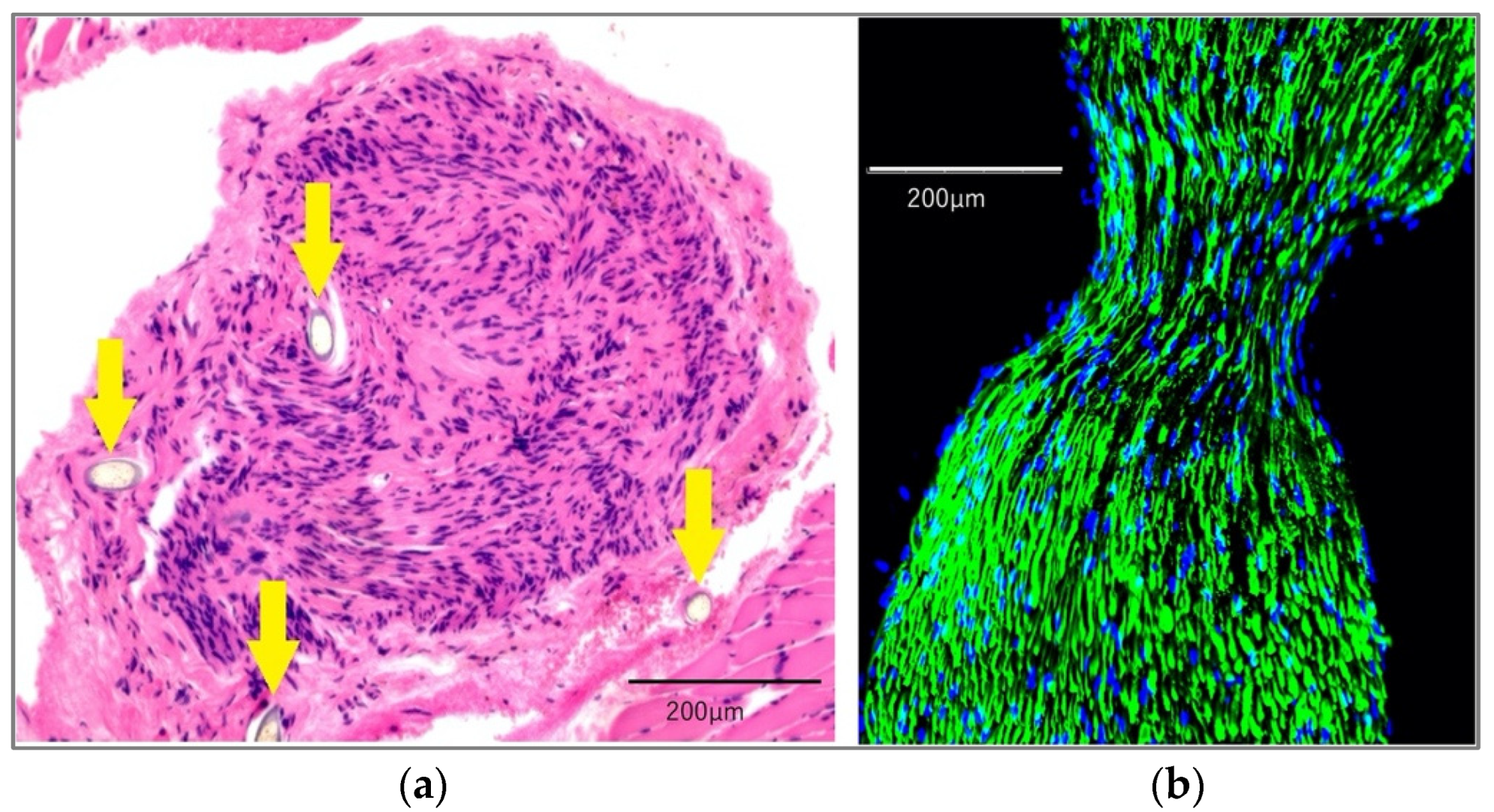
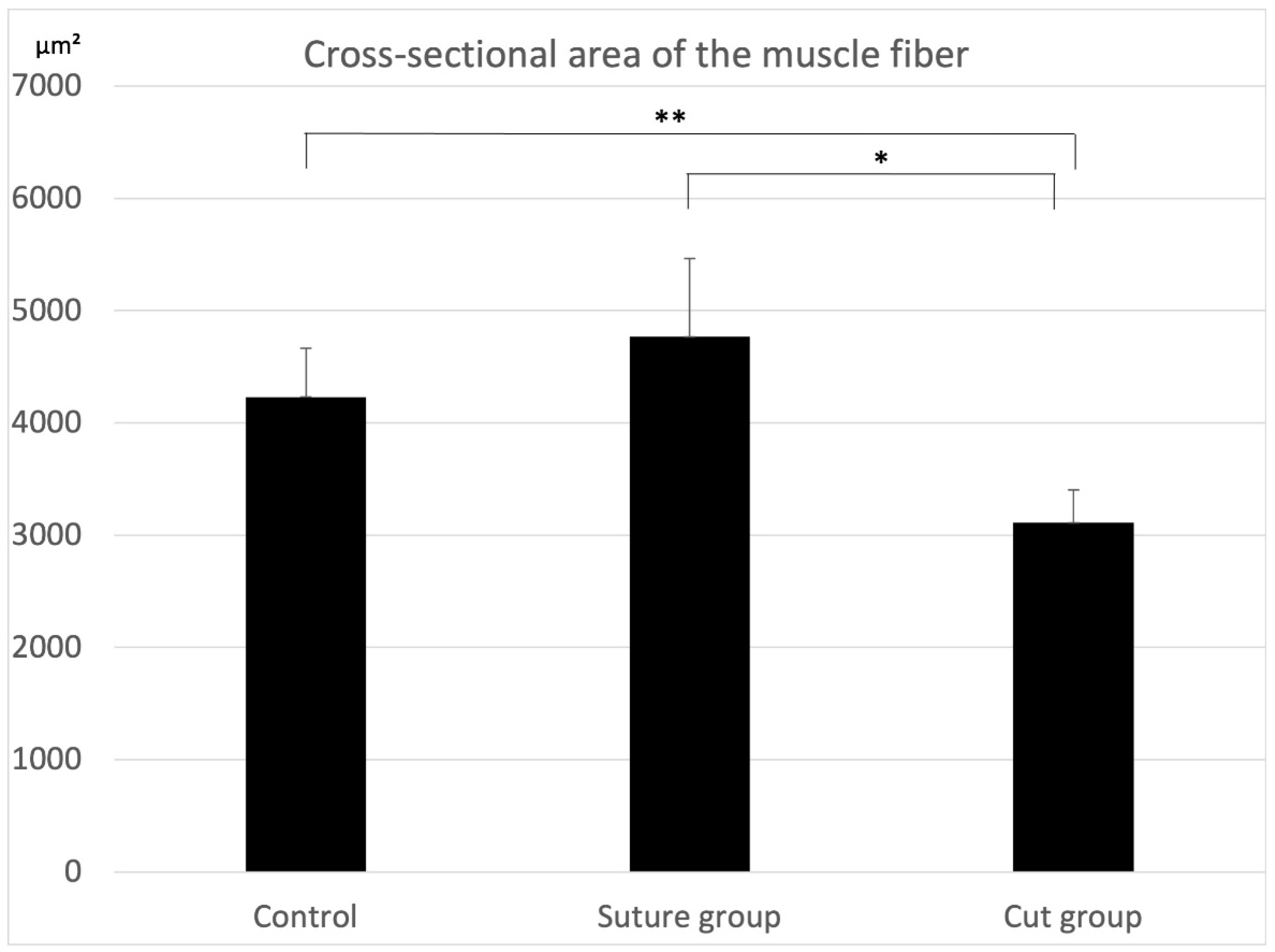
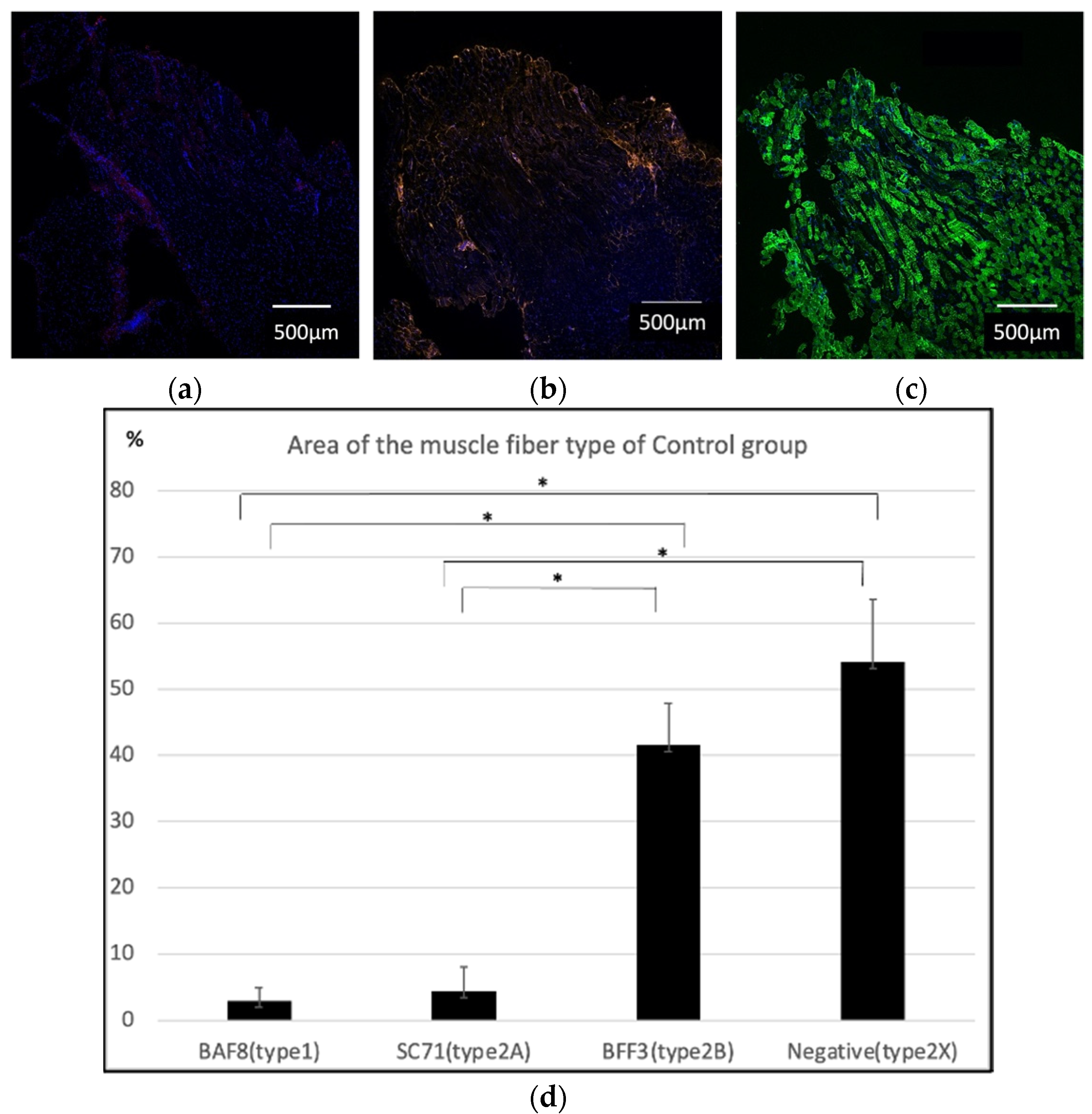
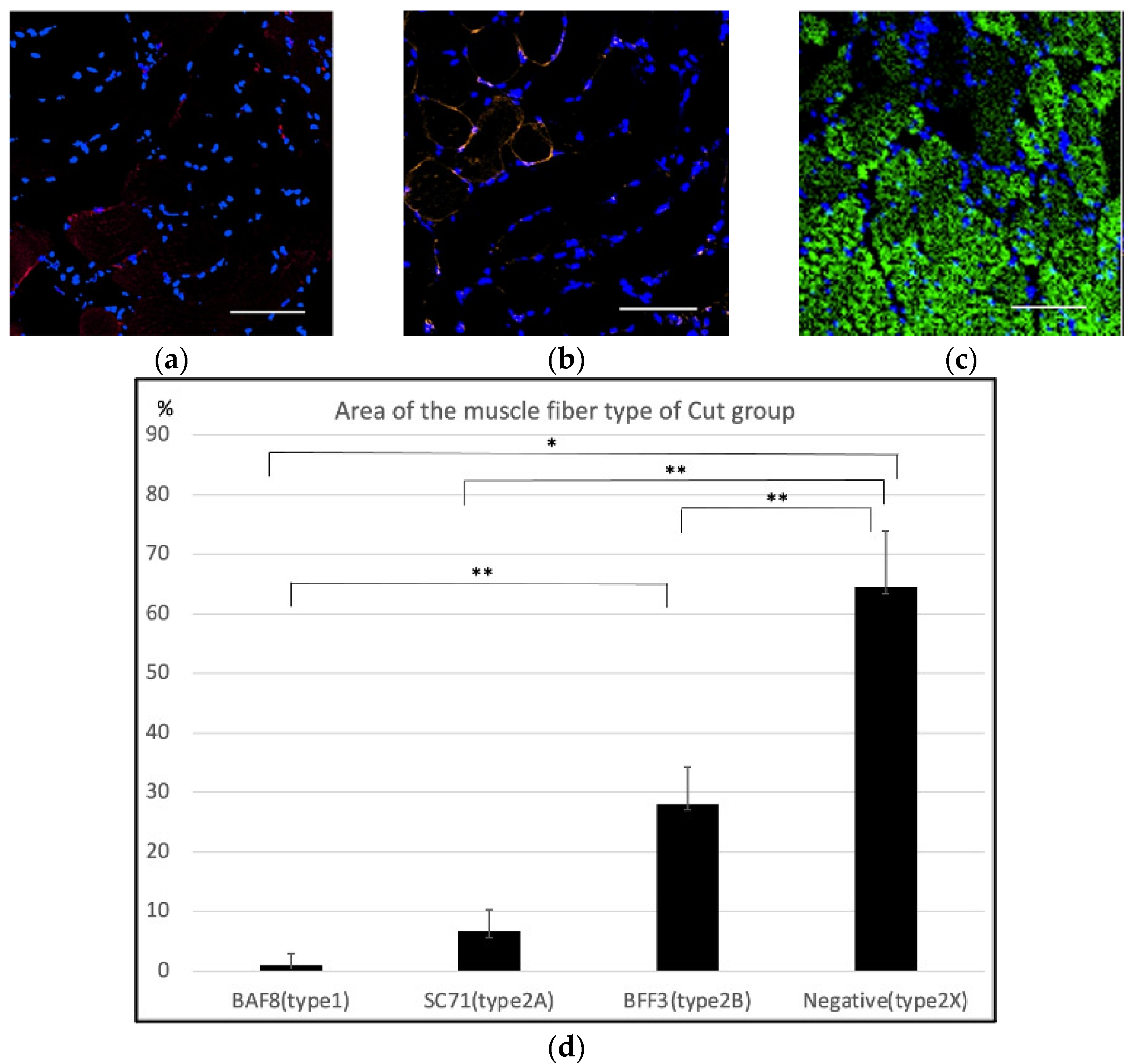
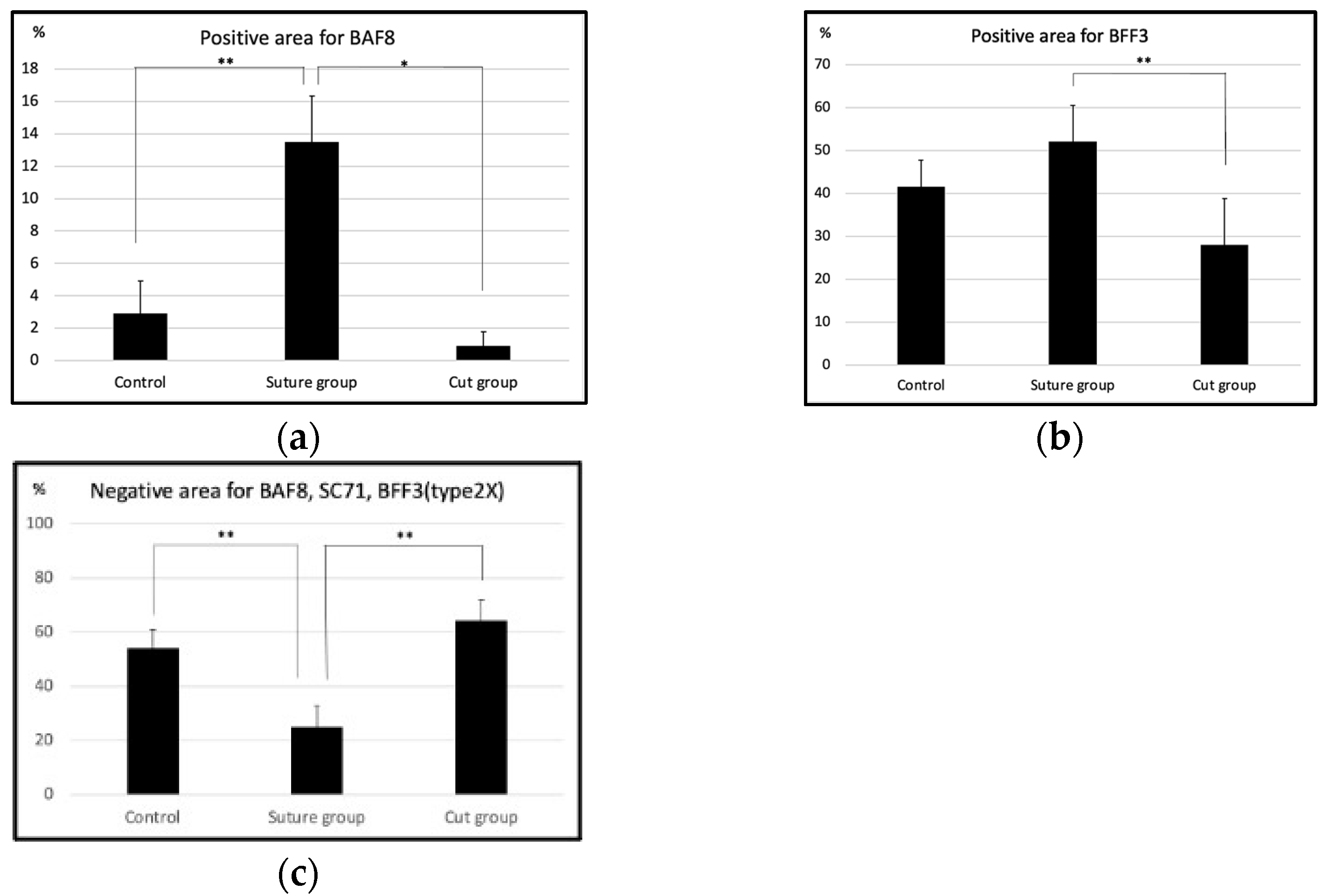
2.1. Histological Findings
2.2. Microarray
2.2.1. Gene Expression in Relation to the Muscle Fiber Type Composition
2.2.2. PPI Network Analysis
3. Discussion
4. Materials and Methods
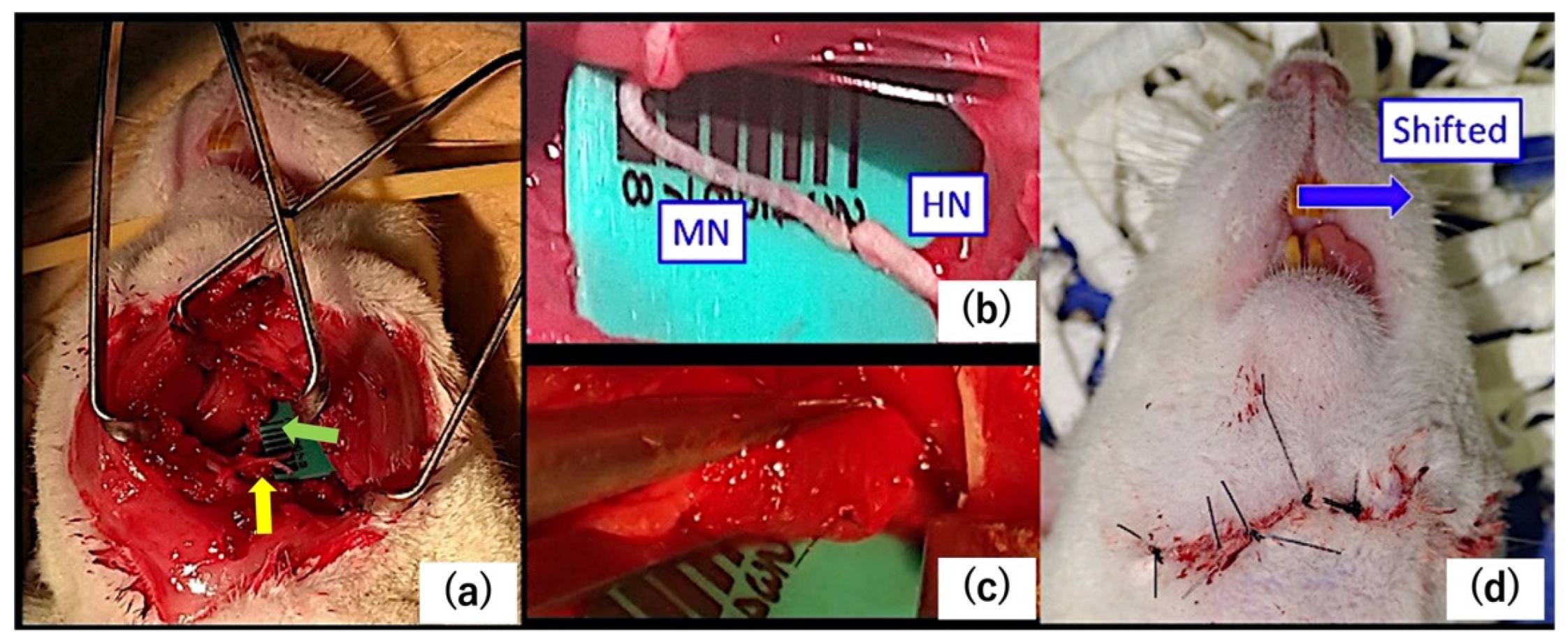
4.1. Surgery
4.2. Histological Analysis
4.3. Microarray
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Harii, K.; Ohmori, K.; Torii, S. Free gracilis muscle transplantation, with microneurovascular anastomoses for the treatment of facial paralysis. A preliminary report. Plast. Reconstr. Surg. 1976, 57, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Koshima, I.; Tsuda, K.; Hamanaka, T.; Moriguchi, T. One-stage reconstruction of established facial paralysis using a rectus abdominis muscle transfer. Plast. Reconstr. Surg. 1997, 99, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Harii, K.; Asato, H.; Yoshimura, K.; Sugawara, Y.; Nakatsuka, T.; Ueda, K. One-stage transfer of the latissimus dorsi muscle for reanimation of a paralyzed face: A new alternative. Plast. Reconstr. Surg. 1998, 102, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, H.; Tanaka, I.; Yazawa, M.; Oh, A. Dual-innervated multivector muscle transfer using two superficial subslips of the serratus anterior muscle for long-standing facial paralysis. Arch. Plast. Surg. 2021, 48, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Kryściak, K.; Grieb, P.; Celichowski, J. Changes in motor unit properties in SOD1 (G93A) rats. Muscle Nerve. 2014, 50, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Robbins, N.; Karpati, G.; Engel, W.K. Histochemical and contractile properties in the cross-innervated guinea pig soleus muscle. Arch. Neurol. 1969, 20, 318–329. [Google Scholar] [CrossRef] [PubMed]
- Romanul, F.C.; Van der Meulen, J.P. Slow and fast muscles after cross innervation. Enzymatic and physiological changes. Arch. Neurol. 1967, 17, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Murgia, M.; Nogara, L.; Baraldo, M.; Reggiani, C.; Mann, M.; Schiaffino, S. Protein profile of fiber types in human skeletal muscle: A single-fiber proteomics study. Skelet. Muscle 2021, 11, 24. [Google Scholar] [CrossRef]
- Cvetko, E.; Erzen, I. Effect of chronic uncontrollable stress on myosin heavy chain expression in rat masseter muscle. Eur. J. Appl. Physiol. 2001, 442, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Sano, R.; Tanaka, E.; Korfage, J.A.M.; Langenbach, G.E.J.; Kawai, N.; van Eidjen, T.M.G.J.; Tanne, K. Heterogenity of fiber characteristics in the rat masseter and digastric muscles. J. Anat. 2007, 211, 464–470. [Google Scholar] [PubMed]
- Patterson, M.F.; Stephenson, G.M.; Stephenson, D.G. Denervation produces different single fiber phenotypes in fast- and slow-twitch hindlimb muscles of the rat. Am. J. Physiol. Cell Physiol. 2006, 291, C518–C528. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.J.; Neufer, P.D. Type II skeletal myofibers possess unique properties that potentiate mitochondrial H(2)O(2) generation. Am. J. Physiol. Cell Physiol. 2006, 290, C844–C851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picard, B.; Cassar-Malek, I. Evidence for expression of IIb myosin heavy chain isoform in some skeletal muscles of Blonde d’Aquitaine bulls. Meat Sci. 2009, 82, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Varuzhanyan, G.; Pham, A.H.; Chan, D.C. Mitochondrial dynamics is a distinguishing feature of skeletal muscle fiber types and regulates organellar compartmentalization. Cell Metab. 2015, 22, 1033–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signes, A.; Fernandez-Vizarra, E. Assembly of mammalian oxidative phosphorylation complexes I-V and supercomplexes. Essays Biochem. 2018, 62, 255–270. [Google Scholar] [PubMed]
- Nehrer-Tairych, G.V.; Rab, M.; Kamolz, L.; Deutinger, M.; Stohr, H.G.; Frey, M. The influence of the donor nerve on the function and morphology of a mimicmuscle after cross innervation: An experimental study in rabbits. Br. J. Plast. Surg. 2000, 53, 669–675. [Google Scholar] [CrossRef]
- D’Amico, D.; Gammazza, A.M.; Macaluso, F.; Paladino, L.; Scalia, F.; Spinoso, G.; Dimauro, I.; Caporossi, D.; Cappello, F.; Di Felice, V.; et al. Sex-based differences after a single bout of exercise on PGC1α isoforms in skeletal muscle: A pilot study. FASEB J. 2021, 35, e21328. [Google Scholar] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, T.; Rasband, W. ImageJ User Guide—ImageJ/Fiji 1.46. Available online: https://imagej.nih.gov/ij/docs/guide/index.html (accessed on 2 October 2012).
- Bergmeister, K.D.; Gröger, M.; Aman, M.; Willensdorfer, A.; Manzano-Szalai, K.; Salminger, S.; Aszmann, O.C. Automated muscle fiber type population analysis with ImageJ of whole rat muscles using rapid myosin heavy chain immunohistochemistry. Muscle Nerve 2016, 54, 292–299. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely-available easy-to-use software “EZR” (Easy R) for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [Green Version]
- STRING (Search Tool for the Retrieval of Interacting Genes/Proteins) Database Version 11.5. Available online: https://string-db.org (accessed on 12 August 2021).


| Gene | vs. Con | vs. Cut | Gene | vs. Con | vs. Cut | Gene | vs. Con | vs. Cut |
|---|---|---|---|---|---|---|---|---|
| Casq2 | Asb2 | Art3 | ||||||
| Lrrc39 | Ppr1r8 | Atp1a1 | ||||||
| Lmod2 | Ptbp1 | Cav1 | ||||||
| Lss | Tfrc | Dysf | ||||||
| Myom3 | Ddah1 | Fkbp1a | ||||||
| Myoz2 | Ak1 | Gnb1 | ||||||
| Pln | Anxa5 | Itgb1 | ||||||
| Tnni1 | Epm2a | Jph2 | ||||||
| Sptlc2 | Gpd1 | Mlec | ||||||
| Acot9 | Gpd2 | P4hb | ||||||
| Ankrd2 | Hsp90aa1 | Psmd2 | ||||||
| Actn2 | Phkb | Rtn4 | ||||||
| Chchd3 | Phkg1 | Slc37a4 | ||||||
| Ckmt2 | Ppkar1a | Smtnl2 | ||||||
| Cpt1b | Psmc4 | Tmem43 | ||||||
| Crat | Ryr3 | |||||||
| Decr1 | Fndc1 | |||||||
| Ech1 | Pgk1 | |||||||
| Hadh | Tnc | |||||||
| Mrps36 | Tpi1 | |||||||
| Mrps7 | Agl | |||||||
| Ndufs7 | Ahnak2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, S.; Ochiai, H.; Sakuma, H.; Mori, T.; Yazawa, M.; Oka, A.; Kishi, K. Muscle Fiber Composition Changes after Selective Nerve Innervation. Int. J. Mol. Sci. 2022, 23, 7856. https://doi.org/10.3390/ijms23147856
Watanabe S, Ochiai H, Sakuma H, Mori T, Yazawa M, Oka A, Kishi K. Muscle Fiber Composition Changes after Selective Nerve Innervation. International Journal of Molecular Sciences. 2022; 23(14):7856. https://doi.org/10.3390/ijms23147856
Chicago/Turabian StyleWatanabe, Shiho, Hiroko Ochiai, Hisashi Sakuma, Taisuke Mori, Masaki Yazawa, Aiko Oka, and Kazuo Kishi. 2022. "Muscle Fiber Composition Changes after Selective Nerve Innervation" International Journal of Molecular Sciences 23, no. 14: 7856. https://doi.org/10.3390/ijms23147856
APA StyleWatanabe, S., Ochiai, H., Sakuma, H., Mori, T., Yazawa, M., Oka, A., & Kishi, K. (2022). Muscle Fiber Composition Changes after Selective Nerve Innervation. International Journal of Molecular Sciences, 23(14), 7856. https://doi.org/10.3390/ijms23147856







