Plasma Promotes Fungal Cellulase Production by Regulating the Levels of Intracellular NO and Ca2+
Abstract
:1. Introduction
2. Results
2.1. Physical and Chemical Properties of Vogel’s Minimal (VM) Media Treated with the Plasma Jet
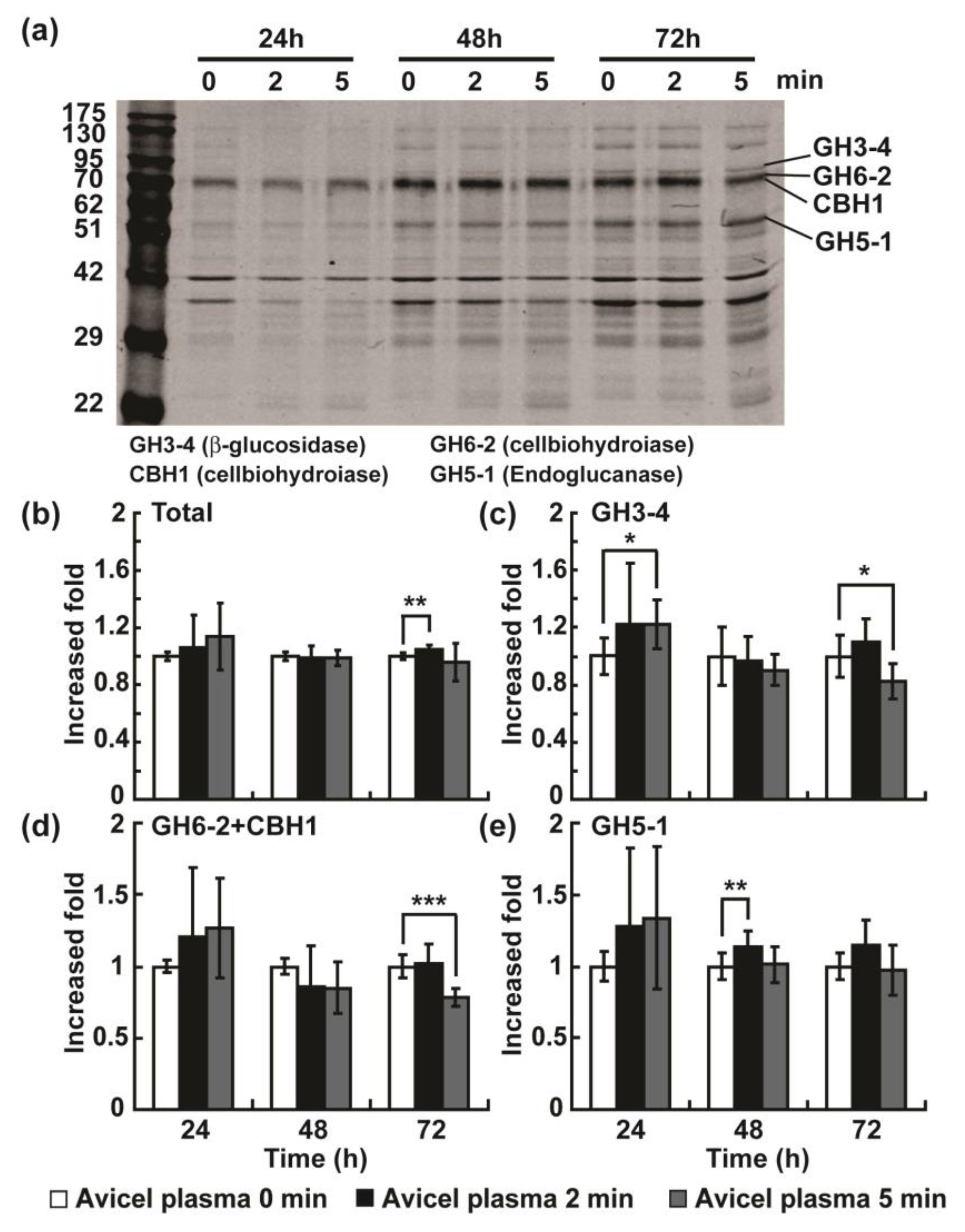
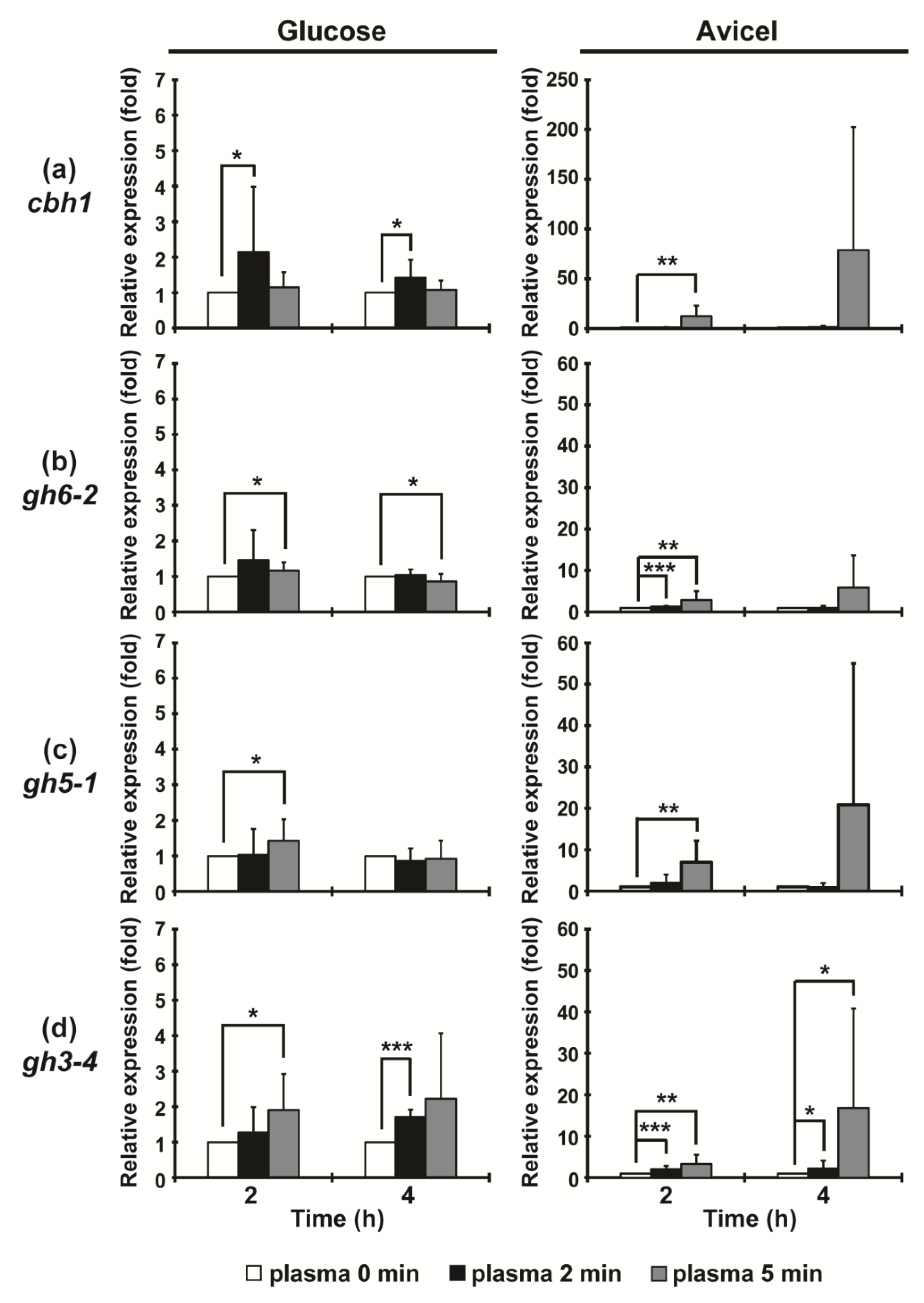
2.2. Plasma Jet Treatment can Enhance the Production of Cellulases in N. crassa
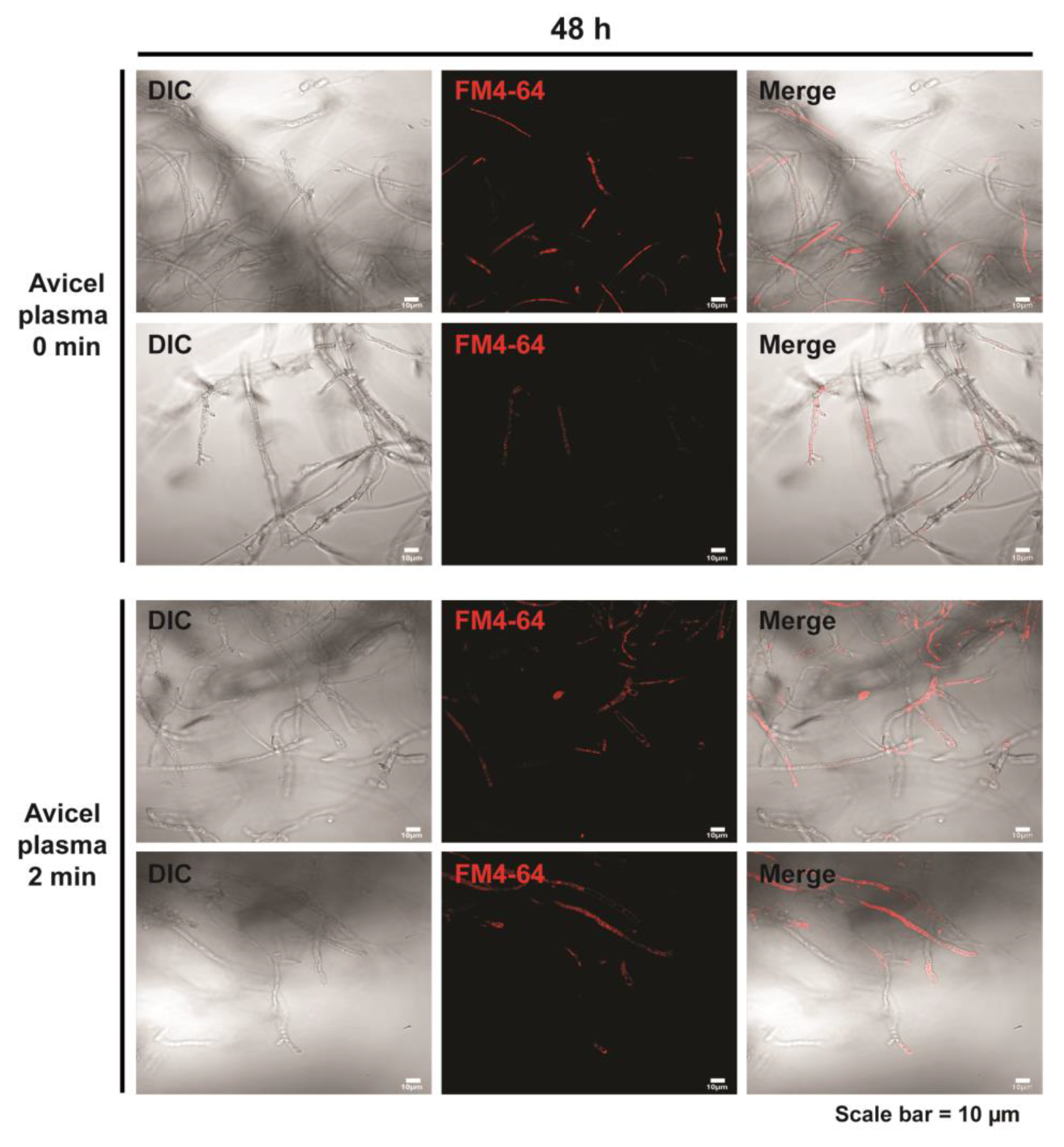
2.3. Plasma Treatment Promotes Slight Vesicle Accumulation in Fungal Hyphae
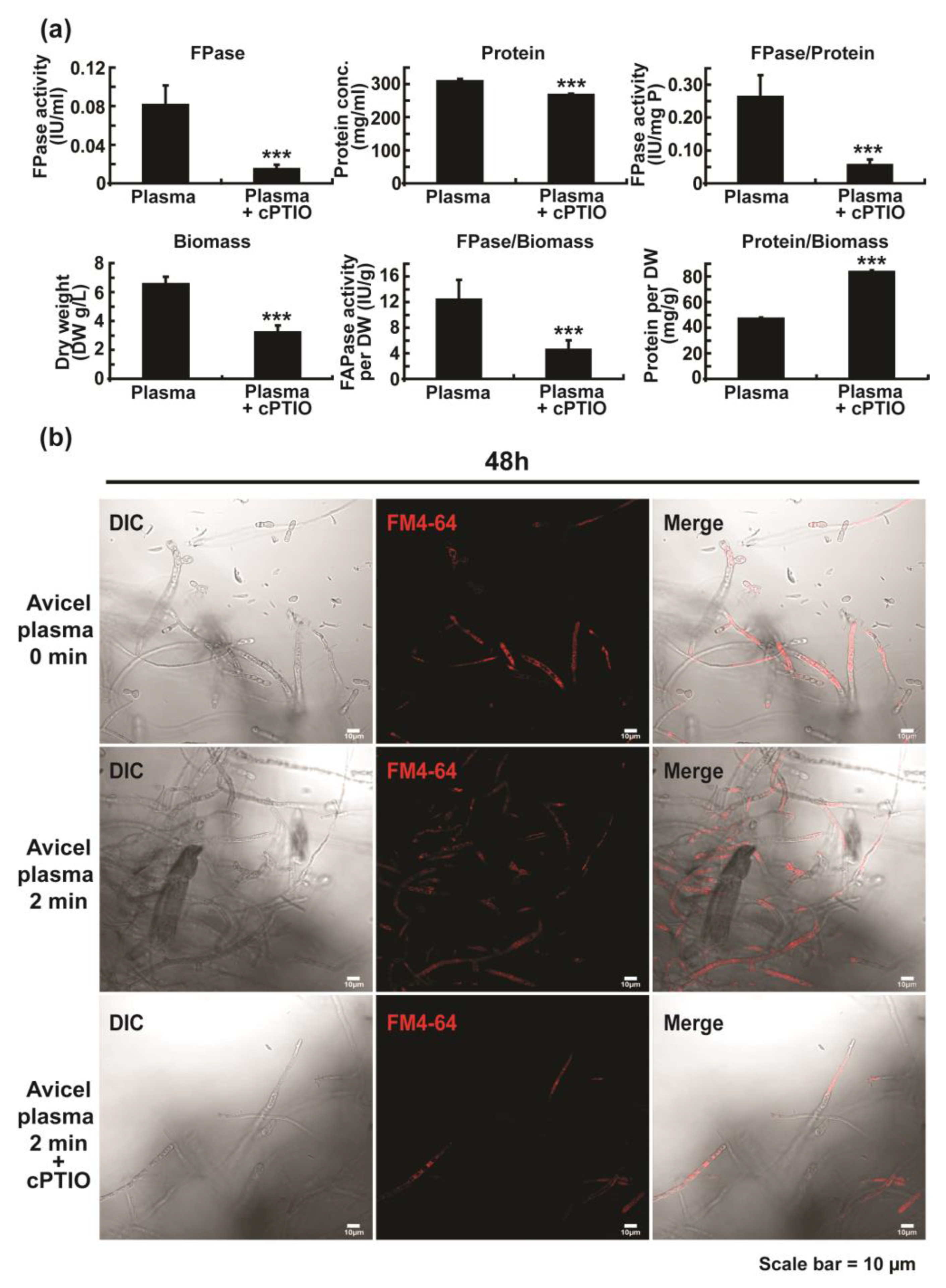
2.4. Plasma-Induced Enzyme Secretion Is Related to Elevated Level of Intracellular NO
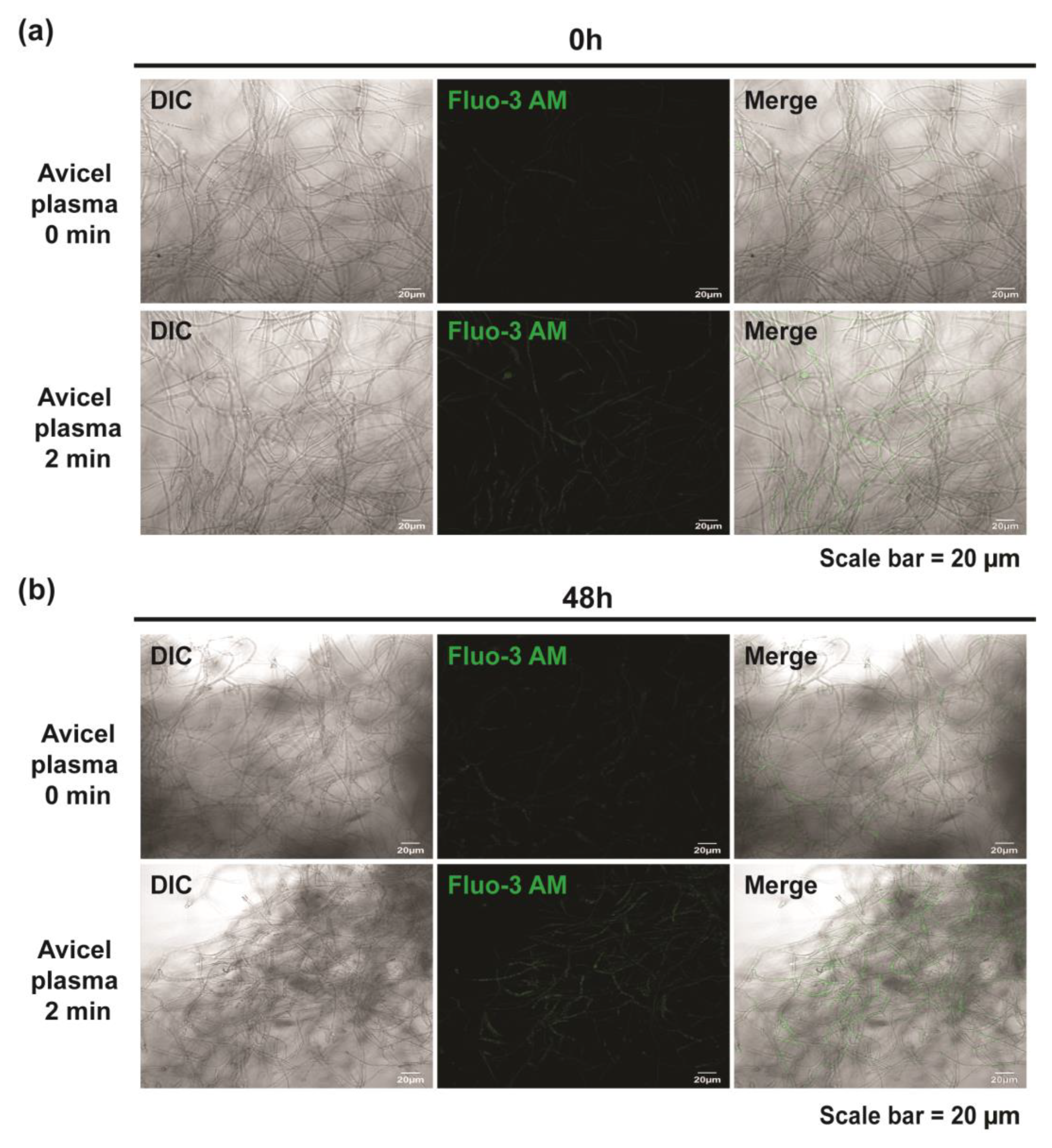
2.5. Plasma Induces Intracellular Calcium Accumulation and Membrane Depolarization
3. Discussion
4. Materials and Methods
4.1. Fungal Strain and Culture Condition
4.2. Plasma Treatment
4.3. Measurement of H2O2, NOX Levels, ORP, and pH in the Media
4.4. Assay for Determining the Activities of Cellulolytic Enzymes
4.5. Quantitative Real-Time PCR Analysis
4.6. Protein Gel Electrophoresis
4.7. Analysis for Intracellular ROS and RNS Levels
4.8. Determination of Membrane Potential, Intracellular Ca2+, and Secretory Vesicles
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Razali, N.A.M.; Mohd Sohaimi, R.; Othman, R.; Abdullah, N.; Demon, S.Z.N.; Jasmani, L.; Yunus, W.; Ya’acob, W.; Salleh, E.M.; Norizan, M.N.; et al. Comparative Study on Extraction of Cellulose Fiber from Rice Straw Waste from Chemo-Mechanical and Pulping Method. Polymers 2022, 14, 387. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, R.C.; Gupta, R.; Singh, A. Microbial cellulases and their industrial applications. Enzym. Res. 2011, 2011, 280696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNamara, J.T.; Morgan, J.L.; Zimmer, J. A molecular description of cellulose biosynthesis. Annu. Rev. Biochem. 2015, 84, 895–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, N.; Kumar, B.; Agrawal, K.; Verma, P. Current perspective on production and applications of microbial cellulases: A review. Bioresour. Bioprocess. 2021, 8, 1–34. [Google Scholar] [CrossRef]
- Imran, M.; Anwar, Z.; Irshad, M.; Asad, M.J.; Ashfaq, H. Cellulase production from species of fungi and bacteria from agricultural wastes and its utilization in industry: A review. Adv. Enzym. Res. 2016, 4, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Lübeck, M.; Lübeck, P.S. Fungal Cell Factories for Efficient and Sustainable Production of Proteins and Peptides. Microorganisms 2022, 10, 753. [Google Scholar] [CrossRef]
- Ribeiro, O.; Magalhaes, F.; Aguiar, T.Q.; Wiebe, M.G.; Penttila, M.; Domingues, L. Random and direct mutagenesis to enhance protein secretion in Ashbya gossypii. Bioengineered 2013, 4, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Sakekar, A.A.; Gaikwad, S.R.; Punekar, N.S. Protein expression and secretion by filamentous fungi. J. Biosci. 2021, 46, 1–18. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, C.; Shen, Y.; Ma, Y.; Wei, D.; Wang, W. N,N-dimethylformamide induces cellulase production in the filamentous fungus Trichoderma reesei. Biotechnol. Biofuels 2019, 12, 1–17. [Google Scholar] [CrossRef]
- Veerana, M.; Yu, N.; Ketya, W.; Park, G. Application of Non-Thermal Plasma to Fungal Resources. J. Fungi 2022, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. Oscillations in ionized gases. Proc. Natl. Acad. Sci. USA 1928, 14, 627. [Google Scholar] [CrossRef] [Green Version]
- Tonks, L. The Birth of “Plasma”. Am. J. Phys. 1967, 35, 857–858. [Google Scholar] [CrossRef]
- Mitra, S.; Veerana, M.; Choi, E.-H.; Park, G. Effects of pre-treatment using plasma on the antibacterial activity of mushroom surfaces. Foods 2021, 10, 1888. [Google Scholar] [CrossRef] [PubMed]
- Veerana, M.; Lim, J.-S.; Choi, E.-H.; Park, G. Aspergillus oryzae spore germination is enhanced by non-thermal atmospheric pressure plasma. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Znameroski, E.A.; Coradetti, S.T.; Roche, C.M.; Tsai, J.C.; Iavarone, A.T.; Cate, J.H.; Glass, N.L. Induction of lignocellulose-degrading enzymes in Neurospora crassa by cellodextrins. Proc. Natl. Acad. Sci. USA 2012, 109, 6012–6017. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Beeson, W.T.; Iavarone, A.T.; Sun, J.; Marletta, M.A.; Cate, J.H.; Glass, N.L. Systems analysis of plant cell wall degradation by the model filamentous fungus Neurospora crassa. Proc. Natl. Acad. Sci. USA 2009, 106, 22157–22162. [Google Scholar] [CrossRef] [Green Version]
- Phillips, C.M.; Iavarone, A.T.; Marletta, M.A. Quantitative proteomic approach for cellulose degradation by Neurospora crassa. J. Proteome Res. 2011, 10, 4177–4185. [Google Scholar] [CrossRef]
- Coradetti, S.T.; Xiong, Y.; Glass, N.L. Analysis of a conserved cellulase transcriptional regulator reveals inducer-independent production of cellulolytic enzymes in Neurospora crassa. Microbiologyopen 2013, 2, 595–609. [Google Scholar] [CrossRef]
- Higuchi, Y. Membrane Traffic in Aspergillus oryzae and Related Filamentous Fungi. J. Fungi 2021, 7, 534. [Google Scholar] [CrossRef]
- Balmant, W.; Sugai-Guerios, M.H.; Coradin, J.H.; Krieger, N.; Furigo Junior, A.; Mitchell, D.A. A model for growth of a single fungal hypha based on well-mixed tanks in series: Simulation of nutrient and vesicle transport in aerial reproductive hyphae. PLoS ONE 2015, 10, e0120307. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Wang, B.; Xu, Y.; Chen, Z.; Cui, Q.; Yang, Y.; Chen, H.; Kong, M.G. Intracellular ROS mediates gas plasma-facilitated cellular transfection in 2D and 3D cultures. Sci. Rep. 2016, 6, 27872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Ho Kang, M.; Sup Uhm, H.; Joon Lee, G.; Ha Choi, E.; Han, I. Effects of atmospheric-pressure non-thermal bio-compatible plasma and plasma activated nitric oxide water on cervical cancer cells. Sci. Rep. 2017, 7, 45781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porrini, C.; Ramarao, N.; Tran, S.L. Dr. NO and Mr. Toxic-the versatile role of nitric oxide. Biol. Chem. 2020, 401, 547–572. [Google Scholar] [CrossRef]
- Martin, J.F. Calcium-containing phosphopeptides pave the secretory pathway for efficient protein traffic and secretion in fungi. Microb. Cell Factories 2014, 13, 117. [Google Scholar] [CrossRef]
- Chen, L.; Zou, G.; Wang, J.; Wang, J.; Liu, R.; Jiang, Y.; Zhao, G.; Zhou, Z. Characterization of the Ca2+-responsive signaling pathway in regulating the expression and secretion of cellulases in Trichoderma reesei Rut-C30. Mol. Microbiol. 2016, 100, 560–575. [Google Scholar] [CrossRef] [Green Version]
- Rusakov, D.A. Ca2+ dependent mechanisms of presynaptic control at central synapses. Neuroscientist 2006, 12, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Tvon Woedtke, T.; Emmert, S.; Metelmann, H.R.; Rupf, S.; Weltmann, K.D. Perspectives on cold atmospheric plasma (CAP) applications in medicine. Phys. Plasmas 2020, 27, 070601. [Google Scholar] [CrossRef]
- Ranieri, P.; Sponsel, N.; Kizer, J.; Rojas-Pierce, M.; Hernández, R.; Gatiboni, L.; Grunden, A.; Stapelmann, K. Plasma agriculture: Review from the perspective of the plant and its ecosystem. Plasma Processes Polym. 2021, 18, 2000162. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.-F.; Li, H.-P.; Wang, L.-Y.; Zhang, C.; Xing, X.-H.; Bao, C.-Y. Atmospheric and room temperature plasma (ARTP) as a new powerful mutagenesis tool. Appl. Microbiol. Biotechnol. 2014, 98, 5387–5396. [Google Scholar] [CrossRef]
- Veerana, M.; Mitra, S.; Ki, S.H.; Kim, S.M.; Choi, E.H.; Lee, T.; Park, G. Plasma-mediated enhancement of enzyme secretion in Aspergillus oryzae. Microb. Biotechnol. 2021, 14, 262–276. [Google Scholar] [CrossRef] [PubMed]
- González, A.; Cabrera, M.D.L.Á.; Henríquez, M.J.; Contreras, R.A.; Morales, B.; Moenne, A. Cross talk among calcium, hydrogen peroxide, and nitric oxide and activation of gene expression involving calmodulins and calcium-dependent protein kinases in Ulva compressa exposed to copper excess. Plant Physiol. 2012, 158, 1451–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response mechanism of plants to drought stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Wu, L.; Yi, H.; Zhang, H. Reactive oxygen species and Ca2+ are involved in sodium arsenite-induced cell killing in yeast cells. FEMS Microbiol. Lett. 2013, 343, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, K.T.; Shinyashiki, M.; Switzer, C.H.; Valentine, J.S.; Gralla, E.B.; Thiele, D.J.; Fukuto, J.M. Effects of nitric oxide on the copper-responsive transcription factor Ace1 in Saccharomyces cerevisiae: Cytotoxic and cytoprotective actions of nitric oxide. Arch. Biochem. Biophys. 2000, 377, 296–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riquelme, M.; Bartnicki-García, S.; González-Prieto, J.M.; Sánchez-León, E.; Verdín-Ramos, J.A.; Beltrán-Aguilar, A.; Freitag, M. Spitzenkorper localization and intracellular traffic of green fluorescent protein-labeled CHS-3 and CHS-6 chitin synthases in living hyphae of Neurospora crassa. Eukaryot. Cell 2007, 6, 1853–1864. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Shen, Y.; Wang, W.; Wei, D. Mn(2+) modulates the expression of cellulase genes in Trichoderma reesei Rut-C30 via calcium signaling. Biotechnol. Biofuels 2018, 11, 54. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, X.; Zhao, X.; Shen, Y.; Xu, X.; Wei, L.; Wang, W.; Wei, D. cAMP activates calcium signalling via phospholipase C to regulate cellulase production in the filamentous fungus Trichoderma reesei. Biotechnol. Biofuels 2021, 14, 62. [Google Scholar] [CrossRef]
- Siddiqi, M.Y.; Glass AD, M.; Ruth, T.J.; Rufty, T.W., Jr. Studies of the Uptake of Nitrate in Barley: I. Kinetics of 13NO3− Influx. Plant Physiol. 1990, 93, 1426–1432. [Google Scholar] [CrossRef] [Green Version]
- McClure, P.R.; Kochian, L.V.; Spanswick, R.M.; Shaff, J.E. Evidence for cotransport of nitrate and protons in maize roots: I. Effects of nitrate on the membrane potential. Plant Physiol. 1990, 93, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Ullrich, W.R.; Novacky, A. Nitrate-dependent membrane potential changes and their induction in Lemna gibba G 1. Plant Sci. Lett. 1981, 22, 211–217. [Google Scholar] [CrossRef]
- Uhrenholt, T.R.; Schjerning, J.; Vanhoutte, P.M.; Jensen, B.L.; Skøtt, O. Intercellular calcium signaling and nitric oxide feedback during constriction of rabbit renal afferent arterioles. Am. J. Physiol. Ren. Physiol. 2007, 292, F1124–F1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loaiza, A.; Carretta, M.D.; Taubert, A.; Hermosilla, C.; Hidalgo, M.A.; Burgos, R.A. Differential intracellular calcium influx, nitric oxide production, ICAM-1 and IL8 expression in primary bovine endothelial cells exposed to nonesterified fatty acids. BMC Vet. Res. 2016, 12, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seol, Y.B.; Kim, J.; Park, S.H.; Young Chang, H. Atmospheric pressure pulsed plasma induces cell death in photosynthetic organs via intracellularly generated ROS. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Adhikari, B.C.; Park, G.; Choi, E.H. Cold plasma seed priming modulates growth, redox homeostasis and stress response by inducing reactive species in tomato (Solanum lycopersicum). Free. Radic. Biol. Med. 2020, 156, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Campos Antoniêto, A.C.; Ramos Pedersoli, W.; dos Santos Castro, L.; da Silva Santos, R.; Cruz, A.H.d.S.; Nogueira, K.M.V.; Silva-Rocha, R.; Rossi, A.; Silva, R.N. Deletion of pH regulator pac-3 affects cellulase and xylanase activity during sugarcane bagasse degradation by Neurospora crassa. PLoS ONE 2017, 12, e0169796. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




| Genes | Primer Sequences |
|---|---|
| actin | Forward- 5′-TGA TCT TAC CGA CTA CCT-3′Reverse- 5′-CAG AGC TTC TCC TTG ATG-3′ |
| cbh-1 | Forward- 5′-ATC TGG GAA GCG AAC AAA G-3′Reverse- 5′-TAG CGG TCG TCG GAA TAG-3′ |
| gh6-2 | Forward- 5′-CCC ATC ACC ACT ACT ACC-3′Reverse- 5′-CCA GCC CTG AAC ACC AAG-3′ |
| gh5-1 | Forward- 5′- GAG TTC ACA TTC CCT GAC A-3′Reverse- 5′-CGA AGC CAA CAC GGA AGA-3′ |
| gh3-4 | Forward- 5′- AAC AAG GTC AAC GGT ACG TGG-3′Reverse- 5′-TCG TCA TAT CCA TAC CAC TGT TTG-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, N.-N.; Ketya, W.; Choi, E.-H.; Park, G. Plasma Promotes Fungal Cellulase Production by Regulating the Levels of Intracellular NO and Ca2+. Int. J. Mol. Sci. 2022, 23, 6668. https://doi.org/10.3390/ijms23126668
Yu N-N, Ketya W, Choi E-H, Park G. Plasma Promotes Fungal Cellulase Production by Regulating the Levels of Intracellular NO and Ca2+. International Journal of Molecular Sciences. 2022; 23(12):6668. https://doi.org/10.3390/ijms23126668
Chicago/Turabian StyleYu, Nan-Nan, Wirinthip Ketya, Eun-Ha Choi, and Gyungsoon Park. 2022. "Plasma Promotes Fungal Cellulase Production by Regulating the Levels of Intracellular NO and Ca2+" International Journal of Molecular Sciences 23, no. 12: 6668. https://doi.org/10.3390/ijms23126668
APA StyleYu, N.-N., Ketya, W., Choi, E.-H., & Park, G. (2022). Plasma Promotes Fungal Cellulase Production by Regulating the Levels of Intracellular NO and Ca2+. International Journal of Molecular Sciences, 23(12), 6668. https://doi.org/10.3390/ijms23126668








