Open Questions in Cold Atmospheric Plasma Treatment in Head and Neck Cancer: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Focused PICO Question
2.4. Selection of Studies
2.5. Data Extraction and Method of Analysis
2.6. Risk of Bias Assessment
2.7. Statistical Analysis
3. Results
3.1. Study Selection
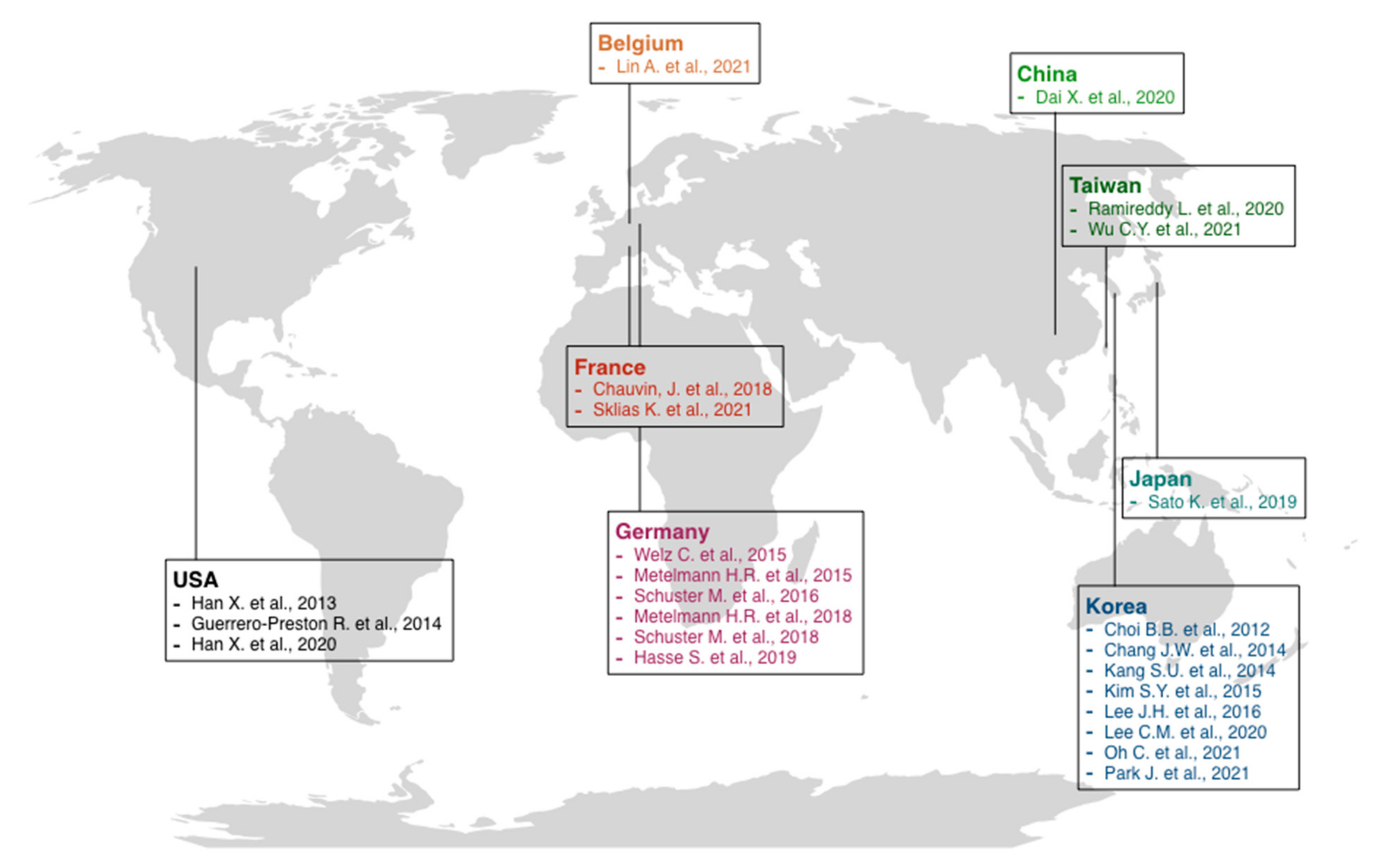
3.2. General Characteristics of Included Studies
- -
- -
3.3. Results of Risk of Bias Assessment
3.4. Preclinical Model: Cell Lines
3.5. Preclinical Models: In Vivo Animal Studies and Ex Vivo Human Studies
3.6. Clinical Model
3.7. Types of CAP Devices and CAP Treatment Parameters
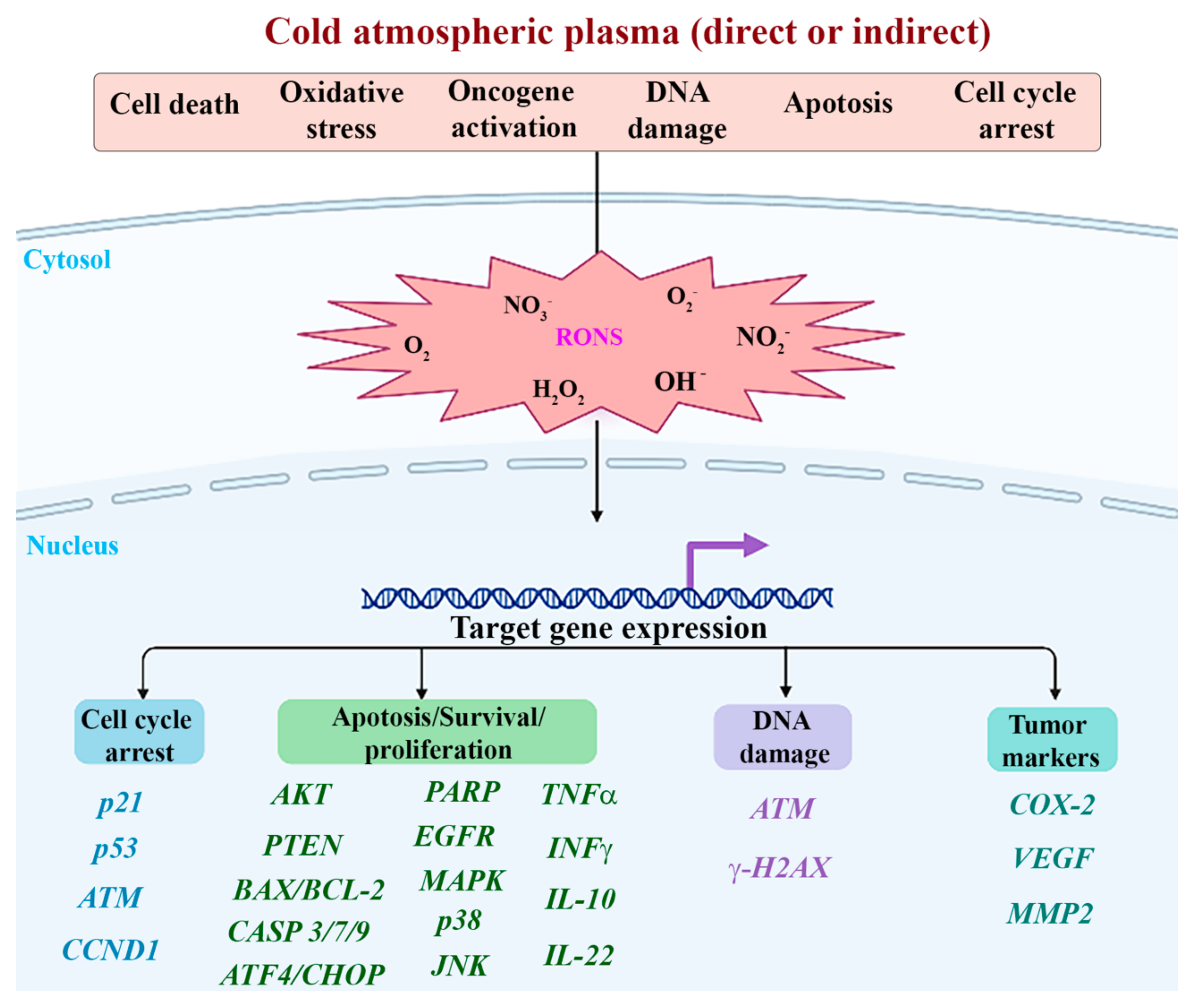
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Chow, L.Q.M. Head and Neck Cancer. N. Engl. J. Med. 2020, 382, 60–72. [Google Scholar] [CrossRef]
- Svahn, M.F.; Munk, C.; Nielsen, T.S.S.; von Buchwald, C.; Frederiksen, K.; Kjaer, S.K. Trends in all-cause five-year mortality after head and neck cancers diagnosed over a period of 33 years. Focus on estimated degree of association with human papillomavirus. Acta Oncol. 2016, 55, 1084–1090. [Google Scholar] [CrossRef]
- National Cancer Institute. Cancer Stat Facts: Oral Cavity and Pharynx Cancer. Available online: https://seer.cancer.gov/statfacts/html/oralcav.html (accessed on 24 June 2022).
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef]
- Chauvin, J.; Judee, F.; Merbahi, N.; Vicendo, P. Effects of Plasma Activated Medium on Head and Neck FaDu Cancerous Cells: Comparison of 3D and 2D Response. Anticancer Agents Med. Chem. 2018, 18, 776–783. [Google Scholar] [CrossRef]
- Bolt, J.; Vo, Q.N.; Kim, W.J.; McWhorter, A.J.; Thomson, J.; Hagensee, M.E.; Friedlander, P.; Brown, K.D.; Gilbert, J. The ATM/p53 pathway is commonly targeted for inactivation in squamous cell carcinoma of the head and neck (SCCHN) by multiple molecular mechanisms. Oral Oncol. 2005, 41, 1013–1020. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef]
- Pierik, A.S.; Leemans, C.R.; Brakenhoff, R.H. Resection Margins in Head and Neck Cancer Surgery: An Update of Residual Disease and Field Cancerization. Cancers 2021, 13, 2635. [Google Scholar] [CrossRef]
- Cavicchi, O.; Piccin, O.; Galasso, F.S.; Marchi, C.; Cantaroni, C.; Latini, G.; Rossi, M.T.; Di Candia, G.; Caliceti, U.; Ceroni, A.R. Multicenter survey through a questionnaire on the prognostic value of surgical resection margin in head and neck tumors. Acta Otorhinolaryngol. Ital. 2000, 20, 413–417. [Google Scholar]
- Lam, L.; Samman, N. Speech and swallowing following tongue cancer surgery and free flap reconstruction—A systematic review. Oral Oncol. 2013, 49, 507–524. [Google Scholar] [CrossRef] [PubMed]
- Brook, I. Late side effects of radiation treatment for head and neck cancer. Radiat. Oncol. J. 2020, 38, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Elaldi, R.; Roussel, L.M.; Gal, J.; Scheller, B.; Chamorey, E.; Schiappa, R.; Lasne-Cardon, A.; Louis, M.Y.; Culié, D.; Dassonville, O.; et al. Correlations between long-term quality of life and patient needs and concerns following head and neck cancer treatment and the impact of psychological distress. A multicentric cross-sectional study. Eur. Arch. Otorhinolaryngol. 2021, 278, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Metelmann, H.R.; Seebauer, C.; Miller, V.; Fridman, A.; Bauer, G.; Graves, D.B.; Pouvesle, J.M.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; et al. Clinical experience with cold plasma in the treatment of locally advanced head and neck cancer. Clin. Plasma Med. 2018, 9, 6–13. [Google Scholar] [CrossRef]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 5. [Google Scholar] [CrossRef]
- Silva-Teixeira, R.; Laranjo, M.; Lopes, B.; Almeida-Ferreira, C.; Gonçalves, A.C.; Rodrigues, T.; Matafome, P.; Sarmento-Ribeiro, A.B.; Caramelo, F.; Botelho, M.F. Plasma activated media and direct exposition can selectively ablate retinoblastoma cells. Free Radic. Biol. Med. 2021, 171, 302–313. [Google Scholar] [CrossRef]
- Bogaerts, A.; Neyts, E.C. Plasma Technology: An Emerging Technology for Energy Storage. ACS Energy Lett. 2018, 3, 1013–1027. [Google Scholar] [CrossRef]
- Witzke, K.; Seebauer, C.; Jesse, K.; Kwiatek, E.; Berner, J.; Semmler, M.-L.; Boeckmann, L.; Emmert, S.; Weltmann, K.-D.; Metelmann, H.-R.; et al. Plasma medical oncology: Immunological interpretation of head and neck squamous cell carcinoma. Plasma Process. Polym. 2020, 17, 1900258. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kINPen—A review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef]
- Stratmann, B.; Costea, T.C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of Cold Atmospheric Plasma Therapy vs Standard Therapy Placebo on Wound Healing in Patients With Diabetic Foot Ulcers: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef]
- Schweigert, I.; Zakrevsky, D.; Gugin, P.; Yelak, E.; Golubitskaya, E.; Troitskaya, O.; Koval, O. Interaction of Cold Atmospheric Argon and Helium Plasma Jets with Bio-Target with Grounded Substrate Beneath. Appl. Sci. 2019, 9, 4528. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Klas, M.; Liu, Y.Y.; Stack, M.S.; Ptasinska, S. DNA damage in oral cancer cells induced by nitrogen atmospheric pressure plasma jets. Appl. Phys. Lett. 2013, 102, 233703. [Google Scholar] [CrossRef]
- Boehm, D.; Bourke, P. Safety implications of plasma-induced effects in living cells—A review of in vitro and in vivo findings. Biol. Chem. 2018, 400, 3–17. [Google Scholar] [CrossRef]
- Assadian, O.; Ousey, K.J.; Daeschlein, G.; Kramer, A.; Parker, C.; Tanner, J.; Leaper, D.J. Effects and safety of atmospheric low-temperature plasma on bacterial reduction in chronic wounds and wound size reduction: A systematic review and meta-analysis. Int. Wound J. 2019, 16, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer 2012, 130, 2185–2194. [Google Scholar] [CrossRef]
- Shakouri, R.; Khani, M.R.; Samsavar, S.; Jezeh, M.A.; Abdollahimajd, F.; Hosseini, S.I.; Dilmaghanian, A.; Ghasemi, E.; Alihoseini, M.R.; Shokri, B. In vivo study of the effects of a portable cold plasma device and vitamin C for skin rejuvenation. Sci. Rep. 2021, 11, 21915. [Google Scholar] [CrossRef] [PubMed]
- Bednarik, M.; Mizera, A.; Manas, M.; Navratil, M.; Huba, J.; Achbergerova, E.; Stoklasek, P. Influence of the β-Radiation/Cold Atmospheric-Pressure Plasma Surface Modification on the Adhesive Bonding of Polyolefins. Materials 2020, 14, 76. [Google Scholar] [CrossRef]
- Carvalho, A.; Barros, D.R.; da Silva, L.S.; Sanches, E.A.; Pinto, C.D.C.; de Souza, S.M.; Clerici, M.; Rodrigues, S.; Fernandes, F.A.N.; Campelo, P.H. Dielectric barrier atmospheric cold plasma applied to the modification of Ariá (Goeppertia allouia) starch: Effect of plasma generation voltage. Int. J. Biol. Macromol. 2021, 182, 1618–1627. [Google Scholar] [CrossRef]
- Dolci, L.S.; Liguori, A.; Panzavolta, S.; Miserocchi, A.; Passerini, N.; Gherardi, M.; Colombo, V.; Bigi, A.; Albertini, B. Non-equilibrium atmospheric pressure plasma as innovative method to crosslink and enhance mucoadhesion of econazole-loaded gelatin films for buccal drug delivery. Colloids Surf. B Biointerfaces 2018, 163, 73–82. [Google Scholar] [CrossRef]
- Hua, D.; Cai, D.; Ning, M.; Yu, L.; Zhang, Z.; Han, P.; Dai, X. Cold atmospheric plasma selectively induces G(0)/G(1) cell cycle arrest and apoptosis in AR-independent prostate cancer cells. J. Cancer 2021, 12, 5977–5986. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Diedrich, S.; Pati, O.; Freund, E.; Flieger, R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Cold Physical Plasma Selectively Elicits Apoptosis in Murine Pancreatic Cancer Cells In Vitro and In Ovo. Anticancer Res. 2018, 38, 5655–5663. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Holmes, B.; Cheng, X.; Zhu, W.; Keidar, M.; Zhang, L.G. Cold atmospheric plasma for selectively ablating metastatic breast cancer cells. PLoS ONE 2013, 8, e73741. [Google Scholar] [CrossRef] [PubMed]
- Semmler, M.L.; Bekeschus, S.; Schäfer, M.; Bernhardt, T.; Fischer, T.; Witzke, K.; Seebauer, C.; Rebl, H.; Grambow, E.; Vollmar, B.; et al. Molecular Mechanisms of the Efficacy of Cold Atmospheric Pressure Plasma (CAP) in Cancer Treatment. Cancers 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Solé-Martí, X.; Espona-Noguera, A.; Ginebra, M.P.; Canal, C. Plasma-Conditioned Liquids as Anticancer Therapies In Vivo: Current State and Future Directions. Cancers 2021, 13, 452. [Google Scholar] [CrossRef]
- Chang, J.W.; Kang, S.U.; Shin, Y.S.; Kim, K.I.; Seo, S.J.; Yang, S.S.; Lee, J.S.; Moon, E.; Baek, S.J.; Lee, K.; et al. Non-thermal atmospheric pressure plasma induces apoptosis in oral cavity squamous cell carcinoma: Involvement of DNA-damage-triggering sub-G(1) arrest via the ATM/p53 pathway. Arch. Biochem. Biophys. 2014, 545, 133–140. [Google Scholar] [CrossRef]
- Choi, B.B.; Choi, Y.S.; Lee, H.J.; Lee, J.K.; Kim, U.K.; Kim, G.C. Nonthermal Plasma-Mediated Cancer Cell Death; Targeted Cancer Treatment. J. Therm. Sci. Technol. 2012, 7, 399–404. [Google Scholar] [CrossRef]
- Lee, C.M.; Jeong, Y.I.; Kook, M.S.; Kim, B.H. Combinatorial Effect of Cold Atmosphere Plasma (CAP) and the Anticancer Drug Cisplatin on Oral Squamous Cell Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 7646. [Google Scholar] [CrossRef]
- Lafontaine, J.; Boisvert, J.S.; Glory, A.; Coulombe, S.; Wong, P. Synergy between Non-Thermal Plasma with Radiation Therapy and Olaparib in a Panel of Breast Cancer Cell Lines. Cancers 2020, 12, 348. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 790–799. [Google Scholar] [CrossRef]
- Huang, S.H.; O’Sullivan, B. Overview of the 8th Edition TNM Classification for Head and Neck Cancer. Curr. Treat. Options Oncol. 2017, 18, 40. [Google Scholar] [CrossRef]
- Mohammad, N.S.; Nazli, R.; Zafar, H.; Fatima, S. Effects of lipid based Multiple Micronutrients Supplement on the birth outcome of underweight pre-eclamptic women: A randomized clinical trial. Pak. J. Med. Sci. 2022, 38, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.M.; Diniz, I.M.; de Cara, S.P.; Pedroni, A.C.; Abe, G.L.; D′Almeida-Couto, R.S.; Lima, P.L.; Tedesco, T.K.; Moreira, M.S. Photobiomodulation of Dental Derived Mesenchymal Stem Cells: A Systematic Review. Photomed. Laser Surg. 2016, 34, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O′Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis; Academic Press: Cambridge, MA, USA, 2000; Available online: https://www.ohri.ca (accessed on 4 August 2022).
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Pannone, G.; Caponio, V.C.A.; De Stefano, I.S.; Ramunno, M.A.; Meccariello, M.; Agostinone, A.; Pedicillo, M.C.; Troiano, G.; Zhurakivska, K.; Cassano, T.; et al. Lung histopathological findings in COVID-19 disease—A systematic review. Infect. Agent Cancer 2021, 16, 34. [Google Scholar] [CrossRef]
- Furusaka, T.; Kida, A.; Aoyagi, M.; Makiyama, K.; Iida, H.; Miyakogawa, M.; Kikuchi, K.; Tomita, H. CAP therapy for advanced, recurrent and/or metastatic malignant tumors of the head and neck. Auris Nasus Larynx 1986, 13 (Suppl. 1), S119–S126. [Google Scholar] [CrossRef]
- Akhmedov, I.N.; Isaev, V.M.; Svistushkin, V.M.; Mustafaev, D.M.; Osipenko, E.V. Endolaryngeal cold-plasma microsurgery for the treatment of benign neoplasms in the larynx and the objective assessment of its functional results. Vestn. Otorinolaringol. 2011, 4, 57–60. [Google Scholar]
- Keidar, M.; Walk, R.; Shashurin, A.; Srinivasan, P.; Sandler, A.; Dasgupta, S.; Ravi, R.; Guerrero-Preston, R.; Trink, B. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br. J. Cancer 2011, 105, 1295–1301. [Google Scholar] [CrossRef]
- Virard, F.; Cousty, S.; Cambus, J.P.; Valentin, A.; Kémoun, P.; Clément, F.; Gallyas, F., Jr. Cold atmospheric plasma induces a predominantly necrotic cell death via the microenvironment. PLoS ONE 2015, 10, e0133120. [Google Scholar] [CrossRef]
- Adhikari, E.R.; Ptasinska, S. Correlation between helium atmospheric pressure plasma jet (APPJ) variables and plasma induced DNA damage. Eur. Phys. J. D 2016, 70, 180. [Google Scholar] [CrossRef]
- Laurita, R.; Alviano, F.; Marchionni, C.; Abruzzo, P.M.; Bolotta, A.; Bonsi, L.; Colombo, V.; Gherardi, M.; Liguori, A.; Ricci, F.; et al. A study of the effect on human mesenchymal stem cells of an atmospheric pressure plasma source driven by different voltage waveforms. J. Phys. D Appl. Phys. 2016, 49, 364003. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, K.N. Effects of a Nonthermal Atmospheric Pressure Plasma Jet on Human Gingival Fibroblasts for Biomedical Application. Biomed. Res. Int. 2016, 2016, 2876916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.U.; Choi, J.W.; Chang, J.W.; Kim, K.I.; Kim, Y.S.; Park, J.K.; Kim, Y.E.; Lee, Y.S.; Yang, S.S.; Kim, C.H. N2 non-thermal atmospheric pressure plasma promotes wound healing in vitro and in vivo: Potential modulation of adhesion molecules and matrix metalloproteinase-9. Exp. Dermatol. 2017, 26, 163–170. [Google Scholar] [CrossRef]
- Han, X.; Liu, Y.; Stack, M.S.; Ptasinska, S. 3D Mapping of plasma effective areas via detection of cancer cell damage induced by atmospheric pressure plasma jets. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2014. [Google Scholar]
- Hajizadeh, K.; Hajisharifi, K.; Mehdian, H. Morphological risk assessment of cold atmospheric plasma-based therapy: Bone marrow mesenchymal stem cells in treatment zone proximity. J. Phys. D Appl. Phys. 2019, 52, 495203. [Google Scholar] [CrossRef]
- Jaiswal, A.S.; Kumar, R.; Thakar, A.; Kumar, R.; Verma, H.; Bhalla, A.S.; Kairo, A.K.; Kakkar, A.; Sharma, S.C.; Sakthivel, P. Plasma ablation-assisted endoscopic excision versus traditional technique of endoscopic excision of juvenile nasopharyngeal angiofibroma. Int. J. Pediatr. Otorhinolaryngol. 2020, 139, 110410. [Google Scholar] [CrossRef]
- Jing, J.; Li, L.; Zheng, L.; Wu, Y.; Jiang, S.; Zhang, L.; Wang, H.; Liu, G. Research on the efficacy of laryngendoscopic low-temperature plasma ablation on early glottic cancer. J. BUON 2020, 25, 2382–2388. [Google Scholar]
- Evert, K.; Kocher, T.; Schindler, A.; Muller, M.; Muller, K.; Pink, C.; Holtfreter, B.; Schmidt, A.; Dombrowski, F.; Schubert, A.; et al. Repeated exposure of the oral mucosa over 12 months with cold plasma is not carcinogenic in mice. Sci. Rep. 2021, 11, 20672. [Google Scholar] [CrossRef]
- Bekeschus, S.; Eisenmann, S.; Sagwal, S.K.; Bodnar, Y.; Moritz, J.; Poschkamp, B.; Stoffels, I.; Emmert, S.; Madesh, M.; Weltmann, K.D.; et al. xCT (SLC7A11) expression confers intrinsic resistance to physical plasma treatment in tumor cells. Redox Biol. 2020, 30, 101423. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Liu, Z.; Xu, D.; Guo, L.; Liu, D.; Kong, M.G. Evaluation of the anticancer effects induced by cold atmospheric plasma in 2D and 3D cell-culture models. Plasma Process. Polym. 2019, 16, 1900072. [Google Scholar] [CrossRef]
- Yan, D.Y.; Lin, L.; Sherman, J.H.; Canady, J.; Trink, B.; Keidar, M. The Correlation Between the Cytotoxicity of Cold Atmospheric Plasma and the Extracellular H2O2-Scavenging Rate. IEEE T R PMS 2018, 2, 618–623. [Google Scholar] [CrossRef]
- Wang, P.; Wang, G. Efficacy of low-temperature plasma ablation combined with low-dose cisplatin chemotherapy in laryngeal carcinoma patients and its influence on tumor markers. J. BUON 2021, 26, 132–137. [Google Scholar]
- Weiss, M.; Barz, J.; Ackermann, M.; Utz, R.; Ghoul, A.; Weltmann, K.D.; Stope, M.B.; Wallwiener, D.; Schenke-Layland, K.; Oehr, C.; et al. Dose-Dependent Tissue-Level Characterization of a Medical Atmospheric Pressure Argon Plasma Jet. ACS Appl. Mater. Interfaces 2019, 11, 19841–19853. [Google Scholar] [CrossRef]
- Tanaka, H.; Nakamura, K.; Mizuno, M.; Ishikawa, K.; Takeda, K.; Kajiyama, H.; Utsumi, F.; Kikkawa, F.; Hori, M. Non-thermal atmospheric pressure plasma activates lactate in Ringer’s solution for anti-tumor effects. Sci. Rep. 2016, 6, 36282. [Google Scholar] [CrossRef]
- Moritz, J.; Metelmann, H.R.; Bekeschus, S. Physical Plasma Treatment of Eight Human Cancer Cell Lines Demarcates Upregulation of CD112 as a Common Immunomodulatory Response Element. IEEE T R PMS 2020, 4, 343–349. [Google Scholar] [CrossRef]
- Metelmann, H.R.; Seebauer, C.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; Metelmann, P. Treating cancer with cold physical plasma: On the way to evidence-based medicine. Contrib. Plasma Phys. 2018, 58, 415–419. [Google Scholar] [CrossRef]
- Ghimire, B.; Szili, E.J.; Lamichhane, P.; Short, R.D.; Lim, J.S.; Attri, P.; Masur, K.; Weltmann, K.D.; Hong, S.H.; Choi, E.H. The role of UV photolysis and molecular transport in the generation of reactive species in a tissue model with a cold atmospheric pressure plasma jet. Appl. Phys. Lett. 2019, 114, 093701. [Google Scholar] [CrossRef]
- Biscop, E.; Lin, A.; Van Boxem, W.; Van Loenhout, J.; De Backer, J.; Deben, C.; Dewilde, S.; Smits, E.; Bogaerts, A. Influence of cell type and culture medium on determining cancer selectivity of cold atmospheric plasma treatment. Cancers 2019, 11, 1287. [Google Scholar] [CrossRef]
- Han, X.; Kapaldo, J.; Liu, Y.; Stack, M.S.; Alizadeh, E.; Ptasinska, S. Large-Scale Image Analysis for Investigating Spatio-Temporal Changes in Nuclear DNA Damage Caused by Nitrogen Atmospheric Pressure Plasma Jets. Int. J. Mol. Sci. 2020, 21, 4127. [Google Scholar] [CrossRef]
- Hasse, S.; Seebauer, C.; Wende, K.; Schmidt, A.; Metelmann, H.R.; von Woedtke, T.; Bekeschus, S. Cold argon plasma as adjuvant tumour therapy on progressive head and neck cancer: A preclinical study. Appl. Sci. 2019, 9, 2061. [Google Scholar] [CrossRef]
- Kang, S.U.; Cho, J.H.; Chang, J.W.; Shin, Y.S.; Kim, K.I.; Park, J.K.; Yang, S.S.; Lee, J.S.; Moon, E.; Lee, K.; et al. Nonthermal plasma induces head and neck cancer cell death: The potential involvement of mitogen-activated protein kinase-dependent mitochondrial reactive oxygen species. Cell Death Dis. 2014, 5, e1056. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.J.; Kang, S.U.; Kim, Y.E.; Park, J.K.; Shin, Y.S.; Kim, Y.S.; Lee, K.; Kim, C.H. Non-thermal plasma induces AKT degradation through turn-on the MUL1 E3 ligase in head and neck cancer. Oncotarget 2015, 6, 33382–33396. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Om, J.Y.; Kim, Y.H.; Kim, K.M.; Choi, E.H.; Kim, K.N. Selective Killing Effects of Cold Atmospheric Pressure Plasma with NO Induced Dysfunction of Epidermal Growth Factor Receptor in Oral Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0150279. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.; Biscop, E.; Gorbanev, Y.; Smits, E.; Bogaerts, A. Toward defining plasma treatment dose: The role of plasma treatment energy of pulsed-dielectric barrier discharge in dictating in vitro biological responses. Plasma Process. Polym. 2021, 19, e2100151. [Google Scholar] [CrossRef]
- Oh, C.; Won, H.R.; Kang, W.S.; Kim, D.W.; Jung, S.N.; Im, M.A.; Liu, L.; Jin, Y.L.; Piao, Y.; Kim, H.J.; et al. Head and Neck Cancer Cell Death due to Mitochondrial Damage Induced by Reactive Oxygen Species from Nonthermal Plasma-Activated Media: Based on Transcriptomic Analysis. Oxid. Med. Cell Longev. 2021, 2021, 9951712. [Google Scholar] [CrossRef]
- Park, J.; Jang, Y.S.; Choi, J.H.; Ryu, M.; Kim, G.C.; Byun, J.H.; Hwang, D.S.; Kim, U.K. Anticancer Efficacy of Nonthermal Plasma Therapy Combined with PD-L1 Antibody Conjugated Gold Nanoparticles on Oral Squamous Cell Carcinoma. Appl. Sci. 2021, 11, 4559. [Google Scholar] [CrossRef]
- Ramireddy, L.; Lai, C.H.; Low, B.S.; Li, C.; Hsieh, J.H.; Lee, J.W.; Wu, H.Y. Cold atmospheric helium plasma induces apoptosis by increasing intracellular reactive oxygen and nitrogen species. Plasma Med. 2020, 10, 243–257. [Google Scholar] [CrossRef]
- Sato, K.; Shi, L.; Ito, F.; Ohara, Y.; Motooka, Y.; Tanaka, H.; Mizuno, M.; Hori, M.; Hirayama, T.; Hibi, H.; et al. Nonthermal plasma specifically kills oral squamous cell carcinoma cells in a catalytic Fe(II)dependent manner. J. Clin. Biochem. Nutr. 2019, 65, 8–15. [Google Scholar] [CrossRef]
- Sklias, K.; Sousa, J.S.; Girard, P.M. Role of short- and long-lived reactive species on the selectivity and anti-cancer action of plasma treatment in vitro. Cancers 2021, 13, 615. [Google Scholar] [CrossRef]
- Welz, C.; Emmert, S.; Canis, M.; Becker, S.; Baumeister, P.; Shimizu, T.; Morfill, G.E.; Harréus, U.; Zimmermann, J.L. Cold Atmospheric Plasma: A Promising Complementary Therapy for Squamous Head and Neck Cancer. PLoS ONE 2015, 10, e0141827. [Google Scholar] [CrossRef]
- Wu, C.Y.; Liao, J.D.; Chen, C.H.; Lee, H.; Wang, S.H.; Liu, B.H.; Lee, C.Y.; Shao, P.L.; Li, E.Y. Non-Thermal Reactive N-2/He Plasma Exposure to Inhibit Epithelial Head and Neck Tumor Cells. Coatings 2021, 11, 1284. [Google Scholar] [CrossRef]
- Dai, X.; Wei, B.; He, Y.; Zhang, Q.; Xi, J. Postoperative efficacy of low-temperature plasma radiofrequency ablation in elderly patients with laryngeal carcinoma and its influences on tumor markers and COX-2 and VEGF expressions in laryngeal carcinoma tissues. J. BUON 2020, 25, 1969–1975. [Google Scholar] [PubMed]
- Metelmann, H.R.; Nedrelow, D.S.; Seebauer, C.; Schuster, M.; von Woedtke, T.; Weltmann, K.D.; Kindler, S.; Metelmann, P.H.; Finkelstein, S.E.; Von Hoff, D.D.; et al. Head and neck cancer treatment and physical plasma. Clin. Plasma Med. 2015, 3, 17–23. [Google Scholar] [CrossRef]
- Schuster, M.; Rutkowski, R.; Hauschild, A.; Shojaei, R.K.; von Woedtke, T.; Rana, A.; Bauer, G.; Metelmann, P.; Seebauer, C. Side effects in cold plasma treatment of advanced oral cancer-Clinical data and biological interpretation. Clin. Plasma Med. 2018, 10, 9–15. [Google Scholar] [CrossRef]
- Schuster, M.; Seebauer, C.; Rutkowski, R.; Hauschild, A.; Podmelle, F.; Metelmann, C.; Metelmann, B.; von Woedtke, T.; Hasse, S.; Weltmann, K.D.; et al. Visible tumor surface response to physical plasma and apoptotic cell kill in head and neck cancer. J. Cranio Maxillofac. Surg. 2016, 44, 1445–1452. [Google Scholar] [CrossRef]
- Kim, C.-H.; Kwon, S.; Bahn, J.H.; Lee, K.; Jun, S.I.; Rack, P.D.; Baek, S.J. Effects of atmospheric nonthermal plasma on invasion of colorectal cancer cells. Appl. Phys. Lett. 2010, 96, 243701. [Google Scholar] [CrossRef]
- Dubuc, A.; Monsarrat, P.; Virard, F.; Merbahi, N.; Sarrette, J.P.; Laurencin-Dalicieux, S.; Cousty, S. Use of cold-atmospheric plasma in oncology: A concise systematic review. Ther. Adv. Med. Oncol. 2018, 10, 1758835918786475. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef]
- Boeckmann, L.; Schäfer, M.; Bernhardt, T.; Semmler, M.L.; Jung, O.; Ojak, G.; Fischer, T.; Peters, K.; Nebe, B.; Müller-Hilke, B.; et al. Cold Atmospheric Pressure Plasma in Wound Healing and Cancer Treatment. Appl. Sci. 2020, 10, 6898. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Xu, M.; Chen, H.; Lu, X.; Ostrikov, K. Cold atmospheric pressure plasmas in dermatology: Sources, reactive agents, and therapeutic effects. Plasma Process. Polym. 2020, 17, e1900218. [Google Scholar] [CrossRef]
- Yan, D.; Sherman, J.H.; Keidar, M. Cold atmospheric plasma, a novel promising anti-cancer treatment modality. Oncotarget 2017, 8, 15977–15995. [Google Scholar] [CrossRef]
- Graves, D.B. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 233001. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Hoan, N.N.; Kim, C.H.; Moon, E.; Choi, K.S.; Yang, S.S.; Lee, J.S. Targeting cancer cells with reactive oxygen and nitrogen species generated by atmospheric-pressure air plasma. PLoS ONE 2014, 9, e86173. [Google Scholar] [CrossRef]
- Keidar, M. Plasma for cancer treatment. Plasma Sources Sci. Technol. 2015, 24, 033001. [Google Scholar] [CrossRef]
- Mitra, S.; Nguyen, L.N.; Akter, M.; Park, G.; Choi, E.H.; Kaushik, N.K. Impact of ROS Generated by Chemical, Physical, and Plasma Techniques on Cancer Attenuation. Cancers 2019, 11, 1030. [Google Scholar] [CrossRef] [Green Version]
- Zubor, P.; Wang, Y.; Liskova, A.; Samec, M.; Koklesova, L.; Dankova, Z.; Dørum, A.; Kajo, K.; Dvorska, D.; Lucansky, V.; et al. Cold Atmospheric Pressure Plasma (CAP) as a New Tool for the Management of Vulva Cancer and Vulvar Premalignant Lesions in Gynaecological Oncology. Int. J. Mol. Sci. 2020, 21, 7988. [Google Scholar] [CrossRef]
- Saadati, F.; Mahdikia, H.; Abbaszadeh, H.A.; Abdollahifar, M.A.; Khoramgah, M.S.; Shokri, B. Comparison of Direct and Indirect cold atmospheric-pressure plasma methods in the B(16)F(10) melanoma cancer cells treatment. Sci. Rep. 2018, 8, 7689. [Google Scholar] [CrossRef] [Green Version]
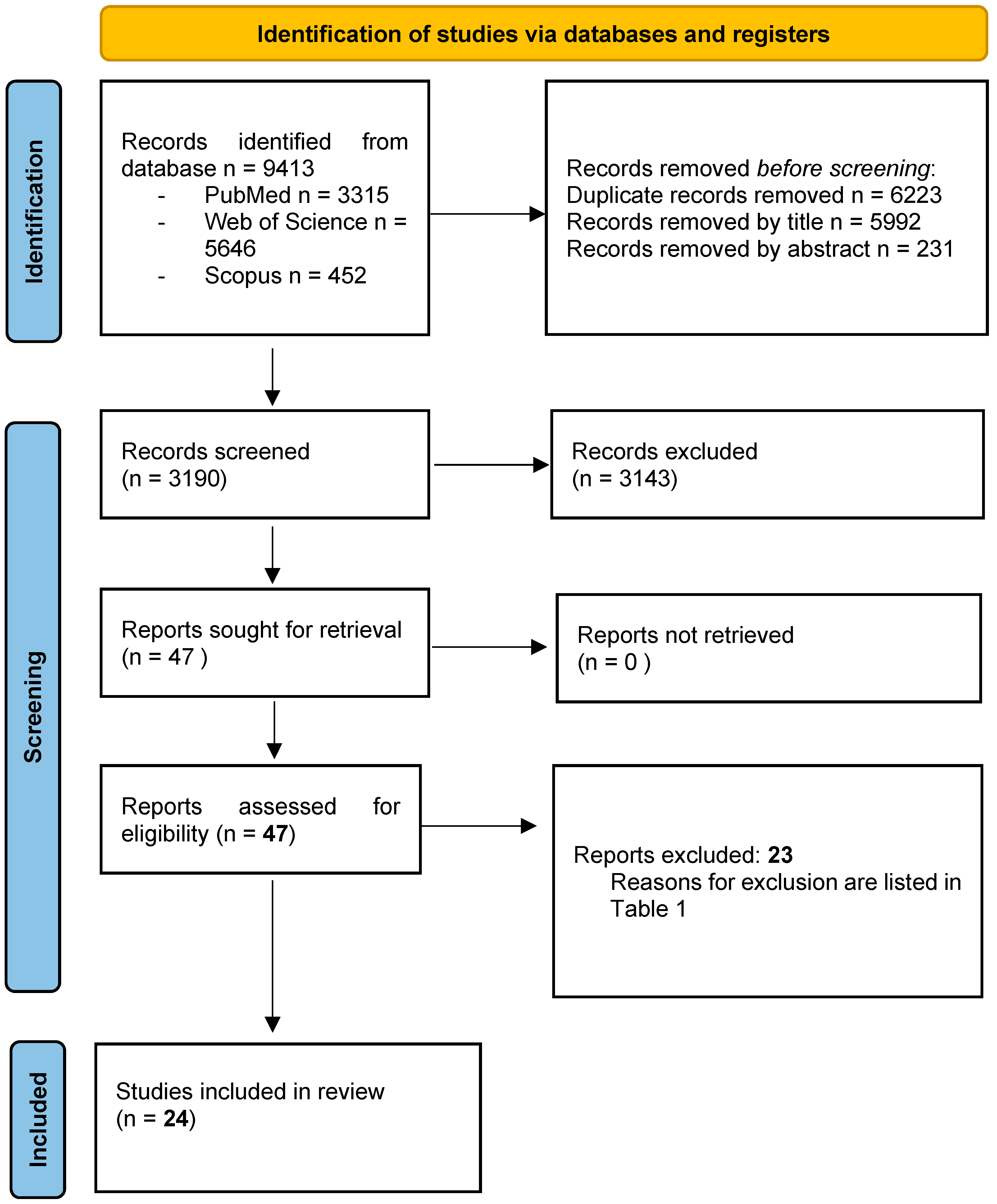
| Study | Reasons for Exclusion |
|---|---|
| Furusaka T. et al., 1986 [48] | No plasma source has been used |
| Akhmedov I.N. et al., 2011 [49] | Article in Russian |
| Keidar M. et al., 2011 [50] | No head and neck in vitro or in vivo experiments |
| Virard F. et al., 2015 [51] | No tumoral cell line, but normal primary human fibroblast cultures isolated from oral tissue |
| Adhikari E.R. et al., 2016 [52] | No head and neck in vitro or in vivo experiments |
| Laurita R. et al., 2016 [53] | No head and neck cell line, but mesenchymal stem cells derived from human fetal membranes (FM-hMSCs) |
| Lee J.H. et al., 2016 [54] | No head and neck cell line, but human gingival fibroblasts |
| Tanaka H. et al., 2016 [66] | U251SP cells (human glioblastoma cell line) and MCF10A cells (human mammary epithelial cell line) as models |
| Kang S.U. et al., 2017 [55] | No head and neck cell line, but primary fibroblast cell line |
| Metelmann H.R. et al., 2018 [68] | Case report |
| Han I. et al., 2018 [56] | Abstract for poster/oral presentation |
| Yan D.Y. et al., 2018 [63] | No head and neck cell lines have been used in the experiments |
| Biscop E. et al., 2019 [70] | No head and neck cell lines have been used in the experiments |
| Ghimire B. et al., 2019 [69] | Agarose has been used as tissue model (no head and neck tissues) |
| Hajizadeh K. et al., 2019 [57] | Bone marrow mesenchymal stem cells, no head and neck tumoral cell model |
| Weiss M. et al., 2019 [65] | Primary human fibroblasts isolated from foreskin samples, no head and neck tumoral cell model |
| Zhang H. et al., 2019 [62] | A549 lung carcinoma cells, no head and neck tumoral cell model |
| Bekeschus S. et al., 2020 [61] | SK-MEL-28, MeWo, MaMel86a, Panc-1, Miapaca2GR, HeLa, MDA-MB-231, PC-3, 501-Mel, OVCAR3, and A375; no head and neck tumoral cell model |
| Jaiswal A.S. et al., 2020 [58] | Nasopharyngeal angiofibroma |
| Jing J. et al., 2020 [59] | LTPA by plasma radiometer |
| Moritz J. et al., 2020 [67] | SK-Mel 28, MNT-1, Capan-1, PANC-01, HT-29, SW 480, MCF-7, and MDA-MB-231, no head and neck tumoral cell model. |
| Evert K. et al., 2021 [60] | Only oral normal mucosa model in vivo (mouse) |
| Wang P. et al., 2021 [64] | LTPA by low-temperature bipolar radiofrequency ablation system |
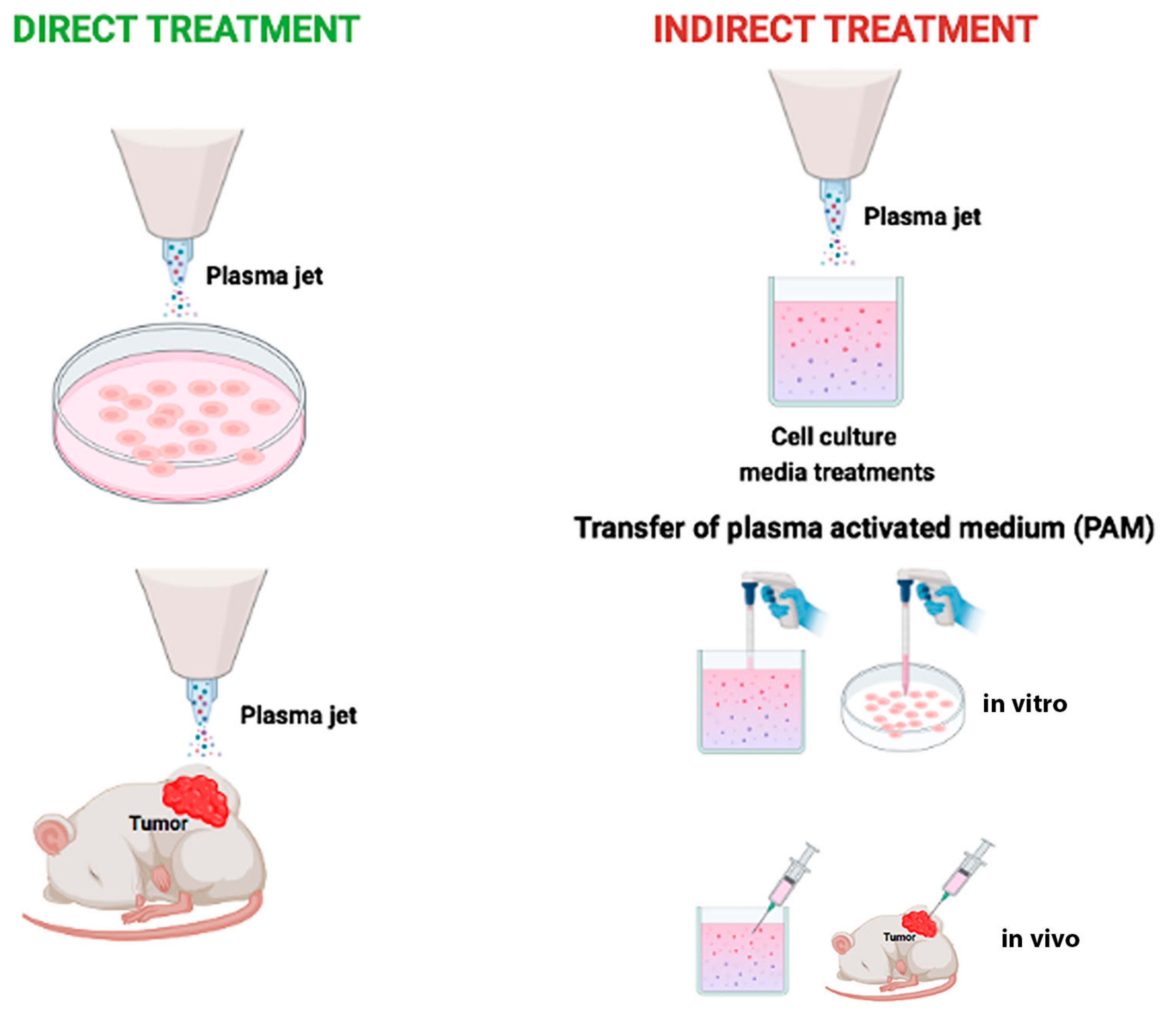
| Authors | Level (In Vitro/In Vivo/Ex Vivo) | Models | Cell Type(s) | Plasma Device | Direct/ Indirect Treatment | Condition of Use | Analyses | Results |
|---|---|---|---|---|---|---|---|---|
| Choi B.B. et al., 2012 [37] | In vitro | Cell lines | Normal: WI-38 Tumor: SCC-25 | air-NTP | Direct | 4 × 104 cells incubated on cover slip (12 mm diameter) for 24 h with the GNP-EGFR, washed with serum free media, and then cover slips placed 2 mm from the plasma source and exposed to treatment. During plasma treatment, cells humidified by 5 μL serum free media. | Biological: -Cell viability; -Protein expression; -Cell morphology. | BIOLOGICAL Cell Viability (Trypan blue): -Plasma death rate 3.61% in WI-38 and 15.7% in SCC-25; -Plasma plus GNP-EGFR death rate 34% in WI-38 and 92% in SCC-25. Protein expression (Western blot): -Basal EGFR more expressed in SCC-25 cells than in WI-38. Cell morphology (SEM): -SCC-25 morphology, after plasma plus GNP-EGFR changed from spindle to round shape, and cell shrinkage and membrane rupture were observed. |
| Han X. et al., 2013 [23] | In vitro | Cell line | Tumor: SCC-25 | N2-APPJ | Direct | Before plasma irradiation, SCC-25 were grown on a grid slide with a marked dot at the center placed inside a P35 cultural dish (3 mm thickness). 2.4 mL PBS was added instead of a culture medium to prevent cell desiccation. Control 1: treated with N2 gas without plasma. Control 2: untreated neither plasma nor gas flow. | Biological: -Identification and quantification of cells with DNA damage. Chemical: -Determination of reactive species. | BIOLOGICAL DNA damage (Immunofluorescence): -60% DSB damage after 30 s irradiation; -Nearly 80% at 2 min. CHEMICAL Determination of reactive species (Optical emission spectroscopy): -Nitrogen oxide bands (NO-ƴ) detected between 200 and 300 nm; -The bands of the second positive system of molecular nitrogen recorded in the range of 300–420 nm; -The dominant peak around 557 nm represents an excimer of ON2 (O(1D)N2). |
| Chang J.W. et al., 2014 [36] | In vitro | Cell lines | Tumor: MSK QLL1 SCC-1483 SCC-15 SCC-25 | He + O2− NTP spray type | Direct | Cells were treated in complete medium. Control: cells treated with He + O2 gas without plasma. | Biological: -Apoptosis; -Cell cycle -DNA damage; -Cell morphology; -Protein expression. | BIOLOGICAL Apoptosis and cell morphology (FACS, fluorescence microscopy): -Rounding, shrinkage, detachment and increase in annexin V, PI and TUNEL positive cells. Cell cycle (FACS): -Sub-G1 cell cycle arrest in wild-type p53 OSCCs (SCC-25), not in mutated p53; -Decrease in sub-G1 phase cells in the ATM siRNA-transfected compared with the control siRNA-transfected. DNA damage (COMET assay, immunocytochemistry): -Intensity-dependent increase in the number of typical comets with elongated tails; -Increased ƴH2AX foci in the nuclei of SCC-25 cells. Protein expression (Western blot): -NTP increased p-ATM, p-p53, p21, cyclin D1, and ƴH2AX expression in wild-type p53, but not mutated p53 cells. |
| Guerrero-Preston R. et al., 2014 [5] | In vitro | Cell lines | Normal: NOKsi OKF-6 Tumor: JHU-022 JHU-028 JHU-029 SCC-25 | He CAP | Direct | Cells were treated in complete medium. Control: cells treated with He gas without plasma. | Biological: -Cell viability; -Colony formation; -Protein expression. | BIOLOGICAL Cell viability (MTT): -Dose-response decrease in SCC-25 and JHU-O28; -Decreased in JHU-O22 and JHU-O29 after 30 and 45 s treatment; -Not affected in OKF-2; -SCC-25 and OKF-6 formed colonies after 10 s treatment; -NOKsi cells formed colonies. Protein expression (Western blot): -No occurrence of PARP cleavage. |
| Kang S.U. et al., 2014 [73] | In vitro | Cell lines | Tumor: FaDu HN-9 SNU-1041 SNU-899 | He + O2-NTP spray type | Direct | 3 mL of cell suspension with a concentration of 1 × 105 cells/mL on the petri dish (diameter 60 mm) treated in complete medium (depth 10 mm). Control: cells treated with He + O2 gas without plasma. | Biological: -Apoptosis; -Protein expression; -Mitochondrial damage. Chemical: -ROS production. | BIOLOGICAL Apoptosis (FACS, TUNEL): -Significant increased apoptosis of treated FaDu, HN-9, SNU-899, and SNU-1041 compared with the control and gas-only groups; -In FaDu, NTP induced apoptosis by MAPK-mediated mitochondrial ROS. Protein expression (Western blot): -Increased expression of p-p38, p-c-JUN N-terminal kinase (JNK), and p-ERK after NTP treatment in FaDu. Mitochondrial damage (FACS—MMP measurement): -Loss of MMP and mitochondrial damage. CHEMICAL ROS production (FACS and confocal microscopy) -Mitochondrial superoxide levels increase in NTP-treated cells. |
| Kang S.U. et al., 2014 [73] | In vivo | FaDu-derived xenograft in 16 male BALB/c nu/nu mice. | Tumor: FaDu | He + O2-NTP spray type | Direct | Daily single treatment, 1 cm apart from the upper margin of tumor for 20 days. Control: untreated BALB/c nu/nu mice | Biological: -Protein expression; -Apoptosis; -Tumor mass measurement. | BIOLOGICAL Protein expression (IHC): -Increased caspase-3 and Nox-3 levels. Apoptosis (TUNEL assay): -Increased TUNEL staining, compared with control. Tumor mass measurement (Sliding caliper): -Inhibition of tumor growth after 11 days of treatments. |
| Kim S.Y. et al., 2015 [74] | In vitro | Cell lines | Normal: MRC-5 HNLF Tumor: AMC-HN6 Human derived cancer cell lines: FaDu SCC-15 SCC-QLL1 SCC-1483 SNU-1041 Murine derived cancer cell line: SCC-7 | LTP produced by He + O2-NTP spray type | Indirect | LTP was applied to HNC cells in the absence of serum. Control Media (CM): air-treated media. | Biological: -Cell viability; -Apoptosis; -Protein expression; -Colony forming assay; -Gene expression; -Detection of AKT ubiquitination. Chemical: -ROS production. | BIOLOGICAL after NTP treatment. Cell Viability (MTT on SCC-15 and SCC-QLL1): -Significant reduction of HNC viability. Apoptosis (TUNEL assay on SCC-15 and SCC-QLL1): -Apoptotic cell death. Detection of AKT ubiquitination (SCC-15): -AKT degradation via lysine 48-linked ubiquitination. RT-PCR (SCC-15) and proximity ligation assay (SCC-15, SCC-QLL1): -Increased level of MUL1 mRNA; -Cellular ROS increase MUL1/AKT binding and cytotoxicity. BIOLOGICAL after LTP treatment. -Decreased SCC-15 viability; -Greater inhibition of colony-forming growth in LTP-treated SCC-15 compared with CM; -LTP reduced AKT or p-AKT levels and increased the level of MUL1; -CM did not change the levels of AKT, p-AKT, or MUL1; -LTP reduced AKT and p-AKT levels, which was prevented by MUL1 knockdown. CHEMICAL Measurement of ROS (FACS): -NTP induced ROS production in human HNC cells. |
| Kim S.Y. et al., 2015 [74] | In vivo | SCC-7-derived syngeneic tumor model in 10 female C3H/HeJ mice. SCC-15-derived xenograft in BALB/c nu/nu mice. | Tumor: SCC-7 SCC-15 | LTP produced by He + O2-NTP spray type | Indirect by injection | Daily treatment of 200 μL of medium or LTP for 6 days by intra-tumoral injection. | Biological: -IHC; -Protein expression. | BIOLOGICAL -Inhibitory effect of tumor development after the fourth treatment and reduction in tumor weight; -Inhibition in tumor volume after the ninth treatment; -Reduction of p-AKT levels and increase in MUL1. |
| Welz C. et al., 2015 [82] | In vitro | Cell lines | Tumor: FaDu OSC-19 | air-CAP (SMD) | Direct | Cell culture medium was removed before the CAP treatment and was added immediately after treatment. Control: cells not exposed to CAP. | Biological: -Cell viability; -DNA damage; -Apoptosis. | BIOLOGICAL Cell viability (MTT): -Time-dependent reduction in OSC-19 and FaDu cells compared to control. DNA damage (COMET assay): -Time-dependent DNA fragmentation. Apoptosis (FACS): -No dose-dependent apoptosis in both cell lines. |
| Lee J.H. et al., 2016 [75] | In vitro | Cell lines | Normal: HGF-1 Tumor: HSC-2 SCC-15 | N2-CAP jet | Direct | Cells were treated in complete medium (concentration of 1 × 104 cells/100 μL). Control: cells not exposed to CAP. | Biological: -Cell viability; -Apoptosis; -Cell cycle; -Protein expression. Chemical: -Intracellular ROS detection; -Thiol detection. | BIOLOGICAL Cell Viability (Tetrazolium salt assay): -Significant decrease in HSC-2 and SCC-15 compared to HGF-1. Apoptosis (FACS): -More apoptotic death in HSC-2 compared to HGF-1. Cell Cycle (FACS): -HSC-2 killed via sub-G1 arrest; -HGF-1 did not show changes in cell cycle components. Protein expression (Immunoblot assay): -Degradation and dephosphorylation of EGFR in HSC-2 cells; ->deactivation of the EGFR pathway. CHEMICAL -ROS measurement by OES. |
| Chauvin J. et al., 2018 [6] | In vitro | Cell lines | Tumor: FaDu monolayer culture FaDu spheroids (MCTS) | PAM produced by He-CAP jet (DBD) | Indirect | PAM was applied to FaDu and FaDu MCTS. Control: cells treated with He gas without plasma. | Biological: -Cell viability. | BIOLOGICAL Cell viability (PrestoBlue®): -PAM exposure dependent cell detachment from MCTS due to the presence of H2O2; -A rapid spheroids regrowth due to a resistance of FaDu cells to H2O2; -Inhibition of cell growth after multiple treatments of MCTS with PAM; -MCTS are brought out when comparing PAM effect on 2D versus MCTS. Inversely, PAM induces cell death in the case of 2D cell culture. |
| Hasse S. et al., 2019 [72] | In vitro Ex vivo | Cell lines | Normal: HaCaT Tumor: HNO-97 -10 patients (6 M/4 F); age range: 43–75 years affected by maxillofacial cancer. | PAM produced by Ar-CAP Ar-CAP | Indirect for cell lines. Direct on tissue samples. | PAM was applied to cells. Tissue samples were directly exposed. Control: untreated tissue sample and human non-malignant mucosa. | Biological: -Cell viability; -Cell cycle; -Protein expression; -Cell motility. -Apoptosis; -DNA fragmentation; -Cytochrome C measurement; -Cytokine detection. | BIOLOGICAL Cell viability (Resazurin-based assay): -After plasma treatment, time-dependent reduction in HaCaT and HNO-97. Cell cycle (FACS): -19.3% of HNO-97 cells in G2/M vs. 30% of HaCaT cells. Global protein expression (Spectrometry): -Time dependent activation of caspase 3/7 in both cell types; -Several protein expression changes. Cell motility (Wound Healing): -Reduction in HNO-97; -Normal cells not affected. Apoptosis/DNA fragmentation (TUNEL assay): -Stronger induction of apoptosis in tumor tissue in situ compared to healthy tissue. Cytochrome C measurement (ELISA): -Three-fold increase in tumor tissue. Cytokine detection (Flow cytometer): -IL-22 increased in healthy tissue; -INF-γ TNF-α and IL-10 found predominantly in tumor tissue compared to non-cancerous tissue. |
| Sato K. et al., 2019 [80] | In vitro | Cell lines | Normal: HS-K IMR-90-SV Tumor: Ca9-22 Ho-1-u-1 HSC-2 HCS-3 HSC-4 Sa-3 SAS | Ar-NTP | Direct | Cells were treated in complete medium with NTP. Control: cells treated with Ar gas without plasma. | Biological: -Cell viability; -Apoptosis; -Quantification and localization of catalytic Fe(II); -Migration and Invasion; -Protein expression; -Colony formation. Chemical -ROS detection. | BIOLOGICAL Cell viability (WST-8 assay): -Time and treatment-dependent decrease in SCC; -Decrease in SAS e CA9-22 viability as the concentration of FAC increased; -Increase as the concentration of DFO increased in co-treatment with NTP. Quantification and localization of Fe(II) (Flow cytometer): -The Fe level in IMR-SV-90 was lower than SAS and CA9-22; -No difference in the Fe levels between SAS and Ca9-22 cells. Apoptosis (FACS, TUNEL): -Apoptotic cells in SAS and treated Ca9-22. Migration and invasion (Wound Healing and transwell) -Suppressed migratory and invasive ability of SAS and Ca9-22 compared with control. Protein expression (Western blot): -Decrease in MMP-2 SCC cells compared to control. Colony formation (Soft agar): -Decreased colony formation in treated SAS cells compared with control. CHEMICAL ROS detection (Flow cytometer): -Increased ROS levels in treated SCC. |
| Han X. et al., 2020 [71] | In vitro | Cell lines | Normal: OKF-6/T Tumor: SCC-25 | N2-APPJ | Direct | Cells were treated in 2.4 mL of PBS (3 mm depth) with N2-APPJ. Control: cells treated with N2 gas without plasma and no treatment. Control: untreated cells | Biological: -DNA double strand breaks | BIOLOGICAL DNA double strand breaks (Immunofluorescence): -DNA damage in cancer cells was maximized at the plasma jet treatment region and declined radially outward; -In cancer cells DNA damage decreased slightly over the first 4 h and rapidly decreasing by approximately 60% at 8 h post-treatment; -Damage observed 2 h after treatment in non-malignant cells; -S phase cells were more susceptible to DNA damage than either G1 or G2 phase cells. |
| Lee C.M. et al., 2020 [38] | In vitro | Cell lines | Normal: HGF-1 Tumor: SCC-15 | Ar-CAP jet | Direct | Cells (1 × 105 cells/100 μL) in medium were treated. Group 1: 1 µM cisplatin + 3 min CAP. Group 2: 3 µM cisplatin + 1 min CAP. | Biological: -Cell viability; -Apoptosis; -Protein expression. Chemical: -ROS generation; -CAP jet measurement. | BIOLOGICAL Cell Viability (MTT) and Apoptosis (fluorescence microscopy/FACS): -Decrease in cell viability by increasing cisplatin concentration and CAP exposure. Protein expression (Western blot): -Increased expression of PTEN and p53 in both cell lines; -Increased expression of cleaved Cas-9 in SCC-15. CHEMICAL ROS generation (Fluorimetric assay): -Group 1 and group 2 reported 300% and 500% increase in SCC-15 vs. 130% and 170% in HGF-1. CAP jet measurement (OES analysis): -Peaks of Ar+, OH and O− ions observed in the UV range (200 nm–400 nm) and visible range (690 nm–950 nm). |
| Ramireddy L. et al., 2020 [79] | In vitro | Cell line | Tumor: SCC-4 | He-CAPP jet | Direct | He-CAPP jet was applied to SCC-4 cells in cell medium. Control: cells treated with He gas without plasma. | Biological: -Cell viability; -Cell cycle; -Protein expression. Chemical: -ROS/RNS detection. | BIOLOGICAL Cell viability (MTT): -Significantly greater cell death (dose-dependent) compared to controls. Cell cycle (FACS): -Dose-dependent increase G1 cell cycle. Protein expression (Western blot): -Downregulation of ATM protein, Bax increase, and Bcl-2 decrease compared to controls. CHEMICAL ROS/RNS detection (Fluorimetric assay): -He-CAPP time-dependent increase in intracellular ROS and RNS levels; -H2O2 increased immediately after He plasma treatment but reached basal level after 3 h. |
| Lin A. et al., 2021 [76] | In vitro | Cell lines | Tumor: A-375 CAL-27 U-87 | Air-microsecond pulsed DBD | Direct | Cells (15 × 104) were treated with DBD after removing the medium. Control: untreated cells. | Biological: -Cell viability. Chemical: -H2O2, NO2− and NO3− measurements | BIOLOGICAL Cell survival (Trypan Blue Assay): -Reduced survival for all cell lines; -No significant difference between the different treatment times and pulse frequencies. CHEMICAL H2O2 (Fluorimetric assay) and NO2− /NO3− (Colorimetric assay) measurement: -H2O2, NO2− and NO3− concentrations linearly increased with pulse frequency and time of application; -The generation of H2O2, NO2− and NO3− depends on the total delivered energy during treatment. |
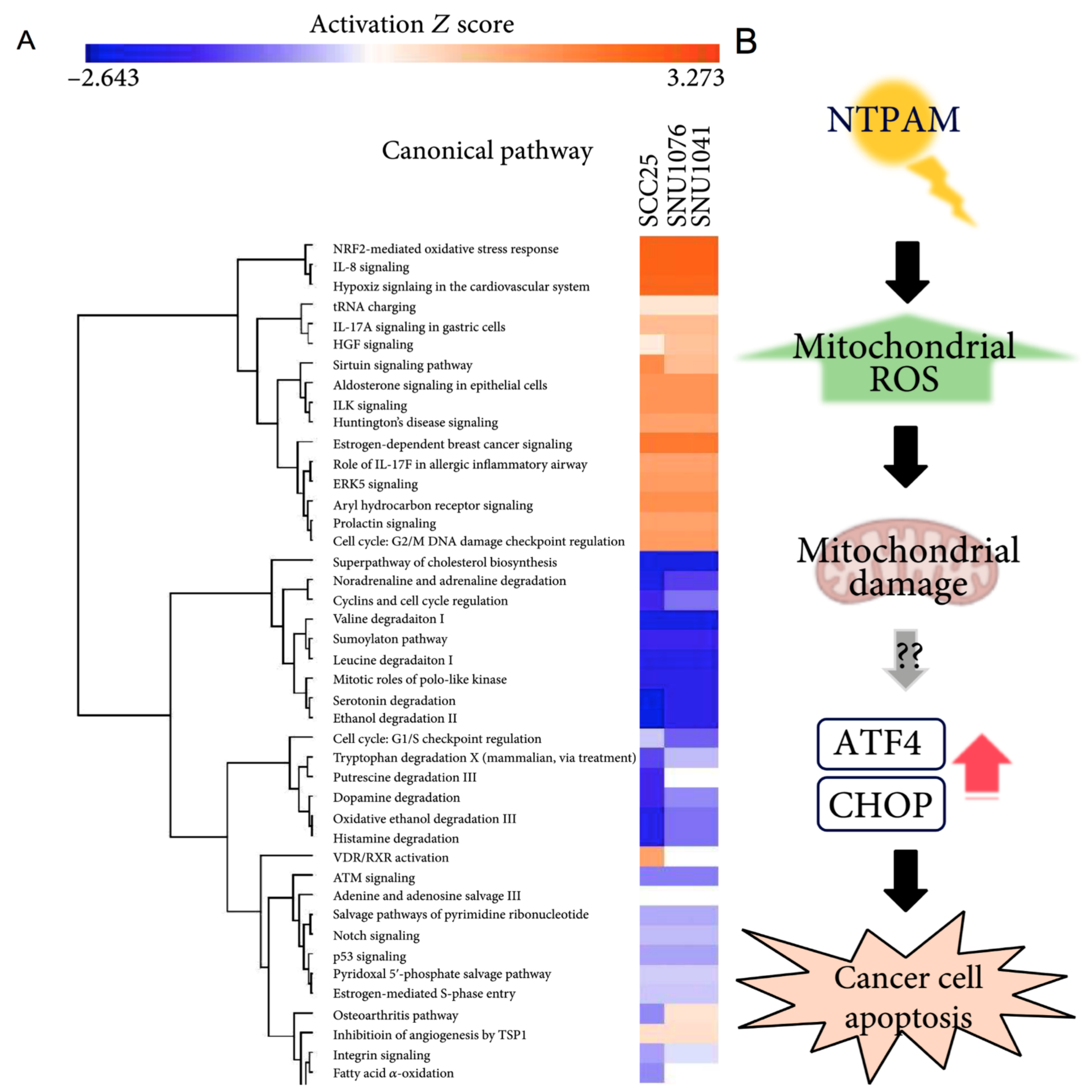
| Oh C. et al., 2021 [77] | In vitro | Cell lines | Normal: Primary normal human fibroblasts Tumor: SCC-25 SNU-1041 SNU-1076 | NTPAM produced by He-O2-PlasmaJet | Indirect | NTPAM preparation: NTP was exposed to the culture media for various activation times. | Biological: -Cell viability; -Apoptosis; -Gene expression; -Protein expression; -Mitochondrial damage; -Cells morphology. Chemical: -ROS detection | BIOLOGICAL Cell viability (WST-1): -Significantly reduced in all HNC cell lines in a manner dependent on NTP activation time compared to normal control cells. Apoptosis (FACS): -Selectively induced apoptosis in HNC cells. Gene expression by qRT-PCR analysis: -NTPAM treatment regulated mechanisms related to cell death and cell cycle; -Significant association with the regulation of PERK, closely related to an altered cell stress response; -ATF4 and CHOP are key regulators of NTPAM induced HNC cell death. Protein expression (Western blot): -Increase in cleaved PARP levels, -Decrease in pro-Caspase 3 and Bcl-2. Mitochondrial damage (OCR, SEM): -Induction of mitochondrial dysfunction in HNC cells through mitochondrial damage. CHEMICAL ROS detection (Fluorescent assay): -Significant increase in mitochondrial peroxide levels. |
| Oh C. et al., 2021 [77] | In vivo | Xenograft Animal Model: 10 healthy male BALB/c nude mice (5 control group, 5 NTPAM treatment group). | Tumor: SNU-1041 | NTPAM | Indirect | SNU1041 cells (5 × 106 cells/mL) were injected subcutaneously into each mouse. Tumor formation was confirmed on the 10th day after injection. 100 μL intra-tumoral injections of medium in the control group and NTPAM in the experimental group was administered once daily for 11 days. | Biological: -Protein expression; -Tumor weight measurement. | BIOLOGICAL Protein expression (Histological and Immunohistochemical analysis): -In tumor tissues, low expression levels of Ki-67 and high expression levels of ATF4 and CHOP were observed in the NTPAM-treated group, compared with the normal control group. Tumor weight measurement: -After NTPAM treatment, the tumor weight was significantly lower than in control tumors. |
| Park J. et al., 2021 [78] | In vitro | Cell lines | Normal: HaCaT Tumor: SCC-25 | Ar-NTP | Direct | A day prior, cells were cultivated in a growth medium with or without PD-L1 Ab + GNP. Immediately prior to treatment, the dishes were rinsed with PBS and later positioned under the end of the plasma jet. | Biological: -Cell viability; -Apoptosis; -Protein expression. | BIOLOGICAL Cell viability (SRB assay, Fluorescent dyes): -The treatment with PD-L1 ab + GNP + NTP significantly increased the number of dead cells in SCC-25 compared to the other treatments. Apoptosis (Western blot, Immunocytochemistry): -Elevated expression levels of cleaved caspase-3 and cleaved PARP in the PD-L1 ab + GNP + NTP group; -AIF and cyt C in the control were clustered in punctuate distribution forms whilst they were disseminated in treated cells. |
| Sklias K. et al., 2021 [81] | In vitro | Cell lines | Normal: 1BR3 hTERT RPE1 (immortalized with hTERT) Tumor: CAL-27 FaDu | He + O2-DBD micro plasma jet | Indirect Direct | Indirect: -PBS in empty well was treated with He/O2 PAP. -Tumor or normal cells were then incubated for 1 h with PAP. Reconstituted buffer (RB): PBS adjusted with plasma-induced concentrations of H2O2, NO2−, NO3− and pH Direct: Cell culture medium was removed, and the cells were washed with PBS. Then, PBS was added to cells and exposed to plasma for 1 h. | Biological: -Cell viability; -Apoptosis. Chemical: -ROS detection; -Lipid peroxidation measurement. | INDIRECT TREATMENT Cell viability (MTT, trypan-blue and CellTiterGlo®): -Lower in FaDu compared to CAL-27; -The strongest cytotoxic effect at a higher concentration of RONS and acidic pH. Apoptosis: Caspase-Glo® 3/7 Assay System. ROS detection (OES): -The highest concentration of H2O2, NO2−, NO3− is obtained after 12 min plasma treatment, at a gas flow of 0.5 slm and at a treatment distance of 8 mm; -These conditions induced the strongest reduction in pH; -H2O2 is a master player in PAP-induced cancer cell death since the addition of catalase during PAP treatment prevents the toxicity of PAP. RECONSTITUTED BUFFER RB is as efficient as PAP to induce lipid peroxidation, intracellular ROS formation, caspase 3/7 activity and cell death in FaDu and CAL-27 cell lines. DIRECT TREATMENT OF CAL27 AND FADU CELL LINES -Greater CAL-27 and FaDu death in direct than indirect plasma treatment DIRECT TREATMENT OF NORMAL CELLS Strong reduction of 1Br3 and RPE-hTERT metabolic activity of normal cell lines direct plasma treatment, while no change after indirect and reconstituted buffer from 6 h up to 72 h post treatment. |
| Wu C.Y. et al., 2021 [83] | In vitro | Cell lines | Tumor: SAS CAL-27 FaDu Detroit 562 | N2+He-NT micro plasma jet | Direct | After cells exposure to plasma, the medium was changed with a new fresh one and incubated for further 24 h. | Biological: -Cell viability; -Apoptosis. Chemical: -ROS and RNS detection | BIOLOGICAL Cell viability (MTS): -Significant decrease with plasma exposure time in SAS, CAL-27 and FaDu; -Significant less decrease in Detroit 562 cells between 30 and 60 s. Apoptosis (FACS/TUNEL): -After plasma exposure for 5 min, 7.8-fold increase in the apoptotic percentage compared to non-treatment group. CHEMICAL ROS and RNS detection (OxiSelect Intracellular Assay kits): -ROS and RNS concentration in medium increased with plasma exposure time. |
| Authors | Tumor Type(s) | Sample Size | Numbers and Characteristics of Groups | Stage | Plasma Device | Direct/Indirect Treatment | Primary OUTCOMES | Secondary Outcomes | Results | Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|
| Metelmann H.R. et al., 2015 [85] | Advanced HNC | 12 | -Female: 6 -Male: 6 -Caucasian -Age range: 50–77 years. -Karnofsky performance status: 60–80. | T 4 N 0–3 M 0–1 | Ar-CAP kINPen MED. | Direct: spot exposure of the ulceration to CAP. | -Change in contamination; -Patient-expressed need of pain medication; -Side effects. | Anti-cancer effects (tumor growth). | -Relief and reduction of fetid odor and pain; -Bacteria decrease in cancer ulcerations; -not severe side effects; -Visible effects after 2 weeks of exposure on tumor surface. | N/P |
| Schuster M. et al., 2016 [87] | Advanced HNC | 21 | -Female: 9 -Male: 12 -Caucasian -Age range: 40–77 years. -Karnofsky performance status: 60–80. Group I: (n = 12) palliative CAP treatment Group II: (n = 9) curative CAP + surgery treatment. | N/P | Ar-CAP kINPen MED. | Direct: spot exposure of the ulceration to CAP. | Tumor surface responses. Type 1: flat area with vascular stimulation; Type 2: contraction of tumor ulceration rims with scabs surrounded by tumor tissue in visible progress. | Evaluation of CAP-induced visible tumor surface in relation to CAP-induced apoptotic cell kill. | -No sign of enhanced or stimulated tumor growth in any patient; -More apoptotic cells in tissue areas treated with CAP than in untreated; -Visible tumor surface response in relation to apoptotic cell. | Tumor surface response evaluated by photographic analysis after 2 weeks of treatments. |
| Metelmann H.R. et al., 2018 [15] | Advanced OPSCC | 6 | -Female: 3 -Male: 3 -Age range: 53–78 years | Locally advanced cancer of the oropharynx (pT4) with contaminated tumor ulcerations. | Ar-CAP kINPen MED. | Direct: spot exposure of the ulceration to CAP. | -Survival time; -Course of disease; -Tumor remission; -Safety of treatment. | Incisional biopsies were performed to verify changes at the cellular level. | -Tumor reduction; -Significant improvement in tumor decontamination (reduction of odor) and tumor mass; -Palliation in terms of quality of life. | Related to the death of participant. |
| Schuster M. et al., 2018 [86] | Advanced HNC | 20 | -Female: 10 -Male: 10 -Caucasian -Age range: 49–84 years -Karnofsky performance status: 60–80. | N/P | Ar-CAP kINPen MED. | Direct: spot exposure of the ulceration to CAP e. | -Evaluation general health condition and side effects; -History of cancer treatment; -State of disease; -Palliation procedure. | N/P | Side effects were mild to moderate and never life threatening. | N/P |
| Dai X. et al., 2020 [84] | LC | 100 | -Female: 42 -Male: 28 -Age range: 60–85 years old Control: 50 treated with conventional surgery Test: 50 treated with CAP | N/P | Unitec low-temperature plasma operation system. | Tumor was ablated with a low-temperature plasma cutter with the extent expanded to 3–5 mm away from the edge of the lesion. | -Postoperative efficacy; -Influence on the tumor markers, COX-2, and VEGF expressions. | -Operation time -VAS pain; -Mucosal recovery scores. | -Few postoperative complications; -Decreased expression levels of postoperative tumor markers (COX-2 and VEGF). | N/P |
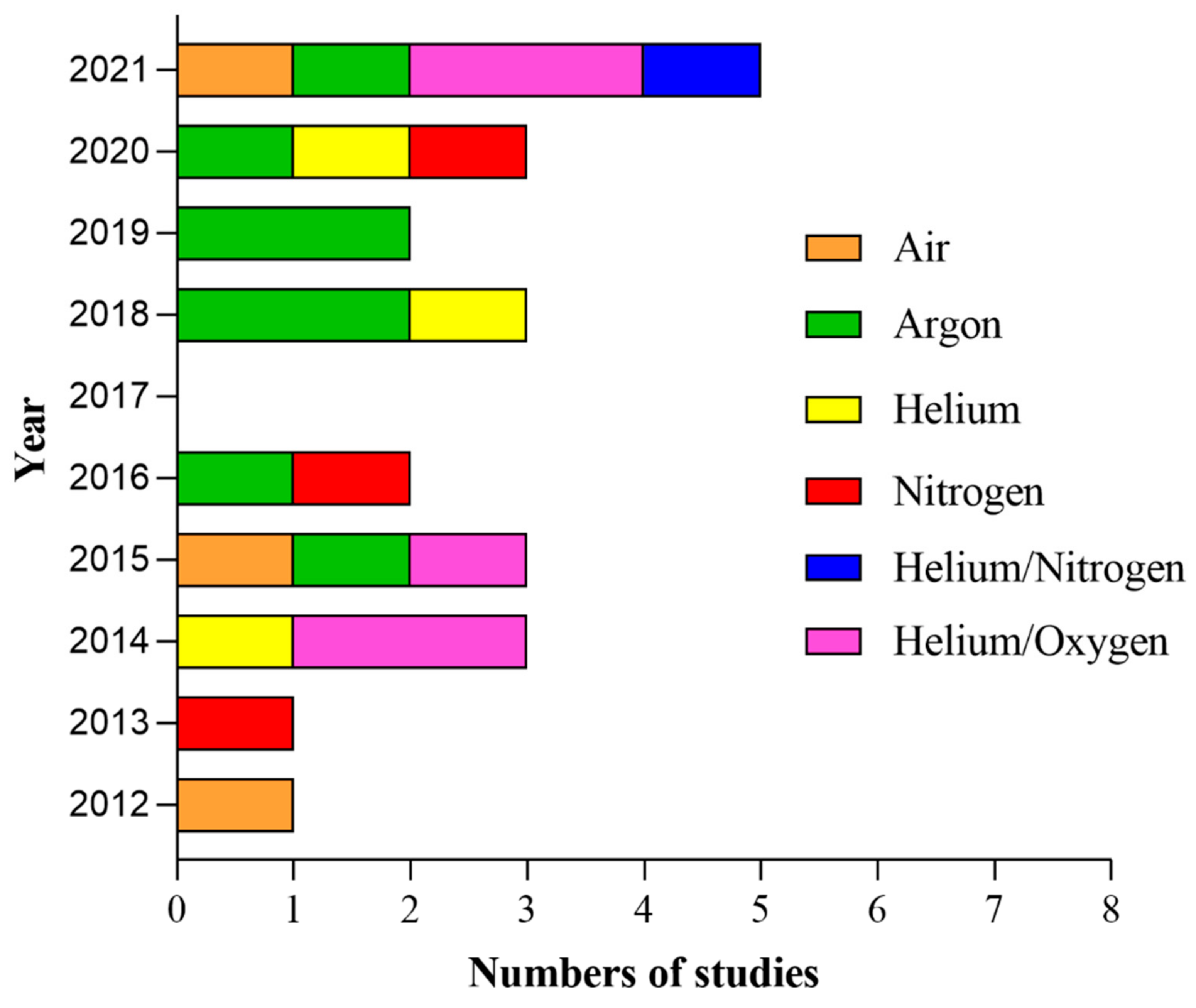
| Authors | Plasma Device Description | Pulse Frequency | Pulse | Flow Rate | Gas | Plasma Temperature | Application Distance | Application Time | Total Energy | Power | Manufacturer |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Choi B.B. et al., 2012 [37] | air-NTP: the size of the device is 10.24 cm2. The mask pattern was etched by wet etching technique on the Cu electrodes which is surrounding both side of PTFE dielectric surface. | 22 kHz | 15 kV | N/P | Air | N/P | 2 mm | 30 s | 9.2 J/cm2 | 3.15 J/s | Pohang University of Science and Technology, Pohang, Korea (Kim et al., 2010 [88]) |
| Han X. et al., 2013 [23] | N-APPJ: Two copper ribbon electrodes of 0.6 mm thick separated by 1.8 mm are wrapping around a quartz tube (outer diameter: 3 mm). One helical electrode is connected to a HV power supply, and the other is grounded. The electrode wrapping zone has a vertical length of 35 mm. | 28 kHz | 22.4 kV (Vrms. 7.75 kV) 59 mA (Irms. 17 mA) | 1.5 L/min | N2 | N/P | 20 mm | 10, 30, 60, and 120 s | N/P | N/P | N/P |
| Chang J.W. et al., 2014 [36] | He+O-NTP spray type: arc-free and antistatic plate to provide a uniform plasma jet. | 20–30 kHz | 2–13 kV | N/P | He/O2 | N/P | N/P | 1 s | N/P | N/P | Pohang University of Science and Technology, Pohang, Korea |
| Guerrero-Preston R. et al., 2014 [5] | He-CAP: The CAP device contains 4 blocks. Block 1 is a DC power supply. Block 2 is a centrally powered electrode with a ground outer electrode wrapped around a quartz tube. Block 3 consists of a capacitor, a transistor, and a timer; block 4 is the He gas supply. | N/P | 8 kV | 10 L/min−1 test and 20 L/min−1 positive treatment control | He | N/P | 3 cm | 10, 30 45 s and 10 s for control | N/P | N/P | School of Engineering and Applied Science of The George Washington University |
| Kang S.U. et al., 2014 [73] | He + O NTP spray-type: NTP with a newly designed arc-free and antistatic plate to provide uniform NTP for biological research applications. The plasma source is equipped with a pair of electrodes that is made of Al2O3 (high voltage and ground electrodes, 1040 mm2 dimension, 2 mm gap between electrodes) that is isolated from direct contact with the plasma by a ceramic barrier. | 20–30 kHz | 2–13 kV | N/P | He/O2 | 35 °C | In vitro: 3 cm In vivo: 1 cm | In vitro: N/P In vivo: 20 s | N/P | N/P | N/P |
| Kim S.Y. et al., 2015 [74] | LTP produced by He + O-NTP spray-type: newly designed arc-free and antistatic plate to provide uniform NTP. The plasma source is equipped with a pair of electrodes made of Al2O3 (high-voltage and ground electrodes, 10 × 40 mm2 in dimension, 2 mm gap between electrodes) isolated from direct contact with the plasma using a ceramic barrier. | 20–30 kHz | 2–13 kV | N/P | He/O2 | 35 °C | 1–2 cm from the culture media | in vitro: 15 min. syngenic tumor model: 1 week. xenograft model: 10 times. | N/P | N/P | N/P |
| Metelmann H.R. et al., 2015 [85] | kINPen MED: Hand-held unit discharges plasma under atmospheric conditions, requiring a DC power unit and Ar gas reservoir. In the center of a ceramic capillary (inner diameter 1.6 mm) a pin -type electrode (1 mm diameter) is mounted. The needle is powered by a miniaturized RF generator. | 1 MHz, modulated with 2.5 kHz and plasma duty cycle of 1:1. | Sinusoidal voltage waveforms range from 2 kV to 3 kV amplitude peak. | 5 slm | Ar | 38 °C | Spot exposure of the ulceration to CAP from 8 mm. | Cycles of 3 single treatments within 1 week, followed by an intermittence of 1 week without CAP exposure. Repeatedly scanning tumor ulceration accessible area for 1 min/cm2. Total treatment time increase to more than 30 min for patients with large ulceration exceeding 30 cm2. | N/P | N/P | Neoplas tools GmbH, Greifswald, Germany |
| Welz C. et al., 2015 [82] | air-CAP: MiniFlatPlaSter is equipped with a high voltage power supply, accumulators, and a SMD electrode for production in air. The SMD electrode consists of a copper foil layer (around 0.2 mm thick), an Epoxy board (1 mm thick), and a stainless-steel mesh of 28 mm in diameter, so that it exactly fits the rim of one well. | 6.75 kHz | 7 kV | N/P | Air | N/P | 17.5 ± 0.5 mm | 30, 60, 90, 120 and 180 s | N/P | N/P | FlatPlaSter, Regensburg University Hospital |
| Lee J.H. et al., 2016 [75] | N-CAP jet: made up of an inner electrode made of tungsten with 1.2 mm depth and 0.2 mm thickness with 3.2 mm depth of quartz as a dielectric. The hole in the outer electrode made of stainless steel was 0.7 mm via the 2 mm height of porous alumina having a 150~200 μm pore size with 35% porosity. | 60 Hz | 1.2 kV | 250; 500; 750; 1000; 1500; 2000 sccm | N2 | N/P | 8 mm | 1 min | 0.51; 0.62; 1.98; 2.91; 2.4; 2.33 W | N/P | Kwangwoon University |
| Schuster M. et al., 2016 [87] | kINPen MED: Hand-held unit discharges plasma under atmospheric conditions, requiring a DC power unit and Ar gas reservoir. In the center of a ceramic capillary (inner diameter 1.6 mm) a pin -type electrode (1 mm diameter) is mounted. The needle is powered by a miniaturized RF generator. | 1 MHz, modulated with 2.5 kHz and plasma duty cycle of 1:1. Amplitude peaks at a frequency of 1 MHz and modulated with 2.5 kHz. | Sinusoidal voltage waveforms range from 2 kV to 3 kV amplitude peak. | N/P | Ar | N/P | Spot exposure of the ulceration to CAP from a distance of 8 mm | Group 1: cycle of 3 single treatments within 1 week for 1 min, followed by an intermittence of 1 week without CAP exposure. Group 2: one-time application for 3 min followed by total resection of the tumor. | N/P | N/P | Neoplas tools GmbH, Greifswald, Germany. |
| Metelmann H.R. et al., 2018 [15] | kINPen MED: Hand-held unit discharges plasma under atmospheric conditions, requiring a DC power unit and Argon gas reservoir. In the center of a ceramic capillary (inner diameter 1.6 mm) a pin -type electrode (1 mm diameter) is mounted. The needle is powered by a miniaturized RF generator. | 1 MHz, modulated with 2.5 kHz and plasma duty cycle of 1:1. | Sinusoidal voltage waveforms range from 2 kV to 3 kV amplitude peak. | 5 slm. | Ar | 38 °C | 8 mm, vertically to naturally moist tissue surface. | Cycles of 3 single treatments within 1 week, followed by an intermittence of 1 week without CAP exposure. Repeatedly scanning tumor ulceration accessible area for 1 min/cm2. Total treatment time increase to more than 30 min for patients with large ulceration exceeding 30 cm2. | N/P | N/P | Neoplas tools GmbH, Greifswald, Germany. |
| Chauvin J. et al., 2018 [6] | PAM produced by He-CAP jet (DBD): made up of Al tape electrodes wrapped on a quartz tube with small diameters (2 mm inner diameter and 4 mm outer diameter) separated by 10 mm space. | 10 kHz | 10 kV square pulses | 3 L/min | He | N/P | 2 cm | 0, 30, 60, 120, 240 s | N/P | N/P | Université de Toulouse-LAPLACE |
| Schuster M. et al., 2018 [86] | kINPen MED: Hand-held unit discharges plasma under atmospheric conditions, requiring a DC power unit and Ar gas reservoir. In the center of a ceramic capillary (inner diameter 1.6 mm) a pin -type electrode (1 mm diameter) is mounted. The needle is powered by a miniaturized RF generator. | 1 MHz, modulated with 2.5 kHz and plasma duty cycle of 1:1. Amplitude peaks at a frequency of 1 MHz and modulated with 2.5 kHz. | Sinusoidal voltage waveforms range from 2 kV to 3 kV amplitude peak | N/P | Ar | N/P | Spot exposure of the ulceration to CAP from a distance of 8 mm. | Cycles of 3 single treatments within 1 week, followed by an intermittence of 1 week without CAP exposure, exceptionally due to the patient’s individual circumstances of 2 to 3 weeks. Repeatedly scanning tumor ulceration accessible area for 1 min/cm2. | N/P | N/P | Neoplas tools GmbH, Greifswald, Germany. |
| Hasse S. et al., 2019 [72] | PAM produced by Ar-CAP kINPen MED made of two electrodes, a pin type high voltage electrode inside a ceramic capillary and one grounded electrode. The plasma is generated at the tip of the pin type electrode and expanded about 1 cm to the surrounding air outside the capillary. | It generates a radiofrequency signal of about 1 MHz. The discharge is switched on at a frequency of 2.5 kHz (50:50). | 2–3 kV | 5 slm | Ar | 35–39 °C | Medium indirect and 8 mm from tissue | In vitro: 20, 40, 80, 150 s Ex vivo: 3 min | N/P | N/P | Neoplas tools, Greifswald, Germany |
| Sato K. et al., 2019 [80] | Ar-NTP: 2 electrodes 20 mm apart. NTP had an ultrahigh electron density and an O density of approximately 4 × 1015 cm3. | 60 Hz | 10 kV | 2 L/min | Ar | 25 °C | 8 mm | 30–120 s | N/P | N/P | Habahiro instrument from Prof. M. Hori, Plasma Nanotechnology Research Center, Nagoya University, Japan. |
| Dai X. et al., 2020 [84] | N/P | N/P | N/P | N/P | N/P | N/P | 3–5 mm | N/P | N/P | N/P | Unitec low-temperature plasma. |
| Han X. et al., 2020 [71] | N-APPJ: 2 copper ribbon electrodes of 0.6 mm thick separated by a distance of 1.8 mm are spirally and alternatively wrapping around a quartz tube, whose outer diameter is 3 mm. One of the helical electrodes is connected to a HV power supply and the other is grounded. The electrode wrapping zone, with a vertical length of 35 mm along the quartz tube, is the major region of plasma ignition. A wider glass tube is sealed outside the quartz tube and a fluid with a high dielectric constant was filled within the volume between these 2 tubes. When N2 is introduced into the quartz tube and HV is applied, plasma is ignited and forms a plasma jet of a few cm long to the open atmosphere. | 28 kHz | 22.4 kV | 1.5 slm | N2 | N/P | 20 mm | 120 s | N/P | N/P | N/P |
| Lee C.M. et al., 2020 [38] | Ar-CAP jet: CAP apparatus P500-SM consists of a gas supply system, MFC, a plasma jet and a high-voltage AC power supply. | 20 kHz | 8.5 kV | 5 slm | Ar | N/P | 3 cm | 10 s–5 min | 45 W | N/P | Sakikake Co. Ltd., Kyoto, Japan |
| Ramireddy L. et al., 2020 [79] | He-CAP jet: Dielectric barrier and quartz tube inner and outer diameters of 2 and 4 mm respectively. Two electrodes copper strips (grounded 2.5 cm and powered 1.5 cm) wrapped around the quartz tube. The powered electrode is 2 mm from the nozzle end and the distance between the grounded and powered electrodes is 1.5 cm. | 10 kHz | 7.5 kV | 5 slm | He | N/P | 3 cm | 1, 3, 5 min | N/P | N/P | N/P |
| Lin A. et al., 2021 [76] | air-microsecond-pulsed DBD: plasma system (custom built): Copper electrode covered with 0.5 mm fused silica diameter 1.2 cm | 0.05 kHz a 0.5 kHz. | 30 kV | N/P | Air | N/P | 1–10 mm | 10–240 s | 9.4 J | N/P | The power supply was custom built (Megaimpulse Ltd.) |
| Oh C. et al., 2021 [77] | NTPAM generated by NTP jet. The device is composed of a quartz tube (diameter: outer 6 mm, inner 4 mm) with two electrodes (an inner stainless-steel tube and an outer ground ring). The inner is also placed as a gas inlet. | 20 kHz | Few kV | He: 4 slpm; O2: 1 sccm. | He/O2 | N/P | In vitro: approximately 1 cm. In vivo: N/A | In vitro: various activation times. In vivo: 100 μL intratumoral injections of NTPAM in the experimental group administered once daily for 11 days. | Discharge power varied from 10 to 24 W. | N/P | N/P |
| Park J. et al., 2021 [78] | Ar-NTP: 1 dielectric and 2 electrodes. The inner is composed of stainless, the external is wrapped with copper tape. The NTP is produced between the inner and outer electrodes, and the plasma flow temperature is kept below 35 °C for 10 min. | N/P | N/P | 2 slm | Ar | N/P | 10 mm | 5 min | N/P | N/P | Feagle Company (Yangsan-si, Kyeongsangnam-do, Korea) |
| Sklias K. et al., 2021 [81] | He+O-DBD micro-plasma jet: Plasma reactor: stainless-steel needle (0.7 mm inner and 1.4 mm outer diameters), inserted inside a dielectric tube made of quartz, and biased electrically by applying high voltage square positive pulses. The distance between the needle’s tip and the reactor’s nozzle is fixed (55 mm). The ground electrode (10 mm width), made of copper, is wrapped around the dielectric tube, centered at the tip of the needle. | 10 kHz | Amplitude of 6 kV, pulse width of 4.8 µs, rise and fall times of around 25 ns. | 0.5 slm or 1 slm | He/O2 | 23 °C | 8 or 20 mm. | N/P | N/P | N/P | N/P |
| Wu C.Y. et al., 2021 [83] | N-NTP jet: Custom-made micro-plasma jet source. One capillary electrode is the jet source to inject additive N2. A stainless-steel capillary tube (diameter of 0.8 mm) at the center of the quartz tube is used as the inner electrode. This is connected to the ground and used as a N2 flow channel. A piece of copper is used as the outer electrode, which is connected to the output of the generator. | 13.56 MHz | 12–14 W | N/P | He/N2 | 37 °C | 4 mm | -30, 90, 120 s. -5 min (apoptosis). | N/P | N/P | RF, ENI ACG-3B, MSK Instruments, Inc., USA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perrotti, V.; Caponio, V.C.A.; Muzio, L.L.; Choi, E.H.; Marcantonio, M.C.D.; Mazzone, M.; Kaushik, N.K.; Mincione, G. Open Questions in Cold Atmospheric Plasma Treatment in Head and Neck Cancer: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 10238. https://doi.org/10.3390/ijms231810238
Perrotti V, Caponio VCA, Muzio LL, Choi EH, Marcantonio MCD, Mazzone M, Kaushik NK, Mincione G. Open Questions in Cold Atmospheric Plasma Treatment in Head and Neck Cancer: A Systematic Review. International Journal of Molecular Sciences. 2022; 23(18):10238. https://doi.org/10.3390/ijms231810238
Chicago/Turabian StylePerrotti, Vittoria, Vito Carlo Alberto Caponio, Lorenzo Lo Muzio, Eun Ha Choi, Maria Carmela Di Marcantonio, Mariangela Mazzone, Nagendra Kumar Kaushik, and Gabriella Mincione. 2022. "Open Questions in Cold Atmospheric Plasma Treatment in Head and Neck Cancer: A Systematic Review" International Journal of Molecular Sciences 23, no. 18: 10238. https://doi.org/10.3390/ijms231810238
APA StylePerrotti, V., Caponio, V. C. A., Muzio, L. L., Choi, E. H., Marcantonio, M. C. D., Mazzone, M., Kaushik, N. K., & Mincione, G. (2022). Open Questions in Cold Atmospheric Plasma Treatment in Head and Neck Cancer: A Systematic Review. International Journal of Molecular Sciences, 23(18), 10238. https://doi.org/10.3390/ijms231810238











