Ibuprofen Favors Binding of Amyloid-β Peptide to Its Depot, Serum Albumin
Abstract
1. Introduction
2. Results and Discussions
2.1. Modulation of HSA Affinity to Monomeric Aβ by IBU
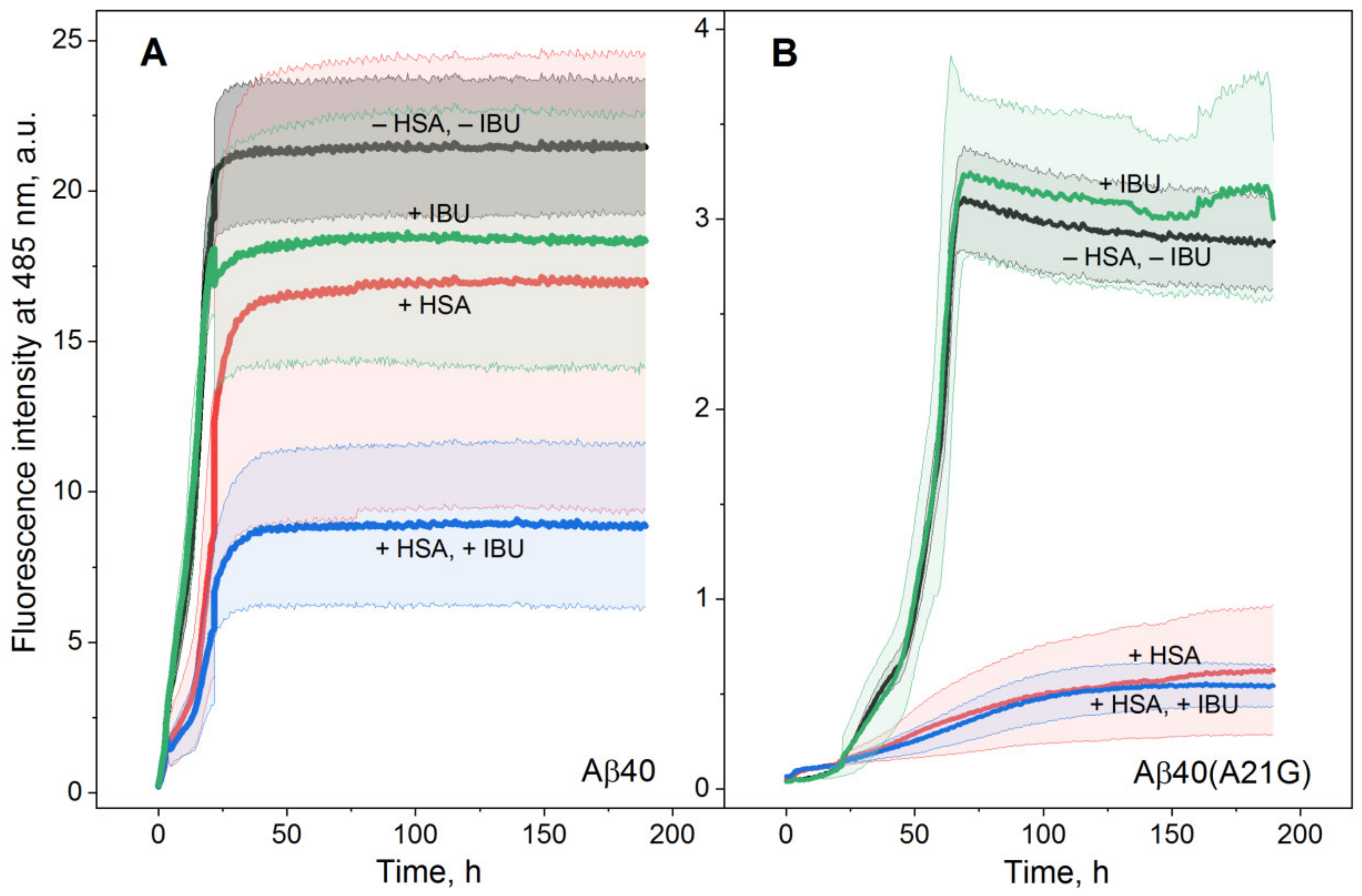
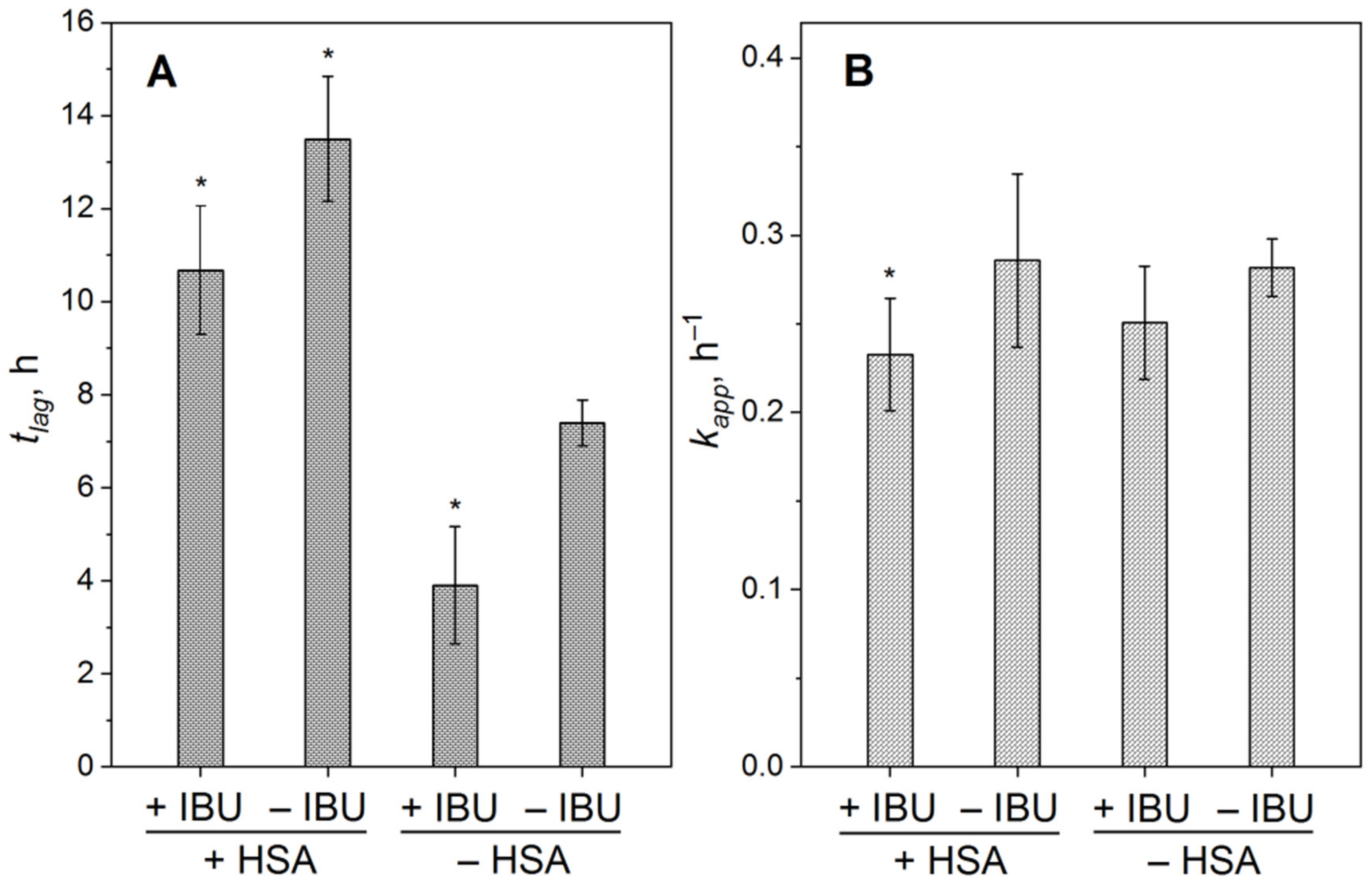
2.2. Influence of IBU on Suppression of Aβ Fibrillation by HSA
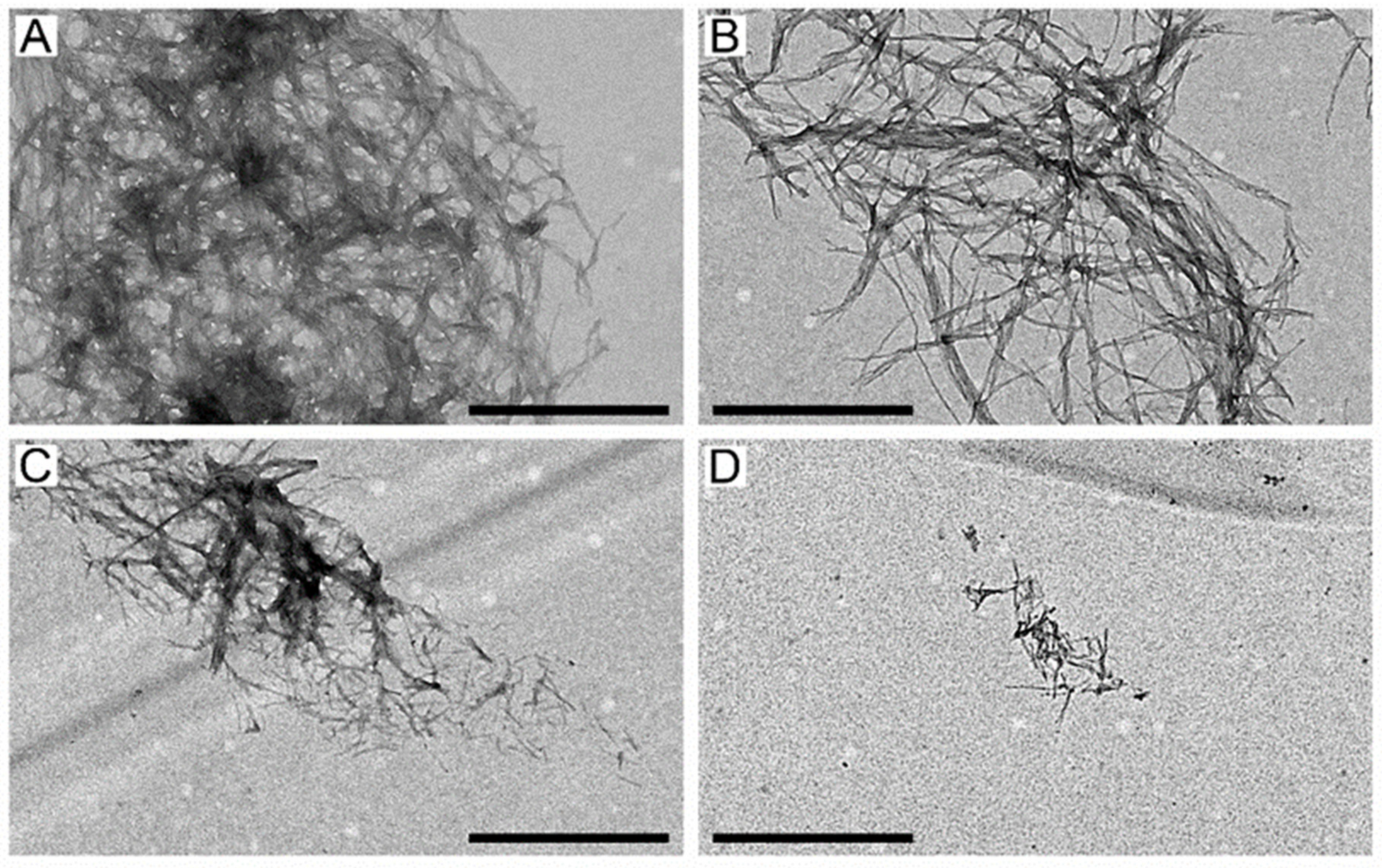
2.3. The Suppression of Aβ40 Fibrillation by HSA/IBU Studied by Transmission Electron Microscopy
2.4. Structural Modeling of HSA–Aβ40/IBU Complexes
3. Materials and Methods
3.1. Materials
3.2. Preparation of Recombinant Human Aβ Samples
3.3. Preparation of Aβ Samples for SPR Experiments
3.4. Surface Plasmon Resonance Studies
3.5. Preparation of Aβ Samples for ThT Fluorescence Assay
3.6. ThT Fluorescence Assay
3.7. Transmission Electron Microscopy
3.8. Structural Modeling of Aβ40/IBU–HSA Complexes
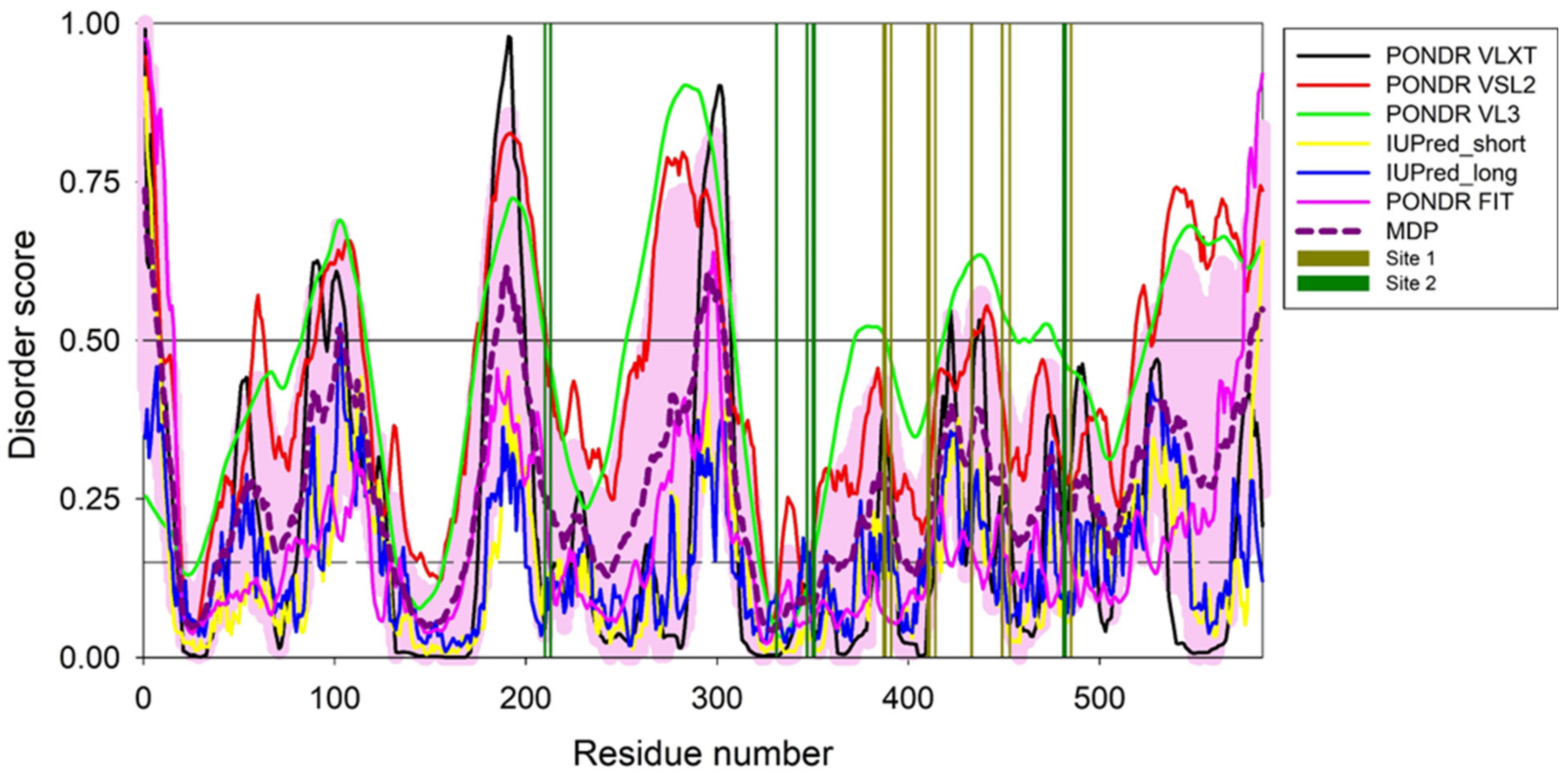
3.9. Per-Residue Intrinsic Disorder Predisposition of HSA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-ME | 2-mercaptoethanol |
| Aβ | amyloid-β peptide |
| Aβ40/Aβ42 | amyloid-β peptide, residues 1-40/42 |
| Aβ40(A21G) | A21G mutant of amyloid-β peptide 1-40, Flemish variant of Aβ40 [41] |
| AD | Alzheimer’s disease |
| a.u. | arbitrary units |
| COX | cyclooxygenase |
| CSF | cerebrospinal fluid |
| DMSO | dimethyl sulfoxide |
| EDTA | ethylenediaminetetraacetic acid |
| HSA | human serum albumin |
| IBU | ibuprofen, 2-[4-(2-methylpropyl)phenyl]propanoic acid (CAS #15687-27-1) |
| PDB | Protein Data Bank |
| RU | resonance unit |
| SDS | sodium dodecyl sulfate |
| SPR | surface plasmon resonance |
| TEM | transmission electron microscopy |
| TFA | trifluoroacetic acid |
| ThT | thioflavin T |
| Tris | tris(hydroxymethyl)aminomethane |
| TWEEN® | polyethylene glycol sorbitan monolaurate |
| Usp2-cc | ubiquitin carboxyl-terminal hydrolase 2, catalytic core |
| WHO | World Health Organization |
References
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000Research 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Tatulian, S.A. Challenges and hopes for Alzheimer’s disease. Drug Discov. Today 2022. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.R.; Serra-Mir, G.; Montoliu-Gaya, L.; Tiessler, L.; Villegas, S. Amyloid-beta peptide and tau protein crosstalk in Alzheimer’s disease. Neural Regen. Res. 2022, 17, 1666–1674. [Google Scholar]
- Reiss, A.B.; Arain, H.A.; Stecker, M.M.; Siegart, N.M.; Kasselman, L.J. Amyloid toxicity in Alzheimer’s disease. Rev. Neurosci. 2018, 29, 613–627. [Google Scholar] [CrossRef]
- Lalli, G.; Schott, J.M.; Hardy, J.; De Strooper, B. Aducanumab: A new phase in therapeutic development for Alzheimer’s disease? EMBO Mol. Med. 2021, 13, e14781. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Xu, T.H.; Yan, Y.; Zhou, Y.R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid beta: Structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Bohrmann, B.; Tjernberg, L.; Kuner, P.; Poli, S.; Levet-Trafit, B.; Naslund, J.; Richards, G.; Huber, W.; Dobeli, H.; Nordstedt, C. Endogenous proteins controlling amyloid beta-peptide polymerization. Possible implications for beta-amyloid formation in the central nervous system and in peripheral tissues. J. Biol. Chem. 1999, 274, 15990–15995. [Google Scholar] [CrossRef]
- Ezra, A.; Rabinovich-Nikitin, I.; Rabinovich-Toidman, P.; Solomon, B. Chapter 11—Multifunctional Effects of Human Serum Albumin Toward Neuroprotection in Alzheimer Disease. In Neuroprotection in Alzheimer’s Disease; Gozes, I., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 217–238. [Google Scholar]
- Biere, A.L.; Ostaszewski, B.; Stimson, E.R.; Hyman, B.T.; Maggio, J.E.; Selkoe, D.J. Amyloid beta-peptide is transported on lipoproteins and albumin in human plasma. J. Biol. Chem. 1996, 271, 32916–32922. [Google Scholar] [CrossRef] [PubMed]
- Seyfert, S.; Faulstich, A.; Marx, P. What determines the CSF concentrations of albumin and plasma-derived IgG? J. Neurol. Sci. 2004, 219, 31–33. [Google Scholar] [CrossRef]
- Stanyon, H.; Viles, J. Human serum albumin can regulate amyloid-β peptide fiber growth in the brain interstitium: Implications for Alzheimer disease. J. Biol. Chem. 2012, 287, 28163–28168. [Google Scholar] [CrossRef]
- Litus, E.A.; Kazakov, A.S.; Sokolov, A.S.; Nemashkalova, E.L.; Galushko, E.I.; Dzhus, U.F.; Marchenkov, V.V.; Galzitskaya, O.V.; Permyakov, E.A.; Permyakov, S.E. The binding of monomeric amyloid beta peptide to serum albumin is affected by major plasma unsaturated fatty acids. Biochem. Biophys. Res. Commun. 2019, 510, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Litus, E.A.; Kazakov, A.S.; Deryusheva, E.I.; Nemashkalova, E.L.; Shevelyova, M.P.; Nazipova, A.A.; Permyakova, M.E.; Raznikova, E.V.; Uversky, V.N.; Permyakov, S.E. Serotonin promotes serum albumin interaction with the monomeric amyloid β peptide. Int. J. Mol. Sci. 2021, 22, 5896. [Google Scholar] [CrossRef]
- Bode, D.C.; Stanyon, H.F.; Hirani, T.; Baker, M.D.; Nield, J.; Viles, J.H. Serum albumin’s protective inhibition of amyloid-β fiber formation is suppressed by cholesterol, fatty acids and warfarin. J. Mol. Biol. 2018, 430, 919–934. [Google Scholar] [CrossRef]
- Boada, M.; Ortiz, P.; Anaya, F.; Hernandez, I.; Munoz, J.; Nunez, L.; Olazaran, J.; Roca, I.; Cuberas, G.; Tarraga, L.; et al. Amyloid-targeted therapeutics in alzheimer’s disease: Use of human albumin in plasma exchange as a novel approach for a beta mobilization. Drug News Perspect. 2009, 22, 325–339. [Google Scholar] [CrossRef]
- Boada, M.; Lopez, O.L.; Olazaran, J.; Nunez, L.; Pfeffer, M.; Paricio, M.; Lorites, J.; Pinol-Ripoll, G.; Gamez, J.E.; Anaya, F.; et al. A randomized, controlled clinical trial of plasma exchange with albumin replacement for Alzheimer’s disease: Primary results of the AMBAR Study. Alzheimers Dement. 2020, 16, 1412–1425. [Google Scholar] [CrossRef] [PubMed]
- Boada, M.; Lopez, O.; Nunez, L.; Szczepiorkowski, Z.M.; Torres, M.; Grifols, C.; Paez, A. Plasma exchange for Alzheimer’s disease Management by Albumin Replacement (AMBAR) trial: Study design and progress. Alzheimers Dement. 2019, 5, 61–69. [Google Scholar] [CrossRef]
- Boada, M.; Ramos-Fernandez, E.; Guivernau, B.; Munoz, F.J.; Costa, M.; Ortiz, A.M.; Jorquera, J.I.; Nunez, L.; Torres, M.; Paez, A. Treatment of Alzheimer disease using combination therapy with plasma exchange and haemapheresis with albumin and intravenous immunoglobulin: Rationale and treatment approach of the AMBAR (Alzheimer Management By Albumin Replacement) study. Neurologia 2016, 31, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Ezra, A.; Rabinovich-Nikitin, I.; Rabinovich-Toidman, P.; Solomon, B. Multifunctional effect of human serum albumin reduces Alzheimer’s disease related pathologies in the 3xTg mouse model. J. Alzheimers Dis. 2016, 50, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Rainsford, K.D. Ibuprofen: Pharmacology, efficacy and safety. Inflammopharmacology 2009, 17, 275–342. [Google Scholar] [CrossRef]
- Bushra, R.; Aslam, N. An overview of clinical pharmacology of Ibuprofen. Oman Med. J. 2010, 25, 155–1661. [Google Scholar] [CrossRef]
- Herzfeldt, C.D.; Kummel, R. Dissociation-constants, solubilities and dissolution rates of some selected non-steroidal antiinflammatories. Drug Dev. Ind. Pharm. 1983, 9, 767–793. [Google Scholar] [CrossRef]
- Avdeef, A.; Berger, C.M.; Brownell, C. pH-Metric Solubility. 2: Correlation Between the acid-base titration and the saturation shake-flask solubility-pH methods. Pharm. Res. 2000, 17, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.F.; Adams, S.S.; Cliffe, E.E.; Dickinson, W.; Nicholson, J.S. The metabolism of ibuprofen. Xenobiotica 1973, 3, 589–598. [Google Scholar] [CrossRef]
- Whitlam, J.B.; Crooks, M.J.; Brown, K.F.; Pedersen, P.V. Binding of nonsteroidal anti-inflammatory agents to proteins—I. Ibuprofen-serum albumin interaction. Biochem. Pharmacol. 1979, 28, 675–678. [Google Scholar] [CrossRef]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef]
- Guglielmelli, A.; Rizzuti, B.; Guzzi, R. Stereoselective and domain-specific effects of ibuprofen on the thermal stability of human serum albumin. Eur. J. Pharm. Sci. 2018, 112, 122–131. [Google Scholar] [CrossRef]
- Heneka, M.T.; Sastre, M.; Dumitrescu-Ozimek, L.; Hanke, A.; Dewachter, I.; Kuiperi, C.; O’Banion, K.; Klockgether, T.; Van Leuven, F.; Landreth, G.E. Acute treatment with the PPARgamma agonist pioglitazone and ibuprofen reduces glial inflammation and Abeta1-42 levels in APPV717I transgenic mice. Brain 2005, 128, 1442–1453. [Google Scholar] [CrossRef]
- McKee, A.C.; Carreras, I.; Hossain, L.; Ryu, H.; Klein, W.L.; Oddo, S.; LaFerla, F.M.; Jenkins, B.G.; Kowall, N.W.; Dedeoglu, A. Ibuprofen reduces Abeta, hyperphosphorylated tau and memory deficits in Alzheimer mice. Brain Res. 2008, 1207, 225–236. [Google Scholar] [CrossRef]
- Choi, J.K.; Jenkins, B.G.; Carreras, I.; Kaymakcalan, S.; Cormier, K.; Kowall, N.W.; Dedeoglu, A. Anti-inflammatory treatment in AD mice protects against neuronal pathology. Exp. Neurol. 2010, 223, 377–384. [Google Scholar] [CrossRef][Green Version]
- Woodling, N.S.; Colas, D.; Wang, Q.; Minhas, P.; Panchal, M.; Liang, X.; Mhatre, S.D.; Brown, H.; Ko, N.; Zagol-Ikapitte, I.; et al. Cyclooxygenase inhibition targets neurons to prevent early behavioural decline in Alzheimer’s disease model mice. Brain 2016, 139, 2063–2081. [Google Scholar] [CrossRef]
- Yan, Q.; Zhang, J.; Liu, H.; Babu-Khan, S.; Vassar, R.; Biere, A.L.; Citron, M.; Landreth, G. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer’s disease. J. Neurosci. 2003, 23, 7504–7509. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.P.; Yang, F.; Chu, T.; Chen, P.; Beech, W.; Teter, B.; Tran, T.; Ubeda, O.; Ashe, K.H.; Frautschy, S.A.; et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer’s disease. J. Neurosci. 2000, 20, 5709–5714. [Google Scholar] [CrossRef] [PubMed]
- Morihara, T.; Teter, B.; Yang, F.; Lim, G.P.; Boudinot, S.; Boudinot, F.D.; Frautschy, S.A.; Cole, G.M. Ibuprofen suppresses interleukin-1beta induction of pro-amyloidogenic alpha1-antichymotrypsin to ameliorate beta-amyloid (Abeta) pathology in Alzheimer’s models. Neuropsychopharmacology 2005, 30, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, B.L.; Cramer, P.E.; Varvel, N.H.; Reed-Geaghan, E.; Jiang, Q.; Szabo, A.; Herrup, K.; Lamb, B.T.; Landreth, G.E. Ibuprofen attenuates oxidative damage through NOX2 inhibition in Alzheimer’s disease. Neurobiol. Aging 2012, 33, 197.e21–197.e32. [Google Scholar] [CrossRef]
- In’T Veld, B.A.; Ruitenberg, A.; Hofman, A.; Launer, L.J.; Van Duijn, C.M.; Stijnen, T.; Breteler, M.M.; Stricker, B.H. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N. Engl. J. Med. 2001, 345, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Vlad, S.C.; Miller, D.R.; Kowall, N.W.; Felson, D.T. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology 2008, 70, 1672–1677. [Google Scholar] [CrossRef]
- Wang, J.; Tan, L.; Wang, H.F.; Tan, C.C.; Meng, X.F.; Wang, C.; Tang, S.W.; Yu, J.T. Anti-inflammatory drugs and risk of Alzheimer’s disease: An updated systematic review and meta-analysis. J. Alzheimers Dis. 2015, 44, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, S.A.; McCay, P.B. Spin trapping of ibuprofen radicals: Evidence that ibuprofen is a hydroxyl radical scavenger. Free Radic. Res. Commun. 1990, 9, 337–442. [Google Scholar] [CrossRef]
- Czirr, E.; Weggen, S. γ-Secretase modulation with Aβ42-lowering nonsteroidal anti-inflammatory drugs and derived compounds. Neurodegener. Dis. 2006, 3, 298–304. [Google Scholar] [CrossRef]
- Hendriks, L.; Vanduijn, C.M.; Cras, P.; Cruts, M.; Vanhul, W.; Vanharskamp, F.; Warren, A.; Mcinnis, M.G.; Antonarakis, S.E.; Martin, J.J.; et al. Presenile-dementia and cerebral-hemorrhage linked to a mutation at codon-692 of the beta-amyloid precursor protein gene. Nat. Genet. 1992, 1, 218–221. [Google Scholar] [CrossRef]
- Travis, J.; Pannell, R. Selective removal of albumin from plasma by affinity chromatography. Clin. Chim. Acta 1973, 49, 49–52. [Google Scholar] [CrossRef]
- Mehlisch, D.R.; Sykes, J. Ibuprofen blood plasma levels and onset of analgesia. Int. J. Clin. Pract. Suppl. 2013, 67, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Mazaleuskaya, L.L.; Theken, K.N.; Gong, L.; Thorn, C.F.; FitzGerald, G.A.; Altman, R.B.; Klein, T.E. PharmGKB summary: Ibuprofen pathways. Pharm. Genom. 2015, 25, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.S.; Lee, H.J.; Han, J.Y.; Lim, M.H.; Kim, H.I. Molecular insights into human serum albumin as a receptor of amyloid-beta in the extracellular region. J. Am. Chem. Soc. 2017, 139, 15437–15445. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, F.; Xu, L.; Jia, L. HSA targets multiple Aβ42 species and inhibits the seeding-mediated aggregation and cytotoxicity of Aβ42 aggregates. RSC Adv. 2016, 6, 71165–71175. [Google Scholar] [CrossRef]
- Naiki, H.; Higuchi, K.; Hosokawa, M.; Takeda, T. Fluorometric determination of amyloid fibrils in vitro using the fluorescent dye, thioflavin T1. Anal. Biochem. 1989, 177, 244–249. [Google Scholar] [CrossRef]
- Xue, C.; Lin, T.Y.; Chang, D.; Guo, Z. Thioflavin T as an amyloid dye: Fibril quantification, optimal concentration and effect on aggregation. R. Soc. Open Sci. 2017, 4, 160696. [Google Scholar] [CrossRef]
- Hirohata, M.; Ono, K.; Naiki, H.; Yamada, M. Non-steroidal anti-inflammatory drugs have anti-amyloidogenic effects for Alzheimer’s β-amyloid fibrils in vitro. Neuropharmacology 2005, 49, 1088–1099. [Google Scholar] [CrossRef]
- Agdeppa, E.D.; Kepe, V.; Petri, A.; Satyamurthy, N.; Liu, J.; Huang, S.C.; Small, G.W.; Cole, G.M.; Barrio, J.R. In vitro detection of (S)-naproxen and ibuprofen binding to plaques in the Alzheimer’s brain using the positron emission tomography molecular imaging probe 2-(1-{6-[(2-[18F]fluoroethyl)(methyl)amino]-2-naphthyl}ethylidene)malononitrile. Neuroscience 2003, 117, 723–730. [Google Scholar] [CrossRef]
- Lockhart, C.; Kim, S.; Klimov, D.K. Explicit solvent molecular dynamics simulations of Aβ peptide interacting with ibuprofen ligands. J. Phys. Chem. B 2012, 116, 12922–12932. [Google Scholar] [CrossRef]
- Raman, E.P.; Takeda, T.; Klimov, D.K. Molecular dynamics simulations of ibuprofen binding to Abeta peptides. Biophys. J. 2009, 97, 2070–2079. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Finder, V.H.; Vodopivec, I.; Nitsch, R.M.; Glockshuber, R. The recombinant amyloid-β peptide Aβ1–42 aggregates faster and is more neurotoxic than synthetic Aβ1–42. J. Mol. Biol. 2010, 396, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Desta, I.T.; Porter, K.A.; Xia, B.; Kozakov, D.; Vajda, S. Performance and its limits in rigid body protein-protein docking. Structure 2020, 28, 1071–1081.e3. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef]
- Catanzariti, A.-M.; Soboleva, T.A.; Jans, D.A.; Board, P.G.; Baker, R.T. An efficient system for high-level expression and easy purification of authentic recombinant proteins. Protein Sci. 2004, 13, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef] [PubMed]
- De Ferrari, G.V.; Mallender, W.D.; Inestrosa, N.C.; Rosenberry, T.L. Thioflavin T is a fluorescent probe of the acetylcholinesterase peripheral site that reveals conformational interactions between the peripheral and acylation sites. J. Biol. Chem. 2001, 276, 23282–23287. [Google Scholar] [CrossRef] [PubMed]
- Zagorski, M.G.; Yang, J.; Shao, H.; Ma, K.; Zeng, H.; Hong, A. Methodological and chemical factors affecting amyloid β peptide amyloidogenicity. Methods Enzymol. 1999, 309, 189–204. [Google Scholar]
- Dawson, R.M.C.; Elliott, D.C.; Elliott, W.H.; Jones, K.M. Data for Biochemical Research, 3rd ed.; Oxford Science Publications: Oxford, UK, 1986; 496p. [Google Scholar]
- Tiiman, A.; Krishtal, J.; Palumaa, P.; Tõugu, V. In vitro fibrillization of Alzheimer’s amyloid-β peptide (1–42). AIP Adv. 2015, 5, 092401. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Schrödinger, L.L.C. The PyMOL Molecular Graphics System, Version 1.8; Schrödinger, LLC: New York, NY, USA, 2015. [Google Scholar]
- Romero, P.; Obradovic, Z.; Li, X.; Garner, E.C.; Brown, C.J.; Dunker, A.K. Sequence complexity of disordered protein. Proteins 2001, 42, 38–48. [Google Scholar] [CrossRef]
- Peng, K.; Radivojac, P.; Vucetic, S.; Dunker, A.K.; Obradovic, Z. Length-dependent prediction of protein intrinsic disorder. BMC Bioinform. 2006, 7, 208. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, Z.; Peng, K.; Vucetic, S.; Radivojac, P.; Dunker, A.K. Exploiting heterogeneous sequence properties improves prediction of protein disorder. Proteins 2005, 61, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Dunbrack, R.L.; Williams, R.W.; Dunker, A.K.; Uversky, V.N. PONDR-FIT: A meta-predictor of intrinsically disordered amino acids. Biochim. Biophys. Acta 2010, 1804, 996–1010. [Google Scholar] [CrossRef]
- Dosztanyi, Z.; Csizmok, V.; Tompa, P.; Simon, I. IUPred: Web server for the prediction of intrinsically unstructured regions of proteins based on estimated energy content. Bioinformatics 2005, 21, 3433–3434. [Google Scholar] [CrossRef]
- Dosztanyi, Z.; Csizmok, V.; Tompa, P.; Simon, I. The pairwise energy content estimated from amino acid composition discriminates between folded and intrinsically unstructured proteins. J. Mol. Biol. 2005, 347, 827–839. [Google Scholar] [CrossRef]
- Erdos, G.; Dosztanyi, Z. Analyzing Protein Disorder with IUPred2A. Curr. Protoc. Bioinform. 2020, 70, e99. [Google Scholar] [CrossRef]
- Meszaros, B.; Erdos, G.; Dosztanyi, Z. IUPred2A: Context-dependent prediction of protein disorder as a function of redox state and protein binding. Nucleic Acids Res. 2018, 46, W329–W337. [Google Scholar] [CrossRef]
- Necci, M.; Piovesan, D.; CAID Predictors; DisProt Curators; Tosatto, S.C.E. Critical assessment of protein intrinsic disorder prediction. Nat. Methods 2021, 18, 472–481. [Google Scholar] [CrossRef]
- Rivers-Auty, J.; Mather, A.E.; Peters, R.; Lawrence, C.B.; Brough, D. Anti-inflammatories in Alzheimer’s disease—potential therapy or spurious correlate? Brain Commun. 2020, 2, fcaa109. [Google Scholar] [CrossRef]
- Baroni, S.; Mattu, M.; Vannini, A.; Cipollone, R.; Aime, S.; Ascenzi, P.; Fasano, M. Effect of ibuprofen and warfarin on the allosteric properties of haem-human serum albumin. A spectroscopic study. Eur. J. Biochem. 2001, 268, 6214–6220. [Google Scholar] [CrossRef]
- Nicoletti, F.P.; Howes, B.D.; Fittipaldi, M.; Fanali, G.; Fasano, M.; Ascenzi, P.; Smulevich, G. Ibuprofen induces an allosteric conformational transition in the heme complex of human serum albumin with significant effects on heme ligation. J. Am. Chem. Soc. 2008, 130, 11677–11688. [Google Scholar] [CrossRef] [PubMed]
- Fitos, I.; Visy, J.; Simonyi, M.; Hermansson, J. Stereoselective allosteric binding interaction on human serum albumin between ibuprofen and lorazepam acetate. Chirality 1999, 11, 115–120. [Google Scholar] [CrossRef]
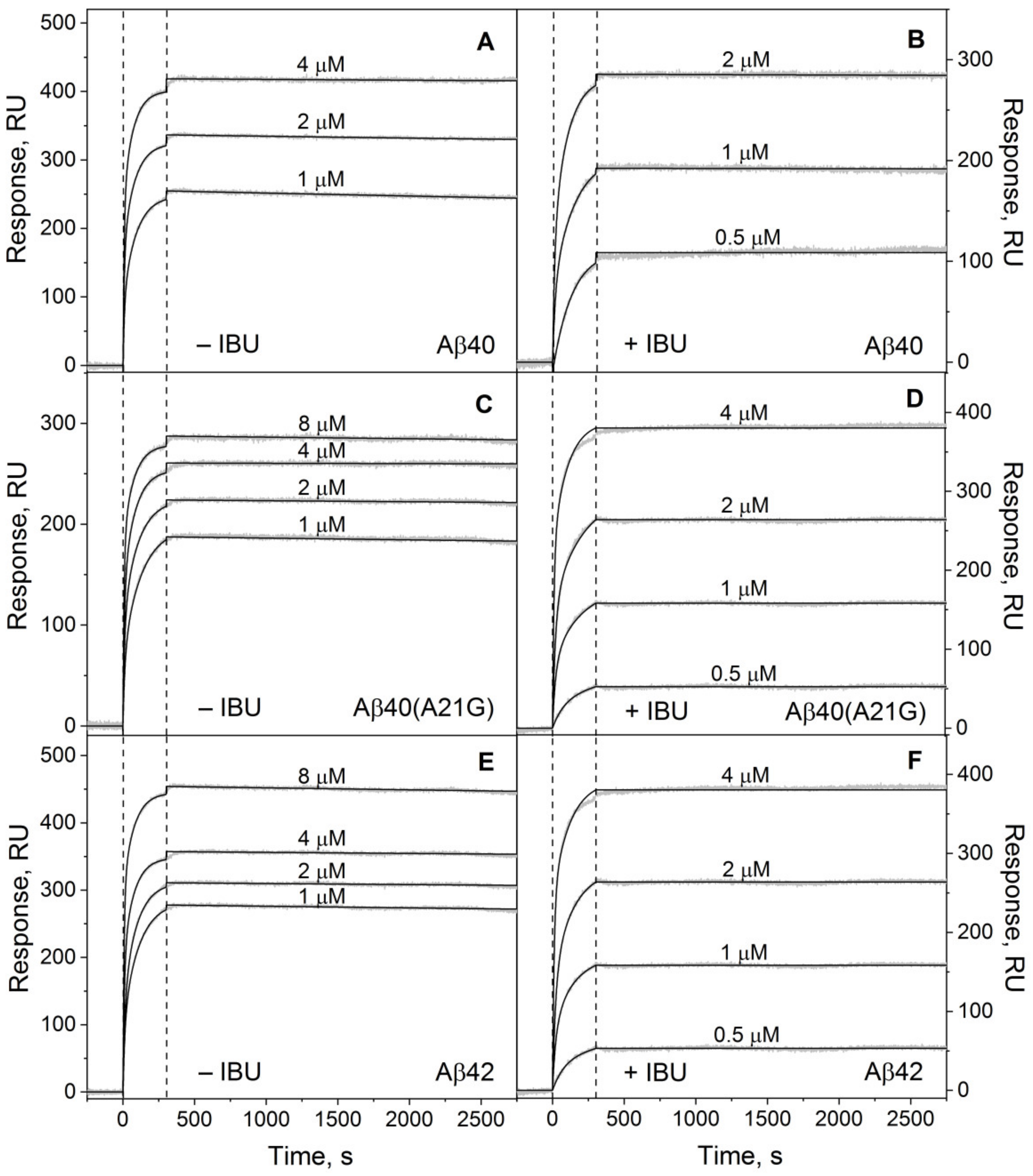


| [IBU], µM | ka1 × 10−3, M−1s−1 | kd1 × 106, s−1 | Kd1 × 1010, M | ka2 × 10−3, M−1s−1 | kd2 × 106, s−1 | Kd2 × 1010, M |
|---|---|---|---|---|---|---|
| Aβ40 | ||||||
| 0 | 65 ± 15 | 3.4 ± 0.6 | 0.52 ± 0.09 | 8.0 ± 0.8 | 7.9 ± 0.6 | 9.9 ± 0.9 |
| 100 | 35 ± 5 | 0.43 ± 0.05 | 0.16 ± 0.02 | 5.7 ± 0.6 | 2.2 ± 0.2 | 3.9 ± 0.4 |
| Aβ40(A21G) | ||||||
| 0 | 30 ± 3 | 0.43 ± 0.06 | 0.14 ± 0.02 | 2.7 ± 0.3 | 1.8 ± 0.2 | 6.7 ± 0.6 |
| 100 | 25 ± 4 | 0.20 ± 0.03 | 0.082 ± 0.012 | 2.4 ± 0.3 | 1.03 ± 0.10 | 4.2 ± 0.6 |
| Aβ42 | ||||||
| 0 | 50 ± 5 | 3.0 ± 0.5 | 0.60 ± 0.06 | 7.2 ± 1.1 | 4.9 ± 0.6 | 6.9 ± 0.7 |
| 100 | 40 ± 4 | 0.46 ± 0.06 | 0.11 ± 0.02 | 8.6 ± 0.8 | 1.4 ± 0.2 | 1.7 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litus, E.A.; Kazakov, A.S.; Deryusheva, E.I.; Nemashkalova, E.L.; Shevelyova, M.P.; Machulin, A.V.; Nazipova, A.A.; Permyakova, M.E.; Uversky, V.N.; Permyakov, S.E. Ibuprofen Favors Binding of Amyloid-β Peptide to Its Depot, Serum Albumin. Int. J. Mol. Sci. 2022, 23, 6168. https://doi.org/10.3390/ijms23116168
Litus EA, Kazakov AS, Deryusheva EI, Nemashkalova EL, Shevelyova MP, Machulin AV, Nazipova AA, Permyakova ME, Uversky VN, Permyakov SE. Ibuprofen Favors Binding of Amyloid-β Peptide to Its Depot, Serum Albumin. International Journal of Molecular Sciences. 2022; 23(11):6168. https://doi.org/10.3390/ijms23116168
Chicago/Turabian StyleLitus, Ekaterina A., Alexey S. Kazakov, Evgenia I. Deryusheva, Ekaterina L. Nemashkalova, Marina P. Shevelyova, Andrey V. Machulin, Aliya A. Nazipova, Maria E. Permyakova, Vladimir N. Uversky, and Sergei E. Permyakov. 2022. "Ibuprofen Favors Binding of Amyloid-β Peptide to Its Depot, Serum Albumin" International Journal of Molecular Sciences 23, no. 11: 6168. https://doi.org/10.3390/ijms23116168
APA StyleLitus, E. A., Kazakov, A. S., Deryusheva, E. I., Nemashkalova, E. L., Shevelyova, M. P., Machulin, A. V., Nazipova, A. A., Permyakova, M. E., Uversky, V. N., & Permyakov, S. E. (2022). Ibuprofen Favors Binding of Amyloid-β Peptide to Its Depot, Serum Albumin. International Journal of Molecular Sciences, 23(11), 6168. https://doi.org/10.3390/ijms23116168








