Lithosepermic Acid Restored the Skin Barrier Functions in the Imiquimod-Induced Psoriasis-like Animal Model
Abstract
:1. Introduction
2. Results
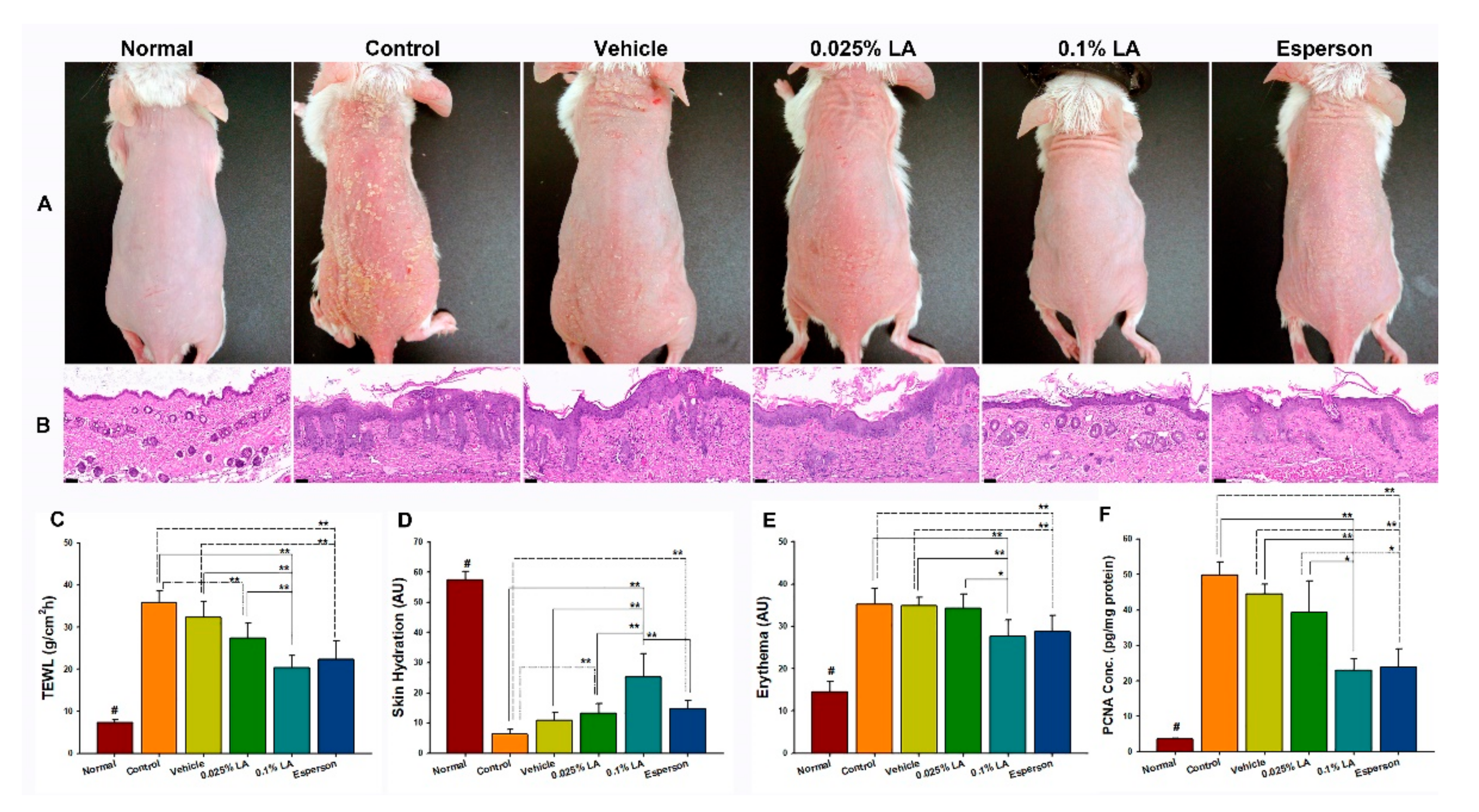
2.1. 0.1% LA and DXM Improved Psoriasis-like Dermatitis
2.2. LA and DXM Restored Barrier Function
2.3. 0.1% LA and DXM Inhibited Hyperproliferation of Keratinocytes
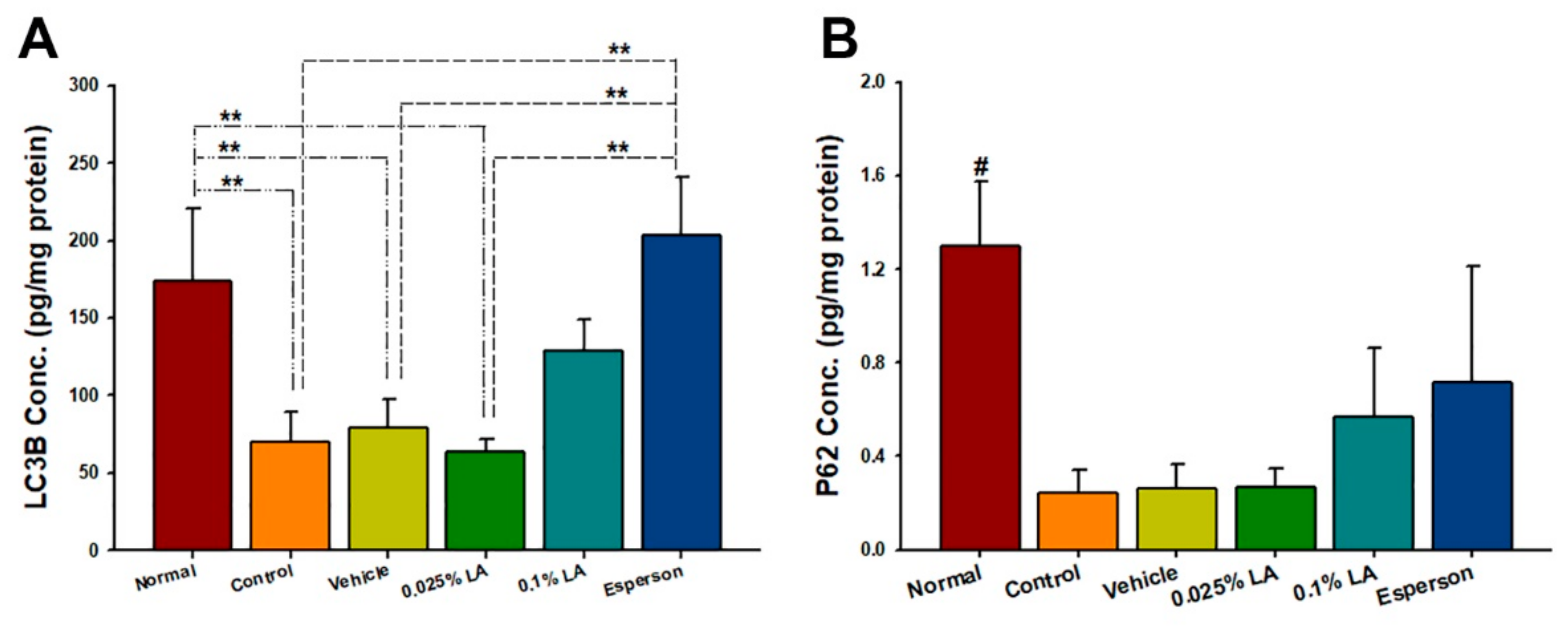
2.4. LA Showed a Dose-Dependent Upregulation of Autophagy-Related Protein Expression in IMQ-Induced Autophagy Dysfunction
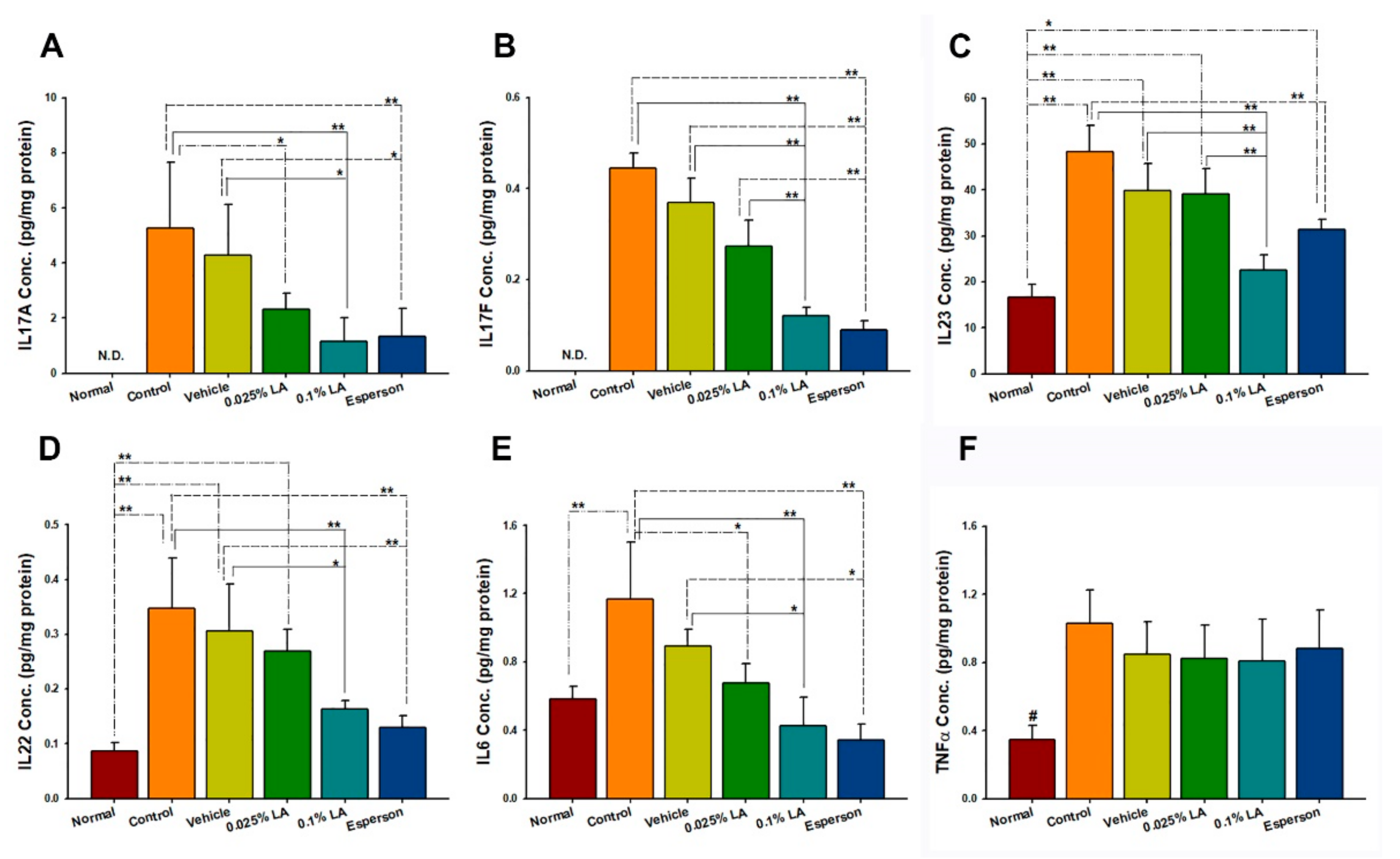
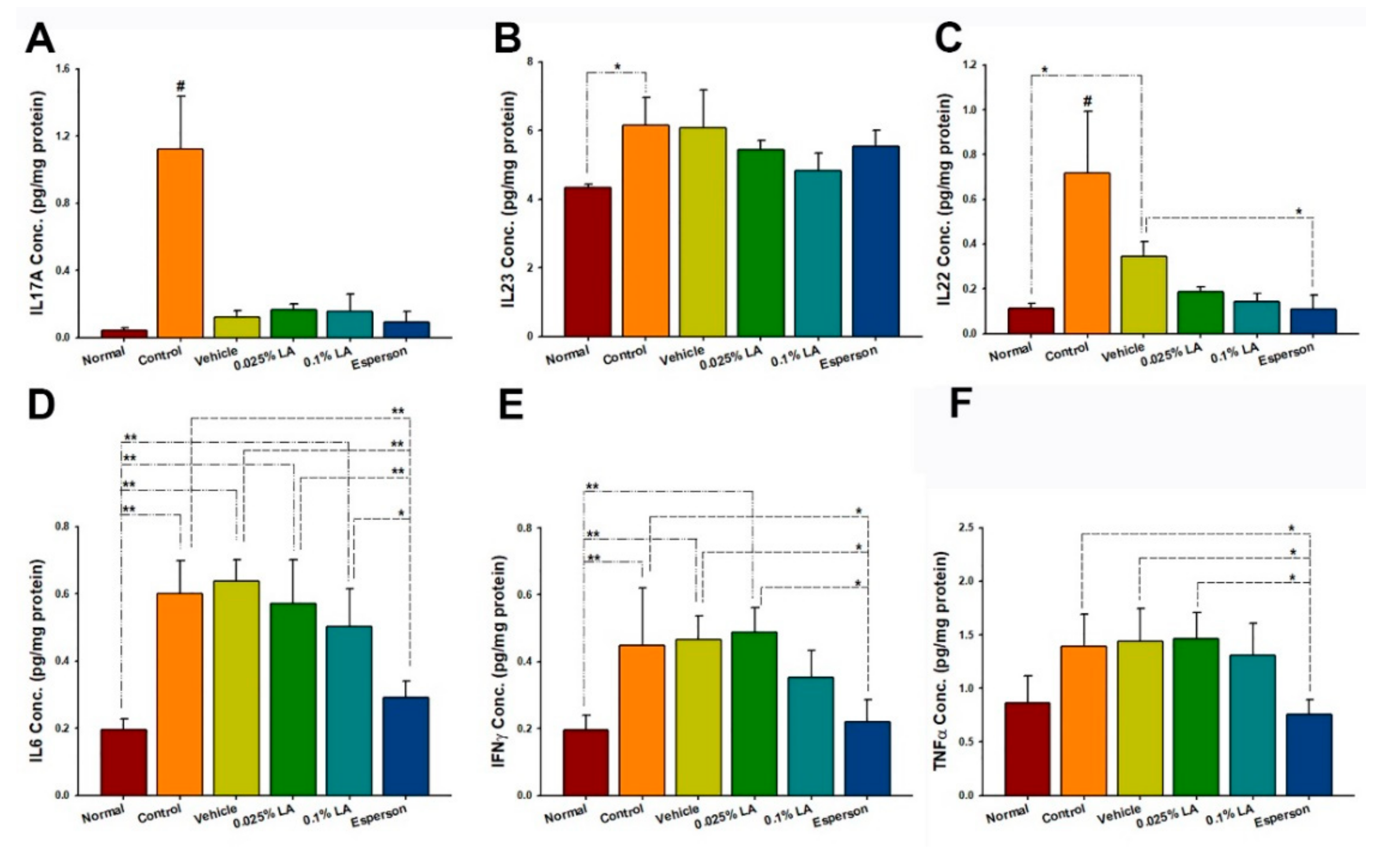
2.5. 0.1% LA and DXM Inhibited IL-17A, IL-17F, IL-22, IL-23, and IL-6 Protein Expression in Psoriasis-like Skin
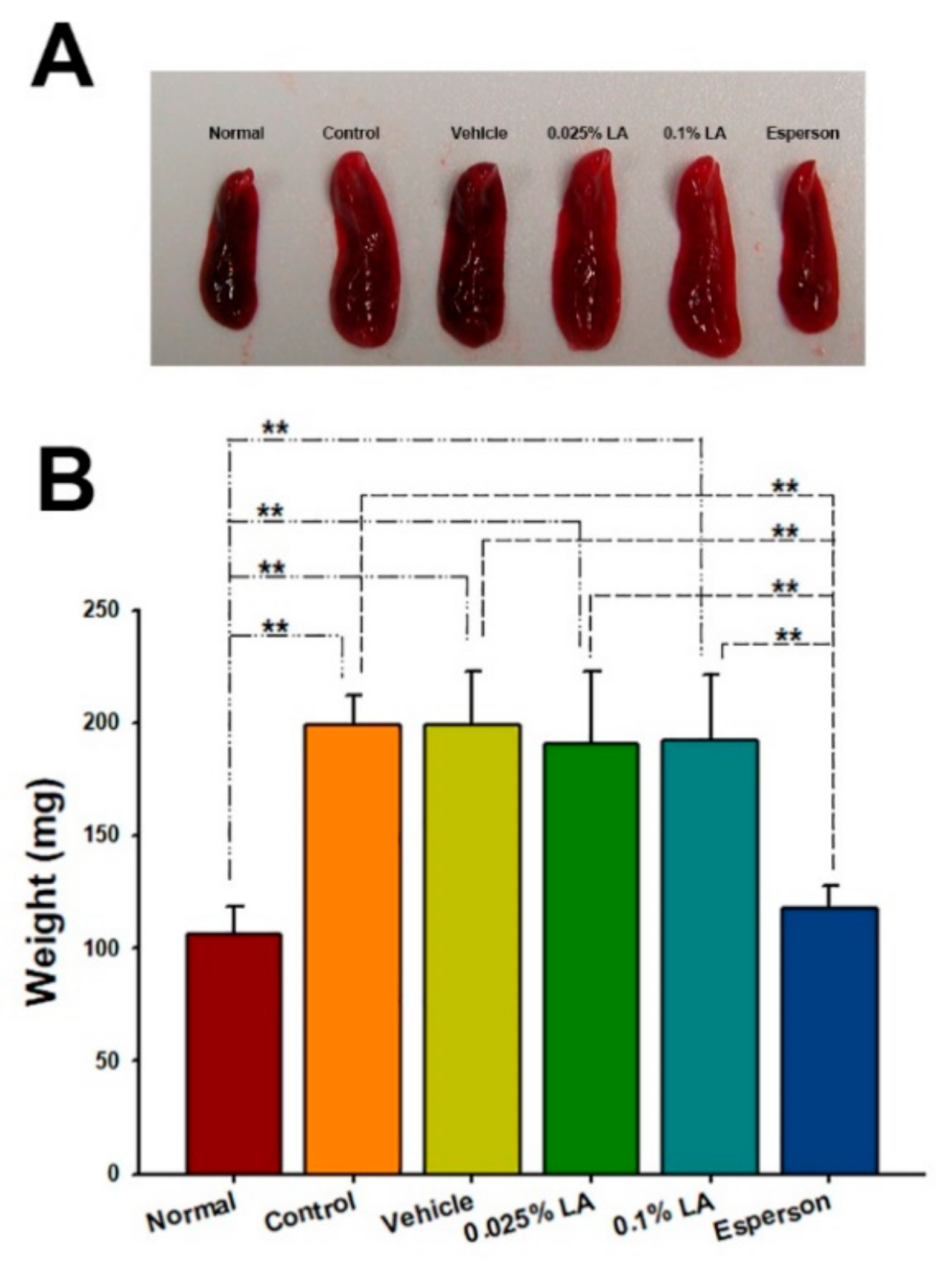
2.6. DXM Inhibited Spleen Enlargement in IMQ-Induced Psoriasis-like Mice
2.7. DXM Inhibited IL-22, IL-6, IFN-γ, and TNF-α Protein Expression of Spleen in IMQ-Induced Psoriasis-like Mice
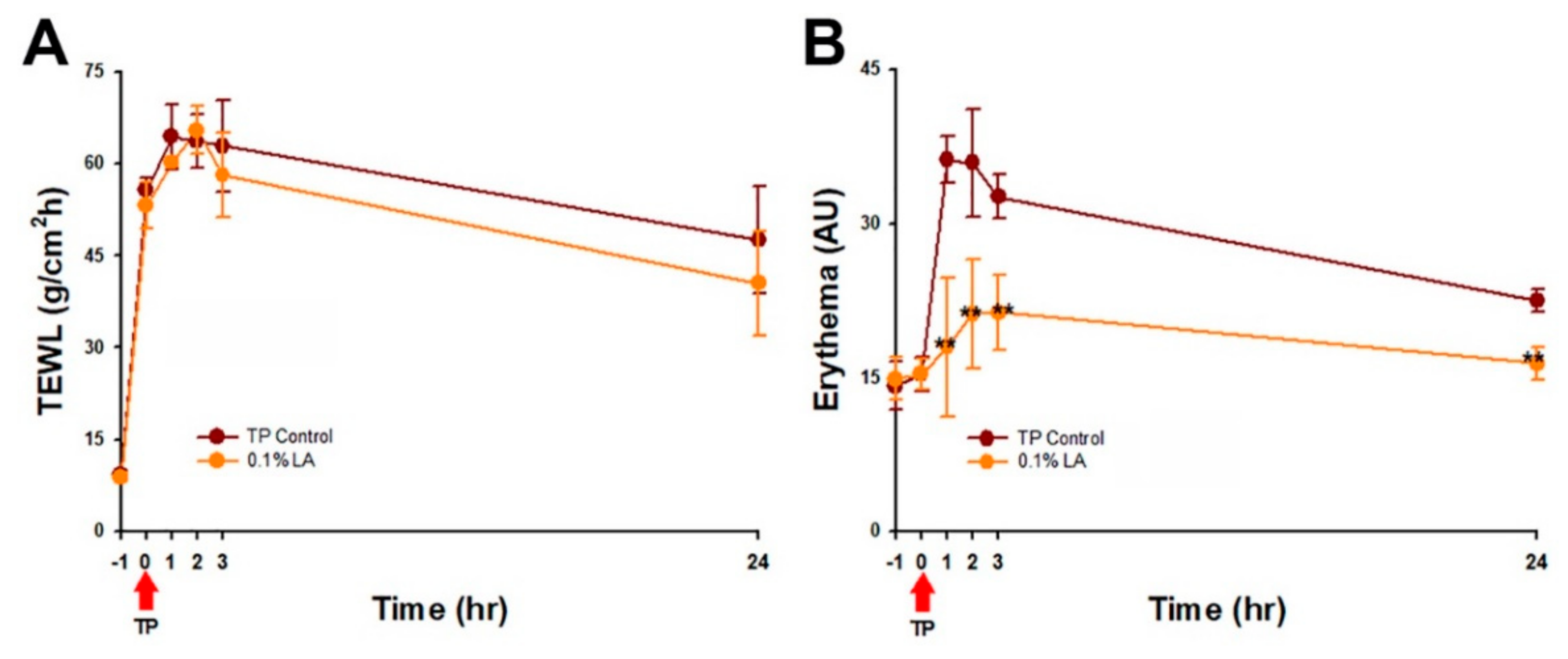
2.8. 0.1% LA Inhibited Tape-Stripping (TP) Induced Skin Erythema
3. Discussion
4. Materials and Methods
4.1. Microemulsion Preparation
4.2. Animals
4.3. Imiquimod (IMQ)-Induced Psoriasis-like Skin Animal Model
4.4. Assessment of Barrier Function
4.5. Collection of Skin and Spleen Specimens
4.6. Autophagy Cytokine Protein Determination
4.7. Inflammatory Cytokine Protein Determination
4.8. Proliferation Cell Nuclear Antigen (PCNA) Protein Determination
4.9. Histological Staining
4.10. Barrier Recovery Study
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamiya, K.; Kishimoto, M.; Sugai, J.; Komine, M.; Ohtsuki, M. Risk Factors for the Development of Psoriasis. Int. J. Mol. Sci. 2019, 20, 4347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, C.E.M.; Armstrong, A.W.; Gudjonsson, J.E.; Barker, J. Psoriasis. Lancet 2021, 397, 1301–1315. [Google Scholar] [CrossRef]
- Wu, W.Y.; Wang, Y.P. Pharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active components. Acta Pharmacol. Sin. 2012, 33, 1119–1130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.W.; Feng, J.N.; Cao, Y.; Meng, L.P.; Wang, S.L. Autophagy prevents autophagic cell death in Tetrahymena in response to oxidative stress. Dongwuxue Yanjiu 2015, 36, 167–173. [Google Scholar]
- Liu, X.; Chen, R.; Shang, Y.; Jiao, B.; Huang, C. Lithospermic acid as a novel xanthine oxidase inhibitor has anti-inflammatory and hypouricemic effects in rats. Chem. Biol. Interact. 2008, 176, 137–142. [Google Scholar] [CrossRef]
- Liu, H.; Ma, S.; Xia, H.; Lou, H.; Zhu, F.; Sun, L. Anti-inflammatory activities and potential mechanisms of phenolic acids isolated from Salvia miltiorrhiza f. alba roots in THP-1 macrophages. J. Ethnopharmacol. 2018, 222, 201–207. [Google Scholar] [CrossRef]
- Guo, J.W.; Cheng, Y.P.; Liu, C.Y.; Thong, H.Y.; Huang, C.J.; Lo, Y.; Wu, C.Y.; Jee, S.H. Salvianolic Acid B in Microemulsion Formulation Provided Sufficient Hydration for Dry Skin and Ameliorated the Severity of Imiquimod-Induced Psoriasis-Like Dermatitis in Mice. Pharmaceutics 2020, 12, 457. [Google Scholar] [CrossRef]
- Bos, J.D.; Meinardi, M.M. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp. Dermatol. 2000, 9, 165–169. [Google Scholar] [CrossRef]
- Benigni, M.; Pescina, S.; Grimaudo, M.A.; Padula, C.; Santi, P.; Nicoli, S. Development of microemulsions of suitable viscosity for cyclosporine skin delivery. Int. J. Pharm. 2018, 545, 197–205. [Google Scholar] [CrossRef]
- Nastiti, C.; Ponto, T.; Abd, E.; Grice, J.E.; Benson, H.A.E.; Roberts, M.S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef]
- Szumala, P.; Macierzanka, A. Topical delivery of pharmaceutical and cosmetic macromolecules using microemulsion systems. Int. J. Pharm. 2022, 615, 121488. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.; Cosgarea, I.; Reynolds, N.; Lovat, P.; Armstrong, J. Research Techniques Made Simple: Analysis of Autophagy in the Skin. J. Investig. Dermatol. 2021, 141, 5–9.e1. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Chae, Y.J.; Choi, J.W.; Chang, J.E. Potential Therapeutic Approaches through Modulating the Autophagy Process for Skin Barrier Dysfunction. Int. J. Mol. Sci. 2021, 22, 7869. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Peng, L.; Zeng, J.; Zhang, M.; Xu, F.; Zhang, X.; Wei, Q. Paeoniflorin suppresses allergic and inflammatory responses by promoting autophagy in rats with urticaria. Exp. Ther. Med. 2021, 21, 590. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, H.R.; Kang, S.Y.; Jung, M.J.; Heo, N.H.; Lee, H.J.; Ryu, A.; Kim, H.O.; Park, C.W.; Chung, B.Y. Aryl Hydrocarbon Receptor and Autophagy-Related Protein Microtubule-Associated Protein Light Chain 3 Expression in Psoriasis. Ann. Dermatol. 2021, 33, 138–146. [Google Scholar] [CrossRef]
- Varshney, P.; Saini, N. PI3K/AKT/mTOR activation and autophagy inhibition plays a key role in increased cholesterol during IL-17A mediated inflammatory response in psoriasis. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1795–1803. [Google Scholar] [CrossRef]
- Tang, Z.L.; Zhang, K.; Lv, S.C.; Xu, G.W.; Zhang, J.F.; Jia, H.Y. Corrigendum to “LncRNA MEG3 suppresses PI3K/AKT/mTOR signalling pathway to enhance autophagy and inhibit inflammation in TNF-alpha-treated keratinocytes and psoriatic mice” [Cytokine 148 (2021) 155657]. Cytokine 2022, 153, 155853. [Google Scholar] [CrossRef]
- Mahil, S.K.; Twelves, S.; Farkas, K.; Setta-Kaffetzi, N.; Burden, A.D.; Gach, J.E.; Irvine, A.D.; Kepiro, L.; Mockenhaupt, M.; Oon, H.H.; et al. AP1S3 Mutations Cause Skin Autoinflammation by Disrupting Keratinocyte Autophagy and Up-Regulating IL-36 Production. J. Investig. Dermatol. 2016, 136, 2251–2259. [Google Scholar] [CrossRef]
- Lv, H.; Liu, X.; Chen, W.; Xiao, S.; Ji, Y.; Han, X.; Li, Y.; Wang, X.; Zhang, G.; Li, P. Yangxue Jiedu Fang Ameliorates Psoriasis by Regulating Vascular Regression via Survivin/PI3K/Akt Pathway. J. Immunol. Res. 2021, 2021, 4678087. [Google Scholar] [CrossRef]
- Korman, N.J. Management of psoriasis as a systemic disease: What is the evidence? Br. J. Dermatol. 2020, 182, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Masson, W.; Lobo, M.; Molinero, G. Psoriasis and Cardiovascular Risk: A Comprehensive Review. Adv. Ther. 2020, 37, 2017–2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fullerton, A.; Fischer, T.; Lahti, A.; Wilhelm, K.P.; Takiwaki, H.; Serup, J. Guidelines for measurement of skin colour and erythema. A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Dermat. 1996, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Serup, J.; Agner, T. Colorimetric quantification of erythema—A comparison of two colorimeters (Lange Micro Color and Minolta Chroma Meter CR-200) with a clinical scoring scheme and laser-Doppler flowmetry. Clin. Exp. Dermatol. 1990, 15, 267–272. [Google Scholar] [CrossRef]
- Ranosz-Janicka, I.; Lis-Swiety, A.; Skrzypek-Salamon, A.; Brzezinska-Wcislo, L. Detecting and quantifying activity/inflammation in localized scleroderma with thermal imaging. Skin Res. Technol. 2019, 25, 118–123. [Google Scholar] [CrossRef]
- Kuo, I.H.; Carpenter-Mendini, A.; Yoshida, T.; McGirt, L.Y.; Ivanov, A.I.; Barnes, K.C.; Gallo, R.L.; Borkowski, A.W.; Yamasaki, K.; Leung, D.Y.; et al. Activation of epidermal toll-like receptor 2 enhances tight junction function: Implications for atopic dermatitis and skin barrier repair. J. Investig. Dermatol. 2013, 133, 988–998. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-C.; Cheng, Y.-P.; Liu, C.-Y.; Guo, J.-W. Lithosepermic Acid Restored the Skin Barrier Functions in the Imiquimod-Induced Psoriasis-like Animal Model. Int. J. Mol. Sci. 2022, 23, 6172. https://doi.org/10.3390/ijms23116172
Chen L-C, Cheng Y-P, Liu C-Y, Guo J-W. Lithosepermic Acid Restored the Skin Barrier Functions in the Imiquimod-Induced Psoriasis-like Animal Model. International Journal of Molecular Sciences. 2022; 23(11):6172. https://doi.org/10.3390/ijms23116172
Chicago/Turabian StyleChen, Li-Ching, Yu-Ping Cheng, Chih-Yi Liu, and Jiun-Wen Guo. 2022. "Lithosepermic Acid Restored the Skin Barrier Functions in the Imiquimod-Induced Psoriasis-like Animal Model" International Journal of Molecular Sciences 23, no. 11: 6172. https://doi.org/10.3390/ijms23116172
APA StyleChen, L.-C., Cheng, Y.-P., Liu, C.-Y., & Guo, J.-W. (2022). Lithosepermic Acid Restored the Skin Barrier Functions in the Imiquimod-Induced Psoriasis-like Animal Model. International Journal of Molecular Sciences, 23(11), 6172. https://doi.org/10.3390/ijms23116172