Macro- and Nanoscale Effect of Ethanol on Bovine Serum Albumin Gelation and Naproxen Release
Abstract
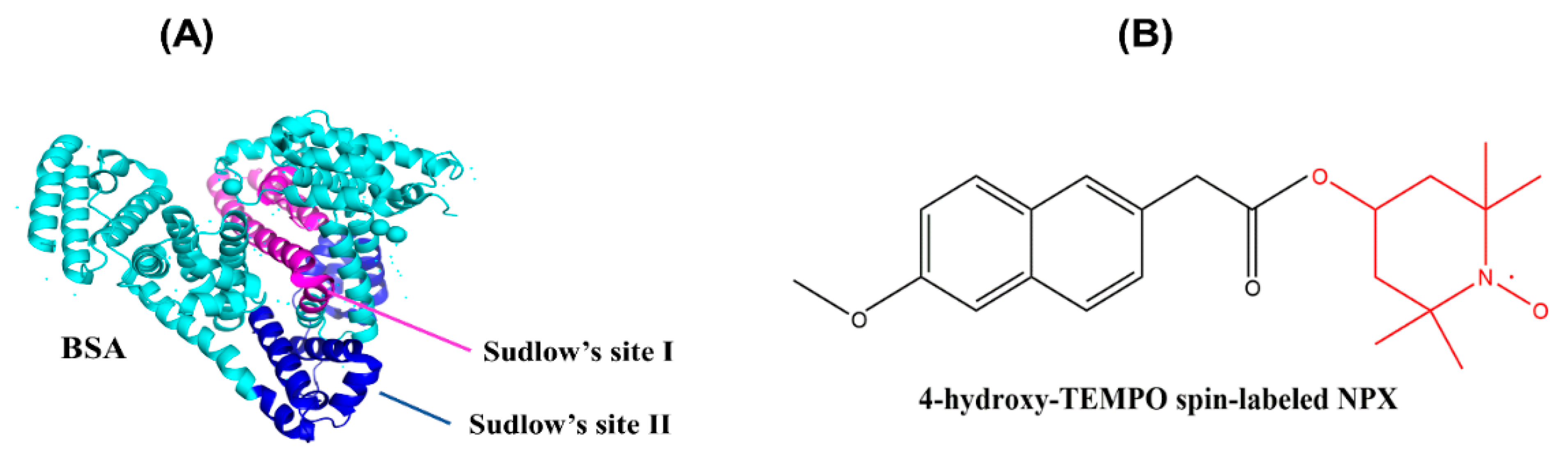
:1. Introduction
2. Results and Discussion
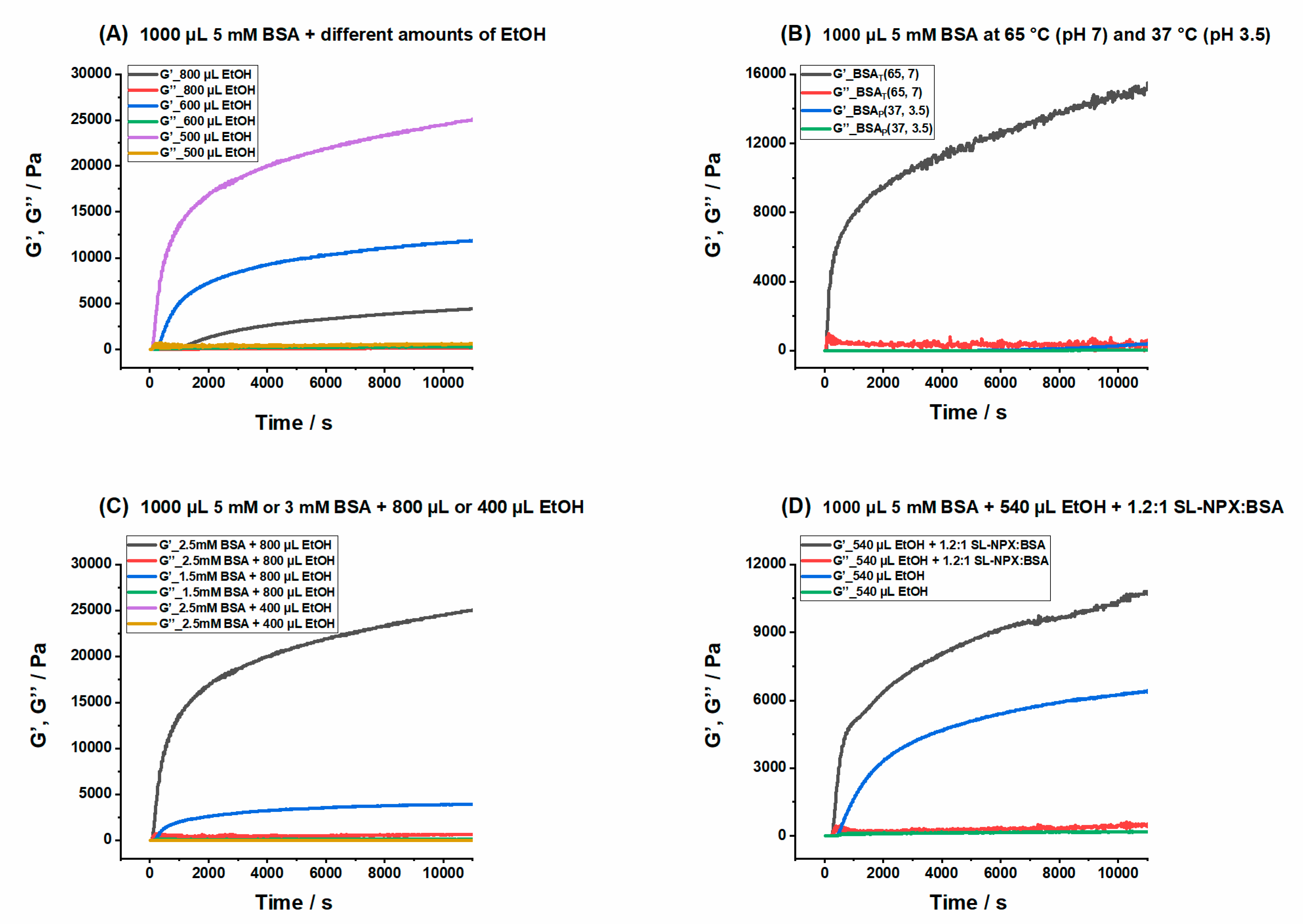
2.1. Measurement of Rheological Properties
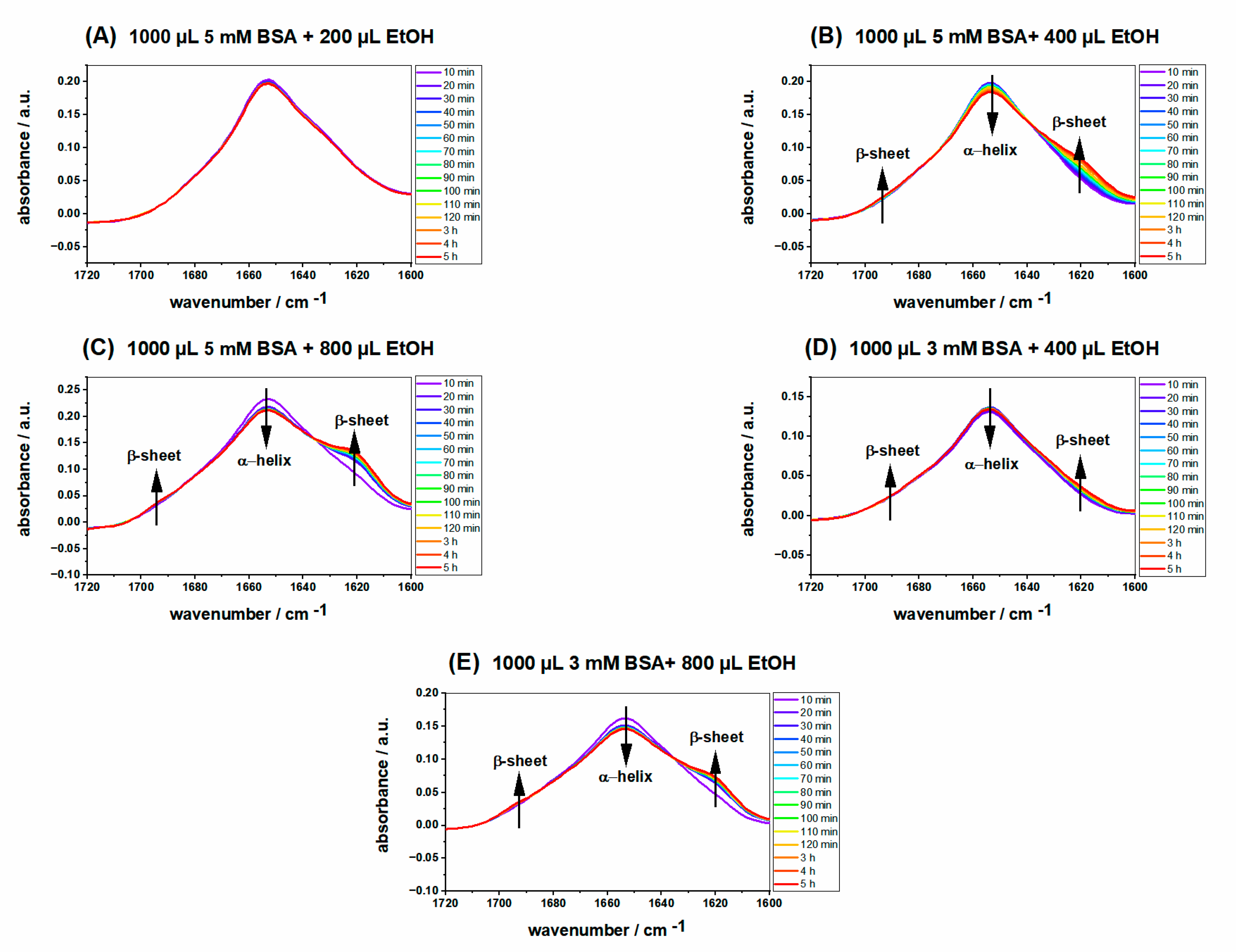
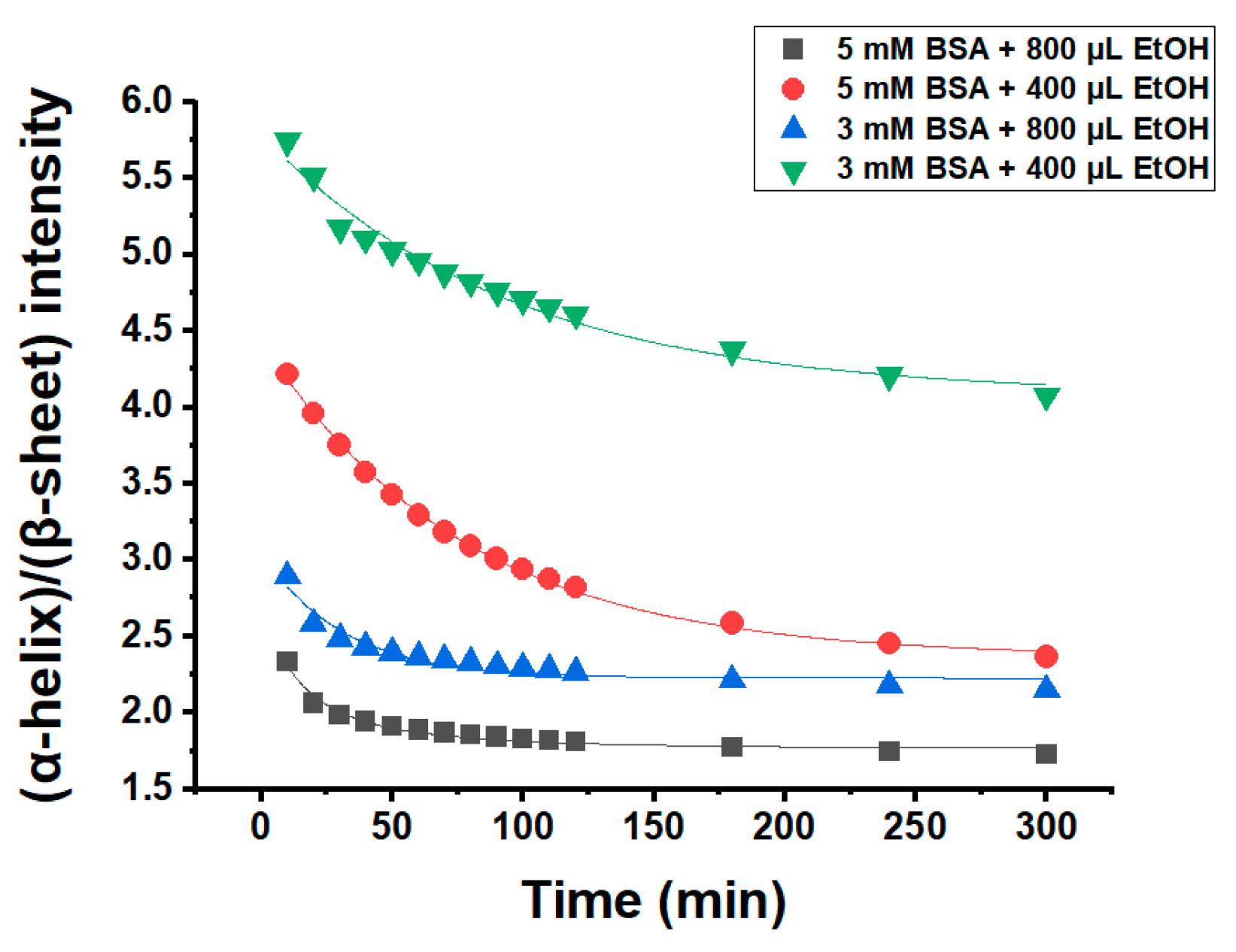
2.2. ATR-IR Spectra of Ethanol-Induced BSA Hydrogels
2.3. CW EPR Spectroscopy
2.3.1. Characterization of BSA Hydrogels
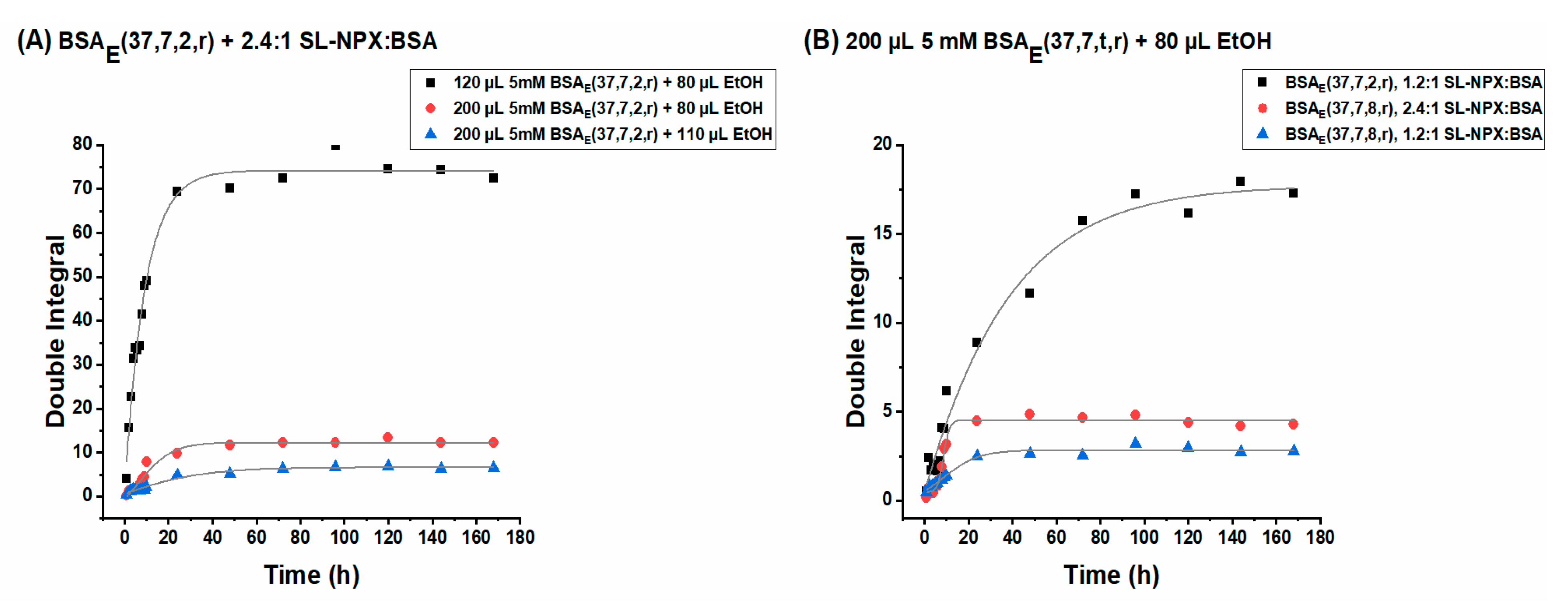
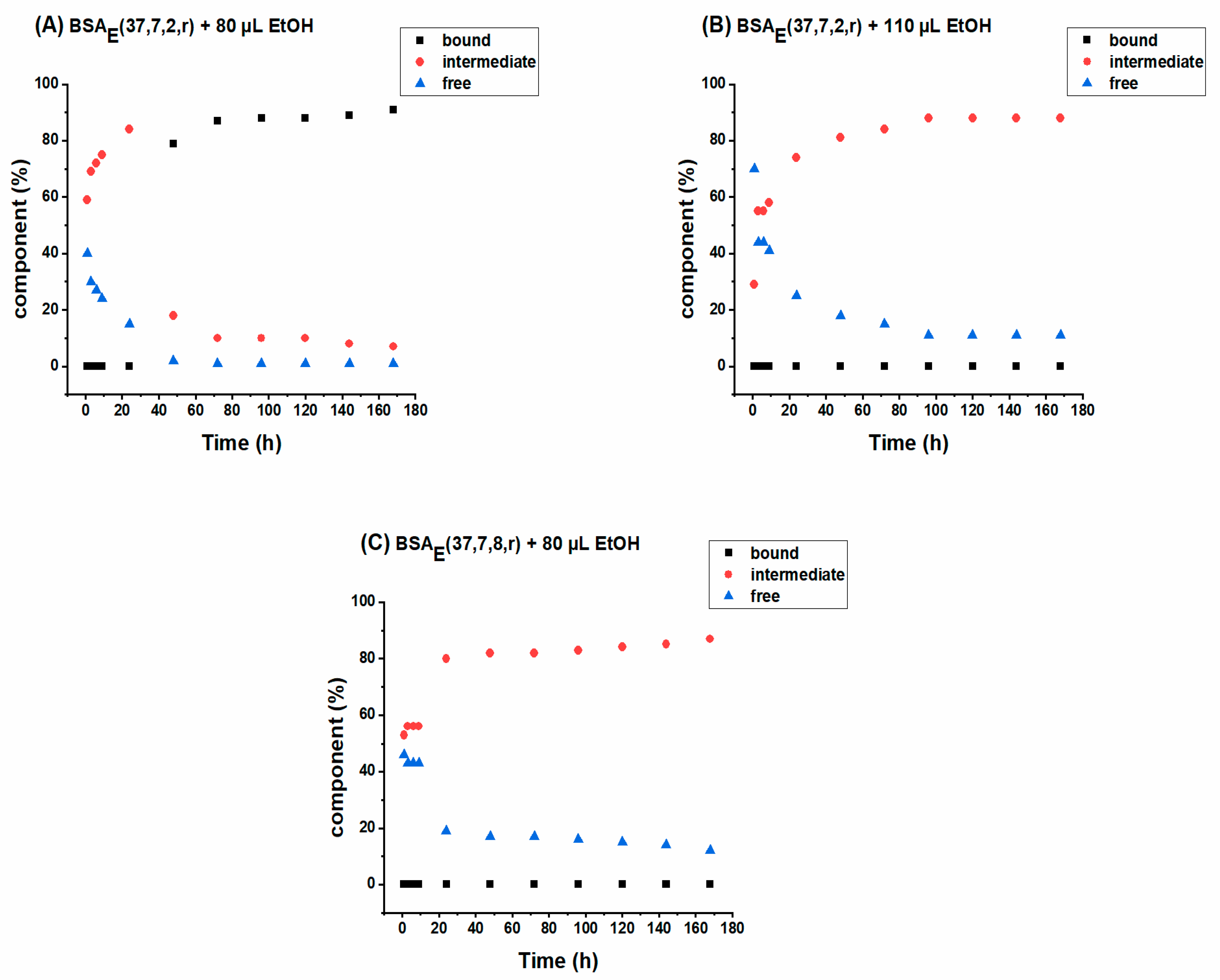
2.3.2. SL-NPX Release Studies from BSA Hydrogels
2.4. Investigation of Released Components by DLS
3. Materials and Methods
3.1. Materials
3.2. Methods
3.3. Rheological Characterization
3.4. ATR IR Spectroscopy
3.5. Continuous Wave Electron Paramagnetic Resonance (CW EPR) Spectroscopy
3.6. Dynamic Light Scattering
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sur, S.; Rathore, A.; Dave, V.; Reddy, K.R.; Chouhan, R.S.; Sadhu, V. Recent developments in functionalized polymer nanoparticles for efficient drug delivery system. Nano-Struct. Nano-Objects 2019, 20, 100397. [Google Scholar] [CrossRef]
- Iqbal, Z.; Babar, A.; Ashraf, M. Controlled-release naproxen using micronized ethyl cellulose by wet-granulation and solid dispersion method. Drug Dev. Ind. Pharm. 2002, 28, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Jung, K.; Li, A.; Liu, J.; Boyer, C. Recent advances in stimuli-responsive polymer systems for remotely controlled drug release. Prog. Polym. Sci. 2019, 99, 101164. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Y.; Wang, Q.; Liu, T.; Sun, J.; Zhang, R. Remote light-responsive nanocarriers for controlled drug delivery: Advances and perspectives. Small 2019, 15, 1903060. [Google Scholar] [CrossRef]
- Zaghloul, A.A.; Vaithiyalingam, S.R.; Faltinek, J.; Reddy, I.K.; Khan, M.A. Response surface methodology to obtain naproxen controlled release tablets from its microspheres with Eudragit L100-55. J. Microencapsul. 2001, 18, 651–662. [Google Scholar]
- Göttel, B.; Silva, J.M.D.S.E.; de Oliveira, C.S.; Syrowatka, F.; Fiorentzis, M.; Viestenz, A.; Viestenz, A.; Mäder, K. Electrospun nanofibers–A promising solid in-situ gelling alternative for ocular drug delivery. Eur. J. Pharm. Biopharm. 2020, 146, 125–132. [Google Scholar] [CrossRef]
- Cleeton, C.; Keirouz, A.; Chen, X.; Radacsi, N. Electrospun nanofibers for drug delivery and biosensing. ACS Biomater. Sci. Eng. 2019, 5, 4183–4205. [Google Scholar] [CrossRef]
- Wang, J.; Kumeria, T.; Bezem, M.T.; Wang, J.; Sailor, M.J. Self-reporting photoluminescent porous silicon microparticles for drug delivery. ACS Appl. Mater. Interfaces 2018, 10, 3200–3209. [Google Scholar] [CrossRef]
- Dhanka, M.; Shetty, C.; Srivastava, R. Methotrexate loaded gellan gum microparticles for drug delivery. Int. J. Biol. Macromol. 2018, 110, 346–356. [Google Scholar] [CrossRef]
- Steiner, J.; Alaneed, R.; Kressler, J.; Mäder, K. Fatty acid-modified poly (glycerol adipate) microparticles for controlled drug delivery. J. Drug Deliv. Sci. Technol. 2021, 61, 102206. [Google Scholar] [CrossRef]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-responsive drug release from smart polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Indermun, S.; Govender, M.; Kumar, P.; Choonara, Y.E.; Pillay, V. Stimuli-responsive polymers as smart drug delivery systems: Classifications based on carrier type and triggered-release mechanism. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier: Amsterdam, The Netherlands, 2018; Volume 1, pp. 43–58. [Google Scholar]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Sanaeifar, N.; Mäder, K.; Hinderberger, D. Nanoscopic Characterization of Stearic Acid Release from Bovine Serum Albumin Hydrogels. Macromol. Biosci. 2020, 20, 2000126. [Google Scholar] [CrossRef] [PubMed]
- Sanaeifar, N.; Mäder, K.; Hinderberger, D. Molecular-Level Release of Coumarin-3-Carboxylic Acid and Warfarin-Derivatives from BSA-Based Hydrogels. Pharmaceutics 2021, 13, 1661. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Wang, W. Phototriggered targeting of nanocarriers for drug delivery. Nano Res. 2018, 11, 5424–5438. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Hanna, D.H.; Abu Elella, M.H.; Mohamed, R.R. Encapsulation of bovine serum albumin within novel xanthan gum based hydrogel for protein delivery. Mater. Sci. Eng. C 2019, 94, 1044–1055. [Google Scholar] [CrossRef]
- Vesković, A.; Nakarada, Đ.; Bijelić, A. A novel methodology for hydrogel water content determination by EPR: The basis for real-time monitoring of controlled drug release and hydrogel swelling and degradation. Polym. Test. 2021, 98, 107187. [Google Scholar] [CrossRef]
- Chen, K.; Chen, X.; Han, X.; Fu, Y. A comparison study on the release kinetics and mechanism of bovine serum albumin and nanoencapsulated albumin from hydrogel networks. Int. J. Biol. Macromol. 2020, 163, 1291–1300. [Google Scholar] [CrossRef]
- Aswathy, H.S.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef]
- Lantigua, D.; Nguyen, M.A.; Wu, X.; Suvarnapathaki, S.; Kwon, S.; Gavin, W.; Camci-Unal, G. Synthesis and characterization of photocrosslinkable albumin-based hydrogels for biomedical applications. Soft Matter 2020, 16, 9242–9252. [Google Scholar] [CrossRef]
- Nnyigide, S.O.; Hyun, K. Effects of anionic and cationic surfactants on the rheological properties and kinetics of bovine serum albumin hydrogel. Rheol. Acta 2018, 57, 563–573. [Google Scholar] [CrossRef]
- Arabi, S.H.; Aghelnejad, B.; Schwieger, C.; Meister, A.; Kerth, A.; Hinderberger, D. Serum albumin hydrogels in broad pH and temperature ranges: Characterization of their self-assembled structures and nanoscopic and macroscopic properties. Biomater. Sci. 2018, 6, 478–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baler, K.; Michael, R.; Szleifer, I.; Ameer, G.A. Albumin hydrogels formed by electrostatically triggered self-assembly and their drug delivery capability. Biomacromolecules 2014, 15, 3625–3633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, J.; Zhao, J.; Justin, A.W.; Markaki, A.E. Albumin-based hydrogels for regenerative engineering and cell transplantation. Biotechnol. Bioeng. 2019, 116, 3457–3468. [Google Scholar] [CrossRef]
- Boye, I.J.; Alli, I.; Ismail, A.A. Interactions involved in the gelation of bovine serum albumin. J. Agric. Food Chem. 1996, 44, 996–1004. [Google Scholar] [CrossRef]
- Arabi, H.S.; Haselberger, D.; Hinderberger, D. The effect of ethanol on gelation, nanoscopic, and macroscopic properties of serum albumin hydrogels. Molecules 2020, 25, 1927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upadhyay, A.; Kandi, R.; Rao, C. Injectable, self-healing, and stress sustainable hydrogel of BSA as a functional biocompatible material for controlled drug delivery in cancer cells. ACS Sustain. Chem. Eng. 2018, 6, 3321–3330. [Google Scholar] [CrossRef]
- Iemma, F.; Spizzirri, U.G.; Puoci, F.; Muzzalupo, R.; Trombino, S.; Cassano, R.; Leta, S.; Picci, N. pH-sensitive hydrogels based on bovine serum albumin for oral drug delivery. Int. J. Pharm. 2006, 312, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Tachibana, A.; Tanabe, T. Recombinant human serum albumin hydrogel as a novel drug delivery vehicle. Mater. Sci. Eng. C 2010, 30, 664–669. [Google Scholar] [CrossRef]
- Ilić-Stojanović, S.; Nikolić, L.; Nikolić, V.; Petrović, S.; Oro, V.; Mitić, Z.; Najman, S. Semi-Crystalline copolymer hydrogels as smart drug carriers: In vitro thermo-responsive naproxen release study. Pharmaceutics 2021, 13, 158. [Google Scholar] [CrossRef]
- Mello, D.V.A.; Ricci-Júnior, E. Encapsulation of naproxen in nanostructured system: Structural characterization and in vitro release studies. Química Nova 2011, 34, 933–939. [Google Scholar] [CrossRef] [Green Version]
- Şanlı, O.; Solak, E.K. Controlled release of naproxen from sodium alginate and poly (vinyl alcohol)/sodium alginate blend beads crosslinked with glutaraldehyde. J. Appl. Polym. Sci. 2009, 112, 2057–2065. [Google Scholar] [CrossRef]
- Hauenschild, T.; Reichenwallner, J.; Enkelmann, V.; Hinderberger, D. Characterizing Active Pharmaceutical Ingredient Binding to Human Serum Albumin by Spin-Labeling and EPR Spectroscopy. Chem. A Eur. J. 2016, 22, 12825–12838. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Pochan, D.J. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem. Soc. Rev. 2010, 39, 3528–3540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, J.; Andreadis, M.; Nikolaidis, A.; Biliaderis, C.G.; Moschakis, T. Effect of ethanol on the microstructure and rheological properties of whey proteins: Acid-induced cold gelation. LWT 2021, 139, 110518. [Google Scholar] [CrossRef]
- Elysée-Collen, B.; Lencki, R.W. Effect of ethanol, ammonium sulfate, fatty acids, and temperature on the solution behavior of bovine serum albumin. Biotechnol. Prog. 1997, 13, 849–856. [Google Scholar] [CrossRef]
- Murayama, K.; Tomida, M. Heat-Induced Secondary Structure and Conformation Change of Bovine Serum Albumin Investigated by Fourier Transform Infrared Spectroscopy. Biochemistry 2004, 43, 11526–11532. [Google Scholar] [CrossRef]
- Takeda, K.; Wada, A.; Yamamoto, K.; Moriyama, Y.; Aoki, K. Conformational change of bovine serum albumin by heat treatment. J. Protein Chem. 1989, 8, 653–659. [Google Scholar] [CrossRef]
- Navarra, G.; Giacomazza, D.; Leone, M.; Librizzi, F.; Militello, V.; Biagio, P.L.S. Thermal aggregation and ion-induced cold-gelation of bovine serum albumin. Eur. Biophys. J. 2009, 38, 437–446. [Google Scholar] [CrossRef]
- Wagner, J.; Biliaderis, C.G.; Moschakis, T. Whey proteins: Musings on denaturation, aggregate formation and gelation. Crit. Rev. Food Sci. Nutr. 2020, 60, 3793–3806. [Google Scholar] [CrossRef]
- Lee, P.; Wu, X. Modifications of human serum albumin and their binding effect. Curr. Pharm. Des. 2015, 21, 1862–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodarahmi, R.; Karimi, S.A.; Kooshk, M.R.A.; Ghadami, S.A.; Ghobadi, S.; Amani, M. Comparative spectroscopic studies on drug binding characteristics and protein surface hydrophobicity of native and modified forms of bovine serum albumin: Possible relevance to change in protein structure/function upon non-enzymatic glycation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 89, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Junk, N.M.J.; Spiess, H.W.; Hinderberger, D. The distribution of fatty acids reveals the functional structure of human serum albumin. Angew. Chem. Int. Ed. 2010, 49, 8755–8759. [Google Scholar] [CrossRef] [PubMed]
- Akdogan, Y.; Reichenwallner, J.; Hinderberger, D. Evidence for water-tuned structural differences in proteins: An approach emphasizing variations in local hydrophilicity. PLoS ONE 2012, 7, e45681. [Google Scholar] [CrossRef] [Green Version]
- Reichenwallner, J.; Hinderberger, D. Using bound fatty acids to disclose the functional structure of serum albumin. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 5382–5393. [Google Scholar] [CrossRef]
- Bouhekka, A.; Bürgi, T. In situ ATR-IR spectroscopy study of adsorbed protein: Visible light denaturation of bovine serum albumin on TiO2. Appl. Surf. Sci. 2012, 261, 369–374. [Google Scholar] [CrossRef]
- Haeri, H.H.; Jerschabek, V.; Sadeghi, A.; Hinderberger, D. Copper–Calcium Poly (Acrylic Acid) Composite Hydrogels as Studied by Electron Paramagnetic Resonance (EPR) Spectroscopy. Macromol. Chem. Phys. 2020, 221, 2000262. [Google Scholar] [CrossRef]
- Widder, K.; MacEwan, S.R.; Garanger, E.; Núñez, V.; Lecommandoux, S.; Chilkoti, A.; Hinderberger, D. Characterisation of hydration and nanophase separation during the temperature response in hydrophobic/hydrophilic elastin-like polypeptide (ELP) diblock copolymers. Soft Matter 2017, 13, 1816–1822. [Google Scholar] [CrossRef] [Green Version]
- Junk, M.J.; Li, W.; Schlüter, A.D.; Wegner, G.; Spiess, H.W.; Zhang, A.; Hinderberger, D. EPR spectroscopic characterization of local nanoscopic heterogeneities during the thermal collapse of thermoresponsive dendronized polymers. Angew. Chem. Int. Ed. 2010, 49, 5683–5687. [Google Scholar] [CrossRef]
- Jans, H.; Liu, X.; Austin, L.; Maes, G.; Huo, Q. Dynamic light scattering as a powerful tool for gold nanoparticle bioconjugation and biomolecular binding studies. Anal. Chem. 2009, 81, 9425–9432. [Google Scholar] [CrossRef]
- Murdock, R.C.; Braydich-Stolle, L.; Schrand, A.M.; Schlager, J.J.; Hussain, S.M. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol. Sci. 2008, 101, 239–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecora, R. Dynamic light scattering measurement of nanometer particles in liquids. J. Nanopart. Res. 2000, 2, 123–131. [Google Scholar] [CrossRef]
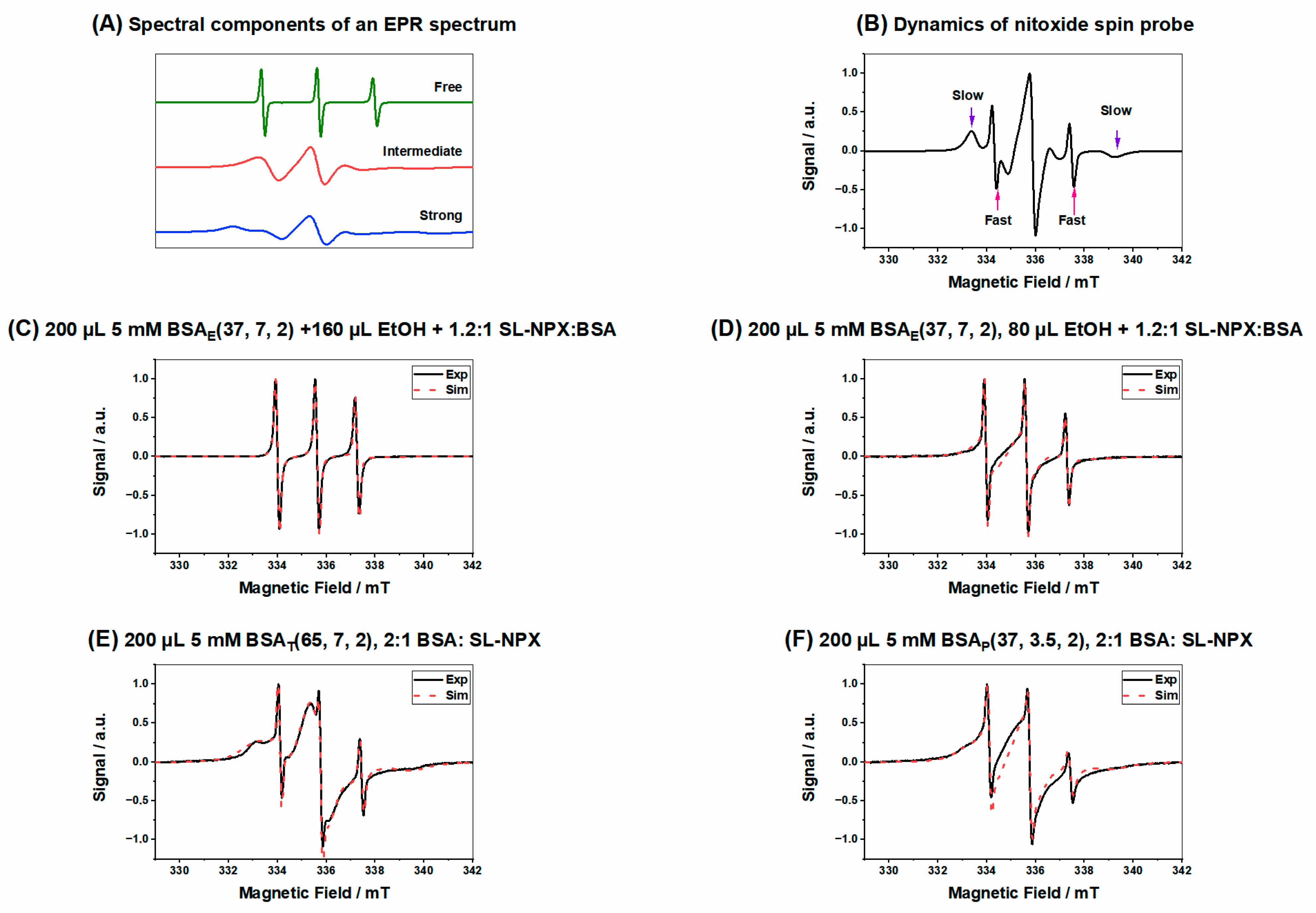


| Figure | Type of Component | Weight Percentage | Correlation Time τc [ns] | Hyperfine Coupling Constant aiso [MHz] |
|---|---|---|---|---|
| Figure 5C | Bound | 0 | 0 | 0 |
| Intermediate | 51% | 2 | 44.5 | |
| Free | 48% | 0.11 | 45.8 | |
| Figure 5D | Bound | 22% | 18 | 46.1 |
| Intermediate | 67% | 2 | 45.2 | |
| Free | 10% | 0.26 | 46.5 | |
| Figure 5E | Bound | 73% | 18 | 46.4 |
| Intermediate | 24% | 3 | 48.2 | |
| Free | 2% | 0.45 | 47.2 | |
| Figure 5F | Bound | 65% | 28 | 42.2 |
| Intermediate | 31% | 2 | 44.2 | |
| Free | 4% | 0.36 | 46.5 |
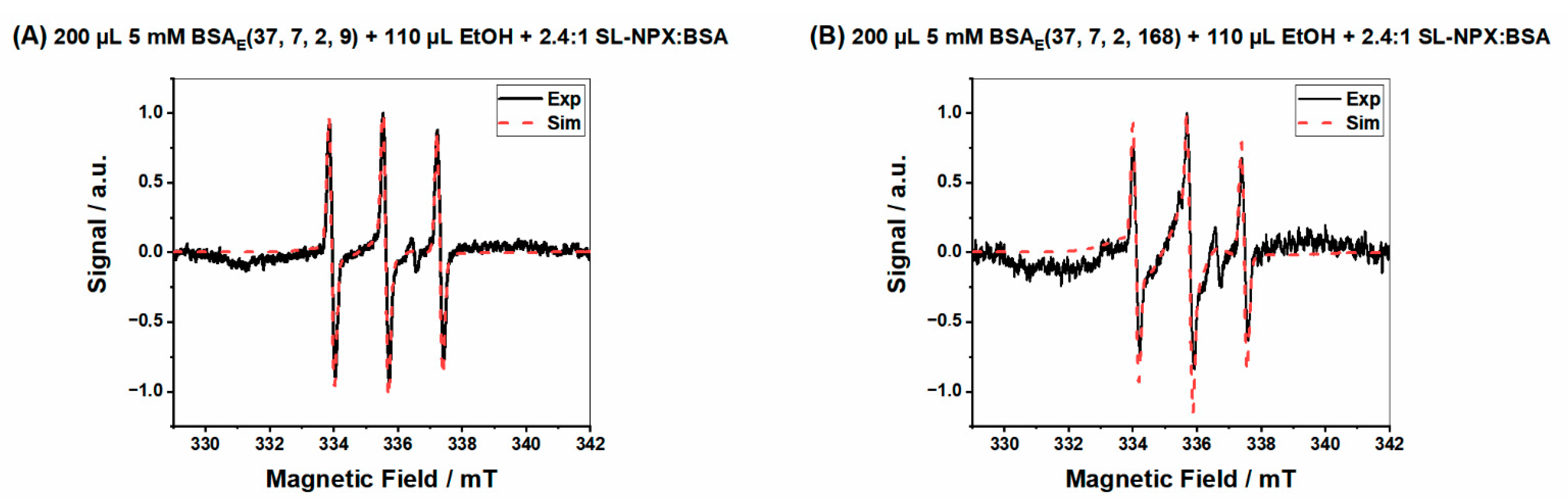
| Figure | Type of Component | Weight Percentage | Correlation Time τc [ns] | Hyperfine Coupling Constant aiso [MHz] |
|---|---|---|---|---|
| Figure 6A | Bound | 7% | 14 | 46.1 |
| Intermediate | 86% | 2.1 | 46.5 | |
| Free | 6% | 0.26 | 46.5 | |
| Figure 6B | Bound | 12% | 14 | 46.1 |
| Intermediate | 86% | 3.8 | 45.5 | |
| Free | 2% | 0.56 | 46.5 | |
| Figure 6C | Bound | 4% | 15 | 44.7 |
| Intermediate | 91% | 8.6 | 46.5 | |
| Free | 5% | 0.42 | 46.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanaeifar, N.; Mäder, K.; Hinderberger, D. Macro- and Nanoscale Effect of Ethanol on Bovine Serum Albumin Gelation and Naproxen Release. Int. J. Mol. Sci. 2022, 23, 7352. https://doi.org/10.3390/ijms23137352
Sanaeifar N, Mäder K, Hinderberger D. Macro- and Nanoscale Effect of Ethanol on Bovine Serum Albumin Gelation and Naproxen Release. International Journal of Molecular Sciences. 2022; 23(13):7352. https://doi.org/10.3390/ijms23137352
Chicago/Turabian StyleSanaeifar, Niuosha, Karsten Mäder, and Dariush Hinderberger. 2022. "Macro- and Nanoscale Effect of Ethanol on Bovine Serum Albumin Gelation and Naproxen Release" International Journal of Molecular Sciences 23, no. 13: 7352. https://doi.org/10.3390/ijms23137352
APA StyleSanaeifar, N., Mäder, K., & Hinderberger, D. (2022). Macro- and Nanoscale Effect of Ethanol on Bovine Serum Albumin Gelation and Naproxen Release. International Journal of Molecular Sciences, 23(13), 7352. https://doi.org/10.3390/ijms23137352







