Crosstalk between the Arabidopsis Glutathione Peroxidase-Like 5 Isoenzyme (AtGPXL5) and Ethylene
Abstract
:1. Introduction
2. Results
2.1. AtGPXL5 Regulates Hypocotyl Hook Development and Seedling Growth
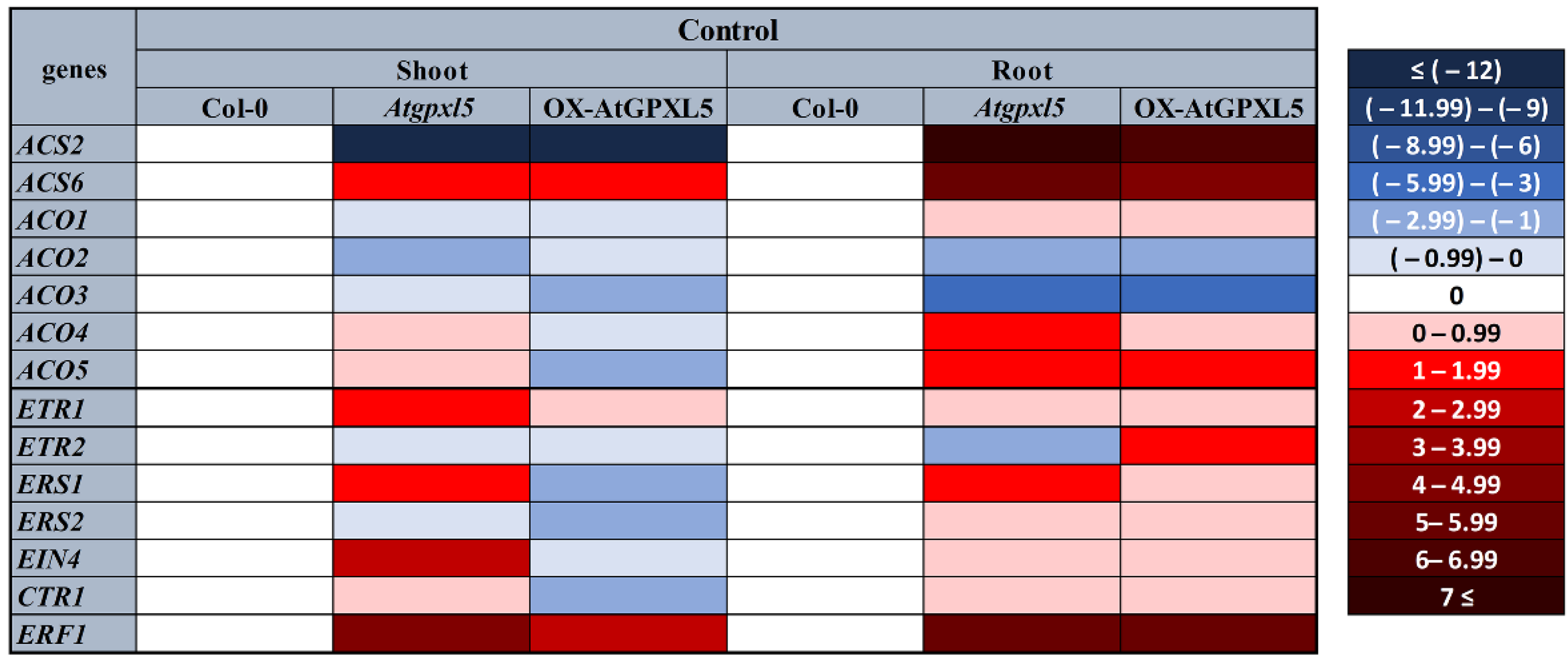
2.2. The Level of the AtGPXL5 Affects the Ethylene Biosynthesis and Expression of Genes Involved in Ethylene Biosynthesis, Sensing and Signalling
2.3. The Ethylene Precursor 1-Aminocyclopropane-1-Carboxylic Acid (ACC) Treatment Affects Differently the Growth of Investigated Plantlets
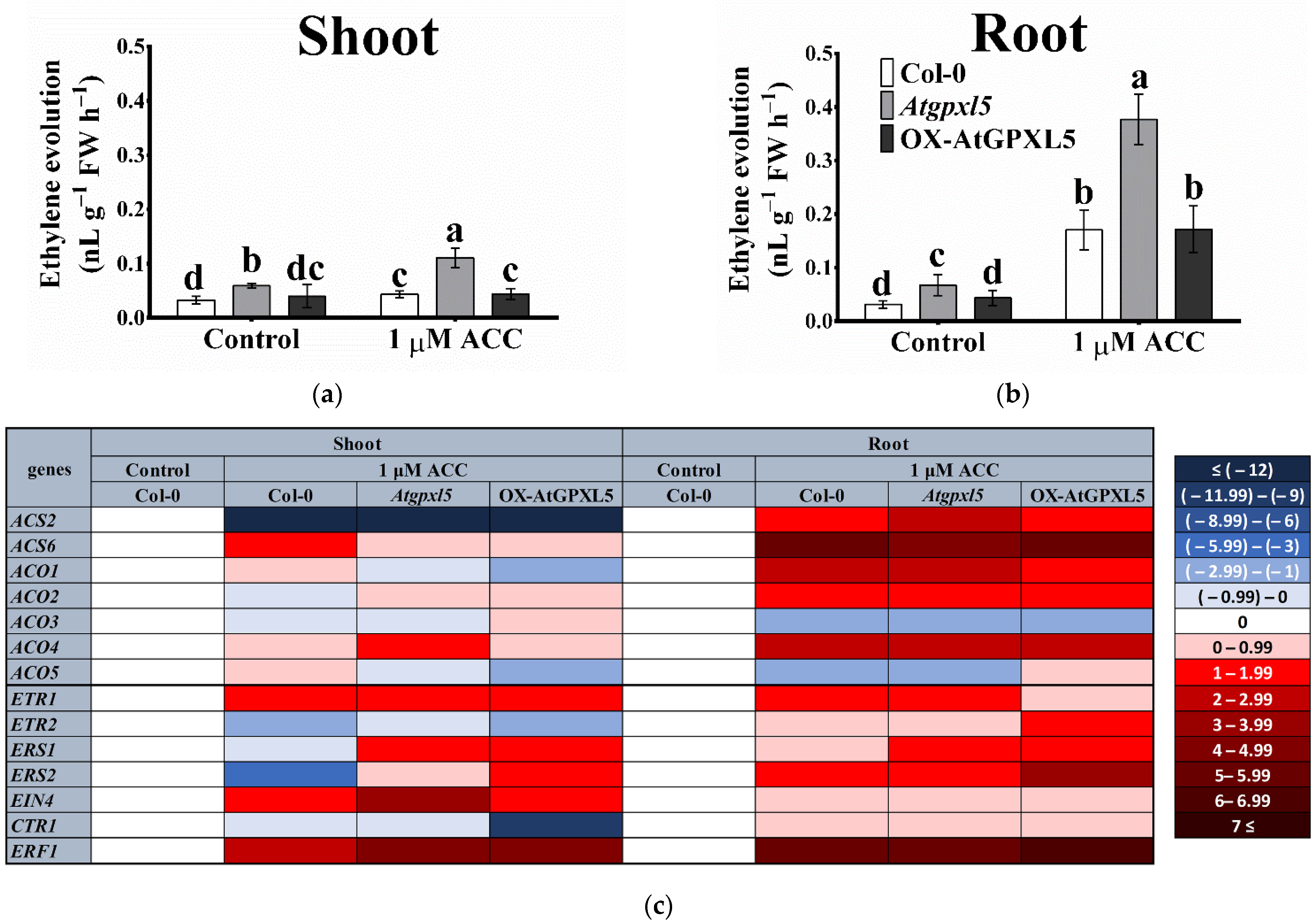
2.4. The ACC Treatment Triggered the Highest ET Production in the Atgpxl5 Mutant
2.5. AtGPXL5 Isoenzyme Affects the Expression of Genes Involved in Ethylene Sensing and Signalling
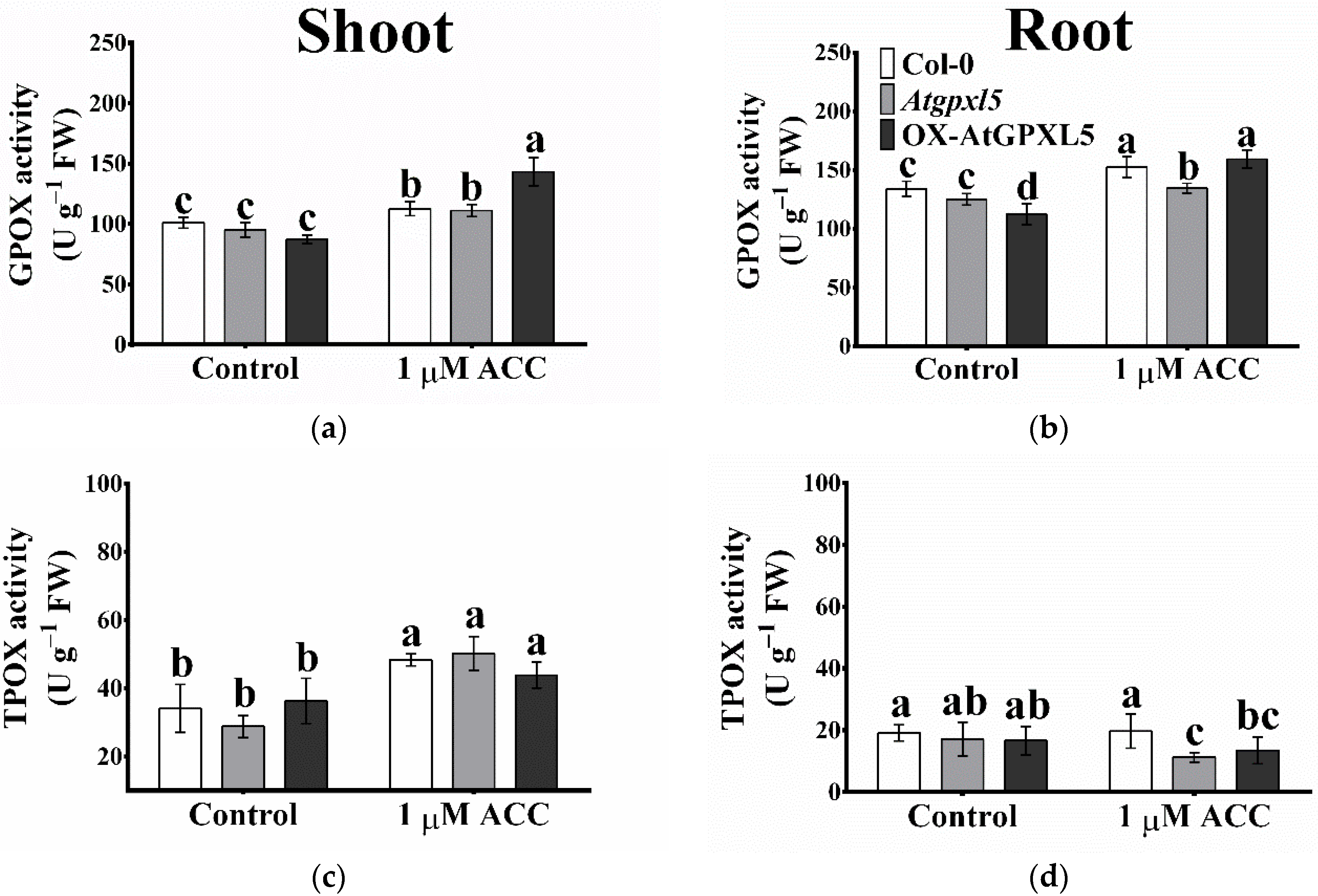
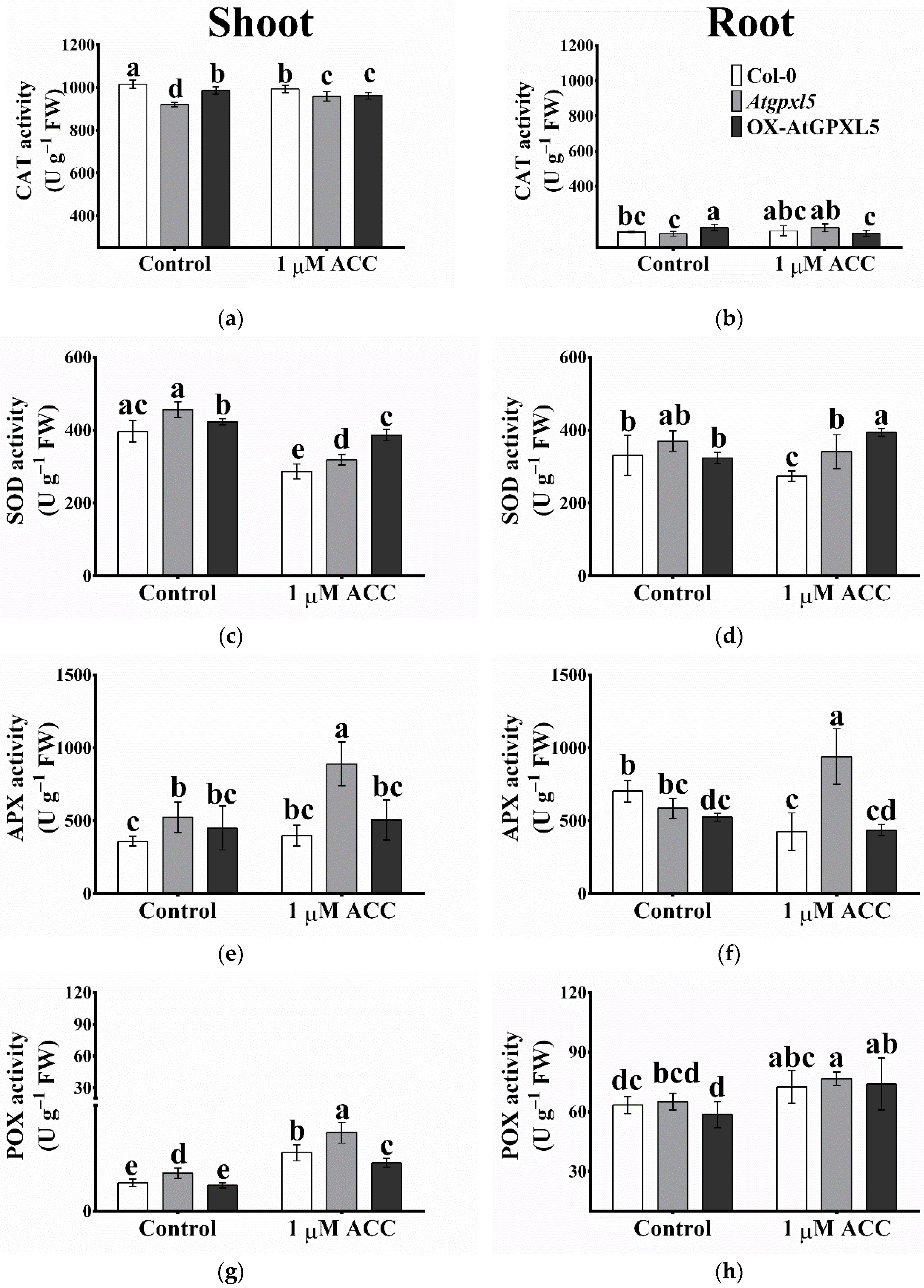
2.6. The Ethylene/ACC Affects the GPOX, TPOX Activities
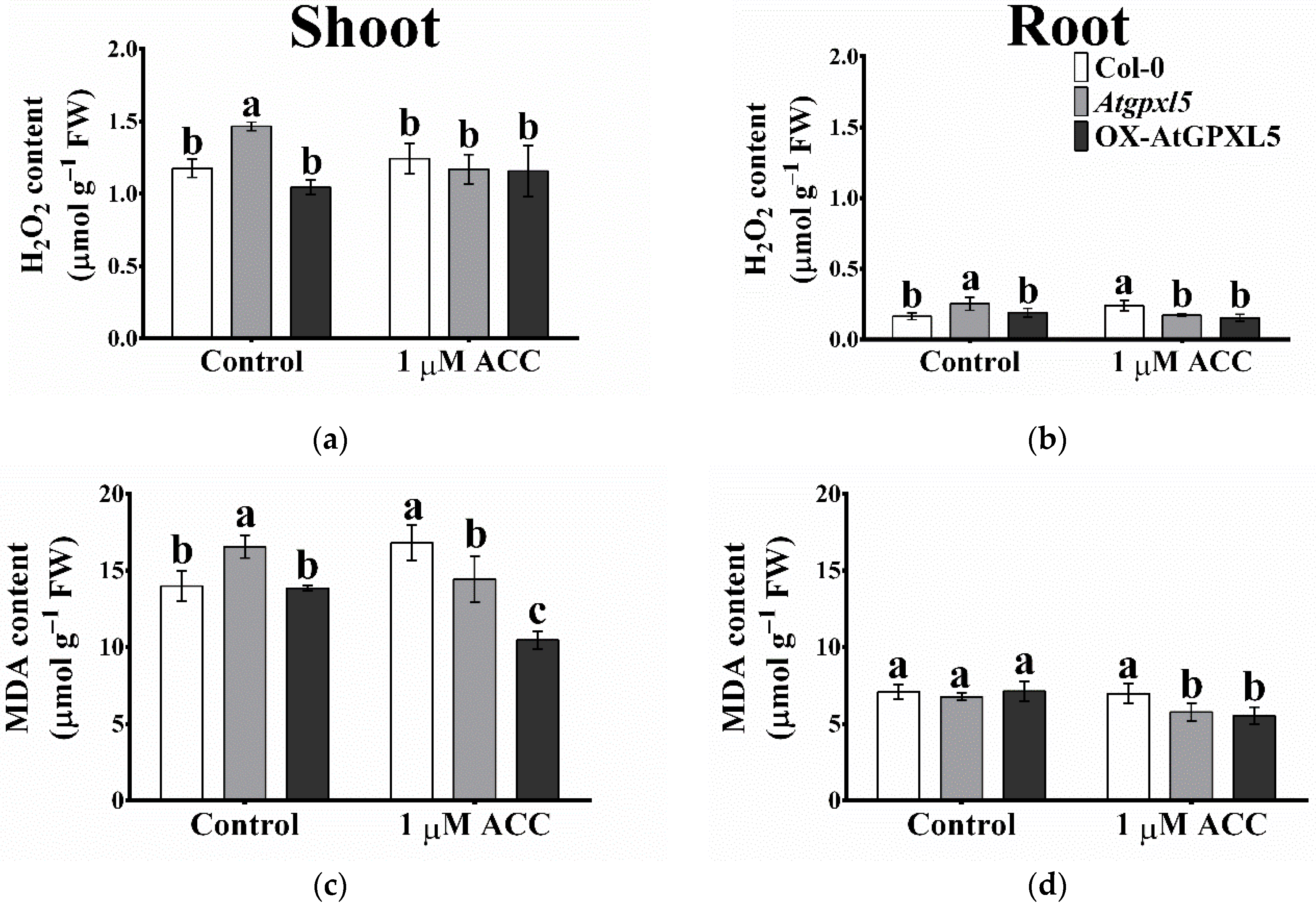
2.7. ACC Treatment Reduced the H2O2 and MDA Accumulation in Atgpxl5 and OX-AtGPXL5 Plants
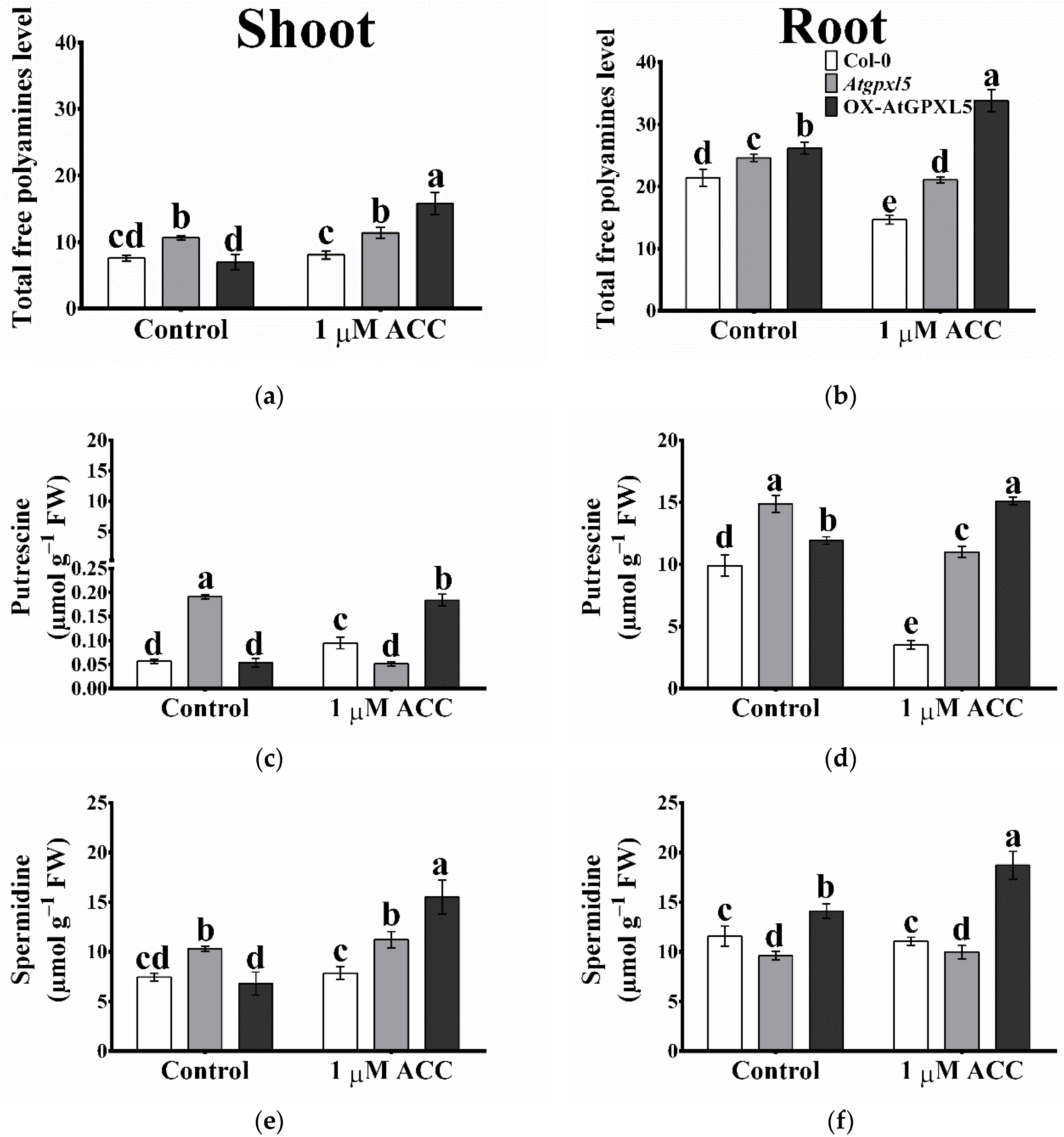
2.8. The Arabidopsis Glutathione Peroxidase-like 5 Interferes with Polyamine Metabolism
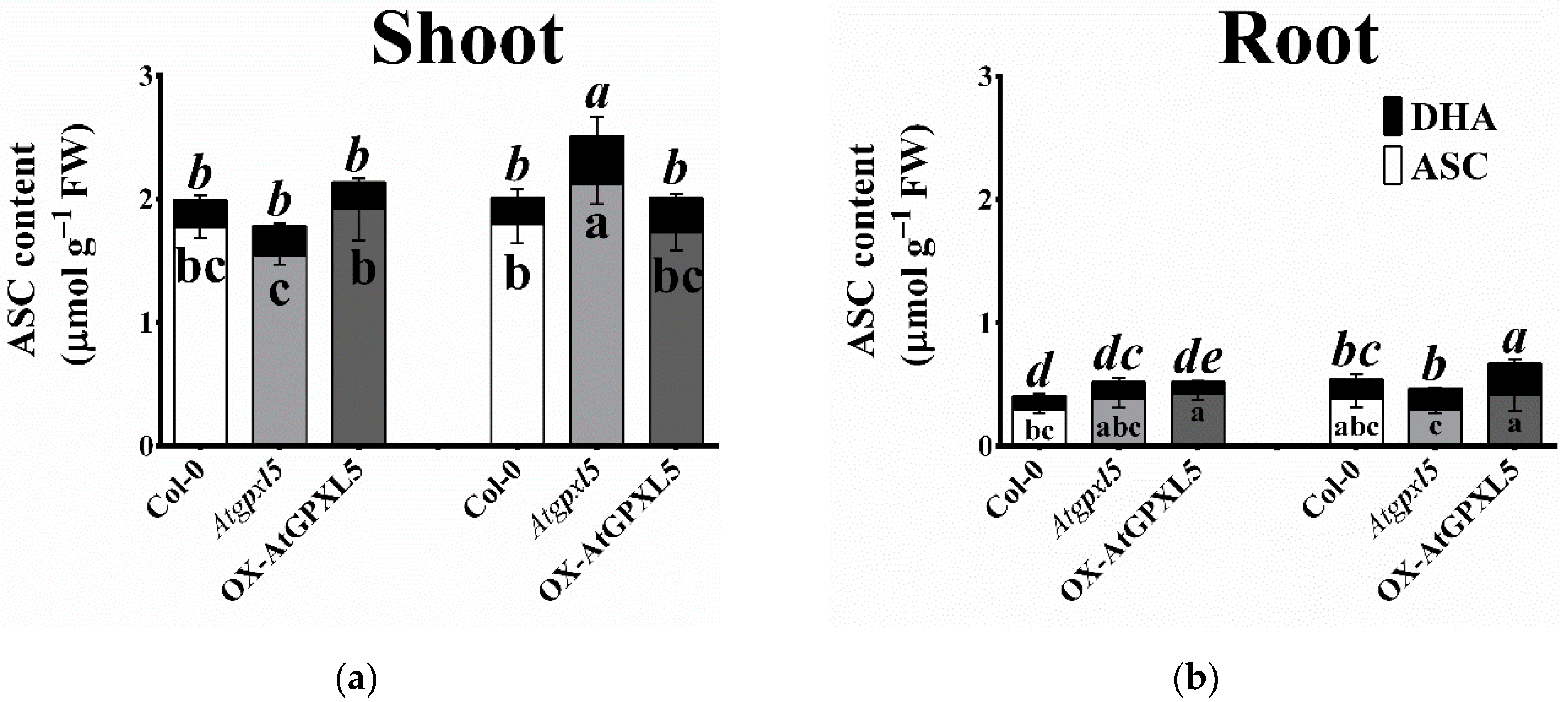
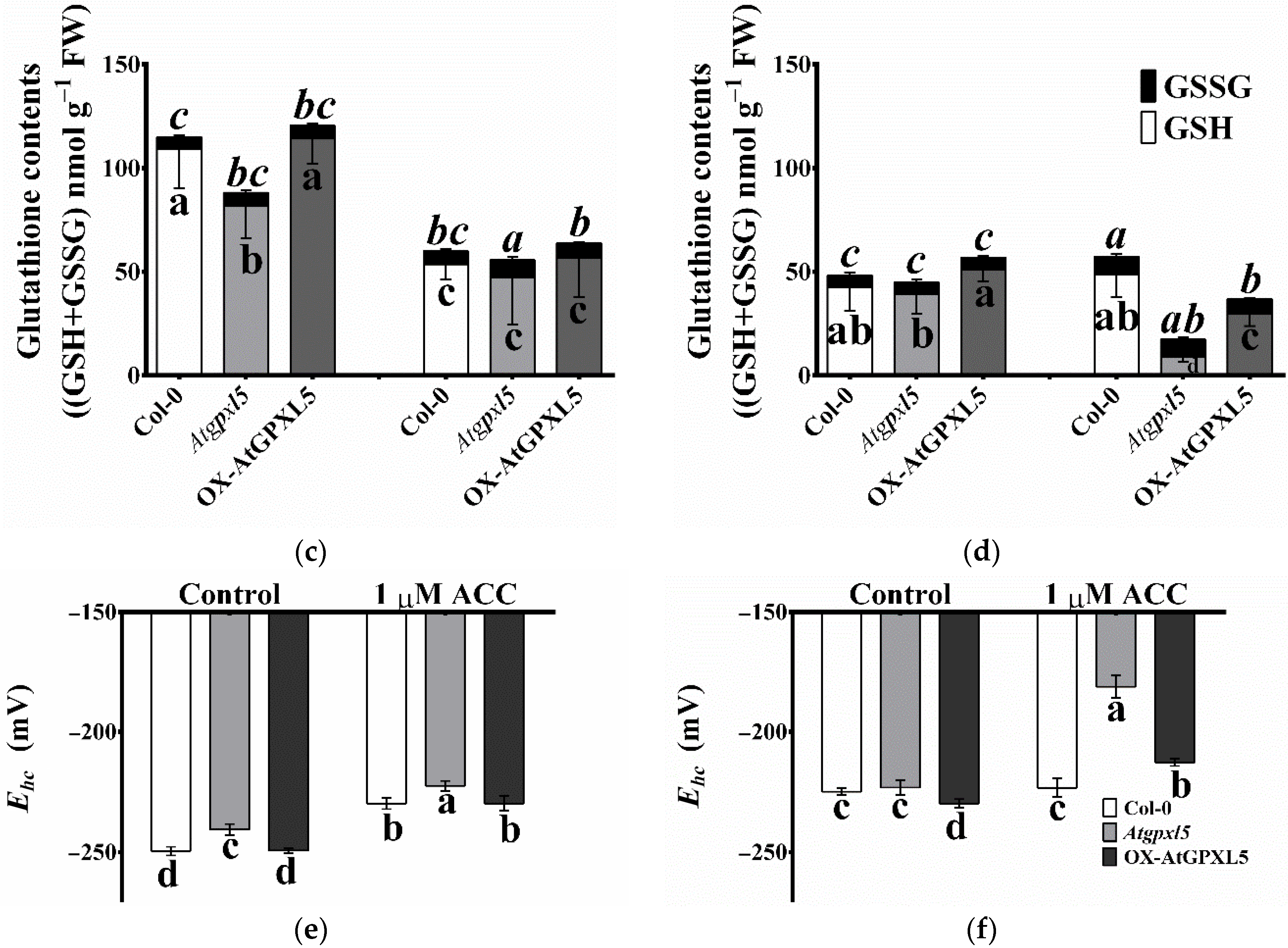
2.9. Atgpxl5 Shoots Had Lower GSH Content and More Oxidised Glutathione Redox Potential Both under Control Conditions and after Application of ACC than Wild Type and OX-AtGPXL5 Plants
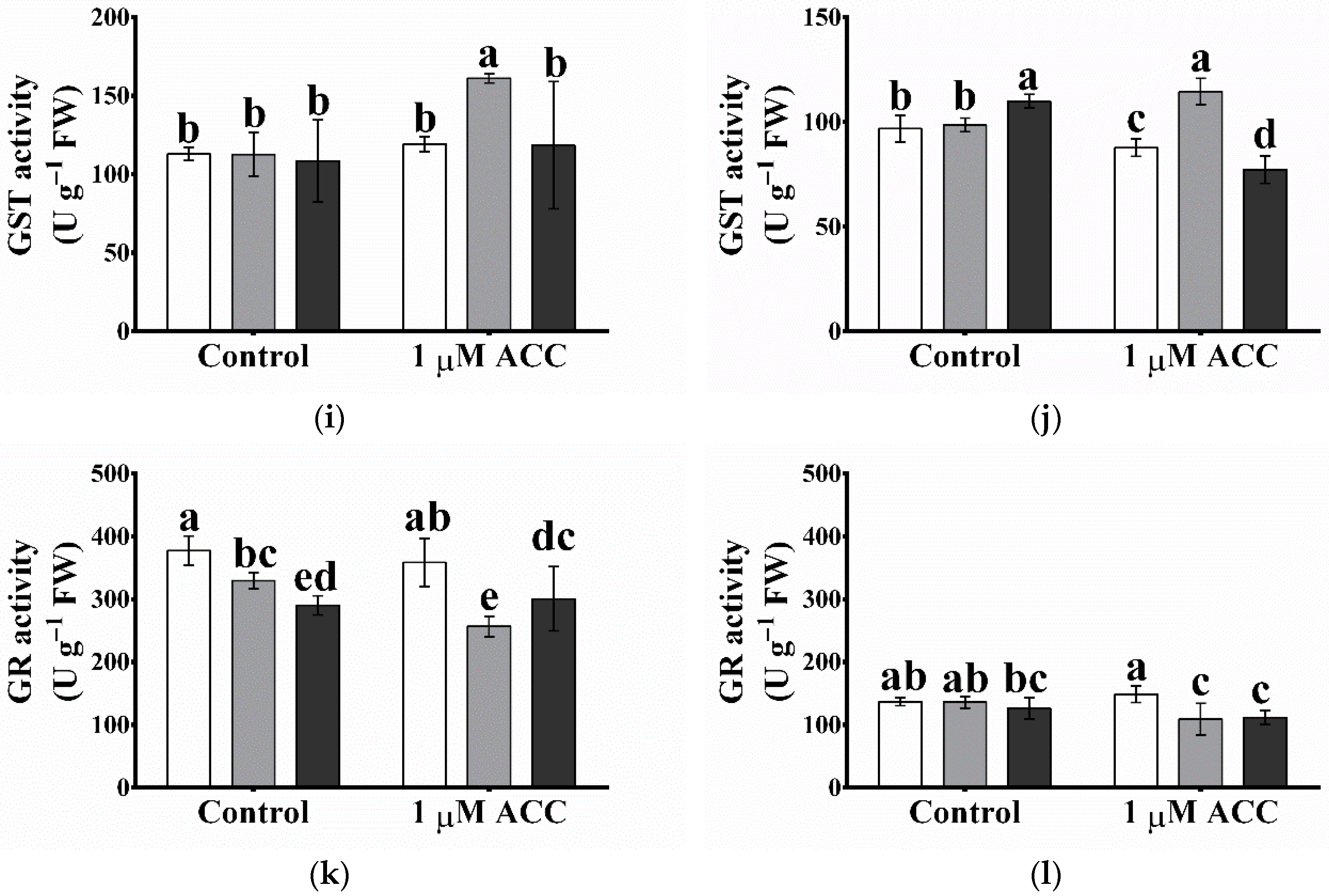
2.10. The ROS-Processing Enzyme Activities Were Elevated Most Cases in Atgpxl5 Plants
3. Discussion
3.1. The Relationship between AtGPXL5, Antioxidant Responses and ET
3.2. The Interplay between ET, AtGPXL5, Redox Regulation and Growth Processes
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Ethylene Evolution Measurement
4.3. Determination of H2O2 Level and Malondialdehyde (MDA) Content
4.4. Measurement of Free Polyamines
4.5. Enzymatic Antioxidant Activities
4.6. Determination of ROS-Processing Enzyme Activities
4.7. Determination of Ascorbate and Glutathione Contents
4.8. RNA Extraction and Gene Expression Analyses
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arthur, J.R. The Glutathione Peroxidases. Cell. Mol. Life Sci. CMLS 2001, 57, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Nakagawa, Y. Biological Significance of Phospholipid Hydroperoxide Glutathione Peroxidase (PHGPx, GPx4) in Mammalian Cells. Free. Radic. Biol. Med. 2003, 34, 145–169. [Google Scholar] [CrossRef]
- Islam, T.; Manna, M.; Kaul, T.; Pandey, S.; Reddy, C.S.; Reddy, M.K. Genome-Wide Dissection of Arabidopsis and Rice for the Identification and Expression Analysis of Glutathione Peroxidases Reveals Their Stress-Specific and Overlapping Response Patterns. Plant Mol. Biol. Rep. 2015, 33, 1413–1427. [Google Scholar] [CrossRef]
- Margis, R.; Dunand, C.; Teixeira, F.K.; Margis-Pinheiro, M. Glutathione Peroxidase Family—An Evolutionary Overview. FEBS J. 2008, 275, 3959–3970. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Yabuta, Y.; Takeda, T.; Nakano, Y.; Shigeoka, S. Hydroperoxide Reduction by Thioredoxin-specific Glutathione Peroxidase Isoenzymes of Arabidopsis thaliana. FEBS J. 2006, 273, 5589–5597. [Google Scholar] [CrossRef]
- Navrot, N.; Collin, V.; Gualberto, J.; Gelhaye, E.; Hirasawa, M.; Rey, P.; Knaff, D.B.; Issakidis, E.; Jacquot, J.-P.; Rouhier, N. Plant Glutathione Peroxidases Are Functional Peroxiredoxins Distributed in Several Subcellular Compartments and Regulated during Biotic and Abiotic Stresses. Plant Physiol. 2006, 142, 1364–1379. [Google Scholar] [CrossRef] [Green Version]
- Attacha, S.; Solbach, D.; Bela, K.; Moseler, A.; Wagner, S.; Schwarzländer, M.; Aller, I.; Müller, S.J.; Meyer, A.J. Glutathione Peroxidase-like Enzymes Cover Five Distinct Cell Compartments and Membrane Surfaces in Arabidopsis thaliana. Plant Cell Environ. 2017, 40, 1281–1295. [Google Scholar] [CrossRef]
- Bela, K.; Riyazuddin, R.; Horváth, E.; Hurton, Á.; Gallé, Á.; Takács, Z.; Zsigmond, L.; Szabados, L.; Tari, I.; Csiszár, J. Comprehensive Analysis of Antioxidant Mechanisms in Arabidopsis Glutathione Peroxidase-like Mutants under Salt-and Osmotic Stress Reveals Organ-Specific Significance of the AtGPXL’s Activities. Environ. Exp. Bot. 2018, 150, 127–140. [Google Scholar] [CrossRef]
- Passaia, G.; Margis-Pinheiro, M. Glutathione Peroxidases as Redox Sensor Proteins in Plant Cells. Plant Sci. 2015, 234, 22–26. [Google Scholar] [CrossRef]
- Delaunay, A.; Pflieger, D.; Barrault, M.-B.; Vinh, J.; Toledano, M.B. A Thiol Peroxidase Is an H2O2 Receptor and Redox-Transducer in Gene Activation. Cell 2002, 111, 471–481. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Lv, D.; Wang, P.; Wang, X.-C.; Chen, J.; Miao, C.; Song, C.-P. An Arabidopsis Glutathione Peroxidase Functions as Both a Redox Transducer and a Scavenger in Abscisic Acid and Drought Stress Responses. Plant Cell 2006, 18, 2749–2766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milla, M.A.R.; Maurer, A.; Huete, A.R.; Gustafson, J.P. Glutathione Peroxidase Genes in Arabidopsis Are Ubiquitous and Regulated by Abiotic Stresses through Diverse Signaling Pathways. Plant J. 2003, 36, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Diao, Y.; Xu, H.; Li, G.; Yu, A.; Yu, X.; Hu, W.; Zheng, X.; Li, S.; Wang, Y.; Hu, Z. Cloning a Glutathione Peroxidase Gene from Nelumbo nucifera and Enhanced Salt Tolerance by Overexpressing in Rice. Mol. Biol. Rep. 2014, 41, 4919–4927. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, J.; Ma, T.; Li, H.; Wang, N.; Li, Z.; Zhang, Z.; Zhou, Y. The Glutathione Peroxidase Gene Family in Thellungiella salsuginea: Genome-Wide Identification, Classification, and Gene and Protein Expression Analysis under Stress Conditions. Int. J. Mol. Sci. 2014, 15, 3319–3335. [Google Scholar] [CrossRef] [Green Version]
- Bela, K.; Bangash, S.A.K.; Csiszár, J. Plant Glutathione Peroxidases: Antioxidant Enzymes in Plant Stress Responses and Tolerance. In Glutathione in Plant Growth, Development, and Stress Tolerance; Springer: New York, NY, USA, 2017; pp. 113–126. [Google Scholar]
- Herbette, S.; de Labrouhe, D.T.; Drevet, J.R.; Roeckel-Drevet, P. Transgenic Tomatoes Showing Higher Glutathione Peroxydase Antioxidant Activity Are More Resistant to an Abiotic Stress but More Susceptible to Biotic Stresses. Plant Sci. 2011, 180, 548–553. [Google Scholar] [CrossRef]
- Zhai, C.-Z.; Zhao, L.; Yin, L.-J.; Chen, M.; Wang, Q.-Y.; Li, L.-C.; Xu, Z.-S.; Ma, Y.-Z. Two Wheat Glutathione Peroxidase Genes Whose Products Are Located in Chloroplasts Improve Salt and H2O2 Tolerances in Arabidopsis. PLoS ONE 2013, 8, e73989. [Google Scholar] [CrossRef] [Green Version]
- Riyazuddin, R.; Bela, K.; Horváth, E.; Rigó, G.; Gallé, Á.; Szabados, L.; Fehér, A.; Csiszár, J. Overexpression of the Arabidopsis Glutathione Peroxidase-like 5 Gene (AtGPXL5) Resulted in Altered Plant Development and Redox Status. Environ. Exp. Bot. 2019, 167, 103849. [Google Scholar] [CrossRef]
- Passaia, G.; Fonini, L.S.; Caverzan, A.; Jardim-Messeder, D.; Christoff, A.P.; Gaeta, M.L.; de Araujo Mariath, J.E.; Margis, R.; Margis-Pinheiro, M. The Mitochondrial Glutathione Peroxidase GPX3 Is Essential for H2O2 Homeostasis and Root and Shoot Development in Rice. Plant Sci. 2013, 208, 93–101. [Google Scholar] [CrossRef]
- Passaia, G.; Queval, G.; Bai, J.; Margis-Pinheiro, M.; Foyer, C.H. The Effects of Redox Controls Mediated by Glutathione Peroxidases on Root Architecture in Arabidopsis thaliana. J. Exp. Bot. 2014, 65, 1403–1413. [Google Scholar] [CrossRef] [Green Version]
- Faltin, Z.; Holland, D.; Velcheva, M.; Tsapovetsky, M.; Roeckel-Drevet, P.; Handa, A.K.; Abu-Abied, M.; Friedman-Einat, M.; Eshdat, Y.; Perl, A. Glutathione Peroxidase Regulation of Reactive Oxygen Species Level Is Crucial for in Vitro Plant Differentiation. Plant Cell Physiol. 2010, 51, 1151–1162. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.C.; Slesak, I.; Jordá, L.; Sotnikov, A.; Melzer, M.; Miszalski, Z.; Mullineaux, P.M.; Parker, J.E.; Karpinska, B.; Karpinski, S. Arabidopsis Chloroplastic Glutathione Peroxidases Play a Role in Cross Talk between Photooxidative Stress and Immune Responses. Plant Physiol. 2009, 150, 670–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima-Melo, Y.; Carvalho, F.E.L.; Martins, M.O.; Passaia, G.; Sousa, R.H.V.; Neto, M.C.L.; Margis-Pinheiro, M.; Silveira, J.A.G. Mitochondrial GPX1 Silencing Triggers Differential Photosynthesis Impairment in Response to Salinity in Rice Plants. J. Integr. Plant Biol. 2016, 58, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Khan, N.A.; Nazar, R.; da Silva, J.A.T. Ethylene-Stimulated Photosynthesis Results from Increased Nitrogen and Sulfur Assimilation in Mustard Types That Differ in Photosynthetic Capacity. Environ. Exp. Bot. 2012, 78, 84–90. [Google Scholar] [CrossRef]
- Nazar, R.; Khan, M.I.R.; Iqbal, N.; Masood, A.; Khan, N.A. Involvement of Ethylene in Reversal of Salt-inhibited Photosynthesis by Sulfur in Mustard. Physiol. Plant 2014, 152, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Houben, M.; Van de Poel, B. 1-Aminocyclopropane-1-Carboxylic Acid Oxidase (ACO): The Enzyme That Makes the Plant Hormone Ethylene. Front. Plant Sci. 2019, 10, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.I.R.; Nazir, F.; Asgher, M.; Per, T.S.; Khan, N.A. Selenium and Sulfur Influence Ethylene Formation and Alleviate Cadmium-Induced Oxidative Stress by Improving Proline and Glutathione Production in Wheat. J. Plant Physiol. 2015, 173, 9–18. [Google Scholar] [CrossRef]
- Negi, S.; Ivanchenko, M.G.; Muday, G.K. Ethylene Regulates Lateral Root Formation and Auxin Transport in Arabidopsis thaliana. Plant J. 2008, 55, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Husain, T.; Fatima, A.; Suhel, M.; Singh, S.; Sharma, A.; Prasad, S.M.; Singh, V.P. A Brief Appraisal of Ethylene Signaling under Abiotic Stress in Plants. Plant Signal. Behav. 2020, 15, 1782051. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Verma, R.; Singh, K.; Nisha, N.; Keisham, M.; Bhati, K.K.; Kim, S.T.; Gupta, R. Ethylene: A Master Regulator of Salinity Stress Tolerance in Plants. Biomolecules 2020, 10, 959. [Google Scholar] [CrossRef]
- Li, D.; Mou, W.; van de Poel, B.; Chang, C. Something Old, Something New: Conservation of the Ethylene Precursor 1-Amino-Cyclopropane-1-Carboxylic Acid as a Signaling Molecule. Curr. Opin. Plant Biol. 2022, 65, 102116. [Google Scholar] [CrossRef]
- Yoon, G.M.; Kieber, J.J. 14-3-3 Regulates 1-Aminocyclopropane-1-Carboxylate Synthase Protein Turnover in Arabidopsis. Plant Cell 2013, 25, 1016–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, G.M.; Kieber, J.J. 1-Aminocyclopropane-1-Carboxylic Acid as a Signalling Molecule in Plants. AoB Plants 2013, 5, plt017. [Google Scholar] [CrossRef] [Green Version]
- Mattoo, A.K.; Cassol, T.; Ra, M.; Goyal, R.; Neelam, A. USDA-ARS Vegetable Laboratory, Henry A. Wallace Beltsville Agricultural Research. Biol. Biotechnol. Plant Horm. Ethyl. III 2003, 349, 359. [Google Scholar]
- Harpaz-Saad, S.; Yoon, G.M.; Mattoo, A.K.; Kieber, J.J. The Formation of ACC and Competition between Polyamines and Ethylene for SAM. Annu. Plant Rev. Plant Horm. Ethyl. 2012, 44, 56. [Google Scholar]
- Sauter, M.; Moffatt, B.; Saechao, M.C.; Hell, R.; Wirtz, M. Methionine Salvage and S-Adenosylmethionine: Essential Links between Sulfur, Ethylene and Polyamine Biosynthesis. Biochem. J. 2013, 451, 145–154. [Google Scholar] [CrossRef] [Green Version]
- Alcázar, R.; Bueno, M.; Tiburcio, A.F. Polyamines: Small Amines with Large Effects on Plant Abiotic Stress Tolerance. Cells 2020, 9, 2373. [Google Scholar] [CrossRef]
- Bleecker, A.B.; Kende, H. Ethylene: A Gaseous Signal Molecule in Plants. Annu. Rev. Cell Dev. Biol. 2000, 16, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Ecker, J.R. The Ethylene Signaling Pathway: New Insights. Curr. Opin. Plant Biol. 2004, 7, 40–49. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Etheridge, N.; Schaller, G.E. Ethylene Signal Transduction. Ann. Bot. 2005, 95, 901–915. [Google Scholar] [CrossRef]
- Kendrick, M.D.; Chang, C. Ethylene Signaling: New Levels of Complexity and Regulation. Curr. Opin. Plant Biol. 2008, 11, 479–485. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.I.R.; Jahan, B.; AlAjmi, M.F.; Rehman, M.T.; Khan, N.A. Ethephon Mitigates Nickel Stress by Modulating Antioxidant System, Glyoxalase System and Proline Metabolism in Indian Mustard. Physiol. Mol. Biol. Plants 2020, 26, 1201–1213. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, M.; Gałgańska, H.; Nowak, W.; Sadowski, J. Exogenously Induced Expression of Ethylene Biosynthesis, Ethylene Perception, Phospholipase D, and Rboh-Oxidase Genes in Broccoli Seedlings. J. Exp. Bot. 2010, 61, 3475–3491. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Hoffman, N.E. Ethylene Biosynthesis and Its Regulation in Higher Plants. Annu. Rev. Plant Physiol. 1984, 35, 155–189. [Google Scholar] [CrossRef]
- Vanderstraeten, L.; Depaepe, T.; Bertrand, S.; Van Der Straeten, D. The Ethylene Precursor ACC Affects Early Vegetative Development Independently of Ethylene Signaling. Front. Plant Sci. 2019, 10, 1591. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues-Pousada, R.A.; De Rycke, R.; Dedonder, A.; Van Caeneghem, W.; Engler, G.; Van Montagu, M.; Van Der Straeten, D. The Arabidopsis 1-Aminocyclopropane-1-Carboxylate Synthase Gene 1 Is Expressed during Early Development. Plant Cell 1993, 5, 897–911. [Google Scholar] [CrossRef]
- Blume, B.; Grierson, D. Expression of ACC Oxidase Promoter—GUS Fusions in Tomato and Nicotiana plumbaginifolia Regulated by Developmental and Environmental Stimuli. Plant J. 1997, 12, 731–746. [Google Scholar] [CrossRef]
- Wang, N.N.; Shih, M.-C.; Li, N. The GUS Reporter-Aided Analysis of the Promoter Activities of Arabidopsis ACC Synthase Genes AtACS4, AtACS5, and AtACS7 Induced by Hormones and Stresses. J. Exp. Bot. 2005, 56, 909–920. [Google Scholar] [CrossRef] [Green Version]
- Datta, R.; Kumar, D.; Sultana, A.; Hazra, S.; Bhattacharyya, D.; Chattopadhyay, S. Glutathione Regulates 1-Aminocyclopropane-1-Carboxylate Synthase Transcription via WRKY33 and 1-Aminocyclopropane-1-Carboxylate Oxidase by Modulating Messenger RNA Stability to Induce Ethylene Synthesis during Stress. Plant Physiol. 2015, 169, 2963–2981. [Google Scholar]
- Park, C.H.; Roh, J.; Youn, J.-H.; Son, S.-H.; Park, J.H.; Kim, S.Y.; Kim, T.-W.; Kim, S.-K. Arabidopsis ACC Oxidase 1 Coordinated by Multiple Signals Mediates Ethylene Biosynthesis and Is Involved in Root Development. Mol. Cells 2018, 41, 923. [Google Scholar]
- Pattyn, J.; Vaughan-Hirsch, J.; van de Poel, B. The Regulation of Ethylene Biosynthesis: A Complex Multilevel Control Circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef]
- Khan, N.A.; Asgher, M.; Per, T.S.; Masood, A.; Fatma, M.; Khan, M.I.R. Ethylene Potentiates Sulfur-Mediated Reversal of Cadmium Inhibited Photosynthetic Responses in Mustard. Front. Plant Sci. 2016, 7, 1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnaubelt, D.; Queval, G.; Dong, Y.; Diaz-Vivancos, P.; Makgopa, M.E.; Howell, G.; De Simone, A.; Bai, J.; Hannah, M.A.; Foyer, C.H. Low Glutathione Regulates Gene Expression and the Redox Potentials of the Nucleus and Cytosol in Arabidopsis thaliana. Plant Cell Environ. 2015, 38, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Argueso, C.T.; Hansen, M.; Kieber, J.J. Regulation of Ethylene Biosynthesis. J. Plant Growth Regul. 2007, 26, 92–105. [Google Scholar] [CrossRef]
- Lin, Z.; Zhong, S.; Grierson, D. Recent Advances in Ethylene Research. J. Exp. Bot. 2009, 60, 3311–3336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; Xu, P.; Li, B.; Li, P.; Wen, X.; An, F.; Gong, Y.; Xin, Y.; Zhu, Z.; Wang, Y. Ethylene Promotes Root Hair Growth through Coordinated EIN3/EIL1 and RHD6/RSL1 Activity in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, 13834–13839. [Google Scholar] [CrossRef] [Green Version]
- Dubois, M.; Van den Broeck, L.; Inzé, D. The Pivotal Role of Ethylene in Plant Growth. Trends Plant Sci. 2018, 23, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Achard, P.; Cheng, H.; de Grauwe, L.; Decat, J.; Schoutteten, H.; Moritz, T.; van der Straeten, D.; Peng, J.; Harberd, N.P. Integration of Plant Responses to Environmentally Activated Phytohormonal Signals. Science 2006, 311, 91–94. [Google Scholar] [CrossRef]
- Shen, X.; Wang, Z.; Song, X.; Xu, J.; Jiang, C.; Zhao, Y.; Ma, C.; Zhang, H. Transcriptomic Profiling Revealed an Important Role of Cell Wall Remodeling and Ethylene Signaling Pathway during Salt Acclimation in Arabidopsis. Plant Mol. Biol. 2014, 86, 303–317. [Google Scholar] [CrossRef]
- Cao, W.; Liu, J.; Zhou, Q.; Cao, Y.; Zheng, S.; Du, B.; Zhang, J.; Chen, S. Expression of Tobacco Ethylene Receptor NTHK1 Alters Plant Responses to Salt Stress. Plant Cell Environ. 2006, 29, 1210–1219. [Google Scholar] [CrossRef]
- Peng, Z.; He, S.; Gong, W.; Sun, J.; Pan, Z.; Xu, F.; Lu, Y.; Du, X. Comprehensive Analysis of Differentially Expressed Genes and Transcriptional Regulation Induced by Salt Stress in Two Contrasting Cotton Genotypes. BMC Genom. 2014, 15, 760. [Google Scholar] [CrossRef] [Green Version]
- Müller, M.; Munné-Bosch, S. Ethylene Response Factors: A Key Regulatory Hub in Hormone and Stress Signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.-C.; Liao, P.-M.; Kuo, W.-W.; Lin, T.-P. The Arabidopsis ETHYLENE RESPONSE FACTOR1 Regulates Abiotic Stress-Responsive Gene Expression by Binding to Different Cis-Acting Elements in Response to Different Stress Signals. Plant Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkindale, J.; Huang, B. Thermotolerance and Antioxidant Systems in Agrostis Stolonifera: Involvement of Salicylic Acid, Abscisic Acid, Calcium, Hydrogen Peroxide, and Ethylene. J. Plant Physiol. 2004, 161, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.; Schellingen, K.; Vangronsveld, J.; Cuypers, A. Ethylene and Metal Stress: Small Molecule, Big Impact. Front. Plant Sci. 2016, 7, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schellingen, K.; van der Straeten, D.; Vandenbussche, F.; Prinsen, E.; Remans, T.; Vangronsveld, J.; Cuypers, A. Cadmium-Induced Ethylene Production and Responses in Arabidopsis thaliana Rely on ACS2 and ACS6 Gene Expression. BMC Plant Biol. 2014, 14, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masood, A.; Iqbal, N.; Khan, N.A. Role of Ethylene in Alleviation of Cadmium-induced Photosynthetic Capacity Inhibition by Sulphur in Mustard. Plant Cell Environ. 2012, 35, 524–533. [Google Scholar] [CrossRef]
- Distéfano, A.M.; Martin, M.V.; Córdoba, J.P.; Bellido, A.M.; D’Ippólito, S.; Colman, S.L.; Soto, D.; Roldán, J.A.; Bartoli, C.G.; Zabaleta, E.J. Heat Stress Induces Ferroptosis-like Cell Death in Plants. J. Cell Biol. 2017, 216, 463–476. [Google Scholar] [CrossRef] [Green Version]
- Meyer, A.J.; Dreyer, A.; Ugalde, J.M.; Feitosa-Araujo, E.; Dietz, K.-J.; Schwarzländer, M. Shifting Paradigms and Novel Players in Cys-Based Redox Regulation and ROS Signaling in Plants-and Where to Go Next. Biol. Chem. 2021, 402, 399–423. [Google Scholar] [CrossRef]
- Riyazuddin, R.; Gupta, R. Plausible Involvement of Ethylene in Plant Ferroptosis: Prospects and Leads. Front. Plant Sci. 2021, 12, 680709. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A. Ethylene Reverses Photosynthetic Inhibition by Nickel and Zinc in Mustard through Changes in PS II Activity, Photosynthetic Nitrogen Use Efficiency, and Antioxidant Metabolism. Protoplasma 2014, 251, 1007–1019. [Google Scholar] [CrossRef]
- Yoshida, S.; Tamaoki, M.; Ioki, M.; Ogawa, D.; Sato, Y.; Aono, M.; Kubo, A.; Saji, S.; Saji, H.; Satoh, S. Ethylene and Salicylic Acid Control Glutathione Biosynthesis in Ozone-exposed Arabidopsis thaliana. Physiol. Plant. 2009, 136, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Asgher, M.; Khan, N.A. Alleviation of Salt-Induced Photosynthesis and Growth Inhibition by Salicylic Acid Involves Glycinebetaine and Ethylene in Mungbean (Vigna radiata L.). Plant Physiol. Biochem. 2014, 80, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox Homeostasis and Antioxidant Signaling: A Metabolic Interface between Stress Perception and Physiological Responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mase, K.; Tsukagoshi, H. Reactive Oxygen Species Link Gene Regulatory Networks During Arabidopsis Root Development. Front. Plant Sci. 2021, 12, 642. [Google Scholar] [CrossRef]
- Bashandy, T.; Guilleminot, J.; Vernoux, T.; Caparros-Ruiz, D.; Ljung, K.; Meyer, Y.; Reichheld, J.-P. Interplay between the NADP-Linked Thioredoxin and Glutathione Systems in Arabidopsis Auxin Signaling. Plant Cell 2010, 22, 376–391. [Google Scholar] [CrossRef] [Green Version]
- Marquez-Garcia, B.; Njo, M.; Beeckman, T.O.M.; Goormachtig, S.; Foyer, C.H. A New Role for Glutathione in the Regulation of Root Architecture Linked to Strigolactones. Plant Cell Environ. 2014, 37, 488–498. [Google Scholar] [CrossRef]
- Jiang, K.; Moe-Lange, J.; Hennet, L.; Feldman, L.J. Salt Stress Affects the Redox Status of Arabidopsis Root Meristems. Front. Plant Sci. 2016, 7, 81. [Google Scholar] [CrossRef] [Green Version]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Baba, A.I.; Andrási, N.; Valkai, I.; Gorcsa, T.; Koczka, L.; Darula, Z.; Medzihradszky, K.F.; Szabados, L.; Fehér, A.; Rigó, G. AtCRK5 Protein Kinase Exhibits a Regulatory Role in Hypocotyl Hook Development during Skotomorphogenesis. Int. J. Mol. Sci. 2019, 20, 3432. [Google Scholar] [CrossRef] [Green Version]
- Poór, P.; Borbély, P.; Kovács, J.; Papp, A.; Szepesi, Á.; Takács, Z.; Tari, I. Opposite Extremes in Ethylene/Nitric Oxide Ratio Induce Cell Death in Suspension Culture and Root Apices of Tomato Exposed to Salt Stress. Acta Biol. Hung. 2014, 65, 428–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Székely, G.; Ábrahám, E.; Cséplő, Á.; Rigó, G.; Zsigmond, L.; Csiszár, J.; Ayaydin, F.; Strizhov, N.; Jásik, J.; Schmelzer, E.; et al. Duplicated P5CS Genes of Arabidopsis Play Distinct Roles in Stress Regulation and Developmental Control of Proline Biosynthesis. Plant J. 2008, 53, 11–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts: I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Szepesi, Á. Interaction between Salicylic Acid and Polyamines and Their Possible Roles in Tomato Hardening Processes. Acta Biol. Szeged. 2011, 55, 165–166. [Google Scholar]
- Marty, L.; Siala, W.; Schwarzländer, M.; Fricker, M.D.; Wirtz, M.; Sweetlove, L.J.; Meyer, Y.; Meyer, A.J.; Reichheld, J.-P.; Hell, R. The NADPH-Dependent Thioredoxin System Constitutes a Functional Backup for Cytosolic Glutathione Reductase in Arabidopsis. Proc. Natl. Acad. Sci. USA 2009, 106, 9109–9114. [Google Scholar] [CrossRef] [Green Version]
- Csiszár, J.; Lantos, E.; Tari, I.; Madosa, E.; Wodala, B.; Vashegyi, A.; Horváth, F.; Pécsváradi, A.; Szabó, M.; Bartha, B. Antioxidant Enzyme Activities in Allium Species and Their Cultivars under Water Stress. Plant Soil Environ. 2007, 53, 517. [Google Scholar] [CrossRef] [Green Version]
- Horváth, E.; Csiszár, J.; Gallé, Á.; Poór, P.; Szepesi, Á.; Tari, I. Hardening with Salicylic Acid Induces Concentration-Dependent Changes in Abscisic Acid Biosynthesis of Tomato under Salt Stress. J. Plant Physiol. 2015, 183, 54–63. [Google Scholar] [CrossRef]
- Görgényi Miklósné Tari, I.; Csiszár, J.; Horváth, E.; Poór, P.; Takács, Z.; Szepesi, Á. The Alleviation of the Adverse Effects of Salt Stress in the Tomato Plant by Salicylic Acid Shows a Time-and Organ-Specific Antioxidant Response. Acta Biol. Crac. Ser. Bot. 2015, 57, 21–30. [Google Scholar]
- Benyó, D.; Horváth, E.; Németh, E.; Leviczky, T.; Takács, K.; Lehotai, N.; Feigl, G.; Kolbert, Z.; Ördög, A.; Gallé, R. Physiological and Molecular Responses to Heavy Metal Stresses Suggest Different Detoxification Mechanism of Populus Deltoides and P. x Canadensis. J. Plant Physiol. 2016, 201, 62–70. [Google Scholar] [CrossRef]
- Csiszár, J.; Brunner, S.; Horváth, E.; Bela, K.; Ködmön, P.; Riyazuddin, R.; Gallé, Á.; Hurton, Á.; Papdi, C.; Szabados, L. Exogenously Applied Salicylic Acid Maintains Redox Homeostasis in Salt-Stressed Arabidopsis Gr1 Mutants Expressing Cytosolic RoGFP1. Plant Growth Regul. 2018, 86, 181–194. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox Environment of the Cell as Viewed through the Redox State of the Glutathione Disulfide/Glutathione Couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
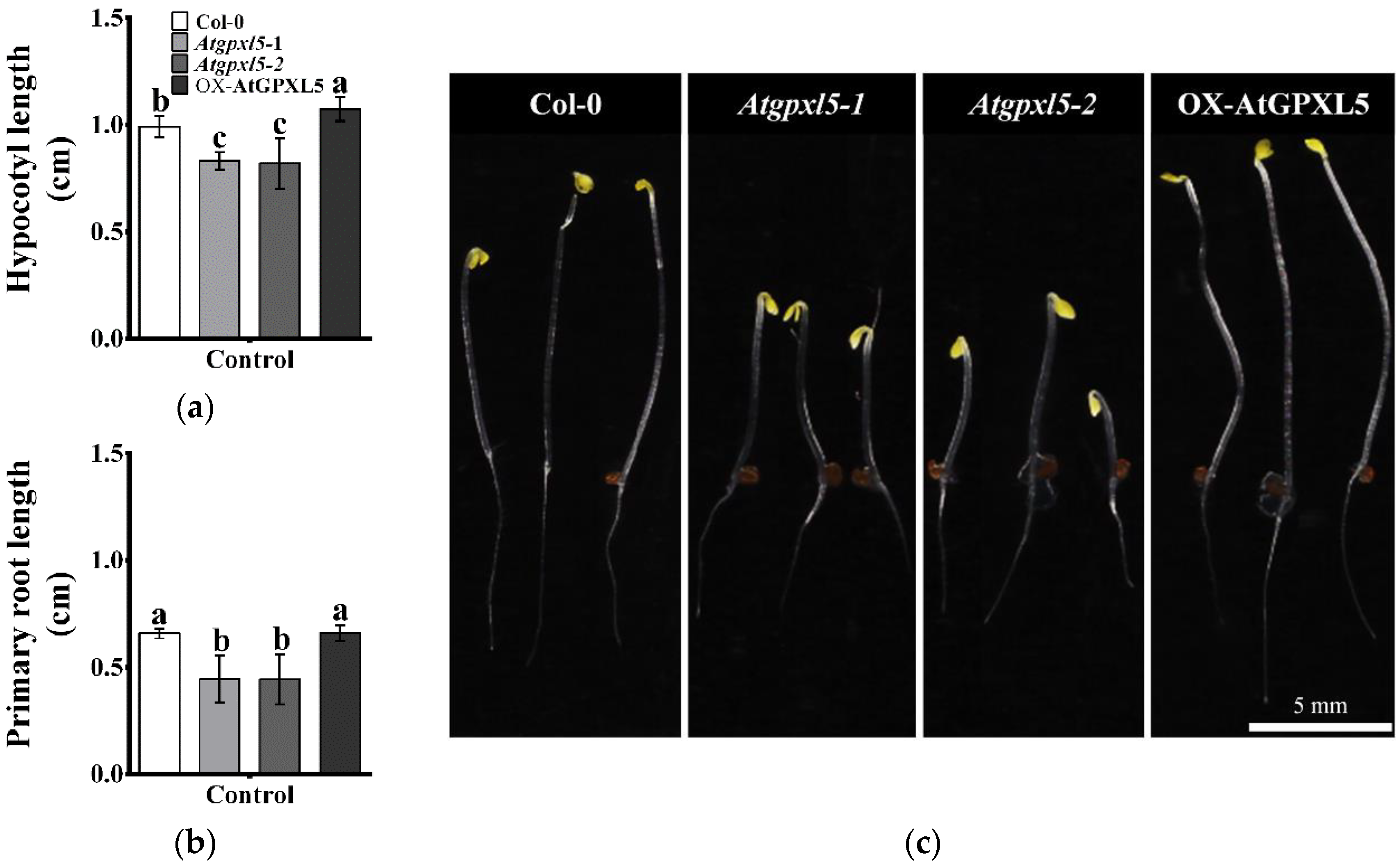
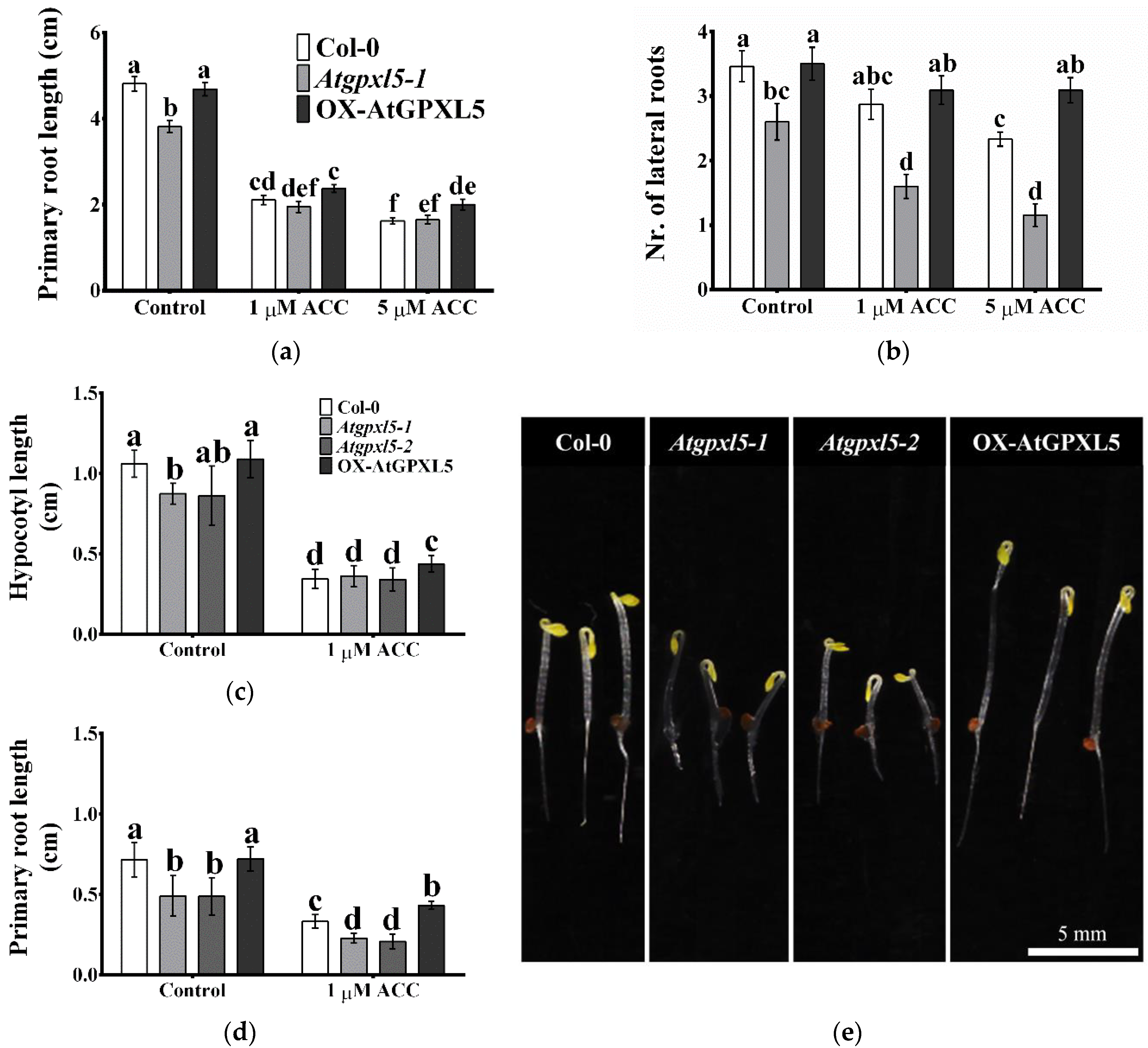

| Growth Parameters | Col-0 | Atgpxl5 | OX-AtGPXL5 |
|---|---|---|---|
| Root length (cm) | 4.81 ± 0.17 a | 3.81 ± 0.14 b | 4.68 ± 0.15 a |
| Number of lateral roots | 3.46 ± 0.24 a | 2.60 ± 0.28 b | 3.50 ± 0.26 a |
| Ethylene Evolution (nL g−1FW h−1) | Col-0 | Atgpxl5 | OX-AtGPXL5 |
|---|---|---|---|
| Shoot | 0.033 ± 0.007 b | 0.059 ± 0.004 a | 0.040 ± 0.021 b |
| Root | 0.031 ± 0.007 c | 0.067 ± 0.020 a | 0.043 ± 0.012 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riyazuddin, R.; Bela, K.; Poór, P.; Szepesi, Á.; Horváth, E.; Rigó, G.; Szabados, L.; Fehér, A.; Csiszár, J. Crosstalk between the Arabidopsis Glutathione Peroxidase-Like 5 Isoenzyme (AtGPXL5) and Ethylene. Int. J. Mol. Sci. 2022, 23, 5749. https://doi.org/10.3390/ijms23105749
Riyazuddin R, Bela K, Poór P, Szepesi Á, Horváth E, Rigó G, Szabados L, Fehér A, Csiszár J. Crosstalk between the Arabidopsis Glutathione Peroxidase-Like 5 Isoenzyme (AtGPXL5) and Ethylene. International Journal of Molecular Sciences. 2022; 23(10):5749. https://doi.org/10.3390/ijms23105749
Chicago/Turabian StyleRiyazuddin, Riyazuddin, Krisztina Bela, Péter Poór, Ágnes Szepesi, Edit Horváth, Gábor Rigó, László Szabados, Attila Fehér, and Jolán Csiszár. 2022. "Crosstalk between the Arabidopsis Glutathione Peroxidase-Like 5 Isoenzyme (AtGPXL5) and Ethylene" International Journal of Molecular Sciences 23, no. 10: 5749. https://doi.org/10.3390/ijms23105749
APA StyleRiyazuddin, R., Bela, K., Poór, P., Szepesi, Á., Horváth, E., Rigó, G., Szabados, L., Fehér, A., & Csiszár, J. (2022). Crosstalk between the Arabidopsis Glutathione Peroxidase-Like 5 Isoenzyme (AtGPXL5) and Ethylene. International Journal of Molecular Sciences, 23(10), 5749. https://doi.org/10.3390/ijms23105749











