Investigation of Brain Function-Related Myokine Secretion by Using Contractile 3D-Engineered Muscle
Abstract
:1. Introduction
2. Results
2.1. Skeletal Muscle Contractile Behavior in 3D-EM
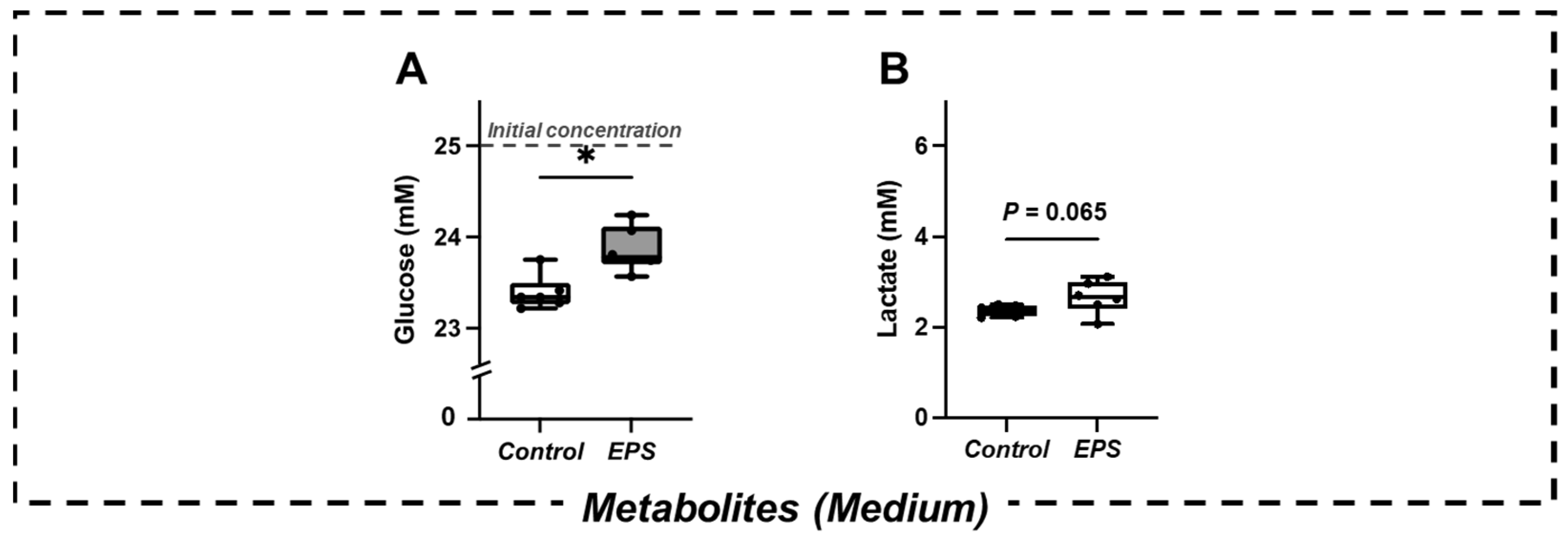
2.2. Changes in Metabolites in the Culture Medium
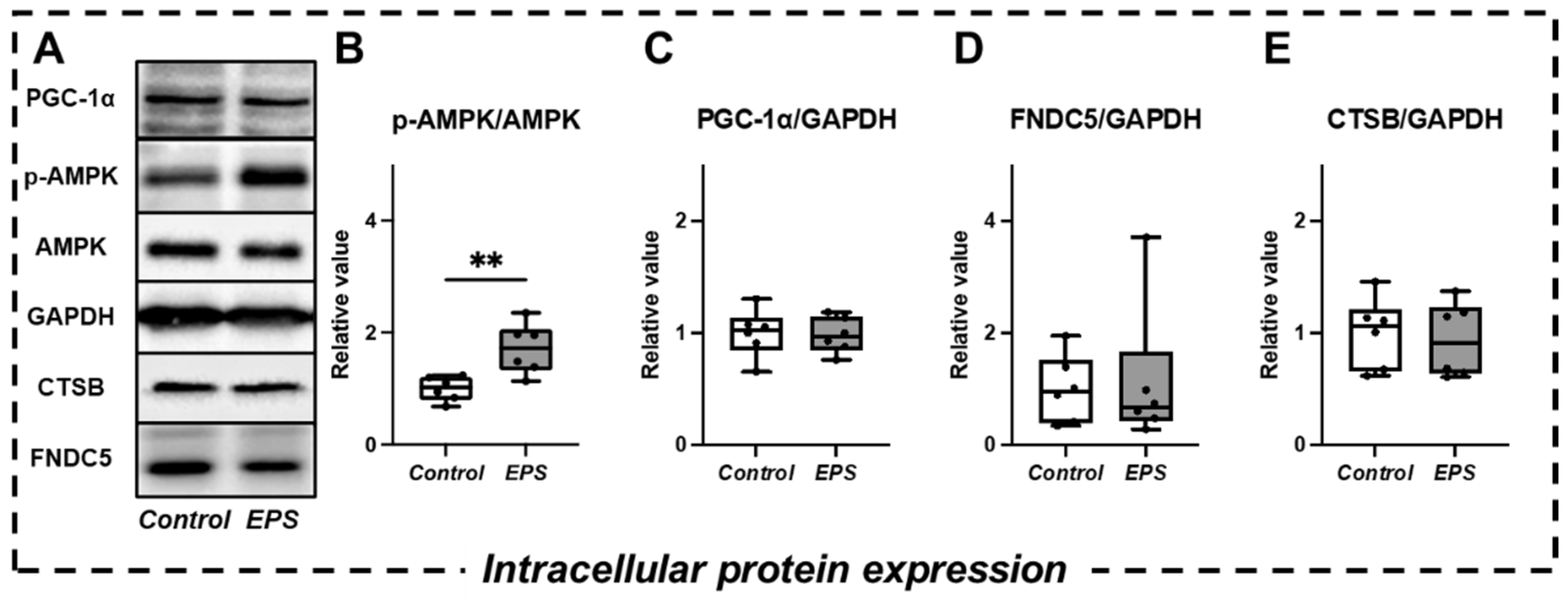
2.3. Changes in Intracellular Signaling and Myokine Expression
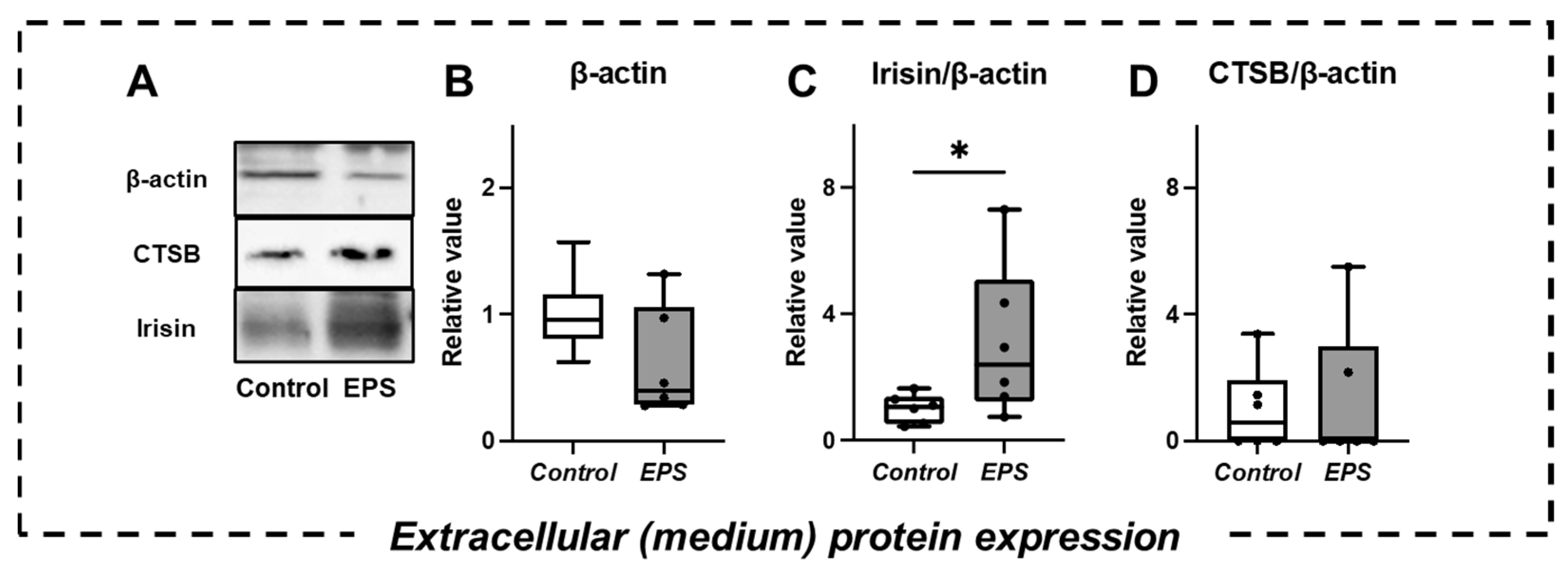
2.4. Changes in Extracellular Protein Expression and Myokine Secretion in the Culture Medium
3. Discussion
4. Materials and Methods
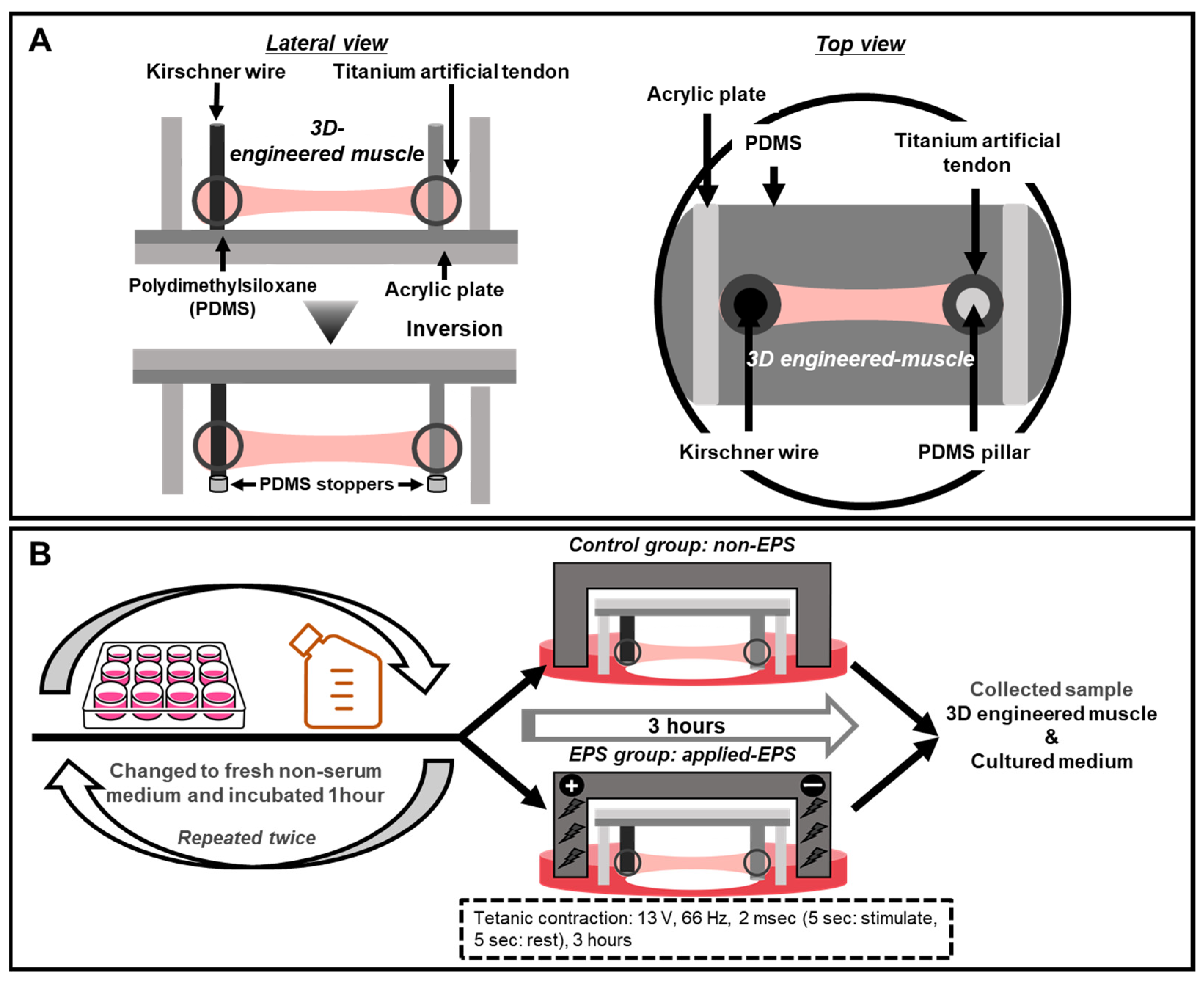
4.1. Molding Devices and Artificial Tendon
4.2. C2C12 Cell Culture
4.3. Three-Dimensional Culture in Collagen Gel
4.4. Electrical Pulse Stimulation (EPS)
4.5. Metabolite Measurement
4.6. Western Blot Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen, B.K.; Akerström, T.C.; Nielsen, A.R.; Fischer, C.P. Role of myokines in exercise and metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Choi, J.Y.; Moon, S.; Park, D.H.; Kwak HB: Kang, J.H. Roles of myokines in exercise-induced improvement of neuropsychiatric function. Pflug. Arch. 2019, 471, 491–505. [Google Scholar] [CrossRef]
- Pedersen, B.K. Physical activity and muscle-brain crosstalk. Nat. Rev. Endocrinol. 2019, 15, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Maudsley, S.; Martin, B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004, 27, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef]
- Kramer, A.F.; Hahn, S.; Cohen, N.J.; Banich, M.T.; McAuley, E.; Harrison, C.R.; Chason, J.; Vakil, E.; Bardell, L.; Boileau, R.A.; et al. Ageing, fitness and neurocognitive function. Nature 1999, 400, 418–419. [Google Scholar] [CrossRef]
- El Hayek, L.; Khalifeh, M.; Zibara, V.; Abi Assaad, R.; Emmanuel, N.; Karnib, N.; El-Ghandour, R.; Nasrallah, P.; Bilen, M.; Ibrahim, P.; et al. Lactate Mediates the Effects of Exercise on Learning and Memory through SIRT1-Dependent Activation of Hippocampal Brain-Derived Neurotrophic Factor (BDNF). J. Neurosci. 2019, 39, 2369–2382. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, T.; Tsukamoto, H.; Takenaka, S.; Olesen, N.D.; Petersen, L.G.; Sørensen, H.; Nielsen, H.B.; Secher, N.H.; Ogoh, S. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J. 2018, 32, 1417–1427. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, T.; Tsukamoto, H.; Ando, S.; Ogoh, S. Effect of Exercise on Brain Health: The Potential Role of Lactate as a Myokine. Metabolites. 2021, 11, 813. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Moon, H.Y.; Becke, A.; Berron, D.; Becker, B.; Sah, N.; Benoni, G.; Janke, E.; Lubejko, S.T.; Greig, N.H.; Mattison, J.A.; et al. Running-Induced Systemic Cathepsin B Secretion Is Associated with Memory Function. Cell Metab. 2016, 24, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolić, N.; Bakke, S.S.; Kase, E.T.; Rudberg, I.; Flo Halle, I.; Rustan, A.C.; Thoresen, G.H.; Aas, V. Electrical pulse stimulation of cultured human skeletal muscle cells as an in vitro model of exercise. PLoS ONE 2012, 7, e33203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, Y.; Kouzaki, K.; Kotani, T.; Nakazato, K. Electrically stimulated contractile activity-induced transcriptomic responses and metabolic remodeling in C2C12 myotubes: Twitch vs. tetanic contractions. Am. J. Physiol. Cell Physiol. 2020, 319, C1029–C1044. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Arevalo, J.A.; Osmond, A.D.; Leija, R.G.; Curl, C.C.; Tovar, A.P. Lactate in contemporary biology: A phoenix risen. J. Physiol. 2022, 600, 1229–1251. [Google Scholar] [CrossRef]
- Lezi, E.; Lu, J.; Selfridge, J.E.; Burns, J.M.; Swerdlow, R.H. Lactate administration reproduces specific brain and liver exercise-related changes. J. Neurochem. 2013, 127, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Pierre, K.; Pellerin, L. Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J. Neurochem. 2005, 94, 1–14. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Johnson, T.K.; Belcher, D.J.; Sousa, C.A.; Carzoli, J.P.; Visavadiya, N.P.; Khamoui, A.V.; Whitehurst, M.; Zourdos, M.C. Low-volume acute multi-joint resistance exercise elicits a circulating brain-derived neurotrophic factor response but not a cathepsin B response in well-trained men. Appl. Physiol. Nutr. Metab. 2020, 45, 1332–1338. [Google Scholar] [CrossRef]
- Nicolini, C.; Michalski, B.; Toepp, S.L.; Turco, C.V.; D’Hoine, T.; Harasym, D.; Gibala, M.J.; Fahnestock, M.; Nelson, A.J. A Single Bout of High-intensity Interval Exercise Increases Corticospinal Excitability, Brain-derived Neurotrophic Factor, and Uncarboxylated Osteolcalcin in Sedentary, Healthy Males. Neuroscience 2020, 437, 242–255. [Google Scholar] [CrossRef]
- Pekkala, S.; Wiklund, P.K.; Hulmi, J.J.; Ahtiainen, J.P.; Horttanainen, M.; Pöllänen, E.; Mäkelä, K.A.; Kainulainen, H.; Häkkinen, K.; Nyman, K.; et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? J. Physiol. 2013, 591, 5393–5400. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.; Nozhenko, Y.; Palou, A.; Rodríguez, A.M. Free fatty acid effects on myokine production in combination with exercise mimetics. Mol. Nutr. Food Res. 2013, 57, 1456–1467. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Solomon, T. In vitro experimental models for examining the skeletal muscle cell biology of exercise: The possibilities, challenges and future developments. Pflug. Arch. 2019, 471, 413–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuichi, Y.; Manabe, Y.; Takagi, M.; Aoki, M.; Fujii, N.L. Evidence for acute contraction-induced myokine secretion by C2C12 myotubes. PLoS ONE 2018, 13, e0206146. [Google Scholar] [CrossRef] [Green Version]
- Nedachi, T.; Fujita, H.; Kanzaki, M. Contractile C2C12 myotube model for studying exercise-inducible responses in skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1191–E1204. [Google Scholar] [CrossRef] [Green Version]
- Pampaloni, F.; Reynaud, E.G.; Stelzer, E.H. The third dimension bridges the gap between cell culture and live tissue. Nat. Rev. Mol. Cell Biol. 2007, 8, 839–845. [Google Scholar] [CrossRef]
- Smith, A.S.; Passey, S.; Greensmith, L.; Mudera, V.; Lewis, M.P. Characterization and optimization of a simple, repeatable system for the long term in vitro culture of aligned myotubes in 3D. J. Cell Biochem. 2012, 113, 1044–1053. [Google Scholar] [CrossRef]
- Sugimoto, T.; Imai, S.; Yoshikawa, M.; Fujisato, T.; Hashimoto, T.; Nakamura, T. Mechanical unloading in 3D-engineered muscle leads to muscle atrophy by suppressing protein synthesis. J. Appl. Physiol. 2022, 132, 1091–1103. [Google Scholar] [CrossRef]
- Liao, H.; Zhou, G.Q. Development and progress of engineering of skeletal muscle tissue. Tissue Eng. Part B Rev. 2009, 15, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, T.; Takagi, S.; Kamon, T.; Yamasaki, K.I.; Fujisato, T. Development and evaluation of a removable tissue-engineered muscle with artificial tendons. J. Biosci. Bioeng. 2017, 123, 265–271. [Google Scholar] [CrossRef]
- Nakamura, T.; Takagi, S.; Okuzaki, D.; Matsui, S.; Fujisato, T. Hypoxia transactivates cholecystokinin gene expression in 3D-engineered muscle. J. Biosci. Bioeng. 2021, 132, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Nikolić, N.; Görgens, S.W.; Thoresen, G.H.; Aas, V.; Eckel, J.; Eckardt, K. Electrical pulse stimulation of cultured skeletal muscle cells as a model for in vitro exercise - possibilities and limitations. Acta Physiol. 2017, 220, 310–331. [Google Scholar] [CrossRef] [PubMed]
- Tit-Oon, P.; Chokchaichamnankit, D.; Khongmanee, A.; Sawangareetrakul, P.; Svasti, J.; Srisomsap, C. Comparative secretome analysis of cholangiocarcinoma cell line in three-dimensional culture. Int. J. Oncol. 2014, 45, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Gourgouvelis, J.; Yielder, P.; Clarke, S.T.; Behbahani, H.; Murphy, B. You can’t fix what isn’t broken: Eight weeks of exercise do not substantially change cognitive function and biochemical markers in young and healthy adults. PeerJ 2018, 6, e4675. [Google Scholar] [CrossRef]
- Nicolini, C.; Toepp, S.; Harasym, D.; Michalski, B.; Fahnestock, M.; Gibala, M.J.; Nelson, A.J. No changes in corticospinal excitability, biochemical markers, and working memory after six weeks of high-intensity interval training in sedentary males. Physiol. Rep. 2019, 7, e14140. [Google Scholar] [CrossRef]
- Burch, N.; Arnold, A.S.; Item, F.; Summermatter, S.; Brochmann Santana Santos, G.; Christe, M.; Boutellier, U.; Toigo, M.; Handschin, C. Electric pulse stimulation of cultured murine muscle cells reproduces gene expression changes of trained mouse muscle. PLoS ONE 2010, 5, e10970. [Google Scholar] [CrossRef] [Green Version]
- Adler, S.; Basketter, D.; Creton, S.; Pelkonen, O.; van Benthem, J.; Zuang, V.; Andersen, K.E.; Angers-Loustau, A.; Aptula, A.; Bal-Price, A.; et al. Alternative (non-animal) methods for cosmetics testing: Current status and future prospects-2010. Arch. Toxicol. 2011, 85, 367–485. [Google Scholar] [CrossRef]
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and potential in organoid research. Nat. Rev. Genet. 2018, 19, 671–687. [Google Scholar] [CrossRef]
- Wang, J.; Khodabukus, A.; Rao, L.; Vandusen, K.; Abutaleb, N.; Bursac, N. Engineered skeletal muscles for disease modeling and drug discovery. Biomaterials 2019, 221, 119416. [Google Scholar] [CrossRef]
- Alave Reyes-Furrer, A.; De Andrade, S.; Bachmann, D.; Jeker, H.; Steinmann, M.; Accart, N.; Dunbar, A.; Rausch, M.; Bono, E.; Rimann, M.; et al. Matrigel 3D bioprinting of contractile human skeletal muscle models recapitulating exercise and pharmacological responses. Commun. Biol. 2021, 4, 1183. [Google Scholar] [CrossRef] [PubMed]
- Fiuza-Luces, C.; Garatachea, N.; Berger, N.A.; Lucia, A. Exercise is the real polypill. Physiology 2013, 28, 330–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabiee, F.; Lachinani, L.; Ghaedi, S.; Nasr-Esfahani, M.H.; Megraw, T.L.; Ghaedi, K. New insights into the cellular activities of Fndc5/Irisin and its signaling pathways. Cell Biosci. 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Lanzi, C.; Perdicaro, D.J.; Gambarte Tudela, J.; Muscia, V.; Fontana, A.R.; Oteiza, P.I.; Vazquez Prieto, M.A. Grape pomace extract supplementation activates FNDC5/irisin in muscle and promotes white adipose browning in rats fed a high-fat diet. Food Funct. 2020, 11, 1537–1546. [Google Scholar] [CrossRef]
- Harada, M.; Nakamura, T.; Yokoyama, S. Design and construction of a continuous quantitative force measurement microdevice for artificial skeletal muscle. Proc. Mech. Eng. Congr. Jpn. 2020. [Google Scholar] [CrossRef]
- Hashimoto, T.; Okada, Y.; Yamanaka, A.; Ono, N.; Uryu, K.; Maru, I. The effect of eleutherococcus senticosus on metabolism-associated protein expression in 3T3-L1 and C2C12 cells. Phys. Act. Nutr. 2020, 24, 13–18. [Google Scholar] [CrossRef]
- Oishi, Y.; Tsukamoto, H.; Yokokawa, T.; Hirotsu, K.; Shimazu, M.; Uchida, K.; Tomi, H.; Higashida, K.; Iwanaka, N.; Hashimoto, T. Mixed lactate and caffeine compound increases satellite cell activity and anabolic signals for muscle hypertrophy. J. Appl. Physiol. 2015, 118, 742–749. [Google Scholar] [CrossRef] [Green Version]
- Sakushima, K.; Yoshikawa, M.; Osaki, T.; Miyamoto, N.; Hashimoto, T. Moderate hypoxia promotes skeletal muscle cell growth and hypertrophy in C2C12 cells. Biochem. Biophys. Res. Commun. 2020, 525, 921–927. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Hosokawa, M.; Miyashita, K.; Fujita, T.; Nishino, H.; Hashimoto, T. Fucoxanthinol attenuates oxidative stress-induced atrophy and loss in myotubes and reduces the triacylglycerol content in mature adipocytes. Mol. Biol. Rep. 2020, 47, 2703–2711. [Google Scholar] [CrossRef]
- Konishi, S.; Hashimoto, T.; Nakabuchi, T.; Ozeki, T.; Kajita, H. Cell and tissue system capable of automated culture, stimulation, and monitor with the aim of feedback control of organs-on-a-chip. Sci. Rep. 2021, 11, 2999. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugimoto, T.; Nakamura, T.; Yokoyama, S.; Fujisato, T.; Konishi, S.; Hashimoto, T. Investigation of Brain Function-Related Myokine Secretion by Using Contractile 3D-Engineered Muscle. Int. J. Mol. Sci. 2022, 23, 5723. https://doi.org/10.3390/ijms23105723
Sugimoto T, Nakamura T, Yokoyama S, Fujisato T, Konishi S, Hashimoto T. Investigation of Brain Function-Related Myokine Secretion by Using Contractile 3D-Engineered Muscle. International Journal of Molecular Sciences. 2022; 23(10):5723. https://doi.org/10.3390/ijms23105723
Chicago/Turabian StyleSugimoto, Takeshi, Tomohiro Nakamura, Sho Yokoyama, Toshia Fujisato, Satoshi Konishi, and Takeshi Hashimoto. 2022. "Investigation of Brain Function-Related Myokine Secretion by Using Contractile 3D-Engineered Muscle" International Journal of Molecular Sciences 23, no. 10: 5723. https://doi.org/10.3390/ijms23105723
APA StyleSugimoto, T., Nakamura, T., Yokoyama, S., Fujisato, T., Konishi, S., & Hashimoto, T. (2022). Investigation of Brain Function-Related Myokine Secretion by Using Contractile 3D-Engineered Muscle. International Journal of Molecular Sciences, 23(10), 5723. https://doi.org/10.3390/ijms23105723





