Antibacterial Vancomycin@ZIF-8 Loaded PVA Nanofiber Membrane for Infected Bone Repair
Abstract
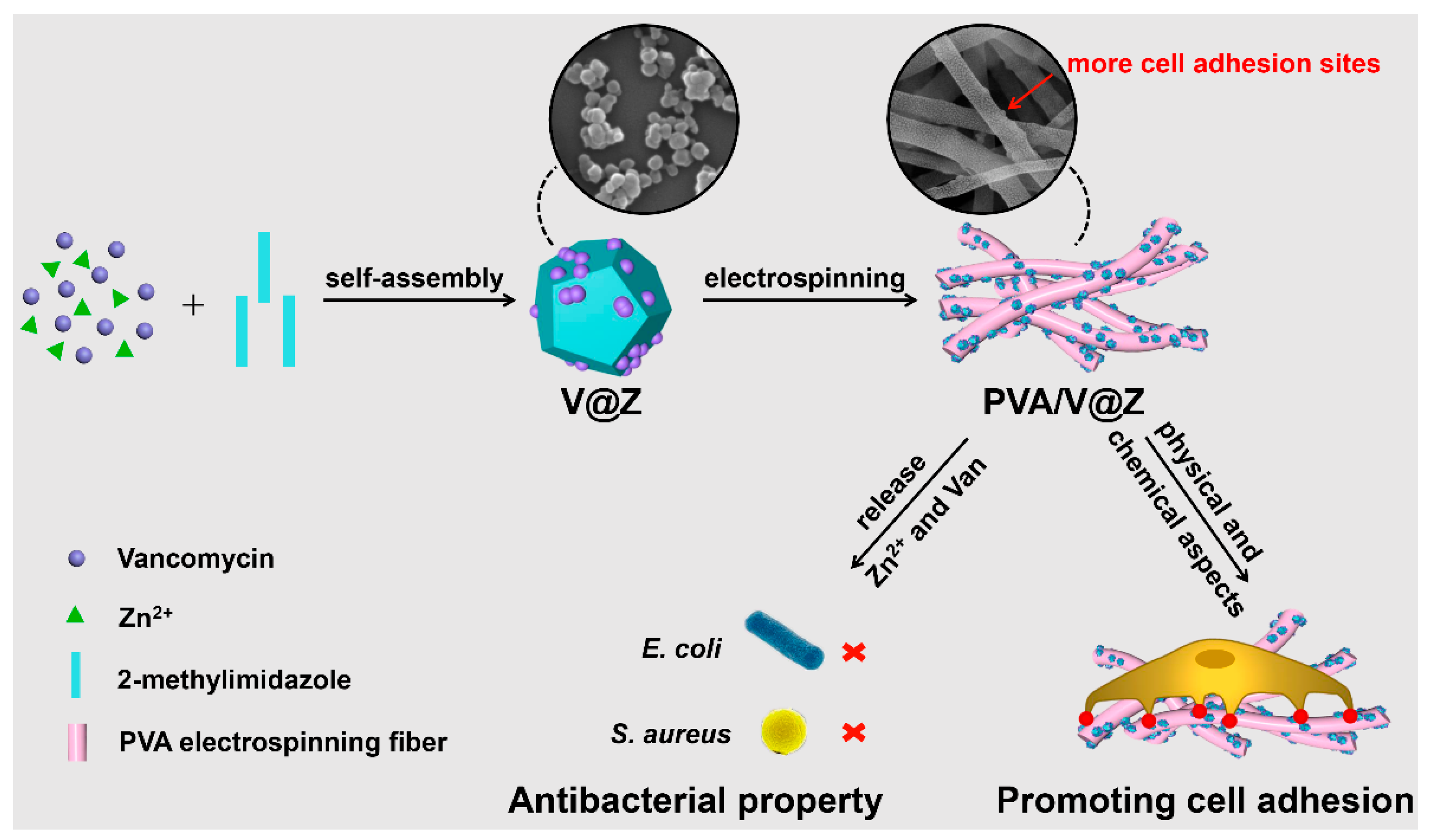
:1. Introduction
2. Results and Discussion
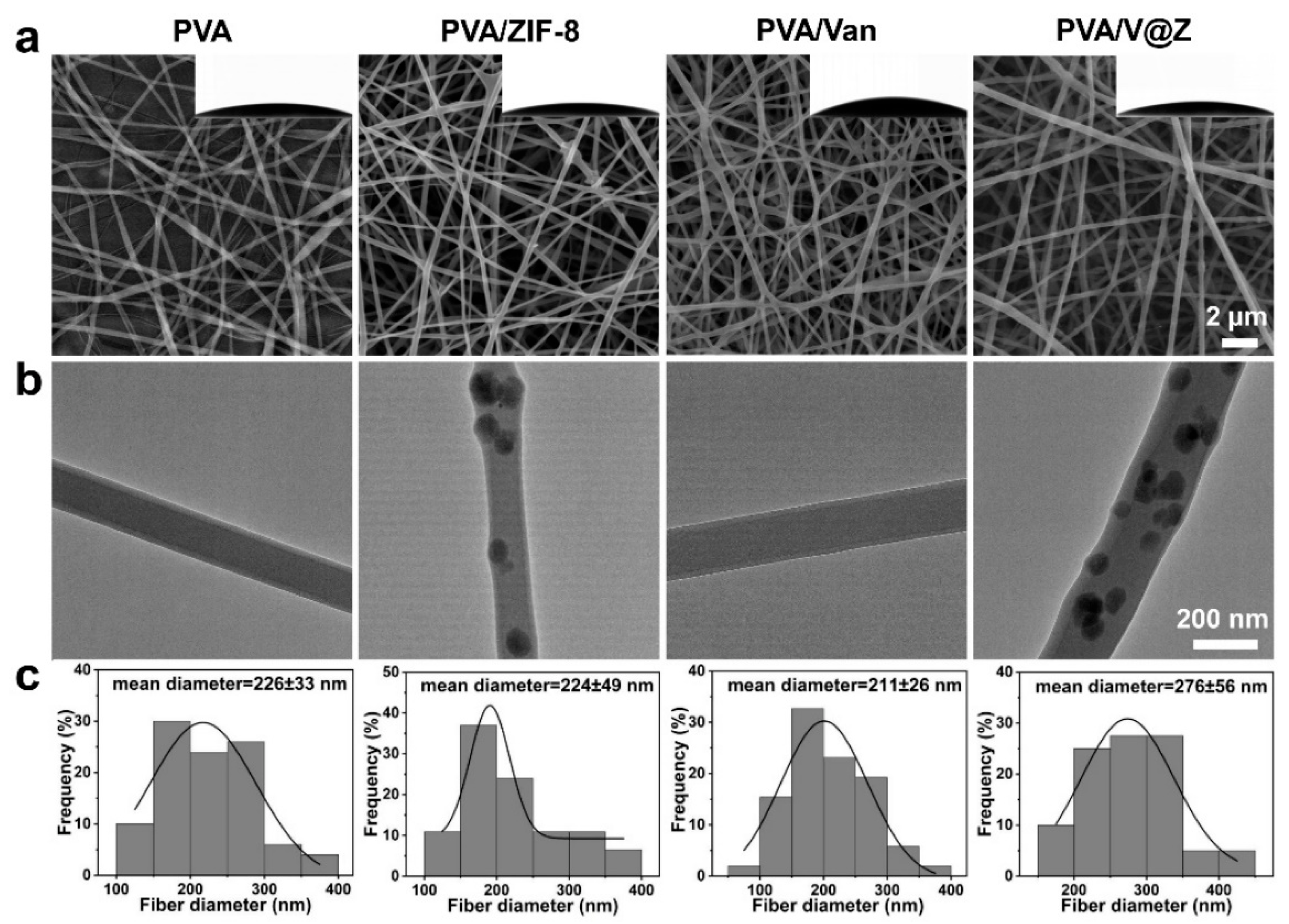
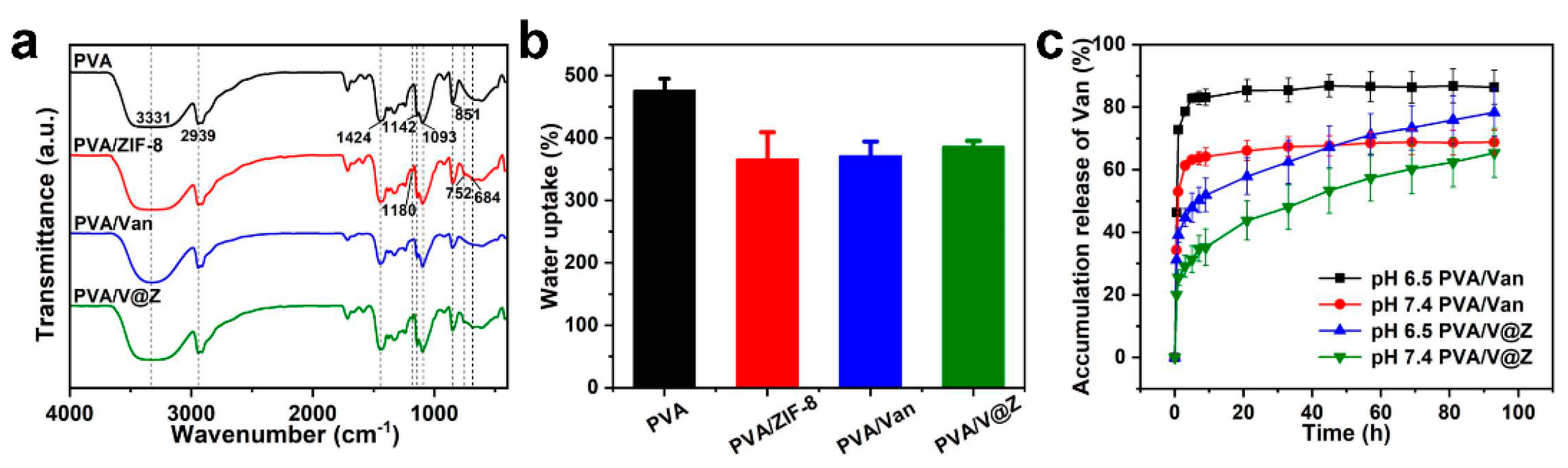
2.1. Materials Characterization
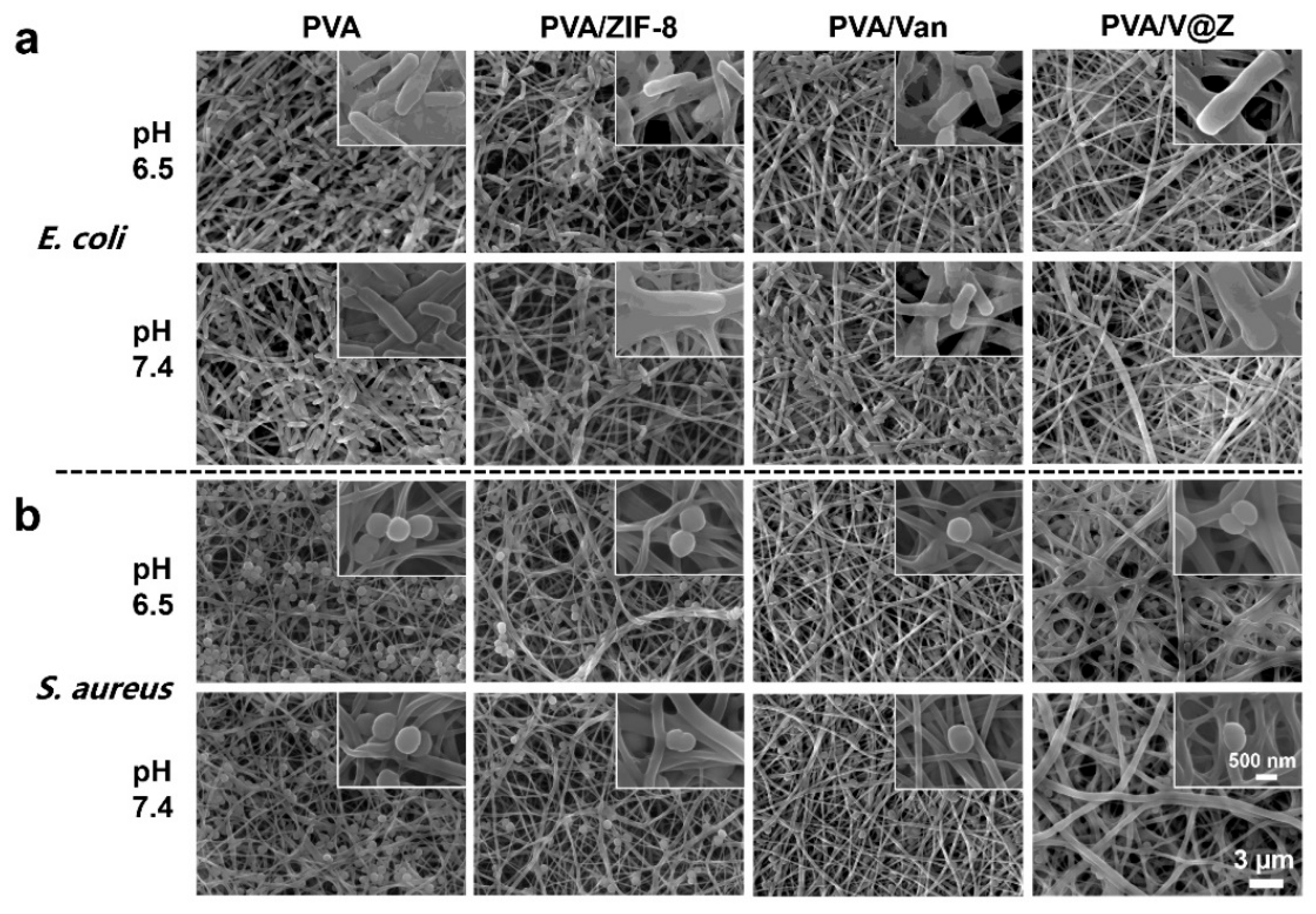
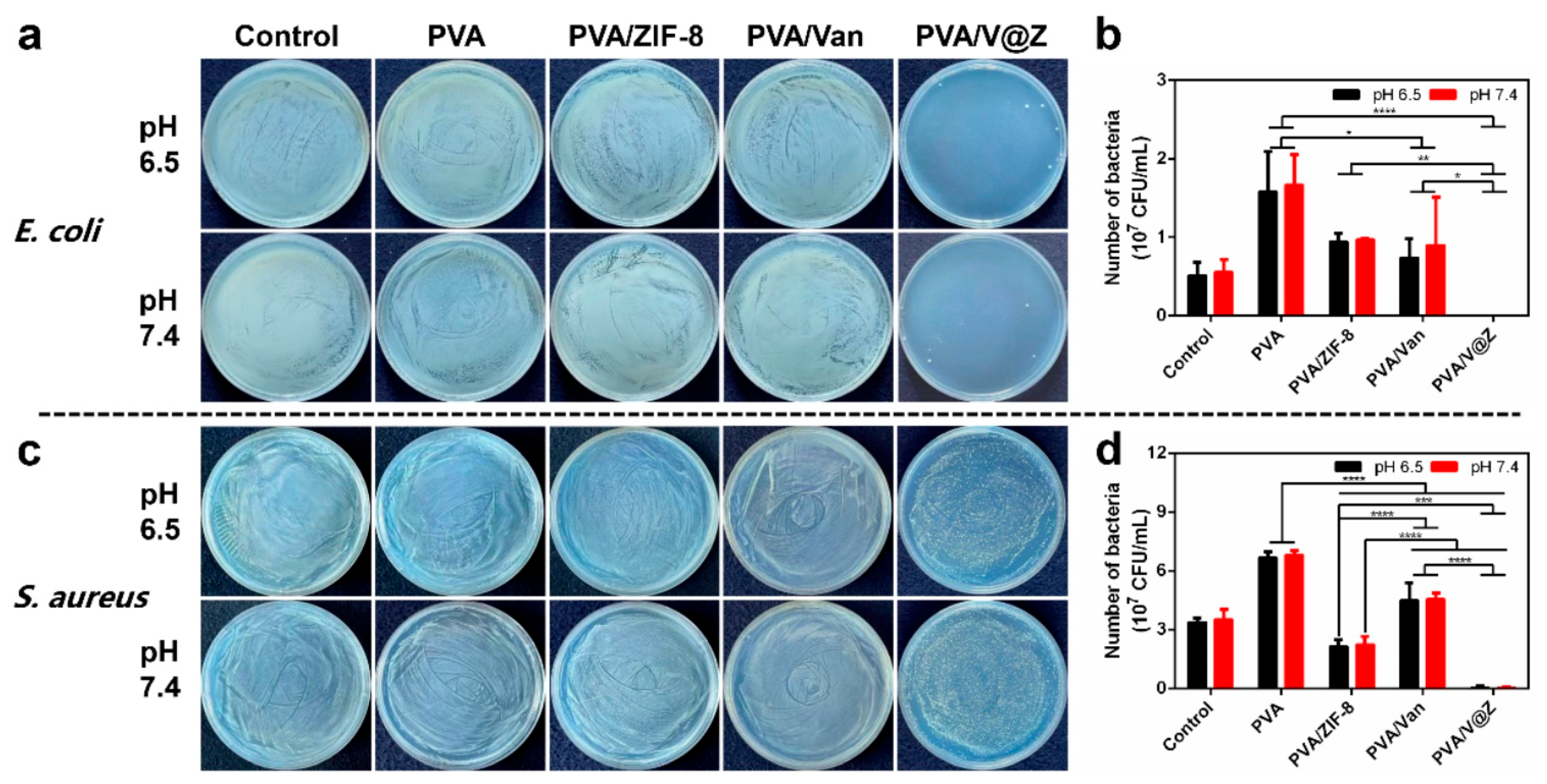
2.2. Antibacterial Properties
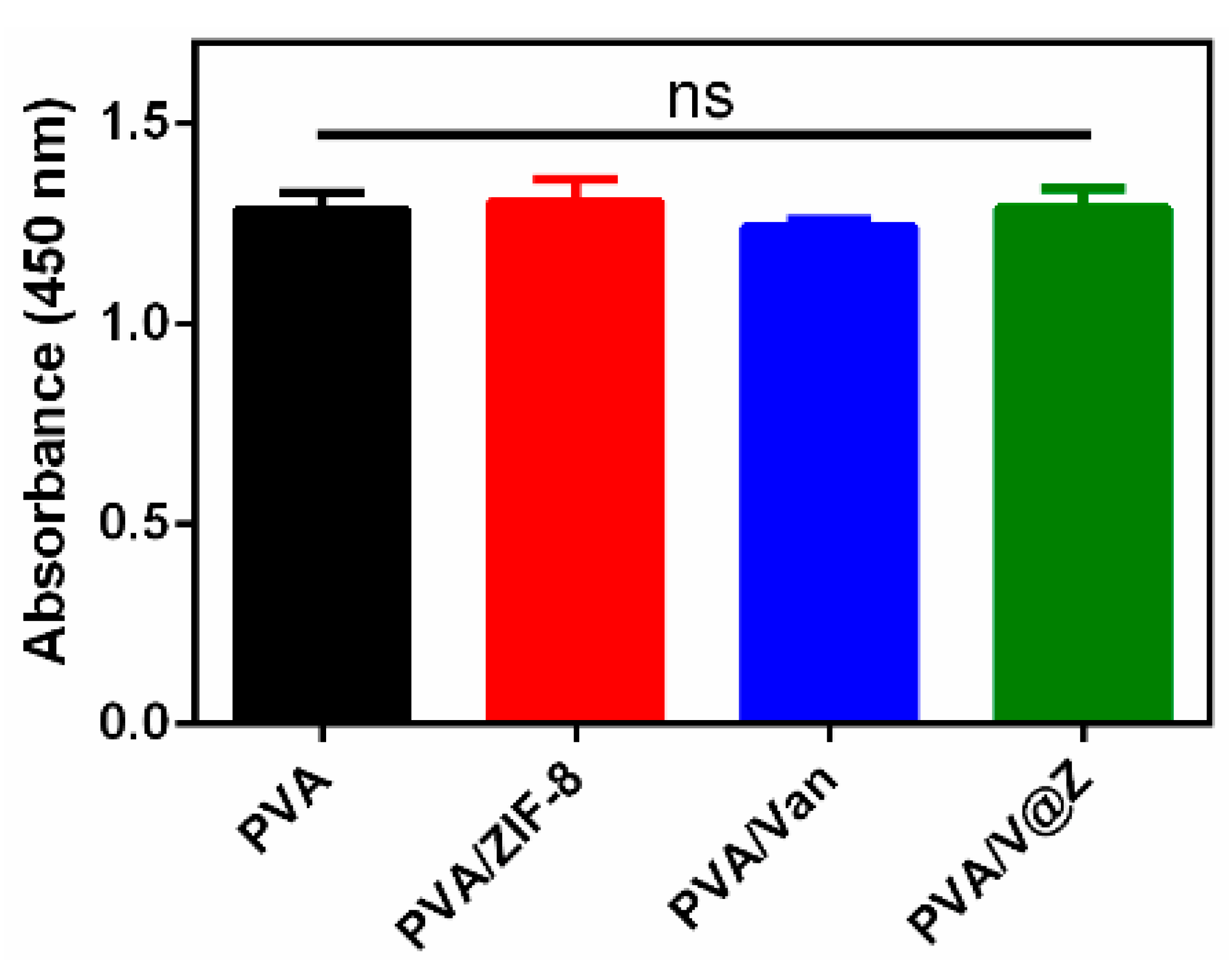
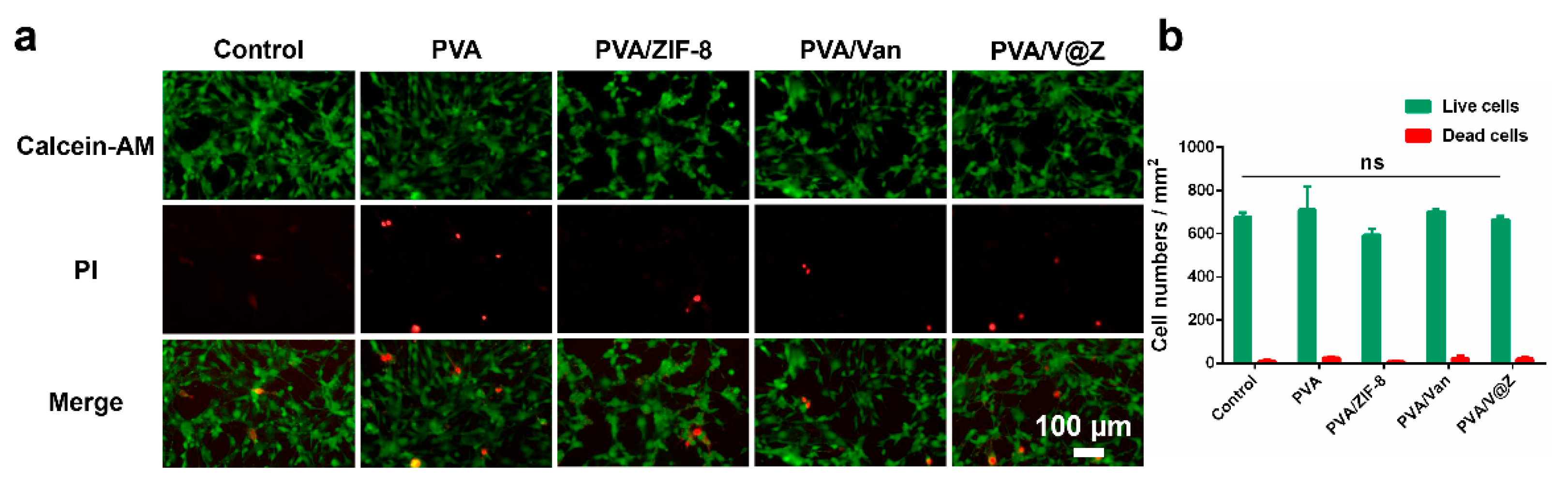
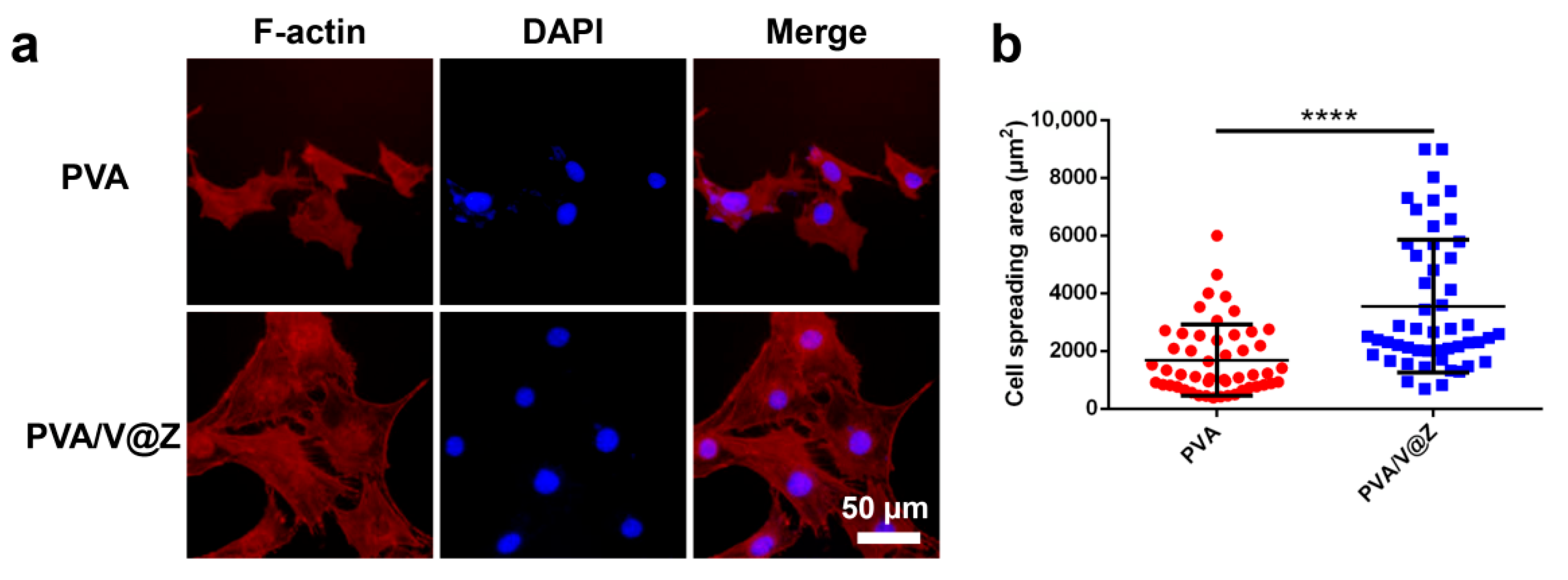
2.3. Cell Studies
3. Materials and Methods
3.1. Material
3.2. Synthesis of ZIF-8 and V@Z Particles
3.3. Preparation of the Electrospun Fibrous Membrane
3.4. Characterization of the Particles and Electrospun Fibrous Membranes
3.5. Drug-Loading Rate (DL) of V@Z Particles
3.6. Water Absorption Rate of the Electrospun Fibrous Membrane
3.7. Van Release from Electrospun Fibrous Membrane
3.8. Antibacterial Activity
3.9. Cell Culture and Bioactivity Characterizations
3.10. Statistic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.H.; Wang, S.L.; Xu, J.Z.; Sun, D.; Shen, J.; Zhao, X. Antibiotic cement plate composite structure internal fixation after debridement of bone infection. Sci. Rep. 2021, 11, 16921. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.N.; Li, Y.; Zhang, Y.Q.; Zheng, Y.F.; Li, Z.Y.; Zhu, S.L.; Li, C.Y.; Cui, Z.D.; Wu, S.L. An engineered pseudo-macrophage for rapid treatment of bacteria-infected osteomyelitis via microwave-excited anti-infection and immunoregulation. Adv. Mater. 2021, 33, 2102926. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Bandyopadhyay, S.; Das, P.; Samanta, I.; Mukherjee, P.; Roy, S.; Kundu, B. Understanding osteomyelitis and its treatment through local drug delivery system. Biotechnol. Adv. 2016, 34, 1305–1317. [Google Scholar] [CrossRef]
- Bidault, P.; Chandad, F.; Grenier, D. Risk of bacterial resistance associated with systemic antibiotic therapy in periodontology. J. Can. Dent. Assoc. 2007, 73, 721–725. [Google Scholar]
- Hanssen, A.D. Local antibiotic delivery vehicles in the treatment of musculoskeletal infection. Clin. Orthop. Relat. Res. 2005, 437, 91–96. [Google Scholar] [CrossRef]
- Lv, M.Z.; Zhou, W.; Tavakoli, H.; Bautista, C.; Xia, J.F.; Wang, Z.H.; Li, X.J. Aptamer-functionalized metal-organic frameworks (MOFs) for biosensing. Biosens. Bioelectron. 2021, 176, 112947. [Google Scholar] [CrossRef]
- Pettinari, C.; Pettinari, R.; Nicola, C.D.; Tombesi, A.; Scuri, S.; Marchetti, F. Antimicrobial MOFs. Coord. Chem. Rev. 2021, 446, 214121. [Google Scholar] [CrossRef]
- Zhi, S.; Shaw, W.L.; Miao, Y.R.; You, S.Z.; Dlott, D.D.; Suslick, K.S. Shock wave chemistry in a metal-organic framework. J. Am. Chem. Soc. 2017, 139, 4619–4622. [Google Scholar]
- Alsaiari, S.K.; Qutub, S.S.; Sun, S.; Baslyman, W.; Aldehaiman, M.; Alyami, M.; Almalik, A.; Halwani, R.; Merzaban, J.; Mao, Z.; et al. Sustained and targeted delivery of checkpoint inhibitors by metal-organic frameworks for cancer immunotherapy. Sci. Adv. 2021, 7, 7174. [Google Scholar] [CrossRef]
- Butonova, S.A.; Ikonnikova, E.V.; Sharsheeva, A.; Chernyshov, I.Y.; Kuchur, O.A.; Mukhin, I.S.; Hey-Hawkins, E.; Vinogradova, A.V.; Morozov, M.I. Degradation kinetic study of ZIF-8 microcrystals with and without the presence of lactic acid. RSC Adv. 2021, 11, 39169–39176. [Google Scholar] [CrossRef]
- Karakecili, A.; Topuz, B.; Korpayev, S.; Erdek, M. Metal-organic frameworks for on-demand pH controlled delivery of vancomycin from chitosan scaffolds. Mater. Sci. Eng. C 2019, 105, 110098. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Zhu, Z.; Pei, X.B.; Zhang, X.; Cheng, X.T.; Hu, S.S.; Gao, X.M.; Wang, J.; Chen, J.Y.; Wan, Q.B. ZIF-8-modified multifunctional bone-adhesive hydrogels promoting angiogenesis and osteogenesis for bone regeneration. ACS Appl. Mater. Interfaces 2020, 12, 36978–36995. [Google Scholar] [CrossRef] [PubMed]
- Eirich, J.; Orth, R.; Sieber, S.A. Unraveling the protein targets of vancomycin in living S. aureus and E. faecalis cells. J. Am. Chem. Soc. 2011, 133, 12144–12153. [Google Scholar] [CrossRef] [PubMed]
- Chowdhuri, A.R.; Das, B.; Kumar, A.; Tripathy, S.; Roy, S.; Sahu, S.K. One-pot synthesis of multifunctional nanoscale metal-organic frameworks as an effective antibacterial agent against multidrug-resistant Staphylococcus aureus. Nanotechnology 2017, 28, 095102. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, C.A.; Barros, J.; Rey-Rico, A.; Redondo, P.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; Monteiro, F.J. Antimicrobial properties and osteogenicity of vancomycin-loaded synthetic scaffolds obtained by supercritical foaming. ACS Appl. Mater. Interfaces 2018, 10, 3349–3360. [Google Scholar] [CrossRef]
- Traub, W.H.; Leonhard, B. Heat stability of the antimicrobial activity of sixty-two antibacterial agents. J. Antimicrob. Chemother. 1995, 35, 149–154. [Google Scholar] [CrossRef]
- Carli, A.V.; Sethuraman, A.S.; Bhimani, S.J.; Ross, F.P.; Bostrom, M.P.G. Selected heat-sensitive antibiotics are not inactivated during polymethylmethacrylate curing and can be used in cement spacers for periprosthetic joint infection. J. Arthroplast. 2018, 33, 1930–1935. [Google Scholar] [CrossRef]
- Fang, Z.Y.; Qiao, K.; Wang, Y.S.; Zheng, Y.D.; He, W.; Xie, Y.J.; Yang, H.Y. Injectable and biodegradable double-network nanocomposite hydrogel with regulable sol-gel transition process and mechanical properties. Polym. Test. 2022, 106, 107452. [Google Scholar] [CrossRef]
- Holzwarth, J.M.; Ma, P.X. Biomimetic nanofibrous scaffolds for bone tissue engineering. Biomaterials 2011, 32, 9622–9629. [Google Scholar] [CrossRef] [Green Version]
- Nie, J.; Zhang, S.; Wu, P.; Liu, Y.G.; Su, Y.J. Electrospinning with lyophilized platelet-rich fibrin has the potential to enhance the proliferation and osteogenesis of MC3T3-E1 cells. Front. Bioeng. Biotechnol. 2020, 8, 595579. [Google Scholar] [CrossRef]
- Cheng, X.; Cheng, G.; Xing, X.; Yin, C.C.; Cheng, Y.; Zhou, X.; Jiang, S.; Tao, F.H.; Deng, H.B.; Li, Z.B. Controlled release of adenosine from core-shell nanofibers to promote bone regeneration through STAT3 signaling pathway. J. Control. Release 2019, 319, 234–245. [Google Scholar] [CrossRef]
- Xia, Y.; Fan, X.; Yang, H.; Li, L.; He, C.; Cheng, C.; Haag, R. ZnO/nanocarbons-modified fibrous scaffolds for stem cell-based osteogenic differentiation. Small 2020, 16, 2003010. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.F.; Sherman, E.; Vajo, J.J. Aqueous room temperature synthesis of cobalt and zinc sodalite zeolitic imidizolate frameworks. Dalton Trans. 2012, 41, 5458–5460. [Google Scholar] [CrossRef]
- Man, T.T.; Xu, C.X.; Liu, X.Y.; Li, D.; Tsung, C.K.; Pei, H.; Wan, Y.; Li, L. Hierarchically encapsulating enzymes with multi-shelled metal-organic frameworks for tandem biocatalytic reactions. Nat. Commun. 2022, 13, 305. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kazemian, H.; Rohani, S.; Huang, Y.N.; Song, Y. In situ high pressure study of ZIF-8 by FTIR spectroscopy. Chem. Commun. 2011, 47, 12694–12696. [Google Scholar] [CrossRef] [PubMed]
- Higashi, S.; Hirai, T.; Matsubara, M.; Hiroaki, Y.; Beniya, A. Dynamic viscosity recovery of electrospinning solution for stabilizing elongated ultrafine polymer nanofiber by TEMPO-CNF. Sci. Rep. 2020, 10, 13427. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Liu, Z.; Qiu, W.; Wen, L. Vacuum evaporated poly(vinyl alcohol) thin films. J. Vac. Sci. Technol. 2004, 24, 359–362. [Google Scholar]
- Yu, D.; Feng, Y.Y.; Xu, J.X.; Kong, B.H.; Liu, Q.; Wang, H. Fabrication, characterization, and antibacterial properties of citric acid crosslinked PVA electrospun microfibre mats for active food packaging. Packag. Technol. Sci. 2021, 34, 361–370. [Google Scholar] [CrossRef]
- Li, B.; Xia, X.M.; Chen, J.T.; Xia, D.; Xu, R.D.; Zou, X.R.; Wang, H.S.; Liang, C.Y. Paclitaxel-loaded lignin particle encapsulated into electrospun pva/pvp composite nanofiber for effective cervical cancer cell inhibition. Nanotechnology 2021, 32, 015101. [Google Scholar] [CrossRef]
- Li, W.C.; Qiao, K.; Zheng, Y.D.; Yan, Y.; Xie, Y.J.; Liu, Y.; Ren, H.M. Preparation, mechanical properties, fatigue and tribological behavior of double crosslinked high strength hydrogel. J. Mech. Behav. Mater. 2022, 126, 105009. [Google Scholar] [CrossRef]
- Wei, J.C.; Hu, J.; Li, M.; Chen, Y.; Chen, Y.W. Multiple drug-loaded electrospun PLGA/gelatin composite nanofibers encapsulated with mesoporous ZnO nanospheres for potential postsurgical cancer treatment. RSC Adv. 2014, 4, 28011–28019. [Google Scholar] [CrossRef]
- Izquierdo-Barba, I.; Sánchez-Salcedo, S.; Colilla, M.; Feito, M.J.; Ramírez-Santillán, C.; Portolés, M.T.; Vallet-Regí, M. Inhibition of bacterial adhesion on biocompatible zwitterionic SBA-15 mesoporous materials. Acta Biomater. 2011, 7, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Mirhosseini, M.; Firouzabadi, F.B. Antibacterial activity of zinc oxide nanoparticle suspensions on food-borne pathogens. Int. J. Dairy Technol. 2013, 66, 291–295. [Google Scholar] [CrossRef]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. Photochem. Photobiol. 2013, 120, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.X.; Liu, Y.; Gao, J.W.; Wang, D.H.; Xia, D.; Liang, C.Y.; Li, N.; Xu, R.D. Synergistic effect of co-delivering ciprofloxacin and tetracycline hydrochloride for promoted wound healing by utilizing coaxial PCL/gelatin nanofiber membrane. Int. J. Mol. Sci. 2022, 23, 1895. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Wang, H.; Zou, X.; Wang, D.; Fan, Y.; Zhao, X.; Li, M.; Yang, L.; Liang, C. Antibacterial Vancomycin@ZIF-8 Loaded PVA Nanofiber Membrane for Infected Bone Repair. Int. J. Mol. Sci. 2022, 23, 5629. https://doi.org/10.3390/ijms23105629
Zhao Y, Wang H, Zou X, Wang D, Fan Y, Zhao X, Li M, Yang L, Liang C. Antibacterial Vancomycin@ZIF-8 Loaded PVA Nanofiber Membrane for Infected Bone Repair. International Journal of Molecular Sciences. 2022; 23(10):5629. https://doi.org/10.3390/ijms23105629
Chicago/Turabian StyleZhao, Yunbo, Hongshui Wang, Xianrui Zou, Donghui Wang, Ying Fan, Xiaoyan Zhao, Mingjun Li, Lei Yang, and Chunyong Liang. 2022. "Antibacterial Vancomycin@ZIF-8 Loaded PVA Nanofiber Membrane for Infected Bone Repair" International Journal of Molecular Sciences 23, no. 10: 5629. https://doi.org/10.3390/ijms23105629
APA StyleZhao, Y., Wang, H., Zou, X., Wang, D., Fan, Y., Zhao, X., Li, M., Yang, L., & Liang, C. (2022). Antibacterial Vancomycin@ZIF-8 Loaded PVA Nanofiber Membrane for Infected Bone Repair. International Journal of Molecular Sciences, 23(10), 5629. https://doi.org/10.3390/ijms23105629




