Solvent-Free Preparation of Tannic Acid Carbon Dots for Selective Detection of Ni2+ in the Environment
Abstract
:1. Introduction
2. Results
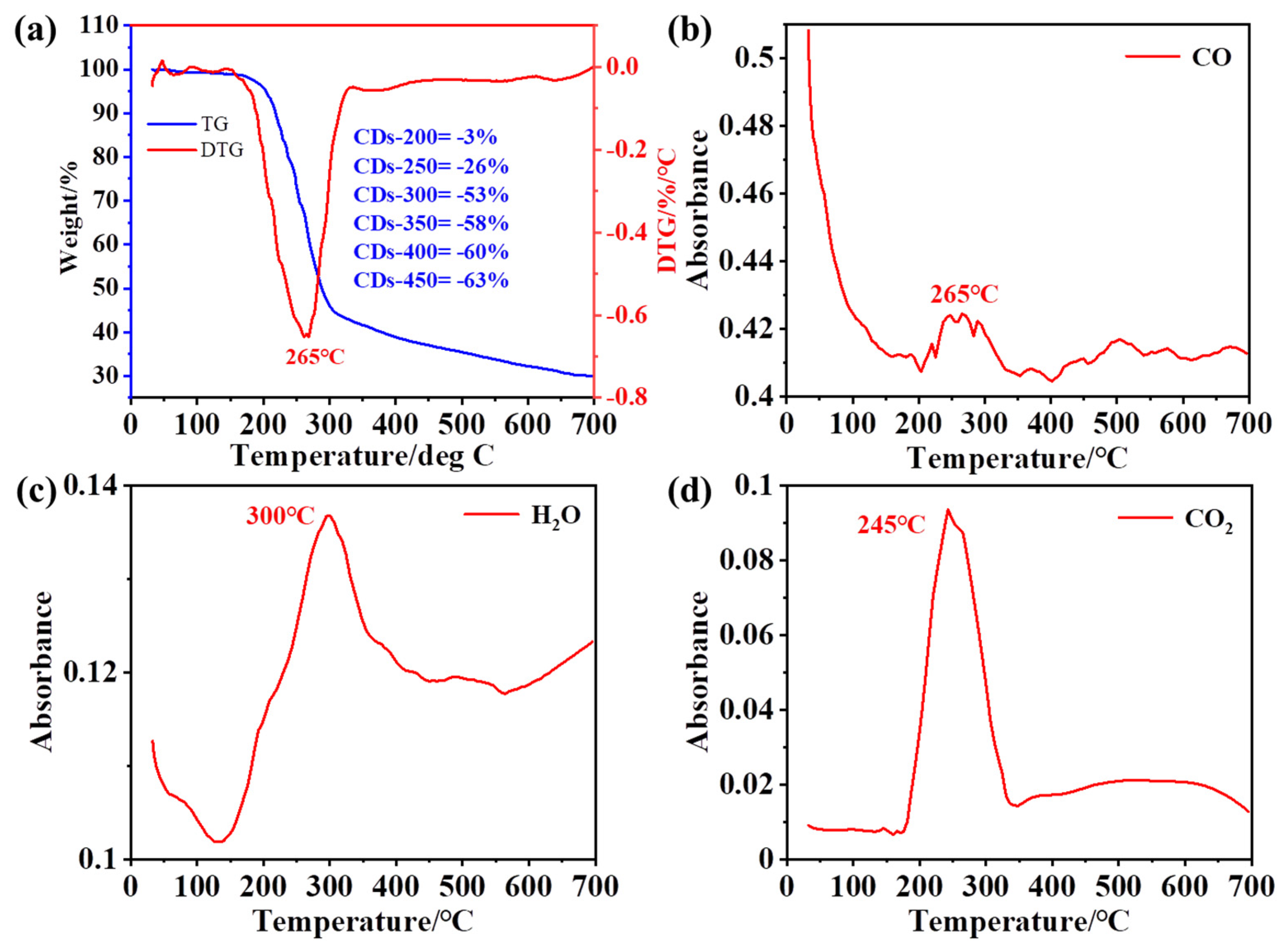
2.1. TGA-MS Analysis of Raw Materials
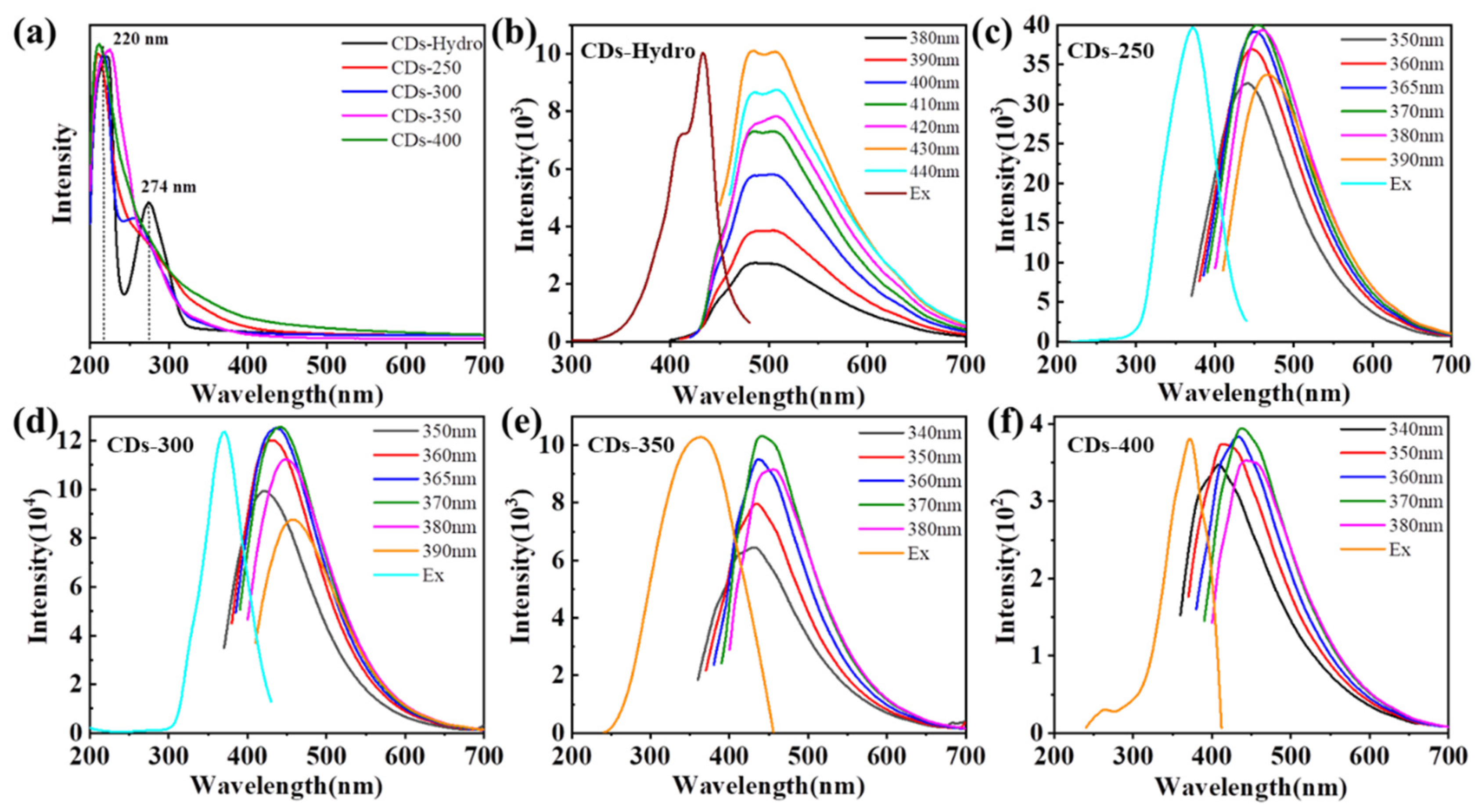
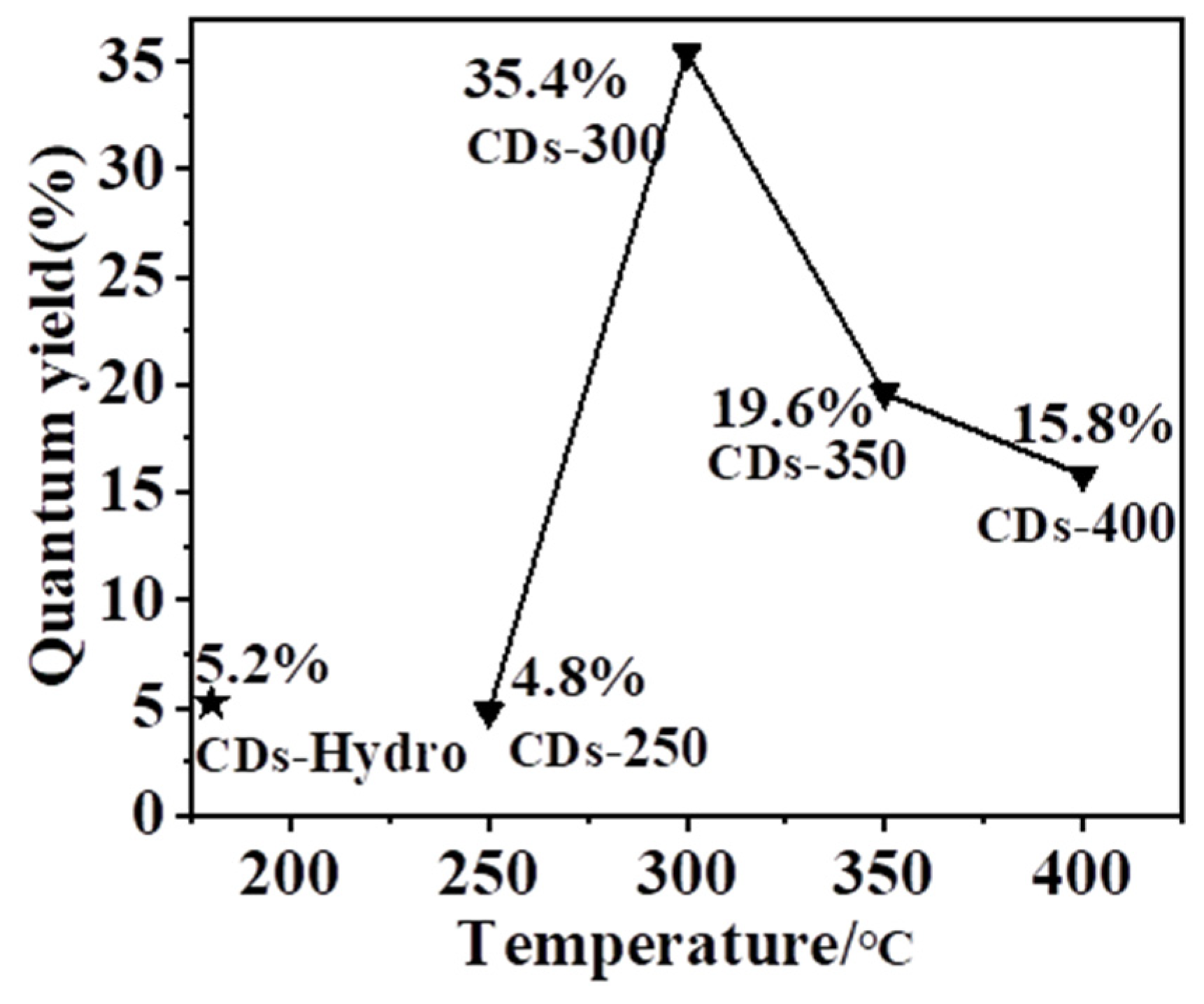
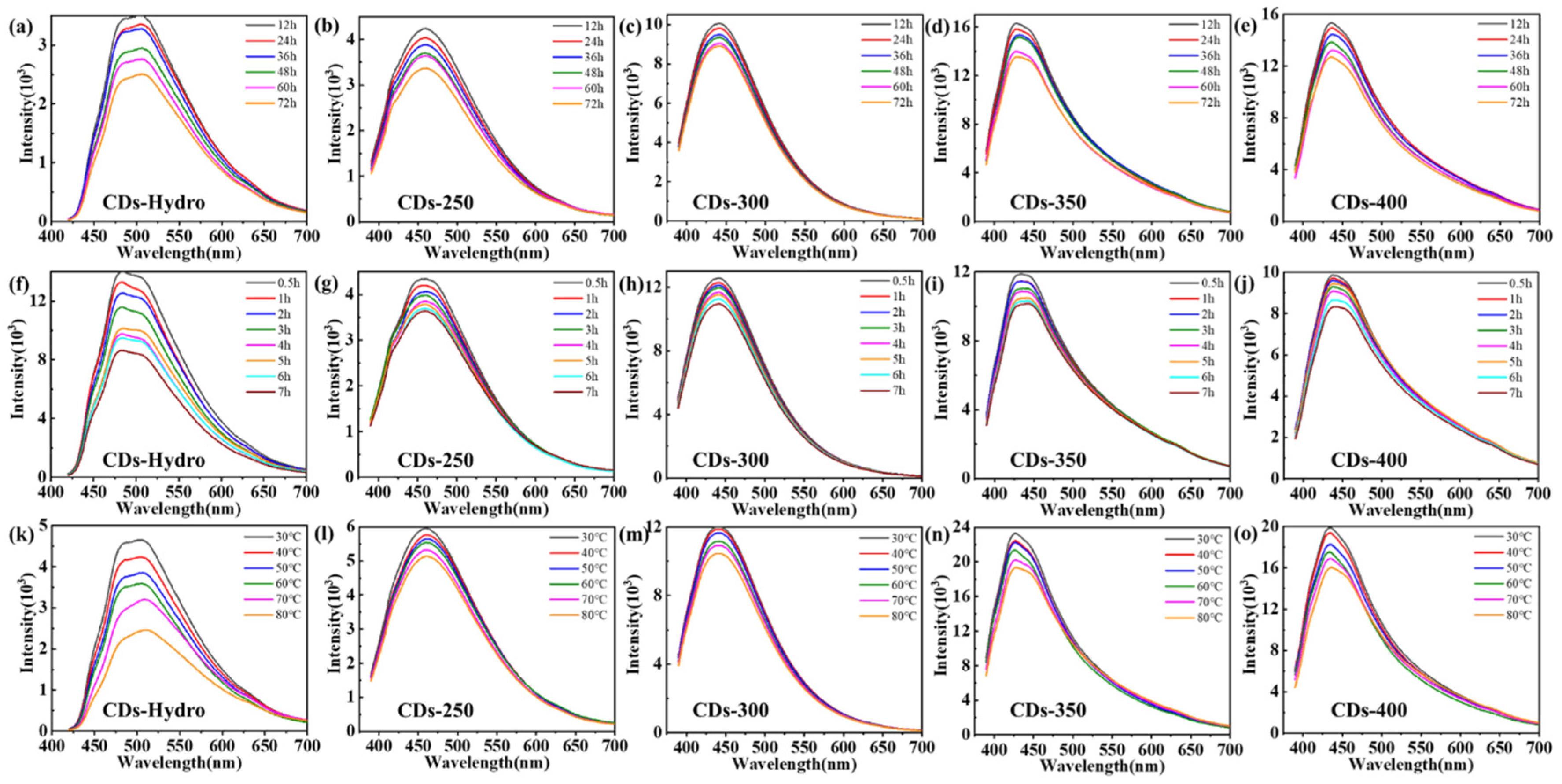
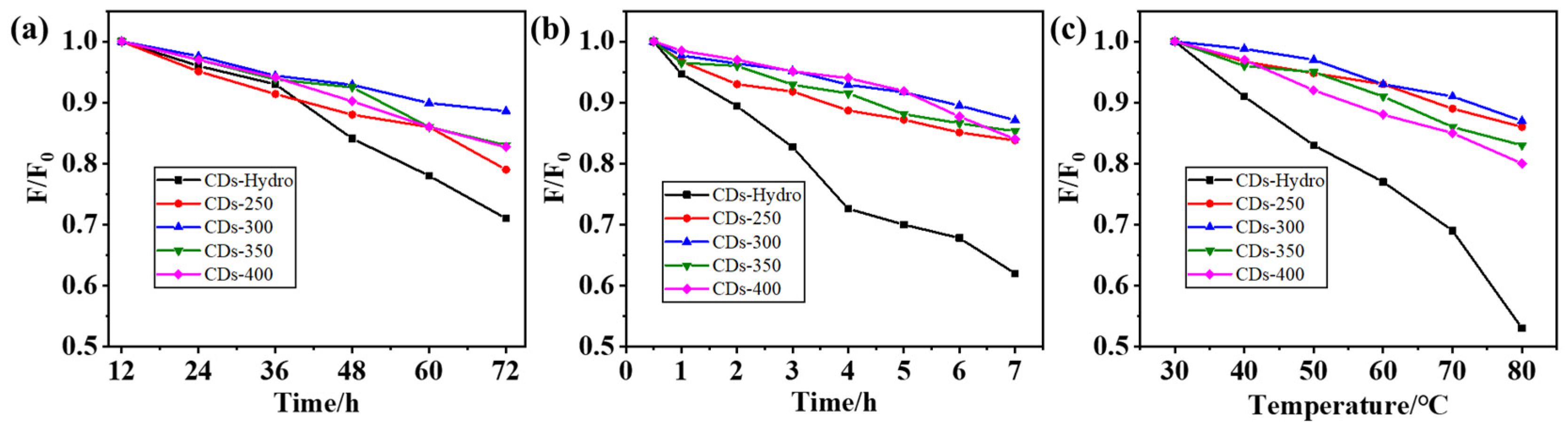
2.2. Optical Properties
2.3. Surface Morphology and Characterizations
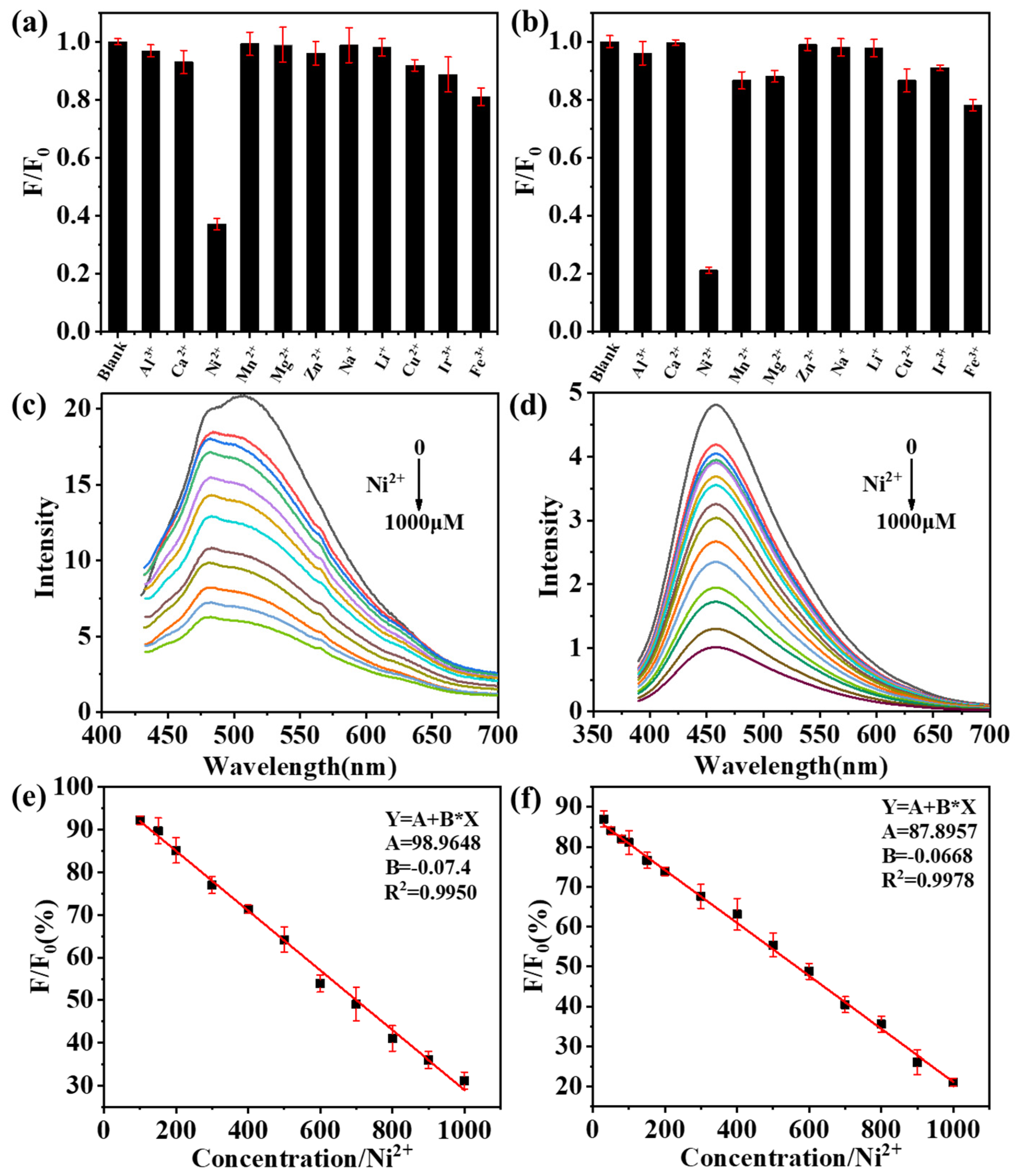
2.4. The Fluorescence Quenching of CDs by Ni2+
3. Discussion
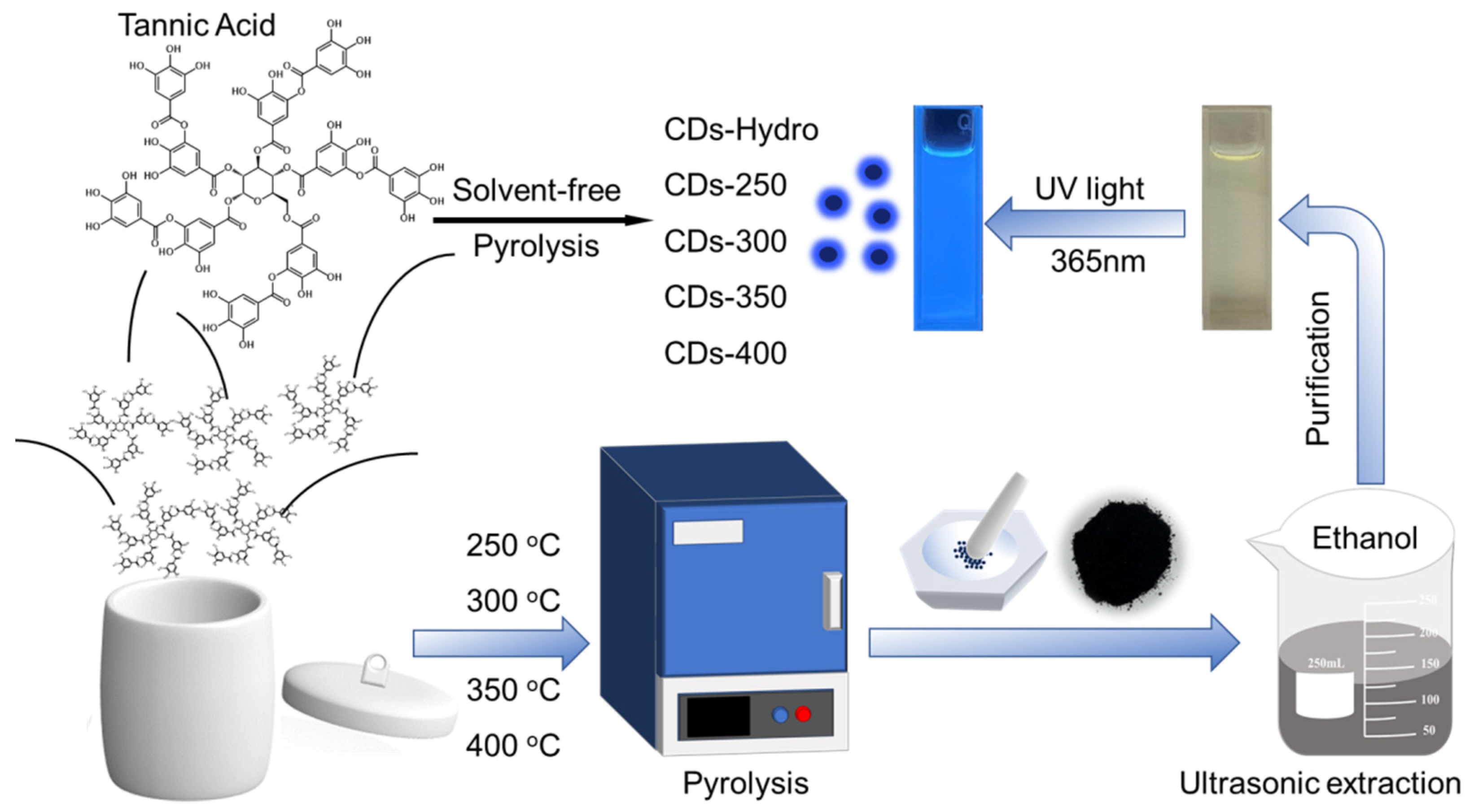
4. Materials and Methods
4.1. Materials
4.2. Methods
4.3. Synthesis of CDs
4.4. Fluorescence Detection of Metal Ions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, C.Y.; Du, B.Y.; Yin, R.S. Isotopic fractionation and source appointment of methylmercury and inorganic mercury in a paddy ecosystem. Environ. Sci. Technol. 2020, 54, 14334–14342. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.Q.; Hu, H.Y.; Meng, B. Methanogenesis Is an Important Process in Controlling MeHg Concentration in Rice Paddy Soils Affected by Mining Activities. Environ. Sci. Technol. 2020, 54, 13517–13526. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, M.A.; Canals, A.; Lopez-Garcia, I. Determination of cadmium in used engine oil, gasoline and diesel by electrothermal atomic absorption spectrometry using magnetic ionic liquid-based dispersive liquid-liquid microextraction. Talanta 2020, 220, 121395. [Google Scholar] [CrossRef]
- Smirnova, S.V.; Lin, D.V.; Pletnev, I.V. Extraction and ICP-OES determination of heavy metals using tetrabutylammonium bromide aqueous biphasic system and oleophilic collector. Talanta 2021, 221, 121485. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Růžička, J.; Hansen, E.H. An efficient flow-injection system with on-line ion-exchange preconcentration for the determination of trace amounts of heavy metals by atomic absorption spectrometry. Analy. Chim. Acta 1984, 164, 23–39. [Google Scholar] [CrossRef]
- Townsend, A.T.; Miller, K.A.; McLean, S.; Aldous, S. The determination of copper, zinc, cadmium and lead in urine by high resolution ICP-MS. J. Anal. At. Spectrom. 1998, 13, 1213–1219. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Peralta-Videa, J.R.; dela Rosa, G.; Parsons, J.G. Phytoremediation of Heavy Metals and Study of the Metal Coordination by X-ray Absorption Spectroscopy, Coordination Chemistry Reviews. Coordin. Chem. Rev. 2005, 249, 1797–1810. [Google Scholar] [CrossRef]
- Eshkeiti, A.; Narakathu, B.B.; Reddy, A.S.G.; Moorthi, A.; Atashbar, M.Z.; Rebrosova, E.; Rebros, M.; Joyce, M. Detection of Heavy Metal Compounds Using a Novel Inkjet Printed Surface Enhanced Raman Spectroscopy (SERS) Substrate. Sensors Actuat. B Chem. 2012, 171, 705–711. [Google Scholar] [CrossRef]
- Pyle, S.M.; Nocerino, J.M.; Deming, S.N.; Palasota, J.A.; Palasota, J.M.; Miller, E.L.; Hillman, D.C.; Kuharic, C.A.; Cole, W.H.; Fitzpatrick, P.M.; et al. Comparison of AAS, ICP-AES, PSA, and XRF in Determining Lead and Cadmium in Soil. Environ. Sci. Technol. 1996, 30, 204–213. [Google Scholar] [CrossRef]
- Nazari, M.; Amini, A.; Eden, N.T. Highly-Efficient Sulfonated UiO-66 (Zr) Optical Fiber for Rapid Detection of Trace Levels of Pb2+. Int. J. Mol. Sci. 2021, 22, 6053. [Google Scholar] [CrossRef]
- Ho, C.K.; Hughes, R.C. In-Situ Chemiresistor Sensor Package for Real-Time Detection of Volatile Organic Compounds in Soil and Groundwater. Sensors 2002, 2, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Yantasee, W.; Lin, Y.; Hongsirikarn, K.; Fryxell, G.E.; Timchalk, C. Electrochemical Sensors for the Detection of Lead and Other Toxic Heavy Metals: The Next Generation of Personal Exposure Biomonitors. Environ. Health. Perspect. 2007, 115, 1683–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhang, Z.; Liu, H.; Mao, X.; Liu, W.; Zhang, S.; Nie, Z.; Lu, X. Ultratrace and robust visual sensor of Cd2+ ions based on the size-dependent optical properties of Au@g-CNQDs nanoparticles in mice models. Biosens. Bioelectron. 2018, 103, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Martín, J.; Delgado-Olidén, A.; Velasco, L. Carbon dots boost dsRNA delivery in plants and increase local and systemic siRNA production. Int. J. Mol. Sci. 2022, 23, 5338. [Google Scholar] [CrossRef] [PubMed]
- Latif, U.; Mujahid, A.; Afzal, A. Dual and tetraelectrode QCMs using imprinted polymers as receptors for ions and neutral analytes. Anal. Bioanal. Chem. 2011, 400, 2507–2515. [Google Scholar] [CrossRef]
- Shamsipur, M.; Poursaberi, T.; Karami, A.R. Development of a new fluorimetric bulk optode membrane based on 2, 5-thiophenylbis (5-tert-butyl-1, 3-benzexazole) for nickel (II) ions. Anal. Chim. Acta 2004, 501, 55–60. [Google Scholar] [CrossRef]
- Mazloum, M.; Niassary, M.S.; Amini, M.K. Pentacyclooctaaza as a neutral carrier in coated-wire ion-selective electrode for nickel (II). Sens. Actuators B 2002, 82, 259–264. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef]
- Xu, X.Y.; Ray, R.; Gu, Y.L.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Sun, Y.P.; Zhou, B.; Lin, Y.; Wang, W.; Fernando, K.A.; Pathak, P.; Meziani, M.J.; Harruff, B.A.; Wang, X.; Wang, H.; et al. Quantum-Sized Carbon Dots for Bright and Colorful Photoluminescence. J. Am. Chem. Soc. 2006, 128, 7756–7757. [Google Scholar] [CrossRef]
- Baker, S.N.; Baker, G.A. Luminescent carbon nanodots: Emergent nanolights. Angew. Chem. Int. Ed. 2010, 49, 6726–6744. [Google Scholar] [CrossRef] [PubMed]
- Hola, K.; Zhang, Y.; Wang, Y.; Giannelis, E.P.; Zboril, R.; Rogach, A.L. Carbon dots-Emerging light emitters for bioimaging, cancer therapy and optoelectronics. Nano Today 2014, 9, 590–603. [Google Scholar] [CrossRef]
- Kong, B.; Zhu, A.; Ding, C.; Zhao, X.; Li, B.; Tian, Y. Carbon dot-based inorganic-organic nanosystem for two-photon imaging and biosensing of pH variation in living cells and tissues. Adv. Mater. 2012, 24, 5844–5848. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Su, W.; Wu, H.; Yuan, T.; Yuan, C.; Liu, J.; Zhou, J. Targeted tumour theranostics in mice via carbon quantum dots structurally mimicking large amino acids. Nature Biomed. Eng. 2020, 4, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Chowdhuri, A.R.; Singh, T.; Ghosh, S.K.; Sahu, S.K. Carbon Dots Embedded Magnetic Nanoparticles @Chitosan @Metal Organic Framework as a Nanoprobe for pH Sensitive Targeted Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 16573–16583. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, D.; Zhang, K.; Yang, M.; Sun, H.; Yang, B. One-step hydrothermal synthesis of nitrogen-doped conjugated carbonized polymer dots with 31% efficient red emission for in vivo imaging. Small 2018, 14, 1703919. [Google Scholar] [CrossRef]
- Lim, S.Y.; Shen, W.; Gao, Z.Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef]
- Ai, L.; Shi, R.; Yang, J.; Zhang, K.; Zhang, T.R.; Lu, S.Y. Efficient Combination of G-C3 N4 and CDs for Enhanced Photocatalytic Performance: A Review of Synthesis, Strategies, and Applications. Small 2021, 17, 2007523. [Google Scholar] [CrossRef]
- Shang, J.; He, D.; He, X. Label-Free Carbon-Dots-Based Ratiometric Fluorescence pH Nanoprobes for Intracellular pH Sensing. Anal. Chem. 2016, 88, 7837–7843. [Google Scholar] [CrossRef]
- Qu, S.; Zhou, D.; Li, D. Toward Efficient Orange Emissive Carbon Nanodots through Conjugated sp(2)-Domain Controlling and Surface Charges Engineering. Adv. Mater. 2016, 28, 3516–3521. [Google Scholar] [CrossRef]
- Chen, Y.J.; Liu, Z.E.; Yang, Q. Nitrogen-Doped Highly Photoluminescent Carbon Dots Derived from Citric Acid and Guanidine Carbonate. J. Nanosci. Nanotechnol. 2019, 19, 4058–4063. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Q. Sensitive pH probe developed with water-soluble fluorescent carbon dots from chocolate by one-step hydrothermal method. Int. J. Environ. Anal. Chem. 2017, 97, 1119–1131. [Google Scholar] [CrossRef]
- Shi, W.; Guo, F.; Han, M. N,S co-doped carbon dots as a stable bio-imaging probe for detection of intracellular temperature and tetracycline. J. Mater. Chem. B 2017, 5, 3293–3299. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kang, B.; Lee, J. Integrative Approach toward Uncovering the Origin of Photoluminescence in Dual Heteroatom-Doped Carbon Nanodots. Chem. Mater. 2016, 28, 6840–6847. [Google Scholar] [CrossRef]
- Lu, S.Y.; Sui, L.Z.; Liu, J.J.; Zhu, S.J.; Chen, A.M.; Jin, M.X.; Yang, B. Near-Infrared Photoluminescent Polymer-Carbon Nanodots with Two-Photon Fluorescence. Adv. Mater. 2017, 29, 1603443. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Wang, J.; Cao, Y. Facile access to B-doped solid-state fluorescent carbon dots toward light emitting devices and cell imaging agents. J. Mater. Chem. C 2015, 3, 6668–6675. [Google Scholar] [CrossRef]
- Han, B.Y.; Yu, M.B.; Pen, T.T.; Li, Y.; Hu, X.X.; Xiang, R.C.; Hou, X.F.; He, G.H. One-step extraction of highly fluorescent carbon quantum dots by a physical method from carbon black. New. J. Chem. 2017, 41, 5267–5270. [Google Scholar] [CrossRef]
- Xia, Z.; Singh, A.; Kiratitanavit, W. Unraveling the mechanism of thermal and thermo-oxidative degradation of tannic acid. Thermochim. Acta 2015, 605, 77–85. [Google Scholar] [CrossRef]
- Sebestyén, Z.; Jakab, E.; Badea, E. Thermal degradation study of vegetable tannins and vegetable tanned leathers. J. Anal. Appl. Pyrolysis 2019, 138, 178–187. [Google Scholar] [CrossRef]
- Sun, C.; Li, W.L.; Chen, X.J.; Li, C.X.; Tan, H.Y.; Zhang, Y.H. Synergistic interactions for saving energy and promoting the co-pyrolysis of polylactic acid and wood flour. Renew. Energ. 2021, 171, 254–265. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.R.; Zheng, Y.; Luo, Z.Y.; Cen, K.F. Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J. Anal. Appl. Pyrolysis 2008, 82, 170–177. [Google Scholar] [CrossRef]
- Jiang, K.; Sun, S.; Zhang, L. Red, green, and blue luminescence by carbon dots: Full-color emission tuning and multicolor cellular imaging. Angew. Chem. Int. Ed. 2015, 54, 5360–5363. [Google Scholar] [CrossRef] [PubMed]
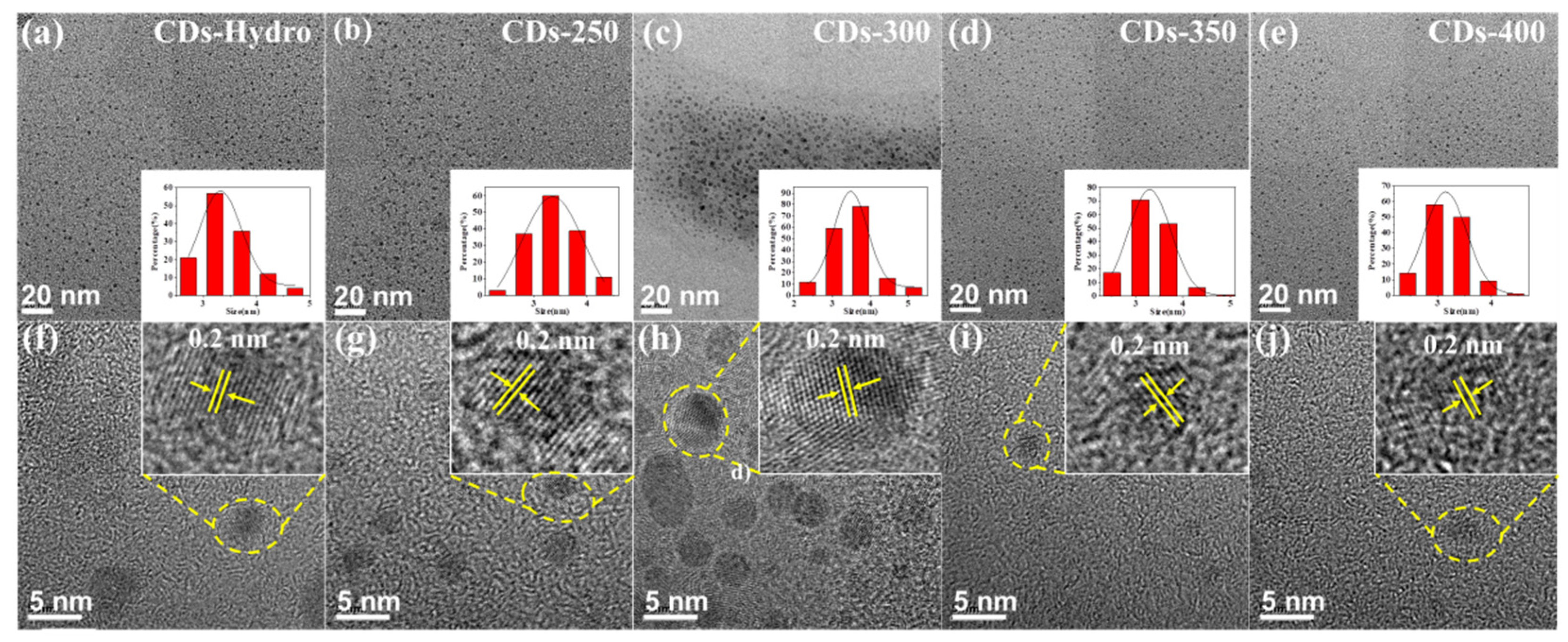
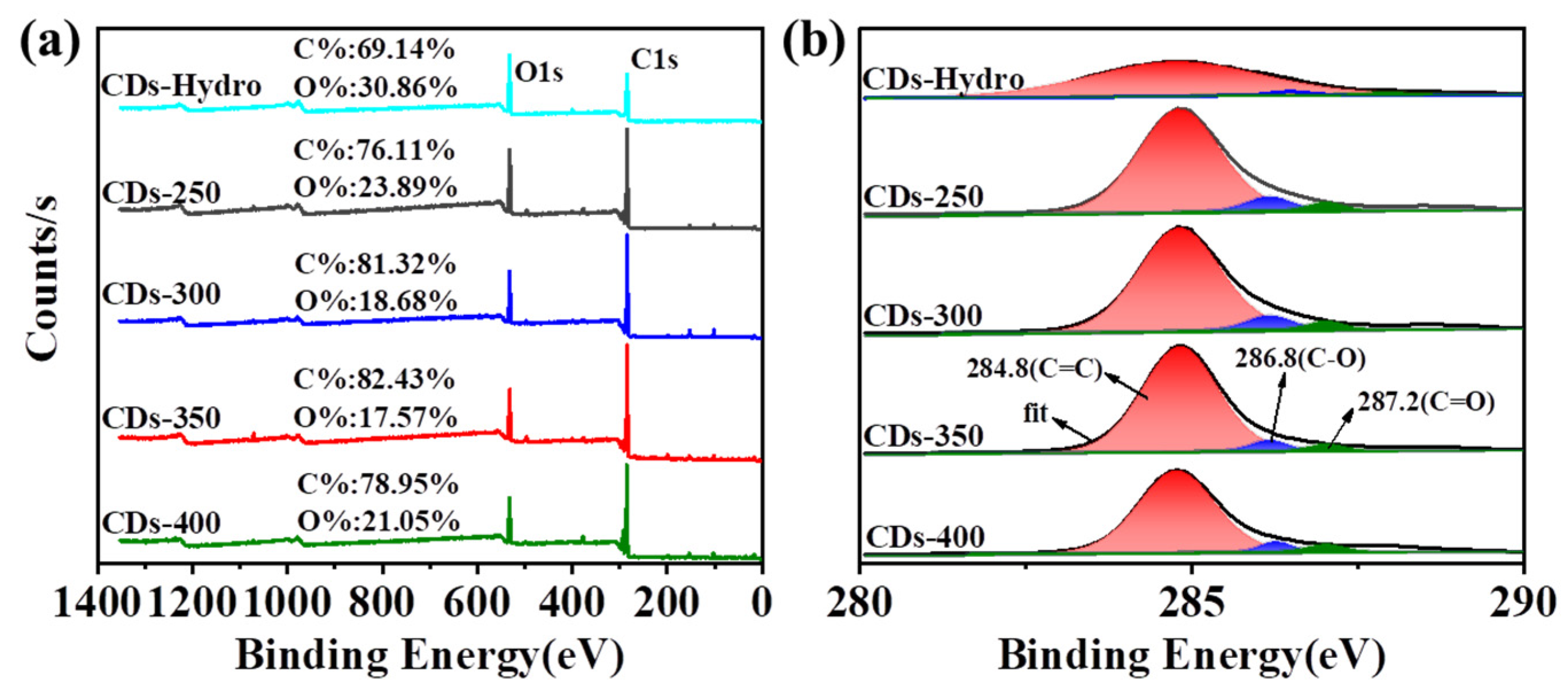

| C1s | O1s | C=C/C-C | C-O | C=O | |
|---|---|---|---|---|---|
| CDs-Hydro | 69.1% | 30.9% | 97.1% | 1.8% | 1.1% |
| CDs-250 | 76.1% | 23.9% | 86.2% | 9.8% | 4.0% |
| CDs-300 | 81.3% | 18.7% | 93.0% | 4.4% | 2.6% |
| CDs-350 | 82.4% | 17.6% | 91.5% | 4.6% | 3.9% |
| CDs-400 | 78.9% | 21.1% | 90.6% | 6.5% | 2.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, C.; Chen, M.; An, Y.; Zheng, Y.; Tian, H.; Shi, R.; He, X.; Lin, X. Solvent-Free Preparation of Tannic Acid Carbon Dots for Selective Detection of Ni2+ in the Environment. Int. J. Mol. Sci. 2022, 23, 6681. https://doi.org/10.3390/ijms23126681
Li Y, Liu C, Chen M, An Y, Zheng Y, Tian H, Shi R, He X, Lin X. Solvent-Free Preparation of Tannic Acid Carbon Dots for Selective Detection of Ni2+ in the Environment. International Journal of Molecular Sciences. 2022; 23(12):6681. https://doi.org/10.3390/ijms23126681
Chicago/Turabian StyleLi, Yan, Can Liu, Menglin Chen, Yulong An, Yunwu Zheng, Hao Tian, Rui Shi, Xiahong He, and Xu Lin. 2022. "Solvent-Free Preparation of Tannic Acid Carbon Dots for Selective Detection of Ni2+ in the Environment" International Journal of Molecular Sciences 23, no. 12: 6681. https://doi.org/10.3390/ijms23126681
APA StyleLi, Y., Liu, C., Chen, M., An, Y., Zheng, Y., Tian, H., Shi, R., He, X., & Lin, X. (2022). Solvent-Free Preparation of Tannic Acid Carbon Dots for Selective Detection of Ni2+ in the Environment. International Journal of Molecular Sciences, 23(12), 6681. https://doi.org/10.3390/ijms23126681






