MicroRNAs: Important Regulatory Molecules in Acute Lung Injury/Acute Respiratory Distress Syndrome
Abstract
:1. Introduction
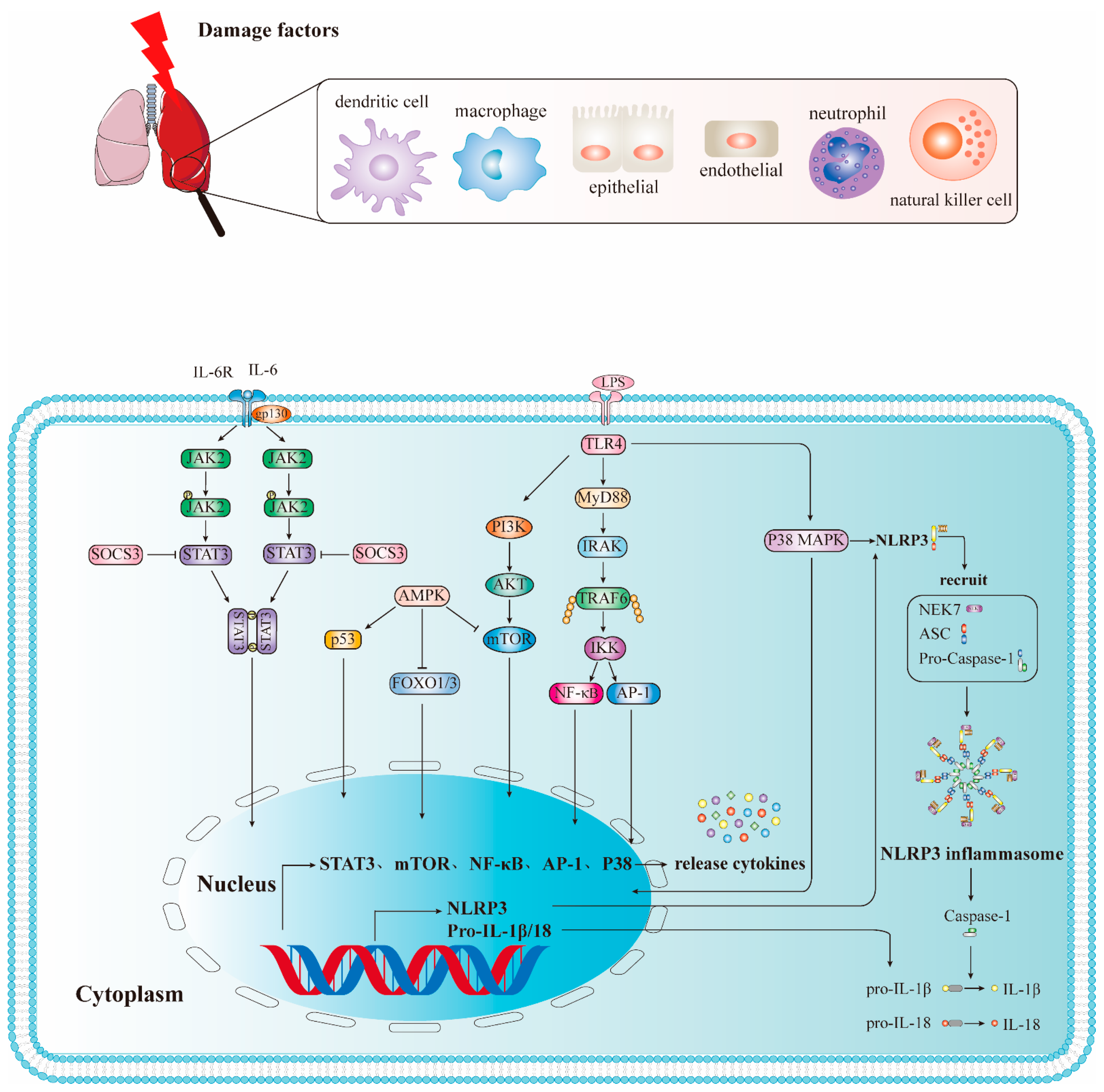
2. Mechanisms Leading to Tissue Damage in ALI/ARDS
2.1. PRRs and Related Molecules in ALI/ARDS
2.1.1. TLRs
2.1.2. NLRs
2.2. Inflammation-Related Pathways of ALI/ARDS
2.2.1. TLRs Mediate NF-κB Signaling Pathway
2.2.2. JAK2/STAT3 Signal Pathway
2.2.3. The Role and Signaling Pathway of the NLRP3 Inflammasome in ALI/ARDS
2.2.4. PI3K/AKT Signaling Pathway
2.2.5. p38 MAPΚ Signaling Pathway
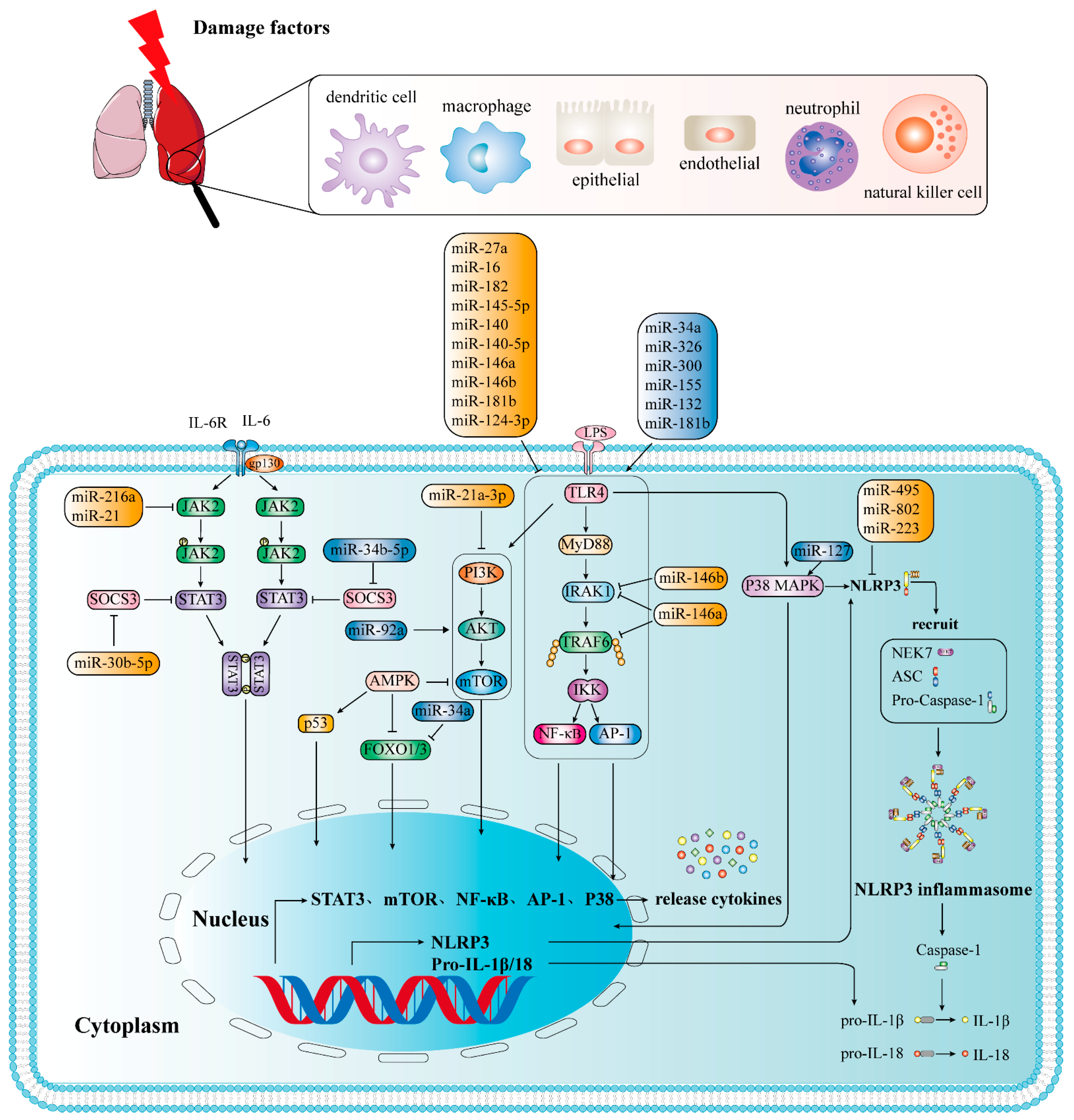
3. The Role of MicroRNAs in LPS-Induced ALI/ARDS
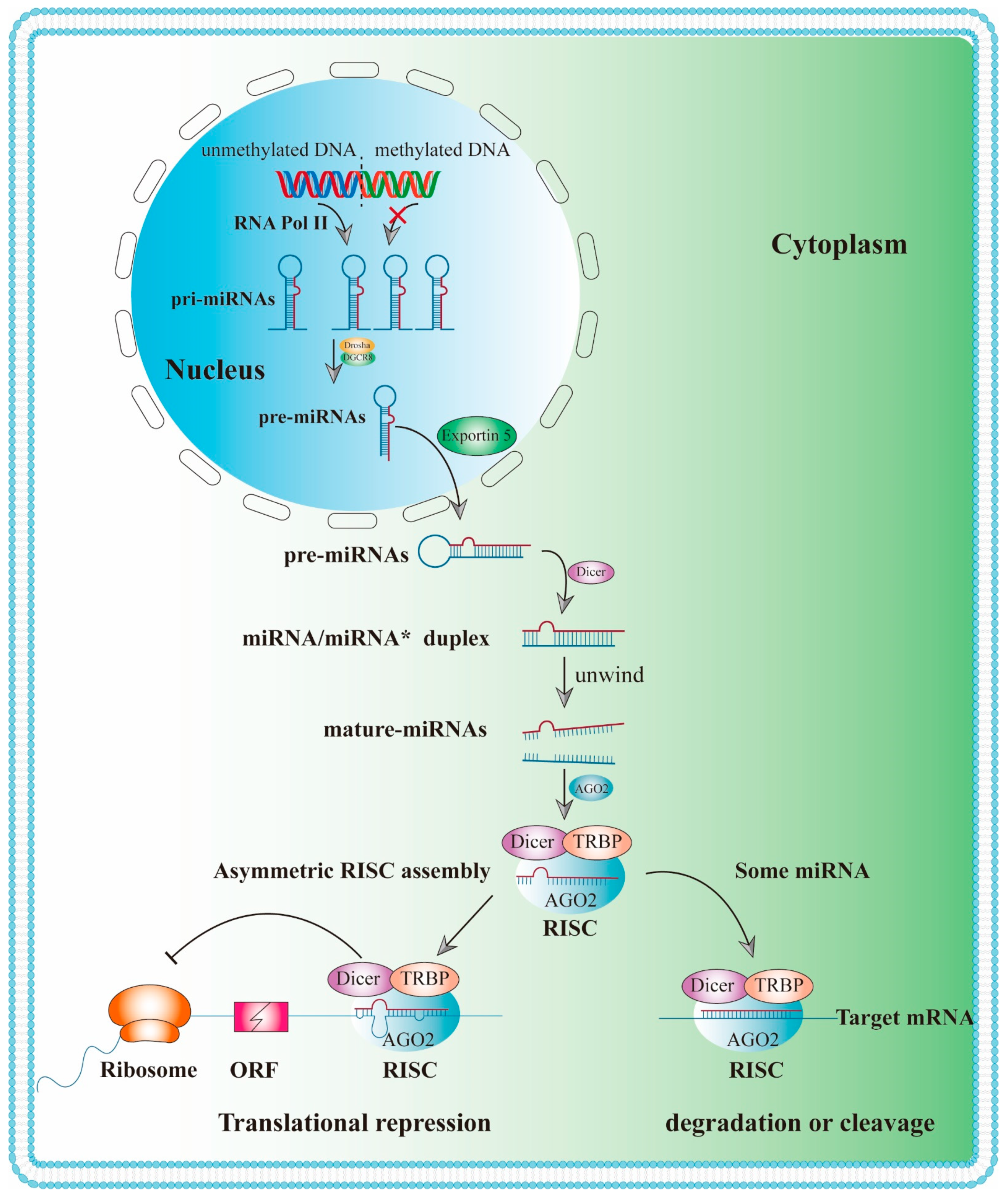
3.1. Biogenesis and Biological Function of MiRNAs
3.2. Adverse Inflammation-Related MiRNAs
3.3. Protective Inflammation-Associated MiRNAs
3.3.1. Targeting ALI/ARDS through NF-κB Signaling Pathway
3.3.2. Targeting ALI/ARDS through JAK2/STAT3 Signaling Pathway
3.3.3. Targeting ALI/ARDS by Regulating NLRP3 Signaling Pathway
3.3.4. Targeting ALI/ARDS through PI3Κ/AKT Signaling Pathway
| Type | MicroRNA | Damage Factors | Target | Expression | Function | Signaling Pathway | Reference |
|---|---|---|---|---|---|---|---|
| Adverse | miR-34a | LPS | FOXO3 | upregulation | Proinflammatory | NF-κB signaling pathway | [84] |
| miR-34a | hyperoxia | Ang1 | upregulation | Proinflammatory | NF-κB signaling pathway | [85] | |
| miR-326 | LPS | BCL2A1 | upregulation | Proinflammatory | NF-κB signaling pathway | [87] | |
| miR-300 | LPS | IκBα | upregulation | Proinflammatory | NF-κB signaling pathway | [88] | |
| miR-132 | SEB | FOXO3 | upregulation | Proinflammatory | NF-κB signaling pathway | [90] | |
| miR-155 | LPS | SOCS1 | upregulation | Proinflammatory | NF-κB signaling pathway | [93] | |
| miR-155 | LPS | C/EBPb and IL-13R | upregulation | Proinflammatory | regulates M1/M2 polarization | [94] | |
| miR-887-3p | LPS | upregulation | Proinflammatory | [96] | |||
| miR-34a-5p | LPS and hyperoxia | SIRT1 | upregulation | Proinflammatory | [121] | ||
| miR-1246 | LPS | ACE2 | upregulation | Proinflammatory | [122] | ||
| miR-92a | LPS | ITGA5 | upregulation | Proinflammatory | PI3Κ/AKT signaling pathway | [119] | |
| miR-34b-5p | LPS | PGRN | upregulation | Proinflammatory | [123] | ||
| miR-199a | LPS | SIRT1 | upregulation | Proinflammatory | [124] | ||
| miR-181b | LPS | P65 | upregulation | Proinflammatory | NF-κB signaling pathway | [108,109] | |
| miR-127 | LPS | BCL6 | upregulation | Proinflammatory | P38 MAPK signaling pathway | [94] | |
| Protective | miR-27a | LPS | TLR4 | downregulation | anti-inflammatory | NF-κB signaling pathway | [99,100] |
| miR-16 | LPS | TLR4 | downregulation | anti-inflammatory | NF-κB signaling pathway | [102] | |
| miR-182 | LPS | downregulation | anti-inflammatory | NF-κB signaling pathway | [103] | ||
| miR-145-5p | LPS | TLR4 | downregulation | anti-inflammatory | NF-κB signaling pathway | [125] | |
| miR-140 | LPS | TLR4 | downregulation | anti-inflammatory | NF-κB signaling pathway | [126] | |
| miR-140-5p | LPS | TLR4 | downregulation | anti-inflammatory | NF-κB signaling pathway | [127] | |
| miR-146a | LPS | IRAK1 TRAF6 | downregulation | anti-inflammatory | NF-κB signaling pathway | [104] | |
| miR-146b | LPS | IRAK1 | downregulation | anti-inflammatory | NF-κB signaling pathway | [105] | |
| miR-181b | LPS | importin-α3 | downregulation | anti-inflammatory | NF-κB signaling pathway | [107] | |
| miR-124-3p | LPS | p65 | downregulation | anti-inflammatory | NF-κB signaling pathway | [106] | |
| miR-21 | LPS | JAK2 | downregulation | anti-inflammatory | JAK2/STAT3 and NF-κB signaling pathways | [110] | |
| miR-216a | LPS | JAK2 | downregulation | anti-inflammatory | JAK2/STAT3 signal pathway | [111] | |
| miR-30b-5p | LPS | SOCS3 | downregulation | anti-inflammatory | JAK2/STAT3 signal pathway | [112] | |
| miR-127 | LPS | CD64 | downregulation | anti-inflammatory | STAT3 signaling pathway | [114] | |
| miR-495 | LPS | NLRP3 | downregulation | anti-inflammatory | NLRP3 signaling pathway | [73] | |
| miR-802 | LPS | Peli2 | downregulation | anti-inflammatory | NLRP3 signaling pathway | [117] | |
| miR-223 | LPS | NLRP3 | downregulation | anti-inflammatory | NLRP3 signaling pathway | [73,118] | |
| miR-21a-3p | LPS | downregulation | anti-inflammatory | PI3K (p110α)/Akt/mTOR pathway | [120] |
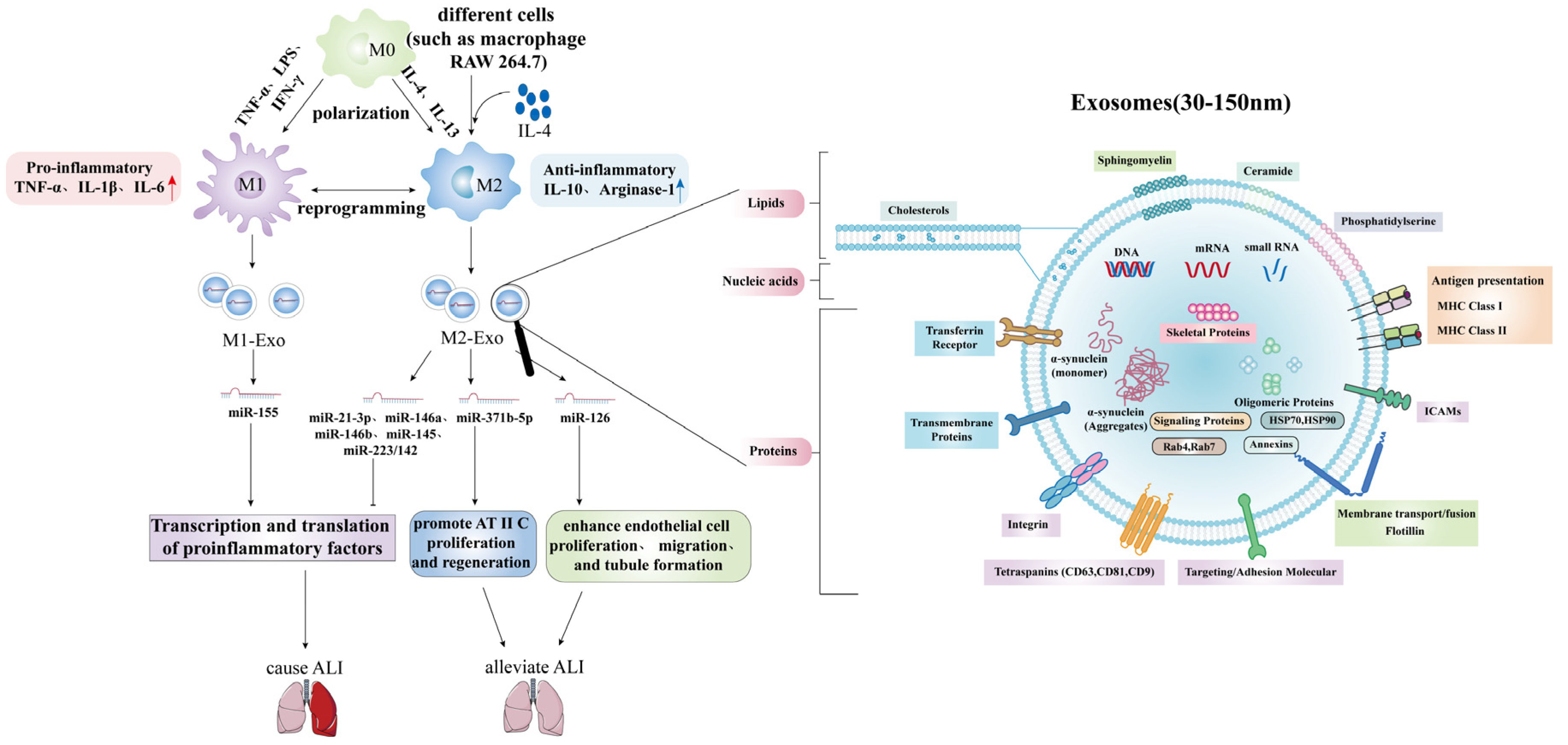
3.4. Acute Lung Injury/Acute Respiratory Distress Syndrome and Exosomal MicroRNAs
4. The Potential Role of MiRNAs in Clinical Treatment of ALI/ARDS
| Name (Company) | Therapeutic Agent | Target Diseases | Trial Details | Clinical Trials.Gov Identifier | Reference |
|---|---|---|---|---|---|
| Mirvirasen (Santaris Pharma A/S and Hoffmann-La Roche) | Anti-miR-122 | Hepatitis C (chronic infections included) | Phase III, completed | NCT01728324 | [78,135] |
| Phase III, completed | NCT01366638 | ||||
| Phase IV, completed | NCT01222611 | ||||
| Phase II, completed | NCT00996476 | ||||
| Phase III, completed | NCT01725529 | ||||
| Phase III, completed | NCT01290731 | ||||
| Phase III, completed | NCT01292239 | ||||
| Phase III, completed | NCT01288209 | ||||
| metastatic soft tissue sarcoma | Phase II, completed | NCT00413192 | [139] | ||
| Cobomarsen or MRG-106 (miRagen Therapeutics) | Anti-miR-155 | cutaneous T-lymphoma, Alibell’s disease, chronic lymphocytic leukemia and adult T-cell leukemia and lymphoma | Phase I, completed | NCT02580552 | [146] |
| CDR132L | miR-132 inhibitor | heart disease | Phase I, completed | NCT04045405 | [149] |
| MRX34 (Mirna Therapeutics) | miR-34 mimic | multiple solid tumours | Multicentre phase I, terminated | NCT01829971 | [141,142,143,144,145] |
| MesomiR-1 (EnGeneIC) | miR-16 mimic | mesothelioma, non-small cell lung cancer | Phase I, completed | NCT02369198 | [135] |
| Acute Lung Injury/Acute Respiratory Distress Syndrome (ARDS) | Recruiting | NCT03766204 |
5. Summary and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mokra, D.; Kosutova, P. Biomarkers in acute lung injury. Respir. Physiol. Neurobiol. 2015, 209, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.; Yan, S.G.; Li, J.T.; Wei, H.L.; Shi, J. Research progress in the treatment of acute lung injury by vasoactive intestinal peptide regulating alveolar macrophage M1/M2 polarization. Chin. J. Immunol. 2021, 1–9. Available online: http://kns.cnki.net/kcms/detail/22.1126.R.20210625.1333.006.html (accessed on 10 April 2022).
- Kumar, V. Pulmonary Innate Immune Response Determines the Outcome of Inflammation During Pneumonia and Sepsis-Associated Acute Lung Injury. Front. Immunol. 2020, 11, 1722. [Google Scholar] [CrossRef] [PubMed]
- Baradaran Rahimi, V.; Rakhshandeh, H.; Raucci, F.; Buono, B.; Shirazinia, R.; Samzadeh Kermani, A.; Maione, F.; Mascolo, N.; Askari, V.R. Anti-Inflammatory and Anti-Oxidant Activity of Portulaca oleracea Extract on LPS-Induced Rat Lung Injury. Molecules 2019, 24, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, S.J.; Bebarta, V.S.; Bonnett, C.J.; Pons, P.T.; Cantrill, S.V. Blast injuries. Lancet 2009, 374, 405–415. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Zhang, L.; Zhao, H. MicroRNA-155 Participates in Smoke-Inhalation-Induced Acute Lung Injury through Inhibition of SOCS-1. Molecules 2020, 25, 1022. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Huang, S.; Meng, X.; Zhang, J.; Yu, S.; Li, J.; Shi, M.; Fan, H.; Zhao, Y. Mechanism of Phosgene-Induced Acute Lung Injury and Treatment Strategy. Int. J. Mol. Sci. 2021, 22, 10933. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef]
- Agarwal, R.; Aggarwal, A.N.; Gupta, D.; Behera, D.; Jindal, S.K. Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in North India. Chest 2006, 130, 724–729. [Google Scholar] [CrossRef]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef]
- Shen, Y.; Song, J.; Wang, Y.; Chen, Z.; Zhang, L.; Yu, J.; Zhu, D.; Zhong, M. M2 macrophages promote pulmonary endothelial cells regeneration in sepsis-induced acute lung injury. Ann. Transl. Med. 2019, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Devaney, J.; Contreras, M.; Laffey, J.G. Clinical review: Gene-based therapies for ALI/ARDS: Where are we now? Crit. Care 2011, 15, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouimet, M.; Koster, S.; Sakowski, E.; Ramkhelawon, B.; van Solingen, C.; Oldebeken, S.; Karunakaran, D.; Portal-Celhay, C.; Sheedy, F.J.; Ray, T.D.; et al. Mycobacterium tuberculosis induces the miR-33 locus to reprogram autophagy and host lipid metabolism. Nat. Immunol. 2016, 17, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Lee, H.; Wang, X.; Groot, M.; Sharma, L.; Dela Cruz, C.S.; Jin, Y. A potential role of microvesicle-containing miR-223/142 in lung inflammation. Thorax 2019, 74, 865–874. [Google Scholar] [CrossRef]
- Elliot, S.; Periera-Simon, S.; Xia, X.; Catanuto, P.; Rubio, G.; Shahzeidi, S.; El Salem, F.; Shapiro, J.; Briegel, K.; Korach, K.S.; et al. MicroRNA let-7 Downregulates Ligand-Independent Estrogen Receptor-mediated Male-Predominant Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2019, 200, 1246–1257. [Google Scholar] [CrossRef]
- Ferragut Cardoso, A.P.; Udoh, K.T.; States, J.C. Arsenic-induced changes in miRNA expression in cancer and other diseases. Toxicol. Appl. Pharmacol. 2020, 409, 115306. [Google Scholar] [CrossRef]
- Hagen, J.W.; Lai, E.C. microRNA control of cell-cell signaling during development and disease. Cell Cycle 2008, 7, 2327–2332. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.R. MicroRNA-223 Attenuates Inflammation in Acute Lung Injury via the NLRP3 Inflammasome and TLR4/NF-κB Signaling Pathway. Ph.D. Thesis, Shandong University, Jinan, China, 2020. [Google Scholar]
- Talukdar, J.; Bhadra, B.; Dattaroy, T.; Nagle, V.; Dasgupta, S. Potential of natural astaxanthin in alleviating the risk of cytokine storm in COVID-19. Biomed. Pharmacother. 2020, 132, 110886. [Google Scholar] [CrossRef]
- Jiang, P.P.; Ye, N.; Xu, B.L. Expression and clinical significance of miR-150 in serum of patients with acute respiratory distress syndrome. J. Clin. Lab. Sci. 2021, 39, 202–205. [Google Scholar]
- Hao, J.X.; Xu, J.X.; Liang, Y.; Chen, Y.; Wu, T.S.; Xiao, C.Q. Evaluation value of miR-122 combined with APACHE II score in patients with ARDS. Chin. Crit. Care Emerg. Med. 2019, 31, 694–698. [Google Scholar]
- Ferruelo, A.; Peñuelas, Ó.; Lorente, J.A. MicroRNAs as biomarkers of acute lung injury. Ann. Transl. Med. 2018, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute Lung Injury: A Clinical and Molecular Review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Goulopoulou, S.; McCarthy, C.G.; Webb, R.C. Toll-like Receptors in the Vascular System: Sensing the Dangers Within. Pharmacol. Rev. 2016, 68, 142–167. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, M. Progress in Toll-like receptor signal transduction pathway and acute lung injury. Chin. J. Immunol. 2021, 37, 115–118. [Google Scholar]
- Smith, S.A.; Jann, O.C.; Haig, D.; Russell, G.C.; Werling, D.; Glass, E.J.; Emes, R.D. Adaptive evolution of Toll-like receptor 5 in domesticated mammals. BMC Evol. Biol. 2012, 12, 122. [Google Scholar] [CrossRef] [Green Version]
- Gong, Q.; He, L.; Wang, M.; Zuo, S.; Gao, H.; Feng, Y.; Du, L.; Luo, Y.; Li, J. Comparison of the TLR4/NF-κB and NLRP3 signalling pathways in major organs of the mouse after intravenous injection of lipopolysaccharide. Pharm. Biol. 2019, 57, 555–563. [Google Scholar] [CrossRef] [Green Version]
- Imai, Y.; Kuba, K.; Neely, G.G.; Yaghubian-Malhami, R.; Perkmann, T.; van Loo, G.; Ermolaeva, M.; Veldhuizen, R.; Leung, Y.H.; Wang, H.; et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008, 133, 235–249. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.D. Inflammasome activation and assembly at a glance. J. Cell Sci. 2017, 130, 3955–3963. [Google Scholar] [CrossRef] [Green Version]
- Sayan, M.; Mossman, B.T. The NLRP3 inflammasome in pathogenic particle and fibre-associated lung inflammation and diseases. Part. Fibre Toxicol. 2016, 13, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Wu, N.; Wang, Y.; Guo, F.; Chen, L.; Zhang, Z.; Jia, D.; Zhao, M. MyD88 gene knockout attenuates paraquat-induced acute lung injury. Toxicol. Lett. 2017, 269, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, J.L. MyD88 in Mycobacterium tuberculosis infection. Med. Microbiol. Immunol. 2017, 206, 187–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, R.; Xu, H.; Jiang, H.; Zhang, Y.; Sun, Y. The role of TLR4 in the pathogenesis of indirect acute lung injury. Front. Biosci. 2013, 18, 1244–1255. [Google Scholar]
- Li, T.T.; Ogino, S.; Qian, Z.R. Toll-like receptor signaling in colorectal cancer: Carcinogenesis to cancer therapy. World J. Gastroenterol. 2014, 20, 17699–17708. [Google Scholar] [CrossRef]
- Chen, O.; Tang, Z.H.; Hu, J.T. The role of Toll-like receptor 4 in sepsis-induced actute lung injury. Guangdong Med. 2019, 40, 1048–1052. [Google Scholar]
- Li, B.; Xi, P.; Wang, Z.; Han, X.; Xu, Y.; Zhang, Y.; Miao, J. PI3K/Akt/mTOR signaling pathway participates in Streptococcus uberis-induced inflammation in mammary epithelial cells in concert with the classical TLRs/NF-ĸB pathway. Vet. Microbiol. 2018, 227, 103–111. [Google Scholar] [CrossRef]
- Diao, Y.R.; Ding, Q.; Shi, Y.Y. Advances in the role of pattern recognition receptors PRRs in the pathogenesis of acute lung injury/acute respiratory distress syndrome. Chin. J. Immunol. 2022, 38, 227–233. [Google Scholar]
- Zhao, J.; Yu, H.; Liu, Y.; Gibson, S.A.; Yan, Z.; Xu, X.; Gaggar, A.; Li, P.K.; Li, C.; Wei, S.; et al. Protective effect of suppressing STAT3 activity in LPS-induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 311, L868–L880. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Ye, W.X.; Huang, Z.J.; Zhang, Q.; He, Y.F. Effect of IL-6-mediated STAT3 signaling pathway on myocardial apoptosis in mice with dilated cardiomyopathy. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3042–3050. [Google Scholar]
- Xu, S.; Pan, X.; Mao, L.; Pan, H.; Xu, W.; Hu, Y.; Yu, X.; Chen, Z.; Qian, S.; Ye, Y.; et al. Phospho-Tyr705 of STAT3 is a therapeutic target for sepsis through regulating inflammation and coagulation. Cell Commun. Signal. 2020, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Lamkanfi, M.; Dixit, V.M. Mechanisms and functions of inflammasomes. Cell 2014, 157, 1013–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tianzhu, Z.; Shihai, Y.; Juan, D. The effects of morin on lipopolysaccharide-induced acute lung injury by suppressing the lung NLRP3 inflammasome. Inflammation 2014, 37, 1976–1983. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Grailer, J.J.; Wang, N.; Wang, M.; Yao, J.; Zhong, R.; Gao, G.F.; Ward, P.A.; Tan, D.X.; et al. Melatonin alleviates acute lung injury through inhibiting the NLRP3 inflammasome. J. Pineal Res. 2016, 60, 405–414. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Liu, S.; Pan, P.; Su, X.; Tan, H.; Wu, D.; Zhang, L.; Song, C.; Dai, M.; et al. Pirfenidone ameliorates lipopolysaccharide-induced pulmonary inflammation and fibrosis by blocking NLRP3 inflammasome activation. Mol. Immunol. 2018, 99, 134–144. [Google Scholar] [CrossRef]
- Cao, H.; Feng, Y.; Ning, Y.; Zhang, Z.; Li, W.; Li, Q. Edaravone protects rats and human pulmonary alveolar epithelial cells against hyperoxia injury: Heme oxygenase-1 and PI3K/Akt pathway may be involved. Exp. Lung Res. 2015, 41, 404–414. [Google Scholar] [CrossRef]
- Chen, H.; Li, N.; Zhan, X.; Zheng, T.; Huang, X.; Chen, Q.; Song, Z.; Yang, F.; Nie, H.; Zhang, Y.; et al. Capsaicin Protects Against Lipopolysaccharide-Induced Acute Lung Injury Through the HMGB1/NF-κB and PI3K/AKT/mTOR Pathways. J. Inflamm. Res. 2021, 14, 5291–5304. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wang, H.C.; Hsu, Y.C.; Cho, C.L.; Yang, M.Y.; Chien, C.Y. Capsaicin Induces Autophagy and Apoptosis in Human Nasopharyngeal Carcinoma Cells by Downregulating the PI3K/AKT/mTOR Pathway. Int. J. Mol. Sci. 2017, 18, 1343. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Li, L.; Lu, S.; Li, K.; Su, Z.; Wang, Y.; Fan, X.; Li, X.; Zhao, G. The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB and PI3K/Akt/mTOR pathways. Mol. Immunol. 2018, 94, 7–17. [Google Scholar] [CrossRef]
- Huang, C.Y.; Deng, J.S.; Huang, W.C.; Jiang, W.P.; Huang, G.J. Attenuation of Lipopolysaccharide-Induced Acute Lung Injury by Hispolon in Mice, Through Regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 Pathways, and Suppressing Oxidative Stress-Mediated ER Stress-Induced Apoptosis and Autophagy. Nutrients 2020, 12, 1742. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, C.; Wang, R.; Li, J.; Zhou, M.; Yan, M.; Xue, X.; Wang, C. Cardioprotective effect of paeonol against epirubicin-induced heart injury via regulating miR-1 and PI3K/AKT pathway. Chem.-Biol. Interact. 2018, 286, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.; Chen, C.; He, W.; Chen, Y.; Li, Y.; Wen, Y.; Zhou, S.; Jiang, Y.; Yang, X.; Zhang, R.; et al. Glycyrrhizic acid ameliorates LPS-induced acute lung injury by regulating autophagy through the PI3K/AKT/mTOR pathway. Am. J. Transl. Res. 2019, 11, 2042–2055. [Google Scholar] [PubMed]
- Deng, W.; Wang, D.X.; Deng, J.; Zhu, T. PI3K/Akt signaling pathway up-regulates the expression of sodium channels in alveolar epithelium of rats with acute lung injury. Basic Med. Clin. Pract. 2012, 32, 1004–1008. [Google Scholar]
- Zhang, P.Y.; Chen, M. Research progress on the role of PI3K/AKT signaling pathway in the occurrence and development of acute lung injury. Shandong Med. 2016, 56, 99–101. [Google Scholar]
- Deng, W.; Li, C.Y.; Tong, J.; Zhang, W.; Wang, D.X. Regulation of ENaC-mediated alveolar fluid clearance by insulin via PI3K/Akt pathway in LPS-induced acute lung injury. Respir. Res. 2012, 13, 29. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Ren, W.; Jiang, Z.; Zhu, L. Regulation of the NLRP3 inflammasome and macrophage pyroptosis by the p38 MAPK signaling pathway in a mouse model of acute lung injury. Mol. Med. Rep. 2018, 18, 4399–4409. [Google Scholar] [CrossRef] [Green Version]
- Xing, J.; Yu, Z.; Zhang, X.; Li, W.; Gao, D.; Wang, J.; Ma, X.; Nie, X.; Wang, W. Epicatechin alleviates inflammation in lipopolysaccharide-induced acute lung injury in mice by inhibiting the p38 MAPK signaling pathway. Int. Immunopharmacol. 2019, 66, 146–153. [Google Scholar] [CrossRef]
- Jiao, C.; Xiang, X.; Jianqin, C.; Ling, J.; Baodi, S.; Wei, Z. Protective effect of taurine on sepsis-induced lung injury via inhibiting the p38/MAPK signaling pathway. Mol. Med. Rep. 2021, 24, 1–10. [Google Scholar]
- Du, T.; Zamore, P.D. Beginning to understand microRNA function. Cell Res. 2007, 17, 661–663. [Google Scholar] [CrossRef]
- Denli, A.M.; Tops, B.B.; Plasterk, R.H.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lee, Y.; Yeom, K.H.; Kim, Y.K.; Jin, H.; Kim, V.N. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004, 18, 3016–3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gon, Y.; Shimizu, T.; Mizumura, K.; Maruoka, S.; Hikichi, M. Molecular techniques for respiratory diseases: MicroRNA and extracellular vesicles. Respirology 2020, 25, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Murchison, E.P.; Hannon, G.J. miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr. Opin. Cell Biol. 2004, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Chendrimada, T.P.; Gregory, R.I.; Kumaraswamy, E.; Norman, J.; Cooch, N.; Nishikura, K.; Shiekhattar, R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature 2005, 436, 740–744. [Google Scholar] [CrossRef]
- Lee, H.Y.; Doudna, J.A. TRBP alters human precursor microRNA processing in vitro. RNA 2012, 18, 2012–2019. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.; Jin, H.; Qian, Q. Argonaute 2: A Novel Rising Star in Cancer Research. J. Cancer 2015, 6, 877–882. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Berg, N.; Lee, J.W.; Le, T.T.; Neudecker, V.; Jing, N.; Eltzschig, H. MicroRNA miR-223 as regulator of innate immunity. J. Leukoc. Biol. 2018, 104, 515–524. [Google Scholar] [CrossRef]
- Haneklaus, M.; Gerlic, M.; O’Neill, L.A.; Masters, S.L. miR-223: Infection, inflammation and cancer. J. Intern. Med. 2013, 274, 215–226. [Google Scholar] [CrossRef]
- Liu, W.; Guo, W.J.; Xu, Q.; Sun, Q. Advances in mechanisms for NLRP3 inflammasomes regulation. Acta Pharm. Sin. 2016, 51, 1505–1512. [Google Scholar]
- Ying, Y.; Mao, Y.; Yao, M. NLRP3 Inflammasome Activation by MicroRNA-495 Promoter Methylation May Contribute to the Progression of Acute Lung Injury. Mol. Ther. Nucleic Acids 2019, 18, 801–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschos, S.A.; Williams, A.E.; Perry, M.M.; Birrell, M.A.; Belvisi, M.G.; Lindsay, M.A. Expression profiling in vivo demonstrates rapid changes in lung microRNA levels following lipopolysaccharide-induced inflammation but not in the anti-inflammatory action of glucocorticoids. BMC Genom. 2007, 8, 240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartel, D.P.; Chen, C.Z. Micromanagers of gene expression: The potentially widespread influence of metazoan microRNAs. Nat. Rev. Genet. 2004, 5, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Doench, J.G.; Sharp, P.A. Specificity of microRNA target selection in translational repression. Genes Dev. 2004, 18, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staszel, T.; Zapała, B.; Polus, A.; Sadakierska-Chudy, A.; Kieć-Wilk, B.; Stępień, E.; Wybrańska, I.; Chojnacka, M.; Dembińska-Kieć, A. Role of microRNAs in endothelial cell pathophysiology. Pol. Arch. Med. Wewn. 2011, 121, 361–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bandiera, S.; Pfeffer, S.; Baumert, T.F.; Zeisel, M.B. miR-122--a key factor and therapeutic target in liver disease. J. Hepatol. 2015, 62, 448–457. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.M.; Ren, G.Q. Research progress of miRNA as biomarkers for sepsis and its complications. Med. Recapitul. 2021, 19, 3796–7801. [Google Scholar]
- Alieva, A.; Filatova, E.V.; Karabanov, A.V.; Illarioshkin, S.N.; Limborska, S.A.; Shadrina, M.I.; Slominsky, P.A. miRNA expression is highly sensitive to a drug therapy in Parkinson’s disease. Parkinsonism Relat. Disord. 2015, 21, 72–74. [Google Scholar] [CrossRef]
- Zhu, Q.L.; Zhan, D.M.; Chong, Y.K.; Ding, L.; Yang, Y.G. MiR-652-3p promotes bladder cancer migration and invasion by targeting KCNN3. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8806–8812. [Google Scholar]
- Li, J.J.; Wang, B.; Kodali, M.C.; Chen, C.; Kim, E.; Patters, B.J.; Lan, L.; Kumar, S.; Wang, X.; Yue, J.; et al. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J. Neuroinflamm. 2018, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Poon, K.S.; Palanisamy, K.; Chang, S.S.; Sun, K.T.; Chen, K.B.; Li, P.C.; Lin, T.C.; Li, C.Y. Plasma exosomal miR-223 expression regulates inflammatory responses during cardiac surgery with cardiopulmonary bypass. Sci. Rep. 2017, 7, 10807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Zhou, F.; Cheng, L.; Hu, M.; He, Y.; Zhang, B.; Liao, D.; Xu, Z. MicroRNA-34a Suppresses Autophagy in Alveolar Type II Epithelial Cells in Acute Lung Injury by Inhibiting FoxO3 Expression. Inflammation 2017, 40, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.; Das, P.; Pawar, A.; Aghai, Z.H.; Kaskinen, A.; Zhuang, Z.W.; Ambalavanan, N.; Pryhuber, G.; Andersson, S.; Bhandari, V. Hyperoxia causes miR-34a-mediated injury via angiopoietin-1 in neonatal lungs. Nat. Commun. 2017, 8, 1173. [Google Scholar] [CrossRef]
- Jin, X.H.; Shi, X.; Lv, X. Research progress on roles of microRNA in acute lung injury. J. Tongji Univ. 2021, 42, 554–561. [Google Scholar]
- Wu, C.T.; Huang, Y.; Pei, Z.Y.; Xi, X.; Zhu, G.F. MicroRNA-326 aggravates acute lung injury in septic shock by mediating the NF-κB signaling pathway. Int. J. Biochem. Cell Biol. 2018, 101, 1–11. [Google Scholar] [CrossRef]
- Cao, W.; Dai, H.; Yang, S.; Liu, Z.; Yi Chen, Q. Increased serum miR-300 level serves as a potential biomarker of lipopolysaccharide-induced lung injury by targeting IκBα. Die Pharm. 2017, 72, 5–9. [Google Scholar]
- Rao, R.; Nagarkatti, P.; Nagarkatti, M. Role of miRNA in the regulation of inflammatory genes in staphylococcal enterotoxin B-induced acute inflammatory lung injury and mortality. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 144, 284–297. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Hron, J.D.; Peng, S.L. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity 2004, 21, 203–213. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, L.A.; Sheedy, F.J.; McCoy, C.E. MicroRNAs: The fine-tuners of Toll-like receptor signalling. Nat. Rev. Immunol. 2011, 11, 163–175. [Google Scholar] [CrossRef]
- O’Connell, R.M.; Taganov, K.D.; Boldin, M.P.; Cheng, G.; Baltimore, D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. USA 2007, 104, 1604–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, K.; Yang, J.; Guo, S.; Zhao, G.; Wu, H.; Deng, G. Peripheral Circulating Exosome-Mediated Delivery of miR-155 as a Novel Mechanism for Acute Lung Inflammation. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 1758–1771. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Kang, Y.; Zhang, H.; Zhao, D.; Xia, J.; Lu, Z.; Wang, H.; Xu, F.; Shi, L. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J. Immunol. 2015, 194, 1239–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Wen, Z.; Qin, A.; Zhou, Y.; Liao, Z.; Liu, Z.; Liang, Y.; Ren, T.; Xu, L. Antisense oligonucleotide treatment enhances the recovery of acute lung injury through IL-10-secreting M2-like macrophage-induced expansion of CD4+ regulatory T cells. J. Immunol. 2013, 190, 4337–4348. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, A.J.; Li, P.; Halushka, P.V.; Cook, J.A.; Sumal, A.S.; Fan, H. Circulating miRNA 887 is differentially expressed in ARDS and modulates endothelial function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L1261–L1269. [Google Scholar] [CrossRef]
- Ke, X.F.; Fang, J.; Wu, X.N.; Yu, C.H. MicroRNA-203 accelerates apoptosis in LPS-stimulated alveolar epithelial cells by targeting PIK3CA. Biochem. Biophys. Res. Commun. 2014, 450, 1297–1303. [Google Scholar] [CrossRef]
- Janku, F.; Hong, D.S.; Fu, S.; Piha-Paul, S.A.; Naing, A.; Falchook, G.S.; Tsimberidou, A.M.; Stepanek, V.M.; Moulder, S.L.; Lee, J.J.; et al. Assessing PIK3CA and PTEN in early-phase trials with PI3K/AKT/mTOR inhibitors. Cell Rep. 2014, 6, 377–387. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Qiu, X.; Liu, J.; Han, Y.; Wei, D.; Ji, G.; Jiang, H. miR-27a protects against acute lung injury in LPS-treated mice by inhibiting NF-κB-mediated inflammatory response. Int. J. Clin. Exp. Pathol. 2018, 11, 2980–2989. [Google Scholar]
- Liu, Q.; Zhang, W.K. Advances in the mechanism of microRNAs regulating acute lung injury in sepsis. Clin. Med. China 2021, 37, 280–284. [Google Scholar]
- Ju, M.; Liu, B.; He, H.; Gu, Z.; Liu, Y.; Su, Y.; Zhu, D.; Cang, J.; Luo, Z. MicroRNA-27a alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis through modulating TLR4/MyD88/NF-κB pathway. Cell Cycle 2018, 17, 2001–2018. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yang, F.; Yu, X.; Wang, B.; Yang, Y.; Zhou, X.; Cheng, R.; Xia, S.; Zhou, X. miR-16 inhibits NLRP3 inflammasome activation by directly targeting TLR4 in acute lung injury. Biomed. Pharmacother. 2019, 112, 108664. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Y.; Jiang, K.; Zhao, G.; Guo, S.; Liu, J.; Yang, Y.; Deng, G. MicroRNA-182 supplies negative feedback regulation to ameliorate lipopolysaccharide-induced ALI in mice by targeting TLR4. J. Cell. Physiol. 2020, 235, 5925–5937. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.; Gong, H.; Li, Y.; Jie, K.; Ding, C.; Shao, Q.; Liu, F.; Zhan, Y.; Nie, C.; Zhu, W.; et al. Upregulation of miR-146a contributes to the suppression of inflammatory responses in LPS-induced acute lung injury. Exp. Lung Res. 2013, 39, 275–282. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Li, Y.; Zhou, L.; Su, X.; Li, Y.; Pan, P.; Hu, C. miR-146b overexpression ameliorates lipopolysaccharide-induced acute lung injury in vivo and in vitro. J. Cell. Biochem. 2019, 120, 2929–2939. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xie, J.; Che, D.; Zhang, C.; Lin, Y.; Feng, L.; Chen, J.; Chen, J.; Chen, L.; Wu, Z. MiR-124-3p helps to protect against acute respiratory distress syndrome by targeting p65. Biosci. Rep. 2020, 40, BSR20192132. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Icli, B.; Wara, A.K.; Belkin, N.; He, S.; Kobzik, L.; Hunninghake, G.M.; Vera, M.P.; Blackwell, T.S.; Baron, R.M.; et al. MicroRNA-181b regulates NF-κB-mediated vascular inflammation. J. Clin. Investig. 2012, 122, 1973–1990. [Google Scholar] [CrossRef]
- Wang, Y.; Mao, G.; Lv, Y.; Huang, Q.; Wang, G. MicroRNA-181b stimulates inflammation via the nuclear factor-κB signaling pathway in vitro. Exp. Ther. Med. 2015, 10, 1584–1590. [Google Scholar] [CrossRef] [Green Version]
- Hayden, M.S.; Ghosh, S. NF-κB, the first quarter-century: Remarkable progress and outstanding questions. Genes Dev. 2012, 26, 203–234. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Lu, Z.; Huo, C.; Chen, Y.; Cao, H.; Xie, P.; Zhou, H.; Liu, D.; Liu, J.; Yu, L. Liang-Ge-San, a Classic Traditional Chinese Medicine Formula, Attenuates Lipopolysaccharide-Induced Acute Lung Injury Through Up-Regulating miR-21. Front. Pharmacol. 2019, 10, 1332. [Google Scholar] [CrossRef]
- Kong, F.; Sun, Y.; Song, W.; Zhou, Y.; Zhu, S. MiR-216a alleviates LPS-induced acute lung injury via regulating JAK2/STAT3 and NF-κB signaling. Hum. Cell 2020, 33, 67–78. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, Y.L. The Functional Mechanisms of miR-30b-5p in Acute Lung Injury in Children. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Chaves de Souza, J.A.; Nogueira, A.V.; Chaves de Souza, P.P.; Kim, Y.J.; Silva Lobo, C.; Pimentel Lopes de Oliveira, G.J.; Cirelli, J.A.; Garlet, G.P.; Rossa, C., Jr. SOCS3 expression correlates with severity of inflammation, expression of proinflammatory cytokines, and activation of STAT3 and p38 MAPK in LPS-induced inflammation in vivo. Mediat. Inflamm. 2013, 2013, 650812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, T.; Liang, J.; Liu, N.; Wang, Q.; Li, Y.; Noble, P.W.; Jiang, D. MicroRNA-127 inhibits lung inflammation by targeting IgG Fcγ receptor I. J. Immunol. 2012, 188, 2437–2444. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Robertson, A.A.; Chae, J.J.; Higgins, S.C.; Muñoz-Planillo, R.; Inserra, M.C.; Vetter, I.; Dungan, L.S.; Monks, B.G.; Stutz, A.; et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015, 21, 248–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, Q.; Wang, J.; Jia, D.; Jiang, L.; Chang, Y.; Li, W. MiR-802 alleviates lipopolysaccharide-induced acute lung injury by targeting Peli2. Inflamm. Res. 2020, 69, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Humphries, F.; Bergin, R.; Jackson, R.; Delagic, N.; Wang, B.; Yang, S.; Dubois, A.V.; Ingram, R.J.; Moynagh, P.N. The E3 ubiquitin ligase Pellino2 mediates priming of the NLRP3 inflammasome. Nat. Commun. 2018, 9, 1560. [Google Scholar] [CrossRef]
- Haneklaus, M.; Gerlic, M.; Kurowska-Stolarska, M.; Rainey, A.A.; Pich, D.; McInnes, I.B.; Hammerschmidt, W.; O’Neill, L.A.; Masters, S.L. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1β production. J. Immunol. 2012, 189, 3795–3799. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Zhou, F. Inhibition of microRNA-92a ameliorates lipopolysaccharide-induced endothelial barrier dysfunction by targeting ITGA5 through the PI3K/Akt signaling pathway in human pulmonary microvascular endothelial cells. Int. Immunopharmacol. 2020, 78, 106060. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Y.; Liang, T.; Hu, Y.; Tang, H.; Song, D.; Fang, H. The regulatory effect of microRNA-21a-3p on the promotion of telocyte angiogenesis mediated by PI3K (p110α)/AKT/mTOR in LPS induced mice ARDS. J. Transl. Med. 2019, 17, 427. [Google Scholar] [CrossRef] [Green Version]
- Shah, D.; Das, P.; Alam, M.A.; Mahajan, N.; Romero, F.; Shahid, M.; Singh, H.; Bhandari, V. MicroRNA-34a Promotes Endothelial Dysfunction and Mitochondrial-mediated Apoptosis in Murine Models of Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2019, 60, 465–477. [Google Scholar] [CrossRef]
- Fang, Y.; Gao, F.; Hao, J.; Liu, Z. microRNA-1246 mediates lipopolysaccharide-induced pulmonary endothelial cell apoptosis and acute lung injury by targeting angiotensin-converting enzyme 2. Am. J. Transl. Res. 2017, 9, 1287–1296. [Google Scholar] [PubMed]
- Xie, W.; Lu, Q.; Wang, K.; Lu, J.; Gu, X.; Zhu, D.; Liu, F.; Guo, Z. miR-34b-5p inhibition attenuates lung inflammation and apoptosis in an LPS-induced acute lung injury mouse model by targeting progranulin. J. Cell. Physiol. 2018, 233, 6615–6631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Guan, H.; Zhang, J.L.; Zheng, Z.; Wang, H.T.; Tao, K.; Han, S.C.; Su, L.L.; Hu, D. Acute downregulation of miR-199a attenuates sepsis-induced acute lung injury by targeting SIRT1. Am. J. Physiol. Cell Physiol. 2018, 314, C449–C455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.L.; Yu, G.; Ding, Z.Y.; Li, S.J.; Fang, Q.Z. Overexpression of miR-145-5p alleviated LPS-induced acute lung injury. J. Biol. Regul. Homeost. Agents 2019, 33, 1063–1072. [Google Scholar]
- Li, X.; Wang, J.; Wu, H.; Guo, P.; Wang, C.; Wang, Y.; Zhang, Z. Reduced peripheral blood miR-140 may be a biomarker for acute lung injury by targeting Toll-like receptor 4 (TLR4). Exp. Ther. Med. 2018, 16, 3632–3638. [Google Scholar]
- Yang, Y.; Liu, D.; Xi, Y.; Li, J.; Liu, B.; Li, J. Upregulation of miRNA-140-5p inhibits inflammatory cytokines in acute lung injury through the MyD88/NF-κB signaling pathway by targeting TLR4. Exp. Ther. Med. 2018, 16, 3913–3920. [Google Scholar] [CrossRef]
- McDonald, M.K.; Tian, Y.; Qureshi, R.A.; Gormley, M.; Ertel, A.; Gao, R.; Aradillas Lopez, E.; Alexander, G.M.; Sacan, A.; Fortina, P.; et al. Functional significance of macrophage-derived exosomes in inflammation and pain. Pain 2014, 155, 1527–1539. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Zhang, C.; Zhang, X.; Chen, Y.; Zhang, H. MiR-145 negatively regulates TGFBR2 signaling responsible for sepsis-induced acute lung injury. Biomed. Pharmacother. 2019, 111, 852–858. [Google Scholar] [CrossRef]
- Yi, X.; Wei, X.; Lv, H.; An, Y.; Li, L.; Lu, P.; Yang, Y.; Zhang, Q.; Yi, H.; Chen, G. Exosomes derived from microRNA-30b-3p-overexpressing mesenchymal stem cells protect against lipopolysaccharide-induced acute lung injury by inhibiting SAA3. Exp. Cell Res. 2019, 383, 111454. [Google Scholar] [CrossRef]
- Quan, Y.; Wang, Z.; Gong, L.; Peng, X.; Richard, M.A.; Zhang, J.; Fornage, M.; Alcorn, J.L.; Wang, D. Exosome miR-371b-5p promotes proliferation of lung alveolar progenitor type II cells by using PTEN to orchestrate the PI3K/Akt signaling. Stem Cell Res. Ther. 2017, 8, 138. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; Hu, L.; Gu, W.; Zhu, L. Exosomes derived from endothelial progenitor cells ameliorate acute lung injury by transferring miR-126. Exp. Cell Res. 2018, 370, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, P.; Goodwin, A.J.; Cook, J.A.; Halushka, P.V.; Chang, E.; Zingarelli, B.; Fan, H. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Crit. Care 2019, 23, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, C.; Li, H.; Bao, M.; Zhuo, R.; Jiang, G.; Wang, W. Alveolar macrophage-derived exosomes modulate severity and outcome of acute lung injury. Aging 2020, 12, 6120–6128. [Google Scholar] [CrossRef] [PubMed]
- Rupaimoole, R.; Slack, F.J. MicroRNA therapeutics: Towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017, 16, 203–222. [Google Scholar] [CrossRef] [PubMed]
- He, C.Z.; Song, J.L.; Hu, S.D.; Teng, D.; Du, X.H. Advances in clinical trials of miRNAs for tumor diagnosis and treatment. J. People’s Lib. Army Med. Coll. 2020, 41, 1265–1269. [Google Scholar]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.X.; Song, W.; Chen, Z.H.; Wei, J.H.; Liao, Y.J.; Lei, J.; Hu, M.; Chen, G.Z.; Liao, B.; Lu, J.; et al. Prognostic and predictive value of a microRNA signature in stage II colon cancer: A microRNA expression analysis. Lancet. Oncol. 2013, 14, 1295–1306. [Google Scholar] [CrossRef]
- Wiemer, E.A.C.; Wozniak, A.; Burger, H.; Smid, M.; Floris, G.; Nzokirantevye, A.; Sciot, R.; Sleijfer, S.; Schöffski, P. Identification of microRNA biomarkers for response of advanced soft tissue sarcomas to eribulin: Translational results of the EORTC 62052 trial. Eur. J. Cancer 2017, 75, 33–40. [Google Scholar] [CrossRef]
- van der Ree, M.H.; de Vree, J.M.; Stelma, F.; Willemse, S.; van der Valk, M.; Rietdijk, S.; Molenkamp, R.; Schinkel, J.; van Nuenen, A.C.; Beuers, U.; et al. Safety, tolerability, and antiviral effect of RG-101 in patients with chronic hepatitis C: A phase 1B, double-blind, randomised controlled trial. Lancet 2017, 389, 709–717. [Google Scholar] [CrossRef]
- Bader, A.G. miR-34—A microRNA replacement therapy is headed to the clinic. Front. Genet. 2012, 3, 120. [Google Scholar] [CrossRef] [Green Version]
- Beg, M.S.; Brenner, A.J.; Sachdev, J.; Borad, M.; Kang, Y.K.; Stoudemire, J.; Smith, S.; Bader, A.G.; Kim, S.; Hong, D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig. New Drugs 2017, 35, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Misso, G.; Di Martino, M.T.; De Rosa, G.; Farooqi, A.A.; Lombardi, A.; Campani, V.; Zarone, M.R.; Gullà, A.; Tagliaferri, P.; Tassone, P.; et al. Mir-34: A new weapon against cancer? Mol. Ther. Nucleic Acids 2014, 3, e194. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.A.; Ivan, C.; Valdecanas, D.; Wang, X.; Peltier, H.J.; Ye, Y.; Araujo, L.; Carbone, D.P.; Shilo, K.; Giri, D.K.; et al. PDL1 Regulation by p53 via miR-34. J. Natl. Cancer Inst. 2016, 108, djv303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahlhut, C.; Slack, F.J. Combinatorial Action of MicroRNAs let-7 and miR-34 Effectively Synergizes with Erlotinib to Suppress Non-small Cell Lung Cancer Cell Proliferation. Cell Cycle 2015, 14, 2171–2180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seto, A.G.; Beatty, X.; Lynch, J.M.; Hermreck, M.; Tetzlaff, M.; Duvic, M.; Jackson, A.L. Cobomarsen, an oligonucleotide inhibitor of miR-155, co-ordinately regulates multiple survival pathways to reduce cellular proliferation and survival in cutaneous T-cell lymphoma. Br. J. Haematol. 2018, 183, 428–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayner, K.J.; Esau, C.C.; Hussain, F.N.; McDaniel, A.L.; Marshall, S.M.; van Gils, J.M.; Ray, T.D.; Sheedy, F.J.; Goedeke, L.; Liu, X.; et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 2011, 478, 404–407. [Google Scholar] [CrossRef] [Green Version]
- Vickers, K.C.; Rader, D.J. Nuclear receptors and microRNA-144 coordinately regulate cholesterol efflux. Circ. Res. 2013, 112, 1529–1531. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.L.; Pu, Y.; Meng, X.P.; Cui, S.Y.; Tao, B.; Fan, S.Z.; Xu, L. Prospects for clinical treatment of heart disease based on microRNA. J. Guangzhou Med. Univ. 2021, 49, 130–136. [Google Scholar]
- Wang, L.; Qin, D.; Shi, H.; Zhang, Y.; Li, H.; Han, Q. MiR-195-5p Promotes Cardiomyocyte Hypertrophy by Targeting MFN2 and FBXW7. BioMed Res. Int. 2019, 2019, 1580982. [Google Scholar] [CrossRef]
- Zeng, M.; Sang, W.; Chen, S.; Chen, R.; Zhang, H.; Xue, F.; Li, Z.; Liu, Y.; Gong, Y.; Zhang, H.; et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol. Lett. 2017, 271, 26–37. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Pattarayan, D.; Rajaguru, P.; Sudhakar Gandhi, P.S.; Thimmulappa, R.K. MicroRNA Regulation of Acute Lung Injury and Acute Respiratory Distress Syndrome. J. Cell. Physiol. 2016, 231, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Yu, S.; Meng, X.; Shi, M.; Huang, S.; Li, J.; Zhang, J.; Liang, Y.; Ji, M.; Zhao, Y.; et al. MicroRNAs: Important Regulatory Molecules in Acute Lung Injury/Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2022, 23, 5545. https://doi.org/10.3390/ijms23105545
Lu Q, Yu S, Meng X, Shi M, Huang S, Li J, Zhang J, Liang Y, Ji M, Zhao Y, et al. MicroRNAs: Important Regulatory Molecules in Acute Lung Injury/Acute Respiratory Distress Syndrome. International Journal of Molecular Sciences. 2022; 23(10):5545. https://doi.org/10.3390/ijms23105545
Chicago/Turabian StyleLu, Qianying, Sifan Yu, Xiangyan Meng, Mingyu Shi, Siyu Huang, Junfeng Li, Jianfeng Zhang, Yangfan Liang, Mengjun Ji, Yanmei Zhao, and et al. 2022. "MicroRNAs: Important Regulatory Molecules in Acute Lung Injury/Acute Respiratory Distress Syndrome" International Journal of Molecular Sciences 23, no. 10: 5545. https://doi.org/10.3390/ijms23105545
APA StyleLu, Q., Yu, S., Meng, X., Shi, M., Huang, S., Li, J., Zhang, J., Liang, Y., Ji, M., Zhao, Y., & Fan, H. (2022). MicroRNAs: Important Regulatory Molecules in Acute Lung Injury/Acute Respiratory Distress Syndrome. International Journal of Molecular Sciences, 23(10), 5545. https://doi.org/10.3390/ijms23105545






