The Comparative Survey of Coordinated Regulation of Steroidogenic Pathway in Japanese Flounder (Paralichthys olivaceus) and Chinese Tongue Sole (Cynoglossus semilaevis)
Abstract
1. Introduction
2. Results and Discussion
2.1. Genomic Landscape, Functional Domain and Phylogeny of Steroidogenic Genes in P. olivaceus and C. semilaevis
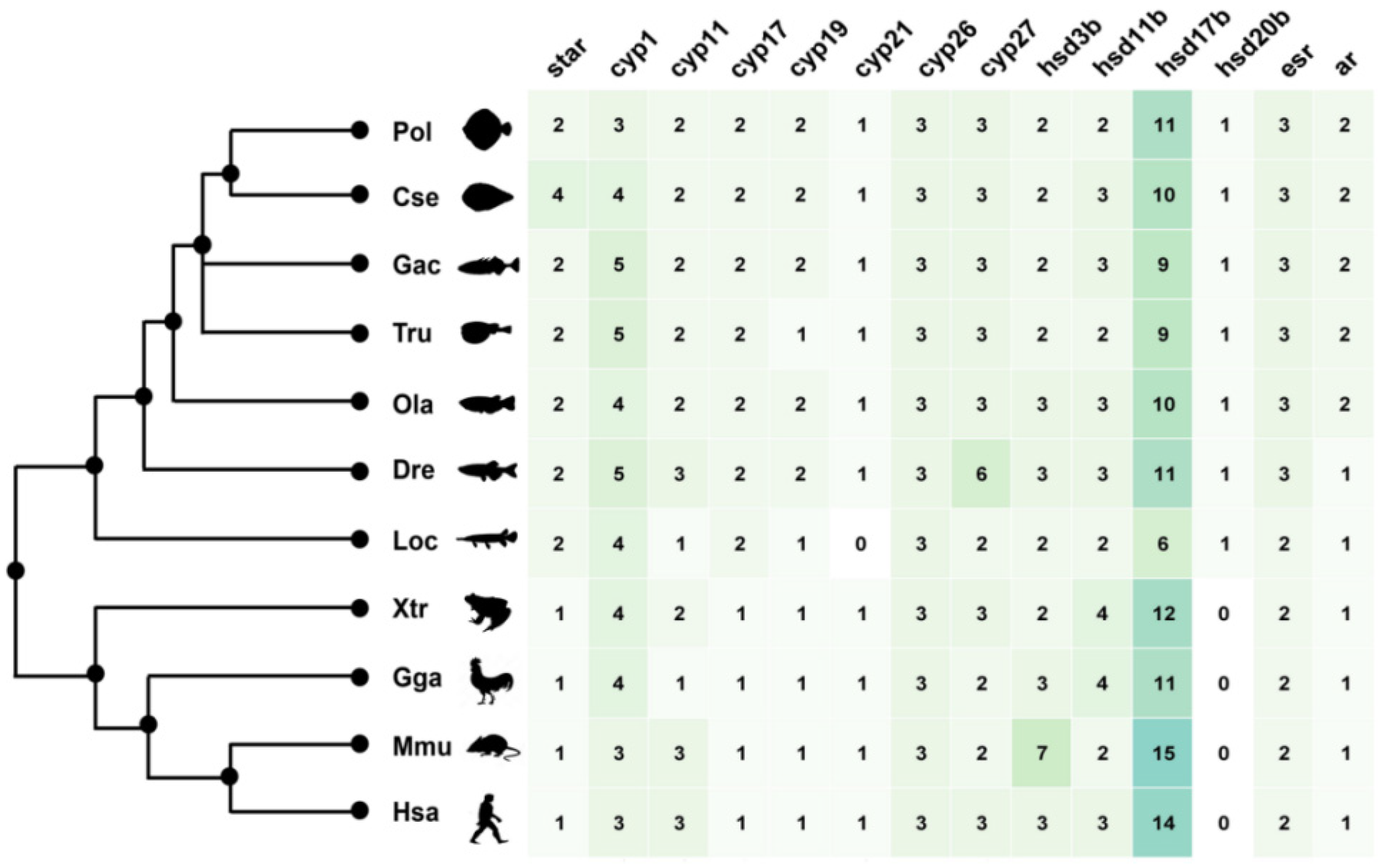
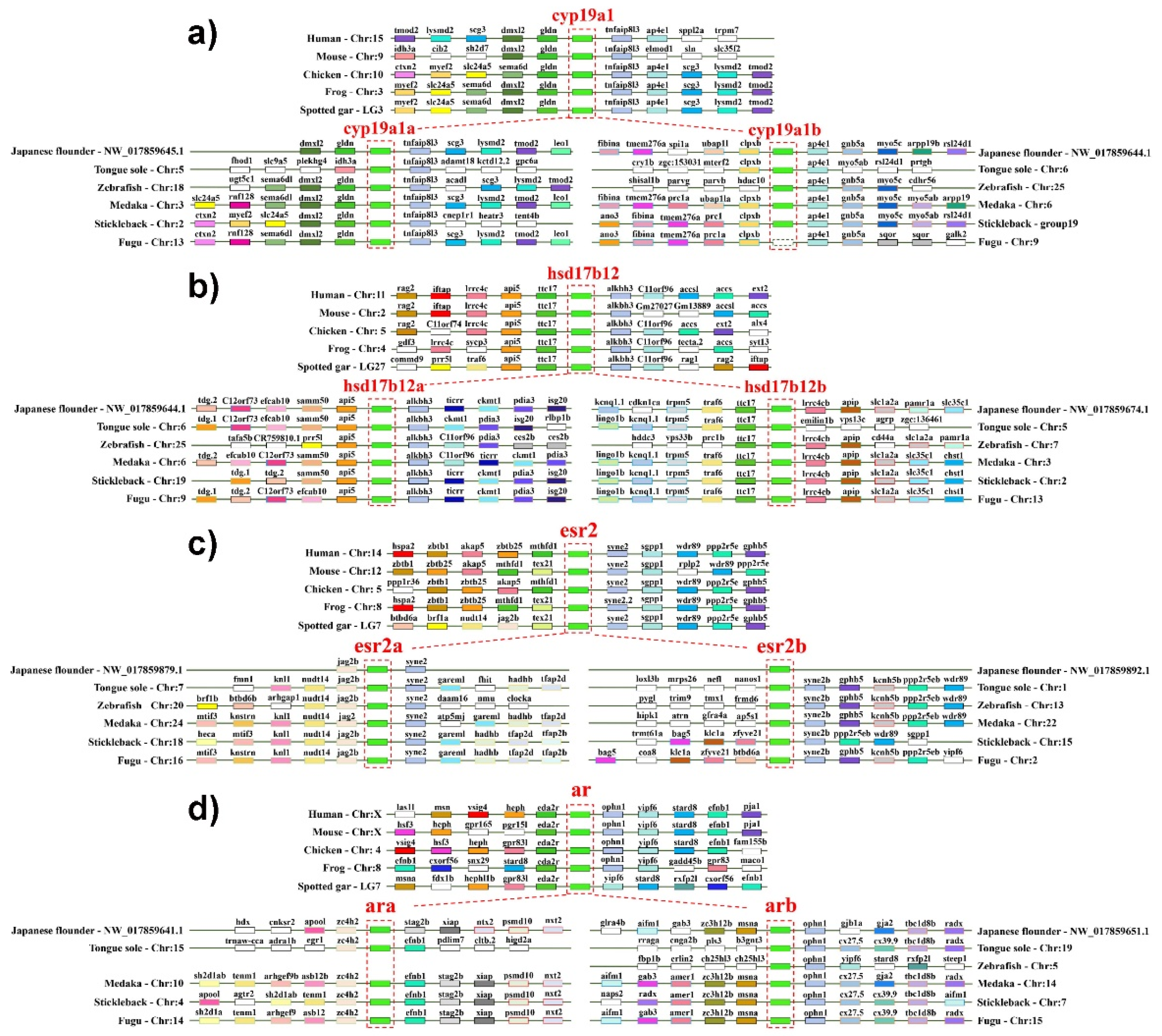
2.2. The Duplication of Steroidogenic Genes in the Teleost Lineages
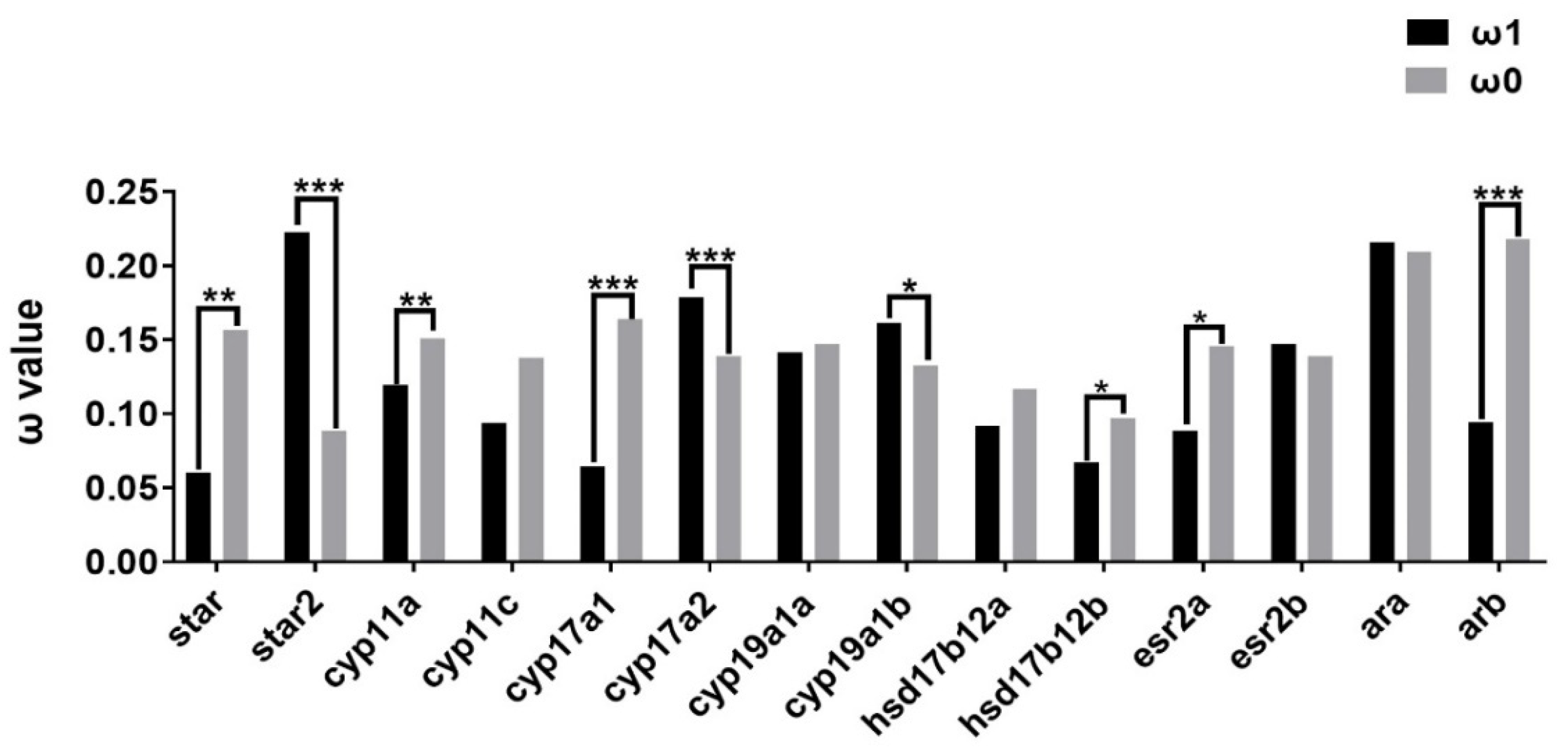
2.3. Evolutionary Dynamics of Steroidogenic Genes in Teleost Lineages
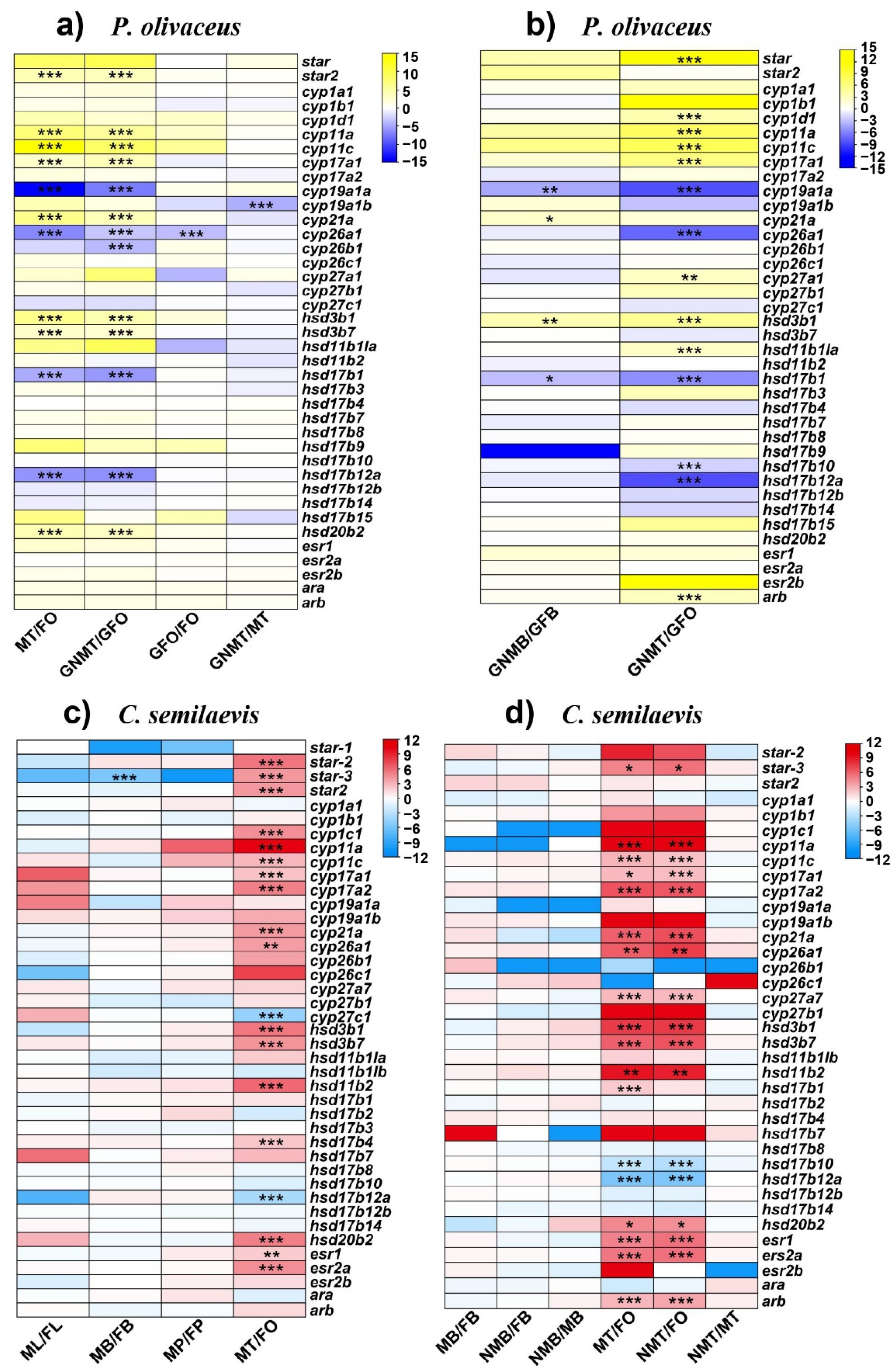
2.4. Expression Profiles of Steroidogenic Genes in P. olivaceus and C. semilaevis
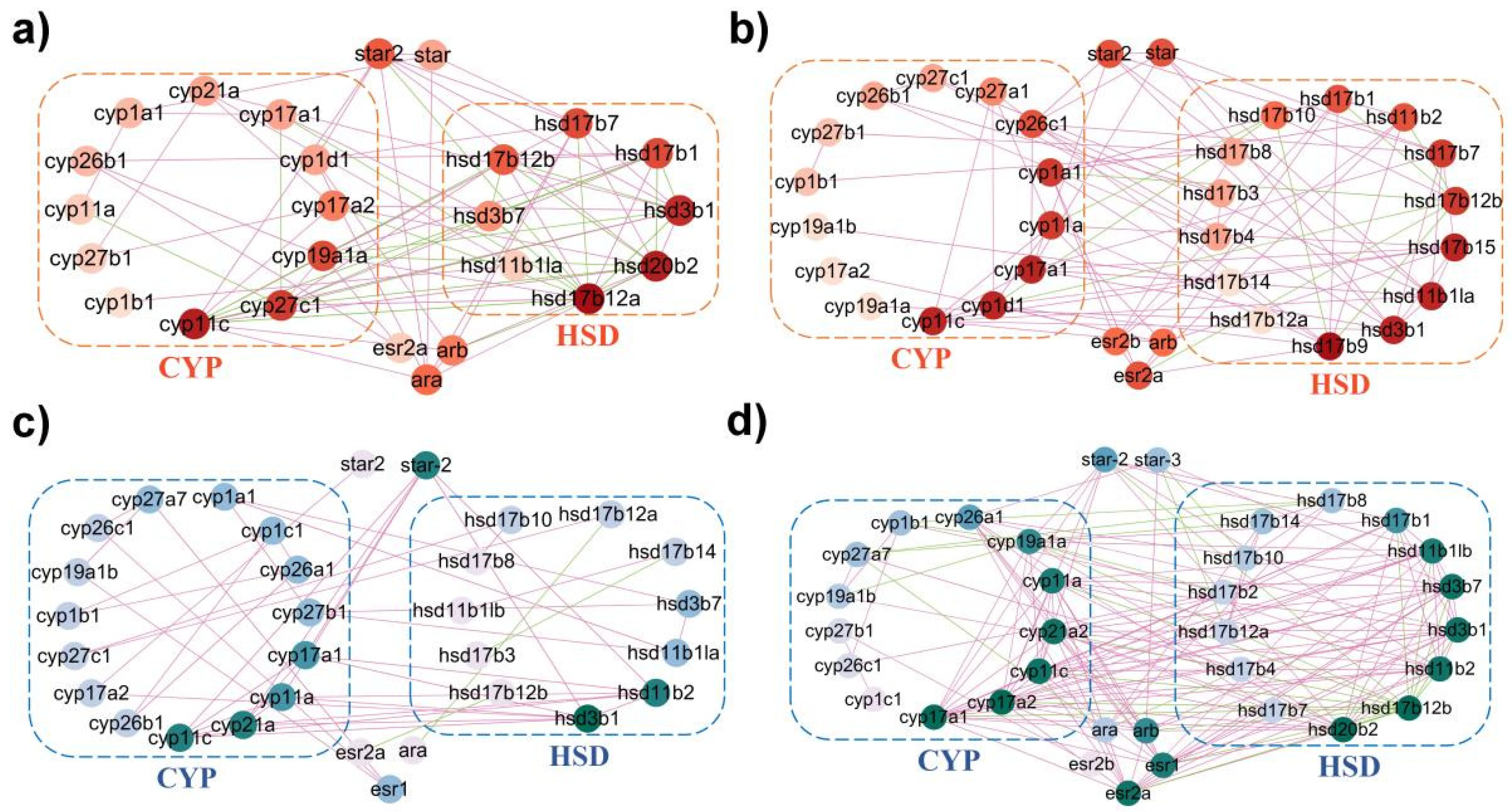
2.5. Coordinated Interaction of Steroidogenic Pathway in P. olivaceus and C. semilaevis
3. Materials and Methods
3.1. Characterization of Steroidogenic Genes in P. olivaceus and C. semilaevis
3.2. Phylogenetic Analysis of Steroidogenic Genes
3.3. Synteny Analysis of Steroidogenic Genes
3.4. Molecular Evolution Analysis
3.5. Expression of Steroidogenic Genes through Transcriptomic Analysis
3.6. Protein-Protein Interaction and Co-Expression Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhat, I.A.; Dar, J.Y.; Ahmad, I.; Mir, I.N.; Bhat, H.; Bhat, R.A.H.; Ganie, P.A.; Sharma, R. Testicular development and spermatogenesis in fish: Insights into molecular aspects and regulation of gene expression by different exogenous factors. Rev. Aquac. 2021, 13, 2142–2168. [Google Scholar] [CrossRef]
- Bliss, S.P.; Navratil, A.M.; Xie, J.; Roberson, M.S. GnRH signaling, the gonadotrope and endocrine control of fertility. Front. Neuroendocrinol. 2010, 31, 322–340. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.N.; Shin, H.S.; Choi, Y.J.; Choi, C.Y. Kisspeptin regulates the hypothalamus-pituitary-gonad axis gene expression during sexual maturation in the cinnamon clownfish, Amphiprion melanopus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 168, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M. The role of the StAR protein in steroidogenesis: Challenges for the future. J. Endocrinol. 2000, 164, 247–253. [Google Scholar] [CrossRef]
- Tenugu, S.; Pranoty, A.; Mamta, S.K.; Senthilkumaran, B. Development and organization of gonadal steroidogenesis in bony fishes—A review. Aquac. Fish. 2021, 6, 223–246. [Google Scholar] [CrossRef]
- Tokarz, J.; Möller, G.; de Angelis, M.H.; Adamski, J. Steroids in teleost fishes: A functional point of view. Steroids 2015, 103, 123–144. [Google Scholar] [CrossRef]
- Godard, C.A.; Leaver, M.J.; Said, M.R.; Dickerson, R.L.; George, S.; Stegeman, J.J. Identification of cytochrome P450 1B-like sequences in two teleost fish species (scup, Stenotomus chrysops and plaice, Pleuronectes platessa) and in a cetacean (striped dolphin, Stenella coeruleo). Mar. Environ. Res. 2000, 50, 7–10. [Google Scholar] [CrossRef]
- McArthur, A.G.; Hegelund, T.; Cox, R.L.; Stegeman, J.J.; Liljenberg, M.; Olsson, U.; Sundberg, P.; Celander, M.C. Phylogenetic analysis of the cytochrome P450 3 (CYP3) gene family. J. Mol. Evol. 2003, 57, 200–211. [Google Scholar] [CrossRef]
- Liang, D.; Fan, Z.; Zou, Y.; Tan, X.; Wu, Z.; Jiao, S.; Li, J.; Zhang, P.; You, F. Characteristics of Cyp11a during Gonad Differentiation of the Olive Flounder Paralichthys olivaceus. Int. J. Mol. Sci. 2018, 19, 2641. [Google Scholar] [CrossRef]
- Meng, L.; Yu, H.; Ni, F.; Niu, J.; Liu, X.; Wang, X. Roles of two cyp11 genes in sex hormone biosynthesis in Japanese flounder (Paralichthys olivaceus). Mol. Reprod. Dev. 2020, 87, 53–65. [Google Scholar] [CrossRef]
- Sun, M.; Jiang, S.; Song, W.; Qu, J.; Qi, J. Molecular characterization and functional analysis of cyp11a and cyp11b in black rockfish (Sebastes schlegelii). J. Fish Biol. 2021, 99, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Wang, D.S.; Shibata, Y.; Paul-Prasanth, B.; Suzuki, A.; Nagahama, Y. Characterization, expression and transcriptional regulation of P450c17-I and -II in the medaka, Oryzias latipes. Biochem. Biophys. Res. Commun. 2007, 362, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L.; Auchus, R.J.; Geller, D.H. The regulation of 17,20 lyase activity. Steroids 1997, 62, 133–142. [Google Scholar] [CrossRef]
- Zhou, L.Y.; Wang, D.S.; Kobayashi, T.; Yano, A.; Paul-Prasanth, B.; Suzuki, A.; Sakai, F.; Nagahama, Y. A novel type of P450c17 lacking the lyase activity is responsible for C21-steroid biosynthesis in the fish ovary and head kidney. Endocrinology 2007, 148, 4282–4291. [Google Scholar] [CrossRef]
- Guiguen, Y.; Fostier, A.; Piferrer, F.; Chang, C.F. Ovarian aromatase and estrogens: A pivotal role for gonadal sex differentiation and sex change in fish. Gen. Comp. Endocrinol. 2010, 165, 352–366. [Google Scholar] [CrossRef]
- Adamski, J.; Jakob, F.J. A guide to 17beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2001, 171, 1–4. [Google Scholar] [CrossRef]
- Rasmussen, M.K.; Ekstrand, B.; Zamaratskaia, G. Regulation of 3β-hydroxysteroid dehydrogenase/Δ⁵-Δ⁴ isomerase: A review. Int. J. Mol. Sci. 2013, 14, 17926–17942. [Google Scholar] [CrossRef]
- Rasheeda, M.K.; Kagawa, H.; Kirubagaran, R.; Dutta-Gupta, A.; Senthilkumaran, B. Cloning, expression and enzyme activity analysis of testicular 11beta-hydroxysteroid dehydrogenase during seasonal cycle and after hCG induction in air-breathing catfish Clarias gariepinus. J. Steroid Biochem. Mol. Biol. 2010, 120, 1–10. [Google Scholar] [CrossRef]
- Tokarz, J.; Lintelmann, J.; Möller, G.; Adamski, J. Substrate multispecificity among 20β-hydroxysteroid dehydrogenase type 2 members. Mol. Cell. Endocrinol. 2020, 510, 110822. [Google Scholar] [CrossRef]
- Mindnich, R.; Haller, F.; Halbach, F.; Moeller, G.; Hrabé de Angelis, M.; Adamski, J. Androgen metabolism via 17beta-hydroxysteroid dehydrogenase type 3 in mammalian and non-mammalian vertebrates: Comparison of the human and the zebrafish enzyme. J. Mol. Endocrinol. 2005, 35, 305–316. [Google Scholar] [CrossRef]
- Ogino, Y.; Tohyama, S.; Kohno, S.; Toyota, K.; Yamada, G.; Yatsu, R.; Kobayashi, T.; Tatarazako, N.; Sato, T.; Matsubara, H.; et al. Functional distinctions associated with the diversity of sex steroid hormone receptors ESR and AR. J. Steroid Biochem. Mol. Biol. 2018, 184, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Senthilkumaran, B. Steroidogenesis and its regulation in teleost-a review. Fish Physiol. Biochem. 2020, 46, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Gao, D.; Lu, J.; Sun, X. Transcriptome Profiling Reveals the Sexual Dimorphism of Gene Expression Patterns during Gonad Differentiation in the Half-Smooth Tongue Sole (Cynoglossus semilaevis). Mar. Biotechnol. 2021, 23, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Wang, L.; Zou, Y.; Wu, Z.; Wang, W.; Liang, S.; Wang, L.; You, F. Characteristics and sex dimorphism of 17β-hydroxysteroid dehydrogenase family genes in the olive flounder Paralichthys olivaceus. J. Steroid Biochem. Mol. Biol. 2020, 199, 105597. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Wang, W.; Ma, L.; Jie, J.; Zhang, Y.; Wang, H.; Li, H. New locus reveals the genetic architecture of sex reversal in the Chinese tongue sole (Cynoglossus semilaevis). Heredity 2018, 121, 319–326. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, W.; Zhang, Y.; Wang, L.; Cui, Y.; Li, H. Integrative analysis reveals pathways associated with sex reversal in Cynoglossus semilaevis. PeerJ 2020, 8, e8801. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Jin, C.; Du, X.; He, Y.; Zhang, Q. Transcriptome Profiling Insights the Feature of Sex Reversal Induced by High Temperature in Tongue Sole Cynoglossus semilaevis. Front. Genet. 2019, 10, 522. [Google Scholar] [CrossRef]
- Si, Y.; Ding, Y.; He, F.; Wen, H.; Li, J.; Zhao, J.; Huang, Z. DNA methylation level of cyp19a1a and foxl2 gene related to their expression patterns and reproduction traits during ovary development stages of Japanese flounder (Paralichthys olivaceus). Gene 2016, 575, 321–330. [Google Scholar] [CrossRef]
- Liang, D.; Fan, Z.; Weng, S.; Jiao, S.; Wu, Z.; Zou, Y.; Tan, X.; Li, J.; Zhang, P.; You, F. Characterization and expression of StAR2a and StAR2b in the olive flounder Paralichthys olivaceus. Gene 2017, 626, 1–8. [Google Scholar] [CrossRef]
- Meng, L.; Yu, L.; Qu, J.; Niu, J.; Ni, F.; Han, P.; Yu, H.; Wang, X. Two cyp17 genes perform different functions in the sex hormone biosynthesis and gonadal differentiation in Japanese flounder (Paralichthys olivaceus). Gene 2019, 702, 17–26. [Google Scholar] [CrossRef]
- Zou, Y.; Peng, L.; Weng, S.; Liang, D.; Fan, Z.; Wu, Z.; Tan, X.; Jiao, S.; You, F. Characterization and expression of androgen receptors in olive flounder. Gene 2019, 683, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wu, L.; Xie, L.; Yang, S.; Charkraborty, T.; Shi, H.; Wang, D.; Zhou, L. Characterization of two paralogous StAR genes in a teleost, Nile tilapia (Oreochromis niloticus). Mol. Cell. Endocrinol. 2014, 392, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Chiang, E.F.; Yan, Y.L.; Guiguen, Y.; Postlethwait, J.; Chung, B. Two Cyp19 (P450 aromatase) genes on duplicated zebrafish chromosomes are expressed in ovary or brain. Mol. Biol. Evol. 2001, 18, 542–550. [Google Scholar] [CrossRef]
- Cheng, J.; Yang, F.; Liu, S.; Zhao, H.; Lu, W.; Zhang, Q. Transcriptomic Analysis Reveals Functional Interaction of mRNA–lncRNA–miRNA in Steroidogenesis and Spermatogenesis of Gynogenetic Japanese Flounder (Paralichthys olivaceus). Biology 2022, 11, 213. [Google Scholar] [CrossRef]
- Strushkevich, N.; MacKenzie, F.; Cherkesova, T.; Grabovec, I.; Usanov, S.; Park, H.W. Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc. Natl. Acad. Sci. USA 2011, 108, 10139–10143. [Google Scholar] [CrossRef]
- Rajakumar, A.; Senthilkumaran, B. Expression analysis of cyp11a1 during gonadal development, recrudescence and after hCG induction and sex steroid analog treatment in the catfish, Clarias batrachus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 176, 42–47. [Google Scholar] [CrossRef]
- Dumont, M.; Luu-The, V.; Dupont, E.; Pelletier, G.; Labrie, F. Characterization, expression, and immunohistochemical localization of 3 beta-hydroxysteroid dehydrogenase/delta 5-delta 4 isomerase in human skin. J. Invest. Dermatol. 1992, 99, 415–421. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sreenivasulu, G.; Senthilkumaran, B.; Sridevi, P.; Rajakumar, A.; Rasheeda, M.K. Expression and immunolocalization of 20β-hydroxysteroid dehydrogenase during testicular cycle and after hCG induction, in vivo in the catfish, Clarias gariepinus. Gen. Comp. Endocrinol. 2012, 175, 48–54. [Google Scholar] [CrossRef]
- Miura, T.; Yamauchi, K.; Takahashi, H.; Nagahama, Y. The role of hormones in the acquisition of sperm motility in salmonid fish. J. Exp. Zool. 1992, 261, 359–363. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Kobayashi, T.; Ge, W.; Kobayashi, H.; Tanaka, M.; Okamoto, M.; Nonaka, Y.; Nagahama, Y. Fish testicular 11beta-hydroxylase: cDNA cloning and mRNA expression during spermatogenesis. FEBS Lett. 1996, 397, 250–252. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Young, G.; Kobayashi, T.; Nagahama, Y. Eel (Anguilla japonica) testis 11beta-hydroxylase gene is expressed in interrenal tissue and its product lacks aldosterone synthesizing activity. Mol. Cell. Endocrinol. 1998, 146, 207–211. [Google Scholar] [CrossRef]
- Xiao, L.; Guo, Y.; Wang, D.; Zhao, M.; Hou, X.; Li, S.; Lin, H.; Zhang, Y. Beta-Hydroxysteroid Dehydrogenase Genes in Orange-Spotted Grouper (Epinephelus coioides): Genome-Wide Identification and Expression Analysis During Sex Reversal. Front. Genet. 2020, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Mills, L.J.; Gutjahr-Gobell, R.E.; Zaroogian, G.E.; Horowitz, D.B.; Laws, S.C. Modulation of aromatase activity as a mode of action for endocrine disrupting chemicals in a marine fish. Aquat. Toxicol. 2014, 147, 140–150. [Google Scholar] [CrossRef]
- Lynch, M.; Force, A. The probability of duplicate gene preservation by subfunctionalization. Genetics 2000, 154, 459–473. [Google Scholar] [CrossRef]
- Goldstone, J.V.; McArthur, A.G.; Kubota, A.; Zanette, J.; Parente, T.; Jönsson, M.E.; Nelson, D.R.; Stegeman, J.J. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genom. 2010, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Diotel, N.; Vaillant, C.; Gabbero, C.; Mironov, S.; Fostier, A.; Gueguen, M.M.; Anglade, I.; Kah, O.; Pellegrini, E. Effects of estradiol in adult neurogenesis and brain repair in zebrafish. Horm. Behav. 2013, 63, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.Y.; Wang, D.S.; Senthilkumaran, B.; Yoshikuni, M.; Shibata, Y.; Kobayashi, T.; Sudhakumari, C.C.; Nagahama, Y. Cloning, expression and characterization of three types of 17beta-hydroxysteroid dehydrogenases from the Nile tilapia, Oreochromis niloticus. J. Mol. Endocrinol. 2005, 35, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L. Molecular Cloning, Gene Expression and Enzyme Activity Characterization of Three Types 17β-hydroxysteroid Dehydrogenases (17β-HSD1, 17β-HSD3, 17β-HSD8) from Nile Tilapia, Oreochromis niloticu. Master’s Thesis, Southwest China Normal University, Chongqing, China, 2004. [Google Scholar]
- Mindnich, R.; Möller, G.; Adamski, J. The role of 17 beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2004, 218, 7–20. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chen, C.; Arab, D.A.; Du, Z.; He, Y.; Ho, S.Y.W. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evol. 2019, 9, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, Z.; Zou, C.; Liang, S.; Zou, Y.; Liu, Y.; You, F. Sex-Dependent RNA Editing and N6-adenosine RNA Methylation Profiling in the Gonads of a Fish, the Olive Flounder (Paralichthys olivaceus). Front. Cell Dev. Biol. 2020, 8, 751. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, R.; Wang, R.; Chen, S. Transcriptomics analysis revealing candidate networks and genes for the body size sexual dimorphism of Chinese tongue sole (Cynoglossus semilaevis). Funct. Integr. Genom. 2018, 18, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Song, H.; Jin, C.; Peng, M.; Gao, C.; Yang, F.; Du, X.; Qi, J.; Zhang, Q.; et al. Evolutionary significance and regulated expression of Tdrd family genes in gynogenetic Japanese flounder (Paralichthys olivaceus). Comp. Biochem. Physiol. D Genom. Proteom. 2019, 31, 100593. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Wang, Y.; Lu, W.; Zong, W.; Zhu, Q.; Cheng, J. The Comparative Survey of Coordinated Regulation of Steroidogenic Pathway in Japanese Flounder (Paralichthys olivaceus) and Chinese Tongue Sole (Cynoglossus semilaevis). Int. J. Mol. Sci. 2022, 23, 5520. https://doi.org/10.3390/ijms23105520
Yang F, Wang Y, Lu W, Zong W, Zhu Q, Cheng J. The Comparative Survey of Coordinated Regulation of Steroidogenic Pathway in Japanese Flounder (Paralichthys olivaceus) and Chinese Tongue Sole (Cynoglossus semilaevis). International Journal of Molecular Sciences. 2022; 23(10):5520. https://doi.org/10.3390/ijms23105520
Chicago/Turabian StyleYang, Fan, Yapeng Wang, Wei Lu, Wenyu Zong, Qing Zhu, and Jie Cheng. 2022. "The Comparative Survey of Coordinated Regulation of Steroidogenic Pathway in Japanese Flounder (Paralichthys olivaceus) and Chinese Tongue Sole (Cynoglossus semilaevis)" International Journal of Molecular Sciences 23, no. 10: 5520. https://doi.org/10.3390/ijms23105520
APA StyleYang, F., Wang, Y., Lu, W., Zong, W., Zhu, Q., & Cheng, J. (2022). The Comparative Survey of Coordinated Regulation of Steroidogenic Pathway in Japanese Flounder (Paralichthys olivaceus) and Chinese Tongue Sole (Cynoglossus semilaevis). International Journal of Molecular Sciences, 23(10), 5520. https://doi.org/10.3390/ijms23105520





