Heparin Enriched-WPI Coating on Ti6Al4V Increases Hydrophilicity and Improves Proliferation and Differentiation of Human Bone Marrow Stromal Cells
Abstract
:1. Introduction
2. Results and Discussion
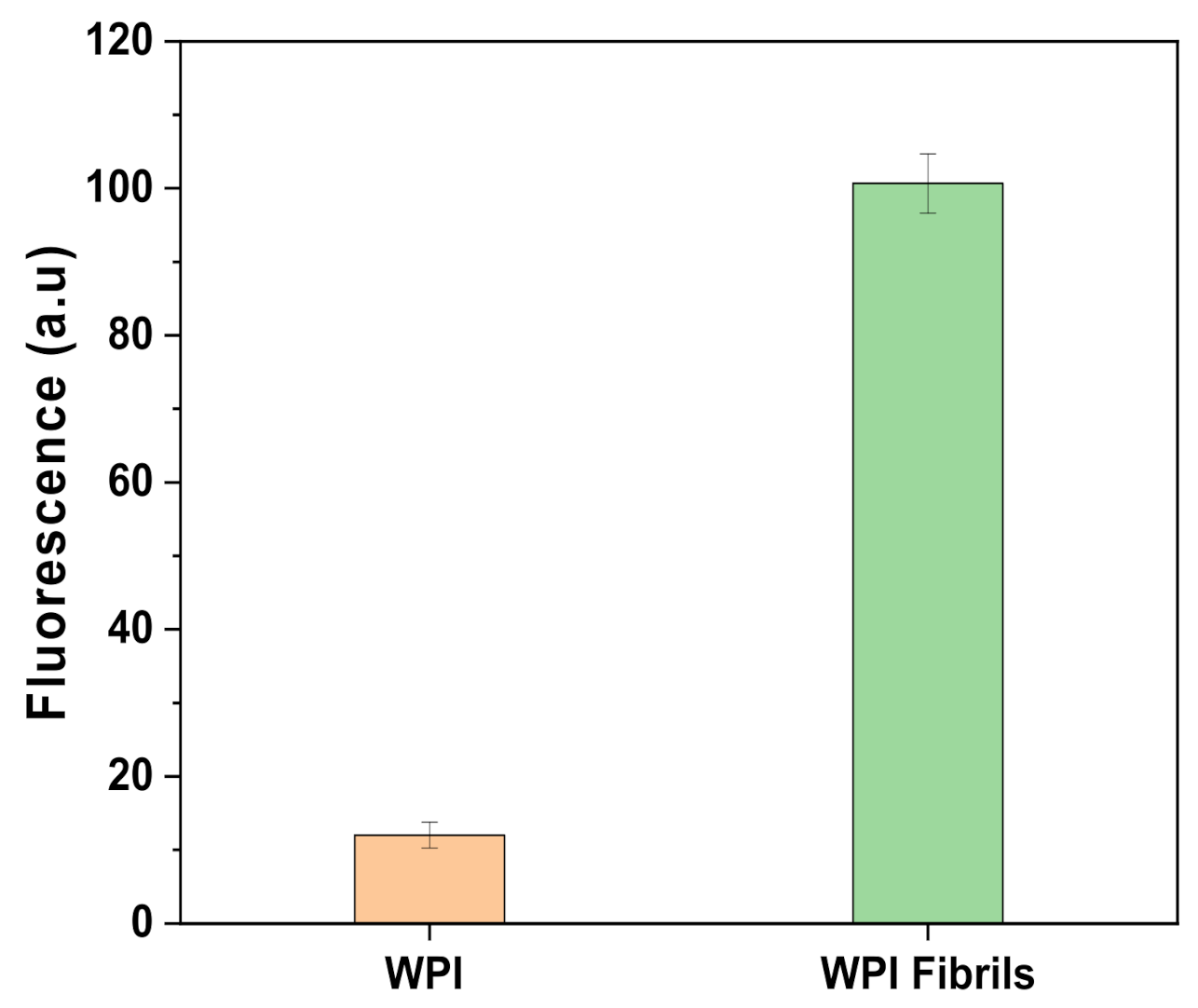
2.1. Fibrils Characterization
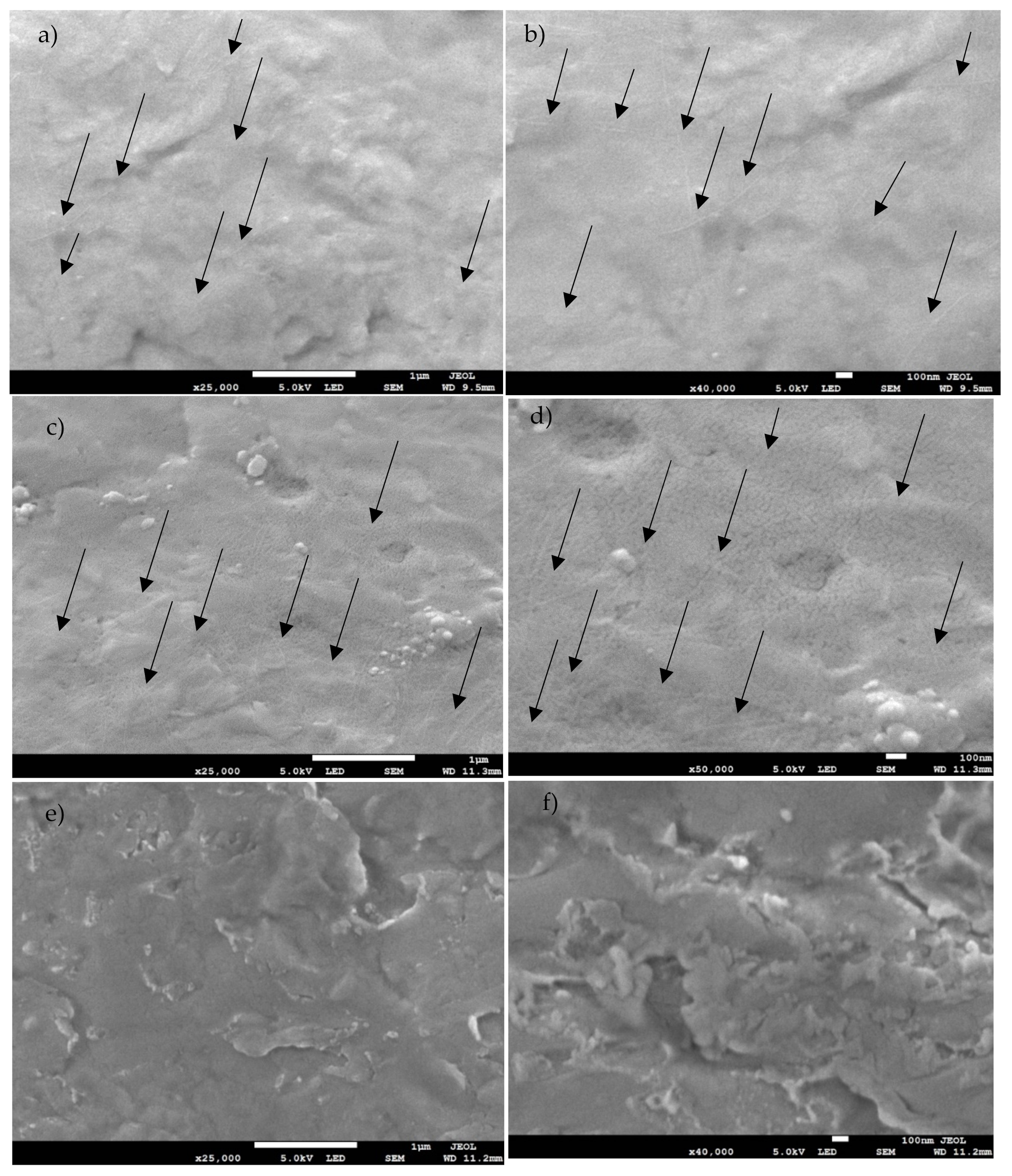
2.2. Coating Characterization
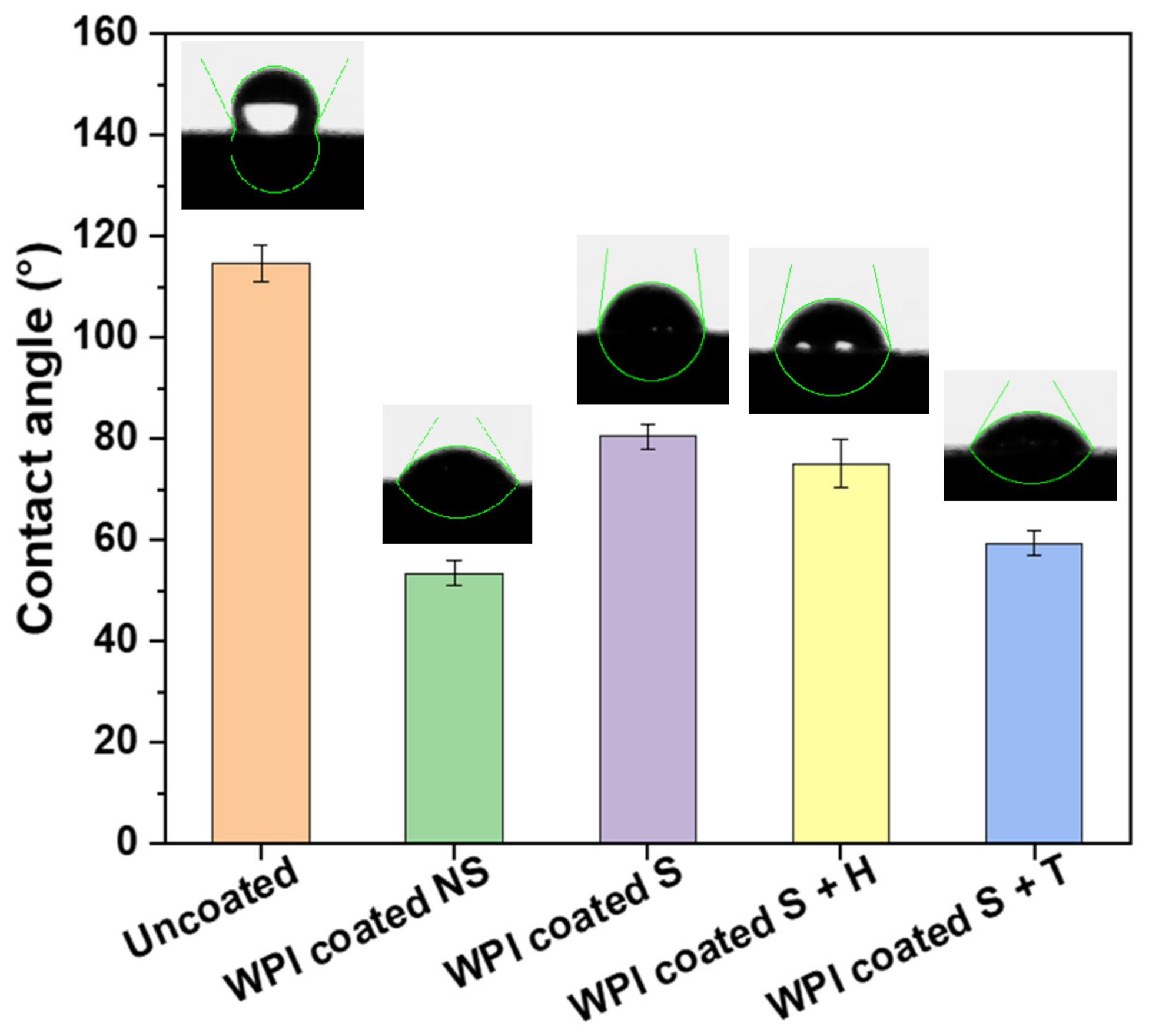
2.2.1. Contact Angle
2.2.2. X-ray Photoelectron Spectroscopy
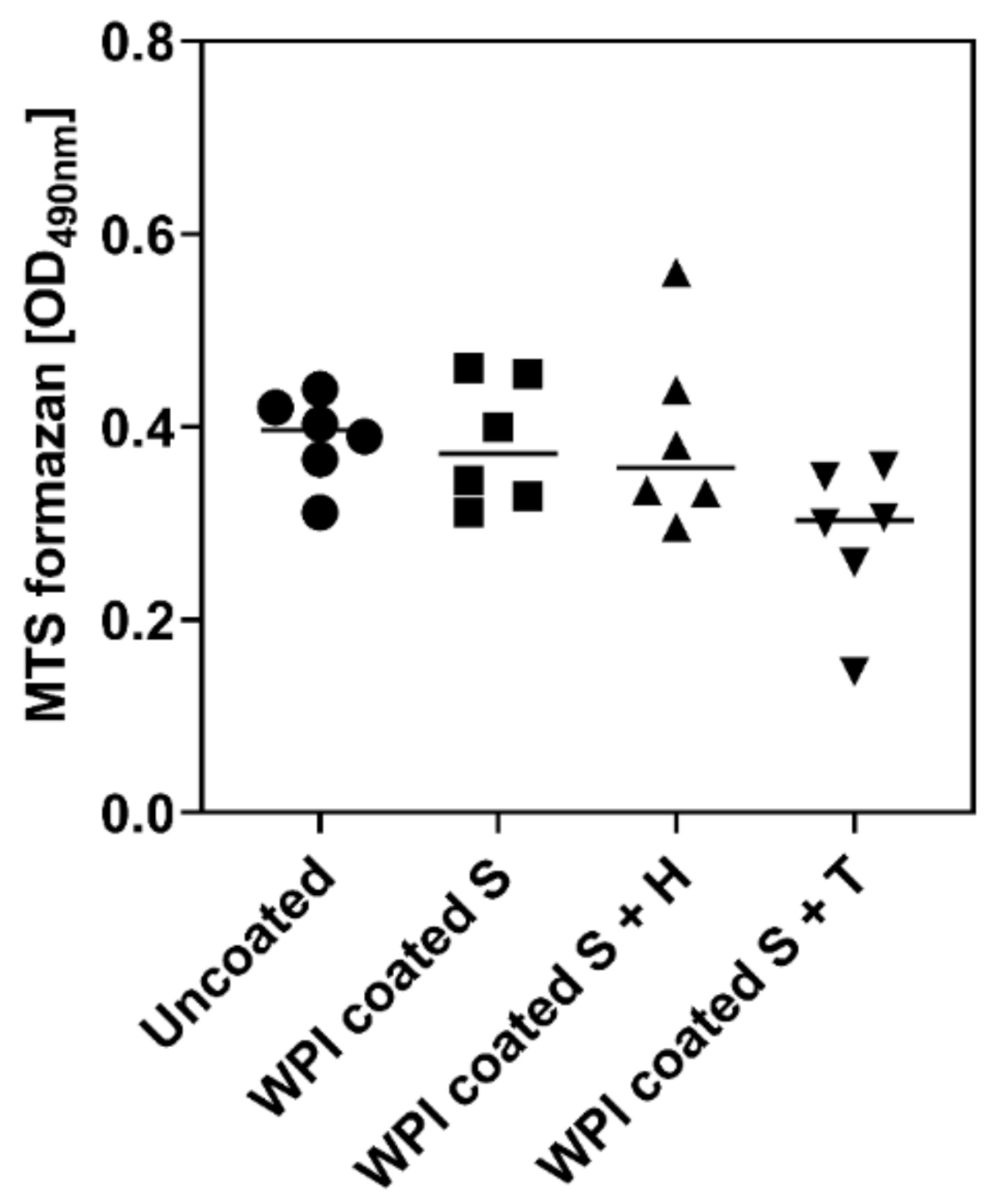
2.3. Influence of Different Ti6Al4V Coating on Metabolic Activity of hBMSC
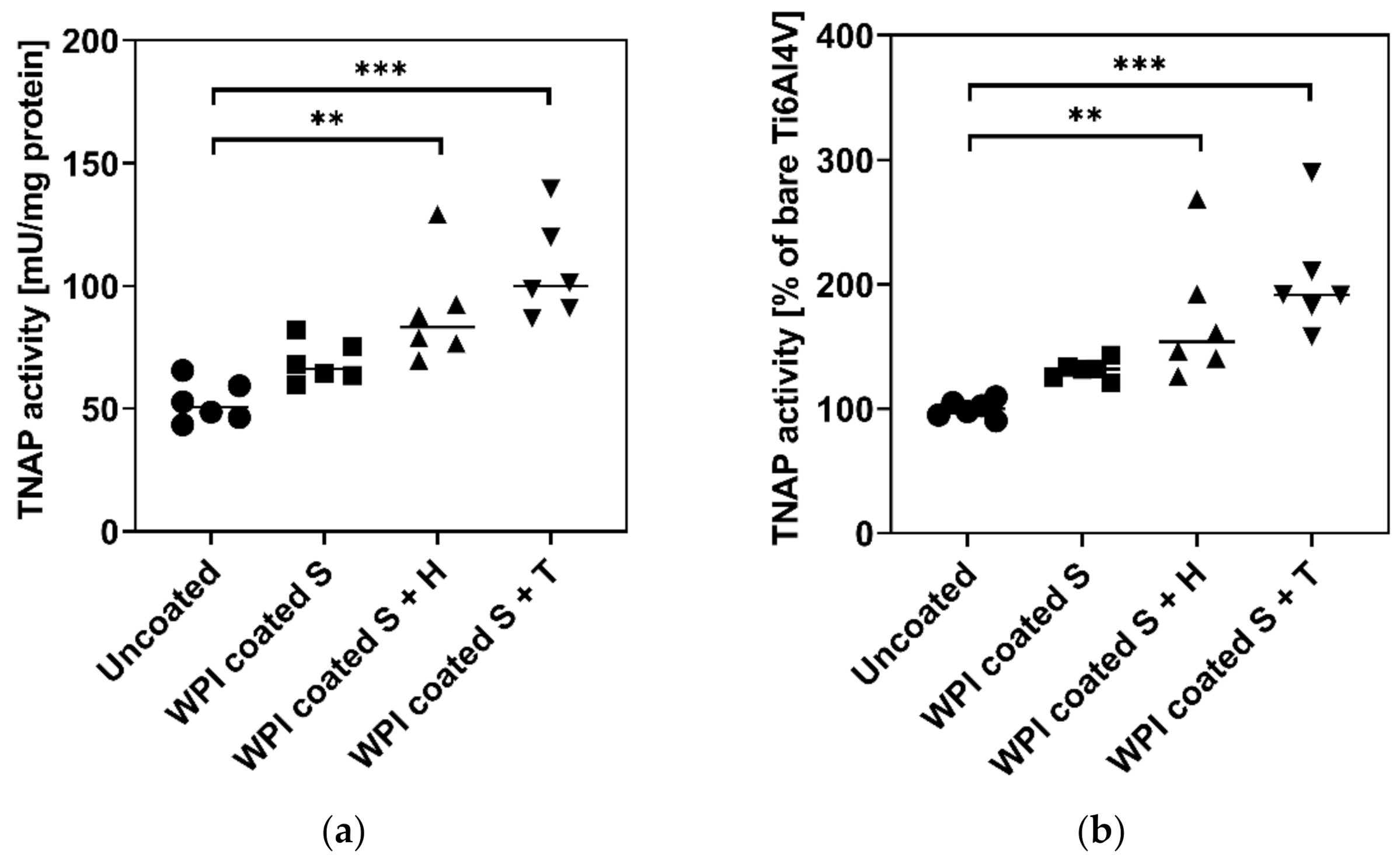
2.4. Influence of Different Ti6Al4V Coatings on TNAP Activity of hBMSC
3. Materials and Methods
3.1. Fibrils Production and Characterization
3.2. Coating Protocol and Characterization
3.3. Isolation and Cultivation of Human Bone Marrow Stromal Cells (hBMSC)
3.4. Determination of Metabolic Activity
3.5. Determination of Tissue Non-Specific Alkaline Phosphatase (TNAP) Enzyme Activity
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Additive Manufacturing |
| ALP | Alkaline phosphatase |
| CA | Contact angle |
| DMEM | Dulbecco’s minimal essential medium |
| FEG-SEM | Field Emission Scanning Electron Microscope |
| GAGs | Glycosaminoglycans |
| hBMSC | human bone marrow stromal cells |
| LMWH | Low molecular weight heparin |
| NS | Not sterile |
| S | Sterile |
| SD | Standard deviation |
| SEOM | Standard error of the mean |
| SEM | Scanning Electron Microscopy |
| ThT | Thioflavin T |
| TNAP | Tissue non-specific alkaline phosphatase |
| WPI | Whey protein isolate |
| β-lg | β-lactoglobulin |
References
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, T.; Heinemann, S.; Mietrach, C.; Hempel, U.; Bierbaum, S.; Scharnweber, D.; Worch, H. Interactions of collagen types I and II with chondroitin sulfates A-C and their effect on osteoblast adhesion. Biomacromolecules 2007, 8, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Douglas, T.; Hempel, U.; Mietrach, C.; Viola, M.; Vigetti, D.; Heinemann, S.; Bierbaum, S.; Scharnweber, D.; Worch, H. Influence of collagen-fibril-based coatings containing decorin and biglycan on osteoblast behavior. J. Biomed. Mater. Res. A 2008, 84, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Mieszkowska, A.; Beaumont, H.; Martocq, L.; Koptyug, A.; Surmeneva, M.A.; Surmenev, R.A.; Naderi, J.; Douglas, T.E.L.; Gurzawska-Comis, K.A. Phenolic-Enriched Collagen Fibrillar Coatings on Titanium Alloy to Promote Osteogenic Differentiation and Reduce Inflammation. Int. J. Mol. Sci. 2020, 21, 6406. [Google Scholar] [CrossRef]
- Norris, K.; Mishukova, O.I.; Zykwinska, A.; Colliec-Jouault, S.; Sinquin, C.; Koptioug, A.; Cuenot, S.; Kerns, J.G.; Surmeneva, M.A.; Surmenev, R.A.; et al. Marine Polysaccharide-Collagen Coatings on Ti6Al4V Alloy Formed by Self-Assembly. Micromachines 2019, 10, 68. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.M.; Gentile, P.; Toumpaniari, S.; Ciardelli, G.; Birch, M.A. Impact of Collagen/Heparin Multilayers for Regulating Bone Cellular Functions. ACS Appl. Mater. Interfaces 2016, 8, 29923–29932. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Vandrovcová, M.; Kročilová, N.; Keppler, J.K.; Zárubová, J.; Skirtach, A.G.; Bačáková, L. Application of whey protein isolate in bone regeneration: Effects on growth and osteogenic differentiation of bone-forming cells. J. Dairy Sci. 2018, 101, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.; Kocot, M.; Tryba, A.M.; Serafim, A.; Stancu, I.C.; Jaegermann, Z.; Pamuła, E.; Reilly, G.C.; Douglas, T.E. Novel naturally derived whey protein isolate and aragonite biocomposite hydrogels have potential for bone regeneration. Mater. Des. 2020, 188, 108408. [Google Scholar] [CrossRef]
- Keppler, J.K.; Martin, D.; Garamus, V.M.; Berton-Carabin, C.; Nipoti, E.; Coenye, T.; Schwarz, K. Functionality of whey proteins covalently modified by allyl isothiocyanate. Part 1 physicochemical and antibacterial properties of native and modified whey proteins at pH 2 to 7. Food Hydrocoll. 2017, 65, 130–143. [Google Scholar] [CrossRef] [Green Version]
- Akkermans, C.; Venema, P.; van der Goot, A.J.; Gruppen, H.; Bakx, E.J.; Boom, R.M.; van der Linden, E. Peptides are building blocks of heat-induced fibrillar protein aggregates of beta-lactoglobulin formed at pH 2. Biomacromolecules 2008, 9, 1474–1479. [Google Scholar] [CrossRef]
- Douglas, T.E.; Hempel, U.; Żydek, J.; Vladescu, A.; Pietryga, K.; Kaeswurm, J.A.; Buchweitz, M.; Surmenev, R.A.; Surmeneva, M.A.; Cotrut, C.M.; et al. Pectin coatings on titanium alloy scaffolds produced by additive manufacturing: Promotion of human bone marrow stromal cell proliferation. Mater. Lett. 2018, 227, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Krebs, M.R.H.; Bromley, E.H.C.; Donald, A.M. The binding of thioflavin-T to amyloid fibrils: Localisation and implications. J. Struct. Biol. 2005, 149, 30–37. [Google Scholar] [CrossRef]
- Heyn, T.R.; Garamus, V.M.; Neumann, H.R.; Uttinger, M.J.; Guckeisen, T.; Heuer, M.; Selhuber-Unkel, C.; Peukert, W.; Keppler, J.K. Influence of the polydispersity of pH 2 and pH 3.5 beta-lactoglobulin amyloid fibril solutions on analytical methods. Eur. Polym. J. 2019, 120, 109211. [Google Scholar] [CrossRef]
- Rabe, R.; Hempel, U.; Martocq, L.; Keppler, J.K.; Aveyard, J.; Douglas, T.E.L. Dairy-Inspired Coatings for Bone Implants from Whey Protein Isolate-Derived Self-Assembled Fibrils. Int. J. Mol. Sci. 2020, 21, 5544. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, D.P.; Wang, W.J.; Feng, Q.L.; Cui, F.Z.; Xu, Y.X.; Song, X.H. Covalent immobilization of chitosan and heparin on PLGA surface. Int. J. Biol. Macromol. 2003, 33, 95–100. [Google Scholar] [CrossRef]
- Chen, J.L.; Li, Q.L.; Chen, J.Y.; Chen, C.; Huang, N. Improving blood-compatibility of titanium by coating collagen–heparin multilayers. Appl. Surf. Sci. 2009, 255, 6894–6900. [Google Scholar] [CrossRef]
- Hempel, U.; Hefti, T.; Dieter, P.; Schlottig, F. Response of human bone marrow stromal cells, MG-63, and SaOS-2 to titanium-based dental implant surfaces with different topography and surface energy. Clin. Oral Implants Res. 2013, 24, 174–182. [Google Scholar] [CrossRef]
- Pittenger, M.F. Mesenchymal stem cells from adult bone marrow. Methods Mol. Biol. 2008, 449, 27–44. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Hempel, U.; Matthäus, C.; Preissler, C.; Möller, S.; Hintze, V.; Dieter, P. Artificial matrices with high-sulfated glycosaminoglycans and collagen are anti-inflammatory and pro-osteogenic for human mesenchymal stromal cells. J. Cell. Biochem. 2014, 115, 1561–1571. [Google Scholar] [CrossRef]
- Jafary, F.; Hanachi, P.; Gorjipour, K. Osteoblast Differentiation on Collagen Scaffold with Immobilized Alkaline Phosphatase. Int. J. Organ Transplant. Med. 2017, 8, 195–202. [Google Scholar]
- Simann, M.; Schneider, V.; Le Blanc, S.; Dotterweich, J.; Zehe, V.; Krug, M.; Jakob, F.; Schilling, T.; Schütze, N. Heparin affects human bone marrow stromal cell fate: Promoting osteogenic and reducing adipogenic differentiation and conversion. Bone 2015, 78, 102–113. [Google Scholar] [CrossRef]
- Mathews, S.; Mathew, S.A.; Gupta, P.K.; Bhonde, R.; Totey, S. Glycosaminoglycans enhance osteoblast differentiation of bone marrow derived human mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2014, 8, 143–152. [Google Scholar] [CrossRef]
- Vogel, S.; Arnoldini, S.; Möller, S.; Schnabelrauch, M.; Hempel, U. Sulfated hyaluronan alters fibronectin matrix assembly and promotes osteogenic differentiation of human bone marrow stromal cells. Sci. Rep. 2016, 6, 36418. [Google Scholar] [CrossRef] [Green Version]
- Keppler, J.K.; Heyn, T.R.; Meissner, P.M.; Schrader, K.; Schwarz, K. Protein oxidation during temperature-induced amyloid aggregation of beta-lactoglobulin. Food Chem. 2019, 289, 223–231. [Google Scholar] [CrossRef]
- Loveday, S.M.; Wang, X.L.; Rao, M.A.; Anema, S.G.; Singh, H. β-Lactoglobulin nanofibrils: Effect of temperature on fibril formation kinetics, fibril morphology and the rheological properties of fibril dispersions. Food Hydrocoll. 2012, 27, 242–249. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Oswald, J.; Boxberger, S.; Jørgensen, B.; Feldmann, S.; Ehninger, G.; Bornhäuser, M.; Werner, C. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 2004, 22, 377–384. [Google Scholar] [CrossRef]
- Hempel, U.; Müller, K.; Preissler, C.; Noack, C.; Boxberger, S.; Dieter, P.; Bornhäuser, M.; Wobus, M. Human Bone Marrow Stromal Cells: A Reliable, Challenging Tool for In Vitro Osteogenesis and Bone Tissue Engineering Approaches. Stem Cells Int. 2016, 2016, 7842191. [Google Scholar] [CrossRef] [Green Version]
- Hempel, U.; Hintze, V.; Möller, S.; Schnabelrauch, M.; Scharnweber, D.; Dieter, P. Artificial extracellular matrices composed of collagen I and sulfated hyaluronan with adsorbed transforming growth factor β1 promote collagen synthesis of human mesenchymal stromal cells. Acta Biomater. 2012, 8, 659–666. [Google Scholar] [CrossRef]

| Sample Name | Treatment |
|---|---|
| Uncoated | Bare Ti6Al4V |
| WPI Coated NS (Not Sterile) | Ti6Al4V coated with WPI fibrils |
| WPI Coated S (Sterile) | Ti6Al4V coated with WPI fibrils and autoclaved |
| WPI Coated S + H (Sterile + Heparin) | Ti6Al4V coated with WPI fibrils, autoclaved and subsequently coated with Heparin |
| WPI Coated S + T (Sterile + Tinzaparin) | Ti6Al4V coated with WPI fibrils, autoclaved and subsequently coated with Tinzaparin (LMWH) |
| Sample | Mean ± SD (°) |
|---|---|
| Uncoated | 114.73 ± 3.61 a |
| WPI Coated NS | 53.49 ± 2.35 be |
| WPI Coated S | 80.40 ± 2.31 cd |
| WPI Coated S + H | 75.11 ± 4.85 cd |
| WPI Coated S + T | 59.33 ± 2.45 be |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facchetti, D.; Hempel, U.; Martocq, L.; Smith, A.M.; Koptyug, A.; Surmenev, R.A.; Surmeneva, M.A.; Douglas, T.E.L. Heparin Enriched-WPI Coating on Ti6Al4V Increases Hydrophilicity and Improves Proliferation and Differentiation of Human Bone Marrow Stromal Cells. Int. J. Mol. Sci. 2022, 23, 139. https://doi.org/10.3390/ijms23010139
Facchetti D, Hempel U, Martocq L, Smith AM, Koptyug A, Surmenev RA, Surmeneva MA, Douglas TEL. Heparin Enriched-WPI Coating on Ti6Al4V Increases Hydrophilicity and Improves Proliferation and Differentiation of Human Bone Marrow Stromal Cells. International Journal of Molecular Sciences. 2022; 23(1):139. https://doi.org/10.3390/ijms23010139
Chicago/Turabian StyleFacchetti, Davide, Ute Hempel, Laurine Martocq, Alan M. Smith, Andrey Koptyug, Roman A. Surmenev, Maria A. Surmeneva, and Timothy E. L. Douglas. 2022. "Heparin Enriched-WPI Coating on Ti6Al4V Increases Hydrophilicity and Improves Proliferation and Differentiation of Human Bone Marrow Stromal Cells" International Journal of Molecular Sciences 23, no. 1: 139. https://doi.org/10.3390/ijms23010139
APA StyleFacchetti, D., Hempel, U., Martocq, L., Smith, A. M., Koptyug, A., Surmenev, R. A., Surmeneva, M. A., & Douglas, T. E. L. (2022). Heparin Enriched-WPI Coating on Ti6Al4V Increases Hydrophilicity and Improves Proliferation and Differentiation of Human Bone Marrow Stromal Cells. International Journal of Molecular Sciences, 23(1), 139. https://doi.org/10.3390/ijms23010139










