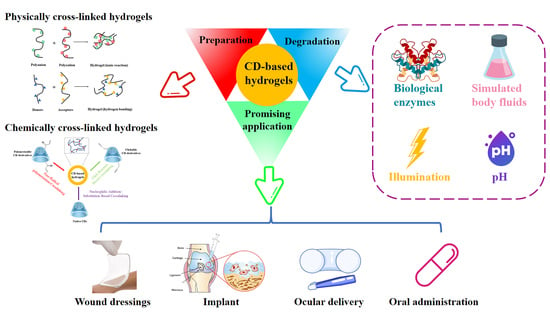
Cyclodextrin-Containing Hydrogels: A Review of Preparation Method, Drug Delivery, and Degradation Behavior
Abstract
:1. Introduction
2. Preparation Methods of CD-Containing Hydrogels
3. The Promising Application of CD-Containing Hydrogels for Drug Delivery
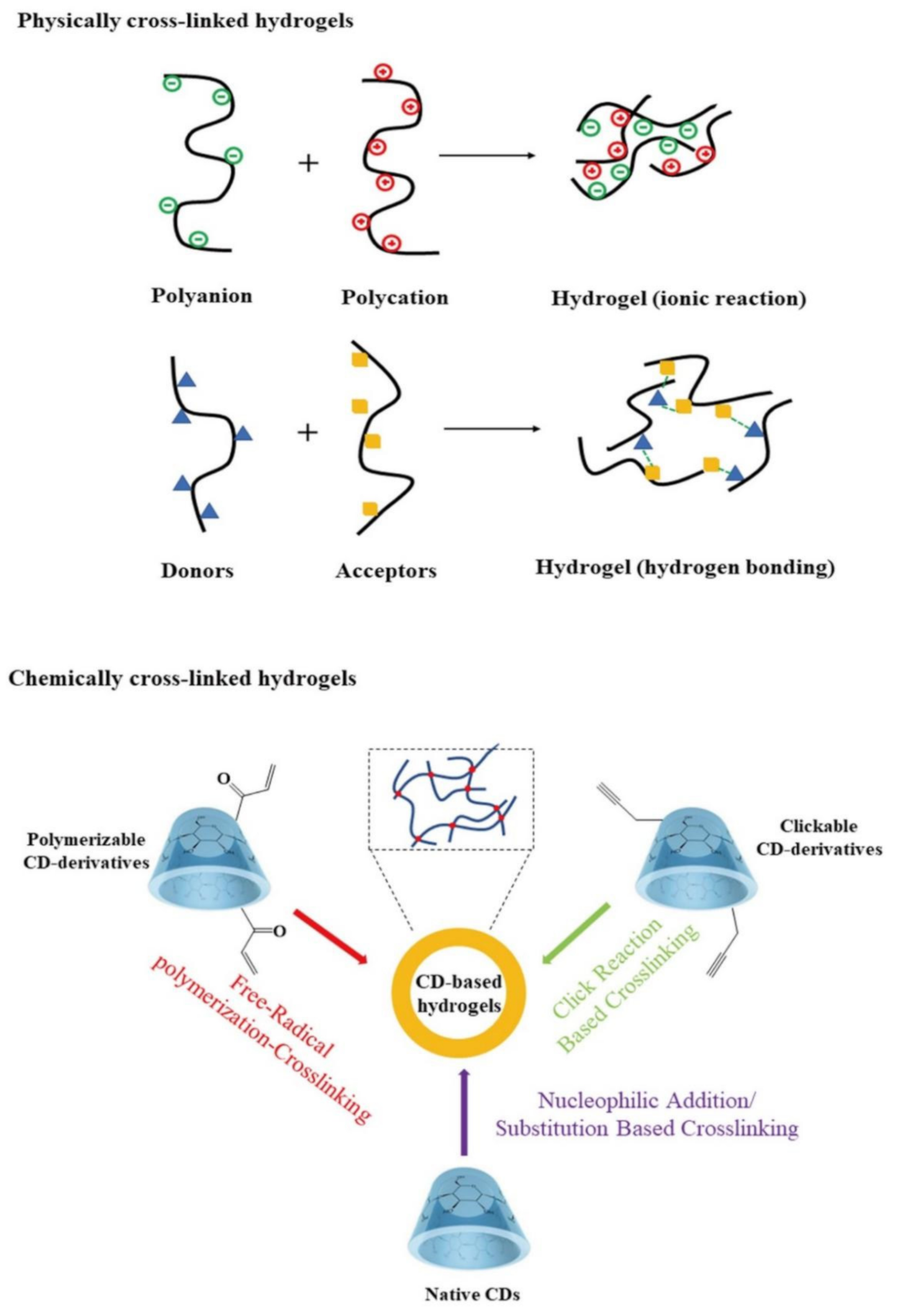
3.1. Physically Cross-Linked CD-Containing Hydrogels
3.2. Chemically Cross-Linked CD-Containing Hydrogels
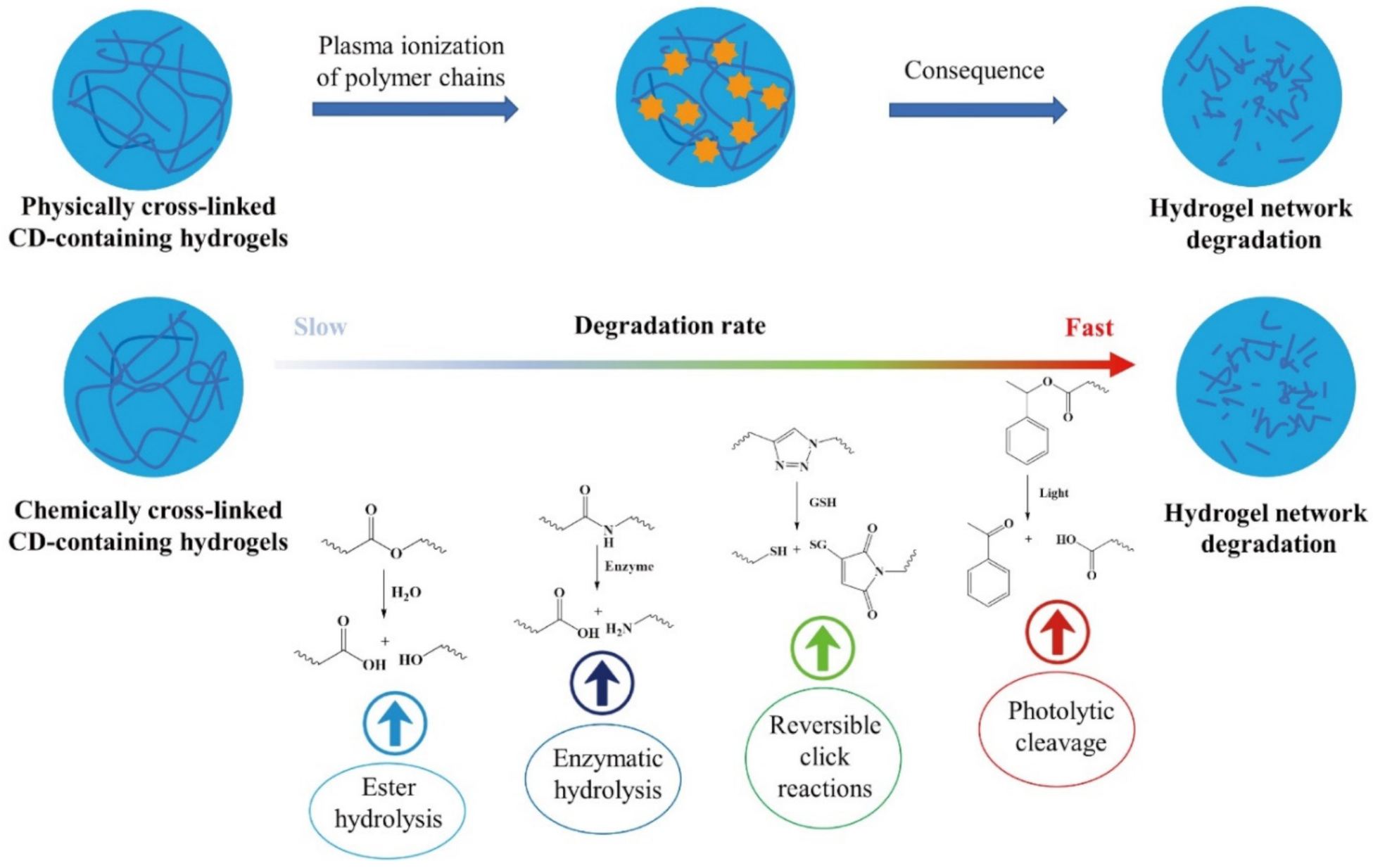
4. Simulated Degradation Behavior for CD-Containing Hydrogels
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| FTIR | Fourier transform infrared spectroscopy |
| NMR | nuclear magnetic resonance |
| SEM | scanning electron microscope |
| XRD | X-Ray diffraction |
| S. aureus | Staphylococcus aureus |
| P. aeruginosa | Pseudomonas aeruginosa |
| E. coli | Escherichia coli |
| C. aureus | Staphylococcus aureus enterotoxin C |
| Panc 1 | Human pancreatic cancer cell |
| U251 | Human brain glioma U251 cell line |
| MSTO | human mesothelioma |
| NHD | Fskin fibroblast cells |
| L929 cells | L929 mouse fibroblast cells |
References
- Champeau, M.; Heinze, D.A.; Viana, T.N.; de Souza, E.R.; Chinellato, A.C.; Titotto, S. 4D printing of hydrogels: A review. Adv. Funct. Mater. 2020, 30, 1910606. [Google Scholar] [CrossRef]
- Arslan, M.; Sanyal, R.; Sanyal, A. Cyclodextrin embedded covalently crosslinked networks: Synthesis and applications of hydrogels with nano-containers. Polym. Chem. 2020, 11, 615–629. [Google Scholar] [CrossRef]
- Tang, W.; Zou, C.; Da, C.; Cao, Y.; Peng, H. A review on the recent development of cyclodextrin-based materials used in oilfield applications. Carbohydr. Polym. 2020, 240, 116321. [Google Scholar] [CrossRef]
- Wang, W.; Yang, Z.; Zhang, A.; Yang, S. Water retention and fertilizer slow release integrated superabsorbent synthesized from millet straw and applied in agriculture. Ind. Crop. Prod. 2020, 160, 113126. [Google Scholar] [CrossRef]
- Mandal, A.; Clegg, J.R.; Anselmo, A.C.; Mitragotri, S. Hydrogels in the clinic. Bioeng. Transl. Med. 2020, 5, e10158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosseini, M.S.; Nabid, M.R. Synthesis of chemically cross-linked hydrogel films based on basil seed (Ocimum basilicum L.) mucilage for wound dressing drug delivery applications. Int. J. Biol. Macromol. 2020, 163, 336–347. [Google Scholar] [CrossRef]
- Cai, T.; Huo, S.; Wang, T.; Sun, W.; Tong, Z. Self-healable tough supramolecular hydrogels crosslinked by poly-cyclodextrin through host-guest interaction. Carbohydr. Polym. 2018, 193, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Ladewig, K.; Fu, Q.; Blencowe, A.; Qiao, G.G. Cyclodextrin-based supramolecular assemblies and hydrogels: Recent advances and future perspectives. Macromol. Rapid Commun. 2014, 35, 1166–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, W.; Wang, M.; Chen, M.; Niu, W.; Li, Y.; Wang, Y.; Luo, M.; Xie, C.; Leng, T.; Lei, B. Injectable Antibacterial Antiinflammatory Molecular Hybrid Hydrogel Dressing for Rapid MDRB-Infected Wound Repair and Therapy. Chem. Eng. J. 2021, 409, 128140. [Google Scholar] [CrossRef]
- Peng, L.; Chang, L.; Si, M.; Lin, J.; Wei, Y.; Wang, S.; Liu, H.; Han, B.; Jiang, L. Hydrogel-coated dental device with adhesion-inhibiting and colony-suppressing properties. ACS Appl. Mater. Interfaces 2020, 12, 9718–9725. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, A. Synthesis and characterization of alginate and sterculia gum based hydrogel for brain drug delivery applications. Int. J. Biol. Macromol. 2020, 148, 248–257. [Google Scholar] [CrossRef]
- Liu, Y.; Du, J.; Peng, P.; Cheng, R.; Lin, J.; Xu, C.; Yang, H.; Cui, W.; Mao, H.; Li, Y.; et al. Regulation of the inflammatory cycle by a controllable release hydrogel for eliminating postoperative inflammation after discectomy. Bioact. Mater. 2020, 6, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. [Google Scholar] [CrossRef]
- Dreiss, C.A. Hydrogel design strategies for drug delivery. Curr. Opin. Colloid Interface Sci. 2020, 48, 1–17. [Google Scholar] [CrossRef]
- Peers, S.; Montembault, A.; Ladavière, C. Chitosan hydrogels for sustained drug delivery. J. Control. Release 2020, 326, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Krukiewicz, K.; Zak, J.K. Biomaterial-based regional chemotherapy: Local anticancer drug delivery to enhance chemotherapy and minimize its side-effects. Mater. Sci. Eng. C 2016, 62, 927–942. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Torres-Luna, C.; Fan, X.; Domszy, R.; Hu, N.; Wang, N.S.; Yang, A. Hydrogel-based ocular drug delivery systems for hydrophobic drugs. Eur. J. Pharm. Sci. 2020, 154, 105503. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Concheiro, A.; Alvarez-Lorenzo, C. Chemically cross-linked and grafted cyclodextrin hydrogels: From nanostructures to drug-eluting medical devices. Adv. Drug Deliv. Rev. 2013, 65, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Morin-Crini, N.; Crini, G. Environmental applications of water-insoluble β-cyclodextrin–epichlorohydrin polymers. Prog. Polym. Sci. 2013, 38, 344–368. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Fundamentals and applications of cyclodextrins. In Cyclodextrin Fundamentals, Reactivity and Analysis; Springer: Cham, Switzerland, 2018; pp. 1–55. [Google Scholar]
- Crini, G. Cyclodextrin–epichlorohydrin polymers synthesis, characterization and applications to wastewater treatment: A review. Enviro. Chem. Lett. 2021, 19, 2383–2403. [Google Scholar] [CrossRef]
- Cova, T.F.; Murtinho, D.; Pais, A.A.; Valente, A.J. Combining cellulose and cyclodextrins: Fascinating designs for materials and pharmaceutics. Front. Chem. 2018, 6, 271. [Google Scholar] [CrossRef]
- Cova, T.F.; Murtinho, D.; Aguado, R.; Pais, A.A.; Valente, A.J. Cyclodextrin polymers and cyclodextrin-containing polysaccharides for water remediation. Polysaccharides 2021, 2, 16–38. [Google Scholar] [CrossRef]
- Pinho, E. Cyclodextrins-based hydrogel. In Plant and Algal Hydrogels for Drug Delivery and Regenerative Medicine; Woodhead Publishing: Sawston, UK, 2021; pp. 113–141. [Google Scholar]
- Machín, R.; Isasi, J.R.; Vélaz, I. β-Cyclodextrin hydrogels as potential drug delivery systems. Carbohydr. Polym. 2012, 87, 2024–2030. [Google Scholar] [CrossRef]
- Liu, G.; Yuan, Q.; Hollett, G.; Zhao, W.; Kang, Y.; Wu, J. Cyclodextrin-based host–guest supramolecular hydrogel and its application in biomedical fields. Polym. Chem. 2018, 9, 3436–3449. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Smart stimuli-responsive drug delivery systems based on cyclodextrin: A review. Carbohydr. Polym. 2021, 251, 116871. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, H.; Moradi, S.; Hudson, S.M.; Tonelli, A.E. Chitosan based hydrogels and their applications for drug delivery in wound dressings: A review. Carbohydr. Polym. 2018, 199, 445–460. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Tian, Y.; Liu, J. Chemical and physical chitosan hydrogels as prospective carriers for drug delivery: A review. J. Mater. Chem. B 2020, 8, 10050–10064. [Google Scholar] [CrossRef] [PubMed]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Guo, X.; Wang, Y.; Qin, Y.; Shen, P.; Peng, Q. Structures, properties and application of alginic acid: A review. Int. J. Biol. Macromol. 2020, 162, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliver. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Patel, S.; Goyal, A. Applications of natural polymer gum arabic: A review. Int. J. Food Prop. 2015, 18, 986–998. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic acid/acrylamide based hydrogels and its properties-A review. Polym. Degrad. Stabil. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Espinosa-Andrews, H.; Velasquillo-Martínez, C.; García-Carvajal, Z.Y. Composite hydrogels based on gelatin, chitosan and polyvinyl alcohol to biomedical applications: A review. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 1–20. [Google Scholar] [CrossRef]
- Timofejeva, A.; D’Este, M.; Loca, D. Calcium phosphate/polyvinyl alcohol composite hydrogels: A review on the freeze-thawing synthesis approach and applications in regenerative medicine. Eur. Polym. J. 2017, 95, 547–565. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, D.; Shang, T.; Guo, L.; Yang, B.; Xu, X. Chain conformation transition induced host-guest assembly between triple helical curdlan and β-CD for drug delivery. Biomater. Sci. 2020, 8, 1638–1648. [Google Scholar] [CrossRef]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef]
- Tian, B.; Liu, Y.; Liu, J. Cyclodextrin as a magic switch in covalent and non-covalent anticancer drug release systems. Carbohydr. Polym. 2020, 242, 116401. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.C.; Yang, H. Hydrogel-based ocular drug delivery systems: Emerging fabrication strategies, applications, and bench-to-bedside manufacturing considerations. J. Control. Release 2019, 306, 29–39. [Google Scholar] [CrossRef]
- Gunathilake, T.M.S.U.; Ching, Y.C.; Chuah, C.H.; Abd Rahman, N.; Nai-Shang, L. Recent advances in celluloses and their hybrids for stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2020, 158, 670–688. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Dave, A.M.; Kumbar, S.G.; Rudzinski, W.E. Stimulus-responsive “smart” hydrogels as novel drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 957–974. [Google Scholar] [CrossRef] [PubMed]
- Mignon, A.; De Belie, N.; Dubruel, P.; Van Vlierberghe, S. Superabsorbent polymers: A review on the characteristics and applications of synthetic, polysaccharide-based, semi-synthetic and ‘smart’derivatives. Eur. Polym. J. 2019, 117, 165–178. [Google Scholar] [CrossRef]
- Zhang, K.; Feng, Q.; Fang, Z.; Gu, L.; Bian, L. Structurally Dynamic Hydrogels for Biomedical Applications: Pursuing a Fine Balance between Macroscopic Stability and Microscopic Dynamics. Chem. Rev. 2021, 121, 11149–11193. [Google Scholar] [CrossRef] [PubMed]
- Kiti, K.; Suwantong, O. Bilayer wound dressing based on sodium alginate incorporated with curcumin-β-cyclodextrin inclusion complex/chitosan hydrogel. Int. J. Biol. Macromol. 2020, 164, 4113–4124. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Barati, A.; Tonelli, A.E.; Hamedi, H. Chitosan-based hydrogels loading with thyme oil cyclodextrin inclusion compounds: From preparation to characterization. Eur. Polym. J. 2020, 122, 109303. [Google Scholar] [CrossRef]
- Barragán, C.A.R.; Balleza, E.R.M.; García-Uriostegui, L.; Ortega, J.A.A.; Toríz, G.; Delgado, E. Rheological characterization of new thermosensitive hydrogels formed by chitosan, glycerophosphate, and phosphorylated β-cyclodextrin. Carbohydr. Polym. 2018, 201, 471–481. [Google Scholar] [CrossRef]
- Das, S.; Subuddhi, U. Cyclodextrin mediated controlled release of naproxen from pH-sensitive chitosan/poly (vinyl alcohol) hydrogels for colon targeted delivery. Ind. Eng. Chem. Res. 2013, 52, 14192–14200. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, A.; Lee, W.; Youn, J.; Rim, M.A.; Kim, W.; Kim, N.; Song, J.E.; Khang, G. Preparation and characterization of an injectable dexamethasone-cyclodextrin complexes-loaded gellan gum hydrogel for cartilage tissue engineering. J. Control. Release 2020, 327, 747–765. [Google Scholar] [CrossRef]
- Bianchi, S.E.; Machado, B.E.; da Silva, M.G.; da Silva, M.M.; Dal Bosco, L.; Marques, M.S.; Horn, A.P.; Persich, L.; Geller, F.C.; Argenta, D.; et al. Coumestrol/hydroxypropyl-β-cyclodextrin association incorporated in hydroxypropyl methylcellulose hydrogel exhibits wound healing effect: In vitro and in vivo study. Eur. J. Pharm. Sci. 2018, 119, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Poudel, A.J.; Huang, L.; Wang, Y.; Abdalla, A.M.; Yang, G. Nanocellulose hyperfine network achieves sustained release of berberine hydrochloride solubilized with β-cyclodextrin for potential anti-infection oral administration. Int. J. Biol. Macromol. 2020, 153, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Chunshom, N.; Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Development of gallic acid/cyclodextrin inclusion complex in freeze-dried bacterial cellulose and poly (vinyl alcohol) hydrogel: Controlled-release characteristic and antioxidant properties. Mater. Chem. Phys. 2019, 232, 294–300. [Google Scholar] [CrossRef]
- Eid, M.; Sobhy, R.; Zhou, P.; Wei, X.; Wu, D.; Li, B. β-cyclodextrin-soy soluble polysaccharide based core-shell bionanocomposites hydrogel for vitamin E swelling controlled delivery. Food Hydrocoll. 2020, 104, 105751. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Li, Z.; Ma, M.; Jia, S.; Li, X. Use of hydroxypropyl β-cyclodextrin as a dual functional component in xanthan hydrogel for sustained drug release and antibacterial activity. Colloid Surf. A 2020, 587, 124368. [Google Scholar] [CrossRef]
- Yu, B.; Zhan, A.; Liu, Q.; Ye, H.; Huang, X.; Shu, Y.; Yang, Y.; Liu, H. A designed supramolecular cross-linking hydrogel for the direct, convenient, and efficient administration of hydrophobic drugs. Int. J. Pharm. 2020, 578, 119075. [Google Scholar] [CrossRef]
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 115011. [Google Scholar] [CrossRef]
- Malik, N.S.; Ahmad, M.; Minhas, M.U. Cross-linked β-cyclodextrin and carboxymethyl cellulose hydrogels for controlled drug delivery of acyclovir. PLoS ONE 2017, 12, e0172727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric acid crosslinked β-cyclodextrin/carboxymethylcellulose hydrogel films for controlled delivery of poorly soluble drugs. Carbohydr. Polym. 2017, 164, 339–348. [Google Scholar] [CrossRef]
- Amiel, A.G.; Palomino-Durand, C.; Maton, M.; Lopez, M.; Cazaux, F.; Chai, F.; Neut, C.; Foligné, B.; Martel, B.; Blanchemain, N. Designed sponges based on chitosan and cyclodextrin polymer for a local release of ciprofloxacin in diabetic foot infections. Int. J. Pharm. 2020, 587, 119677. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Wan, W.; Zhu, S.; Liu, Q. Preparation of crystalline nanocellulose/hydroxypropyl β-cyclodextrin/carboxymethyl cellulose polyelectrolyte complexes and their controlled release of neohesperidin-copper (II) in vitro. Int. J. Biol. Macromol. 2020, 163, 1518–1528. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. Preparation and characterization of polyvinyl alcohol/β-cyclodextrin/GO-Ag nanocomposite with improved antibacterial and strength properties. Polym. Adv. Technol. 2019, 30, 447–456. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Z.; Zhu, J.; Wen, Y.; Zhao, F.; Lei, L.; Phan-Thien, N.; Khoo, B.C.; Li, J. Thermoresponsive hydrogel induced by dual supramolecular assemblies and its controlled release property for enhanced anticancer drug delivery. Biomacromolecules 2020, 21, 1516–1527. [Google Scholar] [CrossRef]
- Li, R.; Guan, X.; Lin, X.; Guan, P.; Zhang, X.; Rao, Z.; Du, L.; Zhao, J.; Rong, J.; Zhao, J. Poly (2-hydroxyethyl methacrylate)/β-cyclodextrin-hyaluronan contact lens with tear protein adsorption resistance and sustained drug delivery for ophthalmic diseases. Acta Biomater. 2020, 110, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, M.A.; Nicoli, S.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C. Cyclosporine-loaded cross-linked inserts of sodium hyaluronan and hydroxypropyl-β-cyclodextrin for ocular administration. Carbohydr. Polym. 2018, 201, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Öztürk-Atar, K.; Kaplan, M.; Çalış, S. Development and evaluation of polymeric micelle containing tablet formulation for poorly water-soluble drug: Tamoxifen citrate. Drug Dev. Ind. Pharm. 2020, 46, 1695–1704. [Google Scholar] [CrossRef]
- Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. The application and prospects of cyclodextrin inclusion complexes and polymers in the food industry: A review. Polym. Int. 2020, 69, 597–603. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent advances in host-guest self-assembled cyclodextrin carriers: Implications for responsive drug delivery and biomedical engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Tejashri, G.; Amrita, B.; Darshana, J. Cyclodextrin based nanosponges for pharmaceutical use: A review. Acta Pharm. 2013, 63, 335–358. [Google Scholar] [CrossRef]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z.G. Cyclodextrin Polymers: Structure, Synthesis, and Use as Drug Carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, J.; Jin, Z. Supramolecular hydrogel formation between chitosan and hydroxypropyl β-cyclodextrin via Diels-Alder reaction and its drug delivery. Int. J. Biol. Macromol. 2018, 114, 381–391. [Google Scholar] [CrossRef]
- Gami, P.; Kundu, D.; Seera, S.D.K.; Banerjee, T. Chemically crosslinked xylan–β-Cyclodextrin hydrogel for the in vitro delivery of curcumin and 5-Fluorouracil. Int. J. Biol. Macromol. 2020, 158, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Fernandez, B.; Lopez-Viota, M.; Concheiro, A.; Alvarez-Lorenzo, C. Synergistic performance of cyclodextrin–agar hydrogels for ciprofloxacin delivery and antimicrobial effect. Carbohydr. Polym. 2011, 85, 765–774. [Google Scholar] [CrossRef]
- Otero-Espinar, F.J.; Torres-Labandeira, J.J.; Alvarez-Lorenzo, C.; Blanco-Méndez, J. Cyclodextrins in drug delivery systems. J. Drug Deliv. Sci. Tec. 2010, 20, 289–301. [Google Scholar] [CrossRef]
- Li, J.; Loh, X.J. Cyclodextrin-based supramolecular architectures: Syntheses, structures, and applications for drug and gene delivery. Adv. Drug Deliver. Rev. 2008, 60, 1000–1017. [Google Scholar] [CrossRef]
- Sharaf, S.; El-Naggar, M.E. Wound dressing properties of cationized cotton fabric treated with carrageenan/cyclodextrin hydrogel loaded with honey bee propolis extract. Int. J. Biol. Macromol. 2019, 133, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, M.G.; Morrison, P.W.; Boostrom, H.M.; Morgan, S.R.; Fallon, M.; Lewis, P.N. In vitro topical delivery of chlorhexidine to the cornea: Enhancement using drug-loaded contact lenses and β-cyclodextrin complexation, and the importance of simulating tear irrigation. Mol. Pharm. 2020, 17, 1428–1441. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Briffa, S.M.; Swingler, S.; Gibson, H.; Kannappan, V.; Adamus, G.; Kowalczuk, M.; Martin, C.; Radecka, I. Synthesis of silver nanoparticles using curcumin-cyclodextrins loaded into bacterial cellulose-based hydrogels for wound dressing applications. Biomacromolecules 2020, 21, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Gularte, M.S.; Quadrado, R.F.; Pedra, N.S.; Soares, M.S.; Bona, N.P.; Spanevello, R.M.; Fajardo, A.R. Preparation, characterization and antitumor activity of a cationic starch-derivative membrane embedded with a β-cyclodextrin/curcumin inclusion complex. Int. J. Biol. Macromol. 2020, 148, 140–152. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Barati, A.; Tonelli, A.E.; Hamedi, H. Effect of clinoptilolite on structure and drug release behavior of chitosan/thyme oil γ-Cyclodextrin inclusion compound hydrogels. J. Appl. Polym. Sci. 2021, 138, 49822. [Google Scholar] [CrossRef]
- Moradi, S.; Barati, A.; Salehi, E.; Tonelli, A.E.; Hamedi, H. Preparation and characterization of chitosan based hydrogels containing cyclodextrin inclusion compounds or nanoemulsions of thyme oil. Polym. Int. 2019, 68, 1891–1902. [Google Scholar] [CrossRef]
- Sajeesh, S.; Bouchemal, K.; Marsaud, V.; Vauthier, C.; Sharma, C.P. Cyclodextrin complexed insulin encapsulated hydrogel microparticles: An oral delivery system for insulin. J. Control. Release 2010, 147, 377–384. [Google Scholar] [CrossRef]
- Okubo, M.; Iohara, D.; Anraku, M.; Higashi, T.; Uekama, K.; Hirayama, F. A thermoresponsive hydrophobically modified hydroxypropylmethylcellulose/cyclodextrin injectable hydrogel for the sustained release of drugs. Int. J. Pharm. 2020, 575, 118845. [Google Scholar] [CrossRef]
- Sarkar, A.; Mackie, A.R. Engineering oral delivery of hydrophobic bioactives in real-world scenarios. Curr. Opin. Colloid Interface Sci. 2020, 48, 40–52. [Google Scholar] [CrossRef]
- Xiang, J.; Shen, L.; Hong, Y. Status and future scope of hydrogels in wound healing: Synthesis, materials and evaluation. Eur. Polym. J. 2020, 130, 109609. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, M.; Zhang, Y.; Pei, R. Recent progress of highly adhesive hydrogels as wound dressings. Biomacromolecules 2020, 21, 3966–3983. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.A.; Penn, C.C.; Negvesky, G.J.; Butrus, S.I. Fungal and parasitic infections of the eye. Clin. Microbiol. Rev. 2000, 13, 662–685. [Google Scholar] [CrossRef]
- Jumelle, C.; Gholizadeh, S.; Annabi, N.; Dana, R. Advances and limitations of drug delivery systems formulated as eye drops. J. Control. Release 2020, 321, 1–22. [Google Scholar] [CrossRef]
- El-Zeiny, H.M.; Abukhadra, M.R.; Sayed, O.M.; Osman, A.H. Ahmed, S.A. Insight into novel β-cyclodextrin-grafted-poly (N-vinylcaprolactam) nanogel structures as advanced carriers for 5-fluorouracil: Equilibrium behavior and pharmacokinetic modeling. Colloid Surf. A 2020, 586, 124197. [Google Scholar] [CrossRef]
- Moncada-Basualto, M.; Matsuhiro, B.; Mansilla, A.; Lapier, M.; Maya, J.D.; Olea-Azar, C. Supramolecular hydrogels of β-cyclodextrin linked to calcium homopoly-l-guluronate for release of coumarins with trypanocidal activity. Carbohydr. Polym. 2019, 204, 170–181. [Google Scholar] [CrossRef]
- Gholibegloo, E.; Mortezazadeh, T.; Salehian, F.; Ramazani, A.; Amanlou, M.; Khoobi, M. Improved curcumin loading, release, solubility and toxicity by tuning the molar ratio of cross-linker to β-cyclodextrin. Carbohydr. Polym. 2019, 213, 70–78. [Google Scholar] [CrossRef]
- Zhou, X.; Luo, Z.; Baidya, A.; Kim, H.J.; Wang, C.; Jiang, X.; Qu, M.; Zhu, J.; Ren, L.; Vajhadin, F.; et al. Biodegradable β-Cyclodextrin Conjugated Gelatin Methacryloyl Microneedle for Delivery of Water-Insoluble Drug. Adv. Healthc. Mater. 2020, 9, 2000527. [Google Scholar] [CrossRef]
- Khalid, Q.; Ahmad, M.; Minhas, M.U.; Batool, F.; Malik, N.S.; Rehman, M. Novel β-cyclodextrin nanosponges by chain growth condensation for solubility enhancement of dexibuprofen: Characterization and acute oral toxicity studies. J. Drug Deliv. Sci. Technol. 2021, 61, 102089. [Google Scholar] [CrossRef]
- Soleimani, K.; Arkan, E.; Derakhshankhah, H.; Haghshenas, B.; Jahanban-Esfahlan, R.; Jaymand, M. A novel bioreducible and pH-responsive magnetic nanohydrogel based on β-cyclodextrin for chemo/hyperthermia therapy of cancer. Carbohydr. Polym. 2020, 252, 117229. [Google Scholar] [CrossRef]
- Fiorica, C.; Palumbo, F.S.; Pitarresi, G.; Puleio, R.; Condorelli, L.; Collura, G.; Giammona, G. A hyaluronic acid/cyclodextrin based injectable hydrogel for local doxorubicin delivery to solid tumors. Int. J. Pharm. 2020, 589, 119879. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Nariya, P.; Joshi, A.; Vohra, A.; Devkar, R.; Seshadri, S.; Thakore, S. Carbon nanotube embedded cyclodextrin polymer derived injectable nanocarrier: A multiple faceted platform for stimulation of multi-drug resistance reversal. Carbohydr. Polym. 2020, 247, 116751. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Le, P.T.; Kwon, H.J.; Park, K.D. Supramolecular assembly of tetronic–adamantane and poly (β-cyclodextrin) as injectable shear-thinning hydrogels. J. Mat. Chem. B 2019, 7, 3374–3382. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Y.; Chen, S.; Cheong, K.L.; Teng, B. Carboxymethyl β-cyclodextrin grafted carboxymethyl chitosan hydrogel-based microparticles for oral insulin delivery. Carbohydr. Polym. 2020, 246, 116617. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.K.; Mia, M.N.H.; Rahman, M.T.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Luo, J.; Shi, X.; Li, L.; Tan, Z.; Feng, F.; Li, J.; Pang, M.; Wang, X.; He, L. An injectable and self-healing hydrogel with controlled release of curcumin to repair spinal cord injury. Bioact. Mater. 2021, 6, 4816–4829. [Google Scholar] [CrossRef] [PubMed]
- Gull, N.; Khan, S.M.; Butt, O.M.; Islam, A.; Shah, A.; Jabeen, S.; Khan, S.U.; Khan, A.; Khan, R.U.; Butt, M.T.Z. Inflammation targeted chitosan-based hydrogel for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2020, 162, 175–187. [Google Scholar] [CrossRef]
- Ata, S.; Rasool, A.; Islam, A.; Bibi, I.; Rizwan, M.; Azeem, M.K.; Iqbal, M. Loading of Cefixime to pH sensitive chitosan based hydrogel and investigation of controlled release kinetics. Int. J. Biol. Macromol. 2020, 155, 1236–1244. [Google Scholar] [CrossRef]
- Jeong, K.; Yu, Y.J.; You, J.Y.; Rhee, W.J.; Kim, J.A. Exosome-mediated microRNA-497 delivery for anti-cancer therapy in a microfluidic 3D lung cancer model. Lab. Chip 2020, 20, 548–557. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, S.; Yu, S.; Li, J.; Tan, G.; Li, S.; Pan, W. A Hybrid Genipin-Cross-Linked Hydrogel/Nanostructured Lipid Carrier for Ocular Drug Delivery: Cellular, ex Vivo, and in Vivo Evaluation. ACS Biomater. Sci. Eng. 2020, 6, 1543–1552. [Google Scholar] [CrossRef]
- Utine, C.A.; Stern, M.; Akpek, E.K. Clinical review: Topical ophthalmic use of cyclosporin A. Ocul. Immunol. Inflamm. 2010, 18, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Periman, L.M.; Mah, F.S.; Karpecki, P.M. A review of the mechanism of action of cyclosporine A: The role of cyclosporine a in dry eye disease and recent formulation developments. Clin. Ophthalmol. 2020, 14, 4187. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Bharadwaj, S.; Lee, K.E.; Kang, S.G. Therapeutic nanoemulsions in ophthalmic drug administration: Concept in formulations and characterization techniques for ocular drug delivery. J. Control. Release 2020, 328, 895–916. [Google Scholar] [CrossRef]
- Campos, P.M.; Petrilli, R.; Lopez, R.F. The prominence of the dosage form design to treat ocular diseases. Int. J. Pharm. 2020, 586, 119577. [Google Scholar] [CrossRef]
- Pradhan, S.; Keller, K.A.; Sperduto, J.L.; Slater, J.H. Fundamentals of laser-based hydrogel degradation and applications in cell and tissue engineering. Adv. Healthc. Mater. 2017, 6, 1700681. [Google Scholar] [CrossRef]
- Tondera, C.; Hauser, S.; Krüger-Genge, A.; Jung, F.; Neffe, A.T.; Lendlein, A.; Klopfleisch, R.; Steinbach, J.; Neuber, C.; Pietzsch, J. Gelatin-based hydrogel degradation and tissue interaction in vivo: Insights from multimodal preclinical imaging in immunocompetent nude mice. Theranostics 2016, 6, 2114. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, X.; Ren, Y.; Xue, W.; Liu, S.; Wang, P.; Zhao, M.; Xu, H.; Chi, B. Injectable adaptive self-healing hyaluronic acid/poly (γ-glutamic acid) hydrogel for cutaneous wound healing. Acta Biomater. 2021, 127, 102–115. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Z.; Liu, X.; Ni, X.; Li, J. Gelatin-based hydrogels with β-cyclodextrin as a dual functional component for enhanced drug loading and controlled release. RSC Adv. 2013, 3, 25041–25049. [Google Scholar] [CrossRef]
- Li, J. Self-assembled supramolecular hydrogels based on polymer–cyclodextrin inclusion complexes for drug delivery. NPG Asia Mater. 2010, 2, 112–118. [Google Scholar] [CrossRef]
- Jalalvandi, E.; Cabral, J.; Hanton, L.R.; Moratti, S.C. Cyclodextrin-polyhydrazine degradable gels for hydrophobic drug delivery. Mater. Sci. Eng. C 2016, 69, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Kersani, D.; Mougin, J.; Lopez, M.; Degoutin, S.; Tabary, N.; Cazaux, F.; Janus, L.; Maton, M.; Chai, F.; Sobocinski, J.; et al. Stent coating by electrospinning with chitosan/poly-cyclodextrin based nanofibers loaded with simvastatin for restenosis prevention. Eur. J. Pharm. Biopharm. 2020, 150, 156–167. [Google Scholar] [CrossRef]
- Yang, N.; Wang, Y.; Zhang, Q.; Chen, L.; Zhao, Y. In situ formation of poly (thiolated chitosan-co-alkylated β-cyclodextrin) hydrogels using click cross-linking for sustained drug release. J. Mater. Sci. 2019, 54, 1677–1691. [Google Scholar] [CrossRef]
- Sheng, J.; Wang, Y.; Xiong, L.; Luo, Q.; Li, X.; Shen, Z.; Zhu, W. Injectable doxorubicin-loaded hydrogels based on dendron-like β-cyclodextrin-poly (ethylene glycol) conjugates. Polym. Chem. 2017, 8, 1680–1688. [Google Scholar] [CrossRef]
- Van de Manakker, F.; Braeckmans, K.; Morabit, N.E.; De Smedt, S.C.; van Nostrum, C.F.; Hennink, W.E. Protein-Release Behavior of Self-Assembled PEG-β-Cyclodextrin/PEG-Cholesterol Hydrogels. Adv. Funct. Mater. 2009, 19, 2992–3001. [Google Scholar] [CrossRef]
- Yu, J.; Fan, H.; Huang, J.; Chen, J. Fabrication and evaluation of reduction-sensitive supramolecular hydrogel based on cyclodextrin/polymer inclusion for injectable drug-carrier application. Soft Matter 2011, 7, 7386–7394. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, S.; Zhang, B.; Wu, Q. Preparation and swelling behavior of a novel self-assembled β-cyclodextrin/acrylic acid/sodium alginate hydrogel. Carbohydr. Polym. 2014, 113, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Maity, P.P.; Dhara, S.; Pal, S. Biocompatible, stimuli-responsive hydrogel of chemically crosslinked β-cyclodextrin as amoxicillin carrier. J. Appl. Polym. Sci. 2018, 135, 45939. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Ni, X.; Wang, X.; Li, H.; Leong, K.W. Self-assembled supramolecular hydrogels formed by biodegradable PEO-PHB-PEO triblock copolymers and α-cyclodextrin for controlled drug delivery. Biomaterials 2006, 27, 4132–4140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, N.; Wang, D.; He, Y.; Chen, L.; Zhao, Y. Poly (MAH-β-cyclodextrin-co-NIPAAm) hydrogels with drug hosting and thermo/pH-sensitive for controlled drug release. Polym. Degrad. Stabil. 2018, 147, 123–131. [Google Scholar] [CrossRef]
- Yoon, S.J.; Hyun, H.; Lee, D.W.; Yang, D.H. Visible light-cured glycol chitosan hydrogel containing a beta-cyclodextrin-curcumin inclusion complex improves wound healing in vivo. Molecules 2017, 22, 1513. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Dong, X.; Wei, C.; Ma, G.; Liu, T.; Kong, D.; Lv, F. A visible and controllable porphyrin-poly (ethylene glycol)/α-cyclodextrin hydrogel nanocomposites system for photo response. Carbohydr. Polym. 2017, 175, 440–449. [Google Scholar] [CrossRef]
| Material for Forming Hydrogel | Cyclodextrin | Chitosan | Cellulose | Alginic Acid | Gum Arabic | Polyacryl Amide | Polyvinyl Alcohol |
|---|---|---|---|---|---|---|---|
| Source | Starch | Chitin | Plant | Alga | Acacia trees | Acrylonitrile | Vinyl acetate |
| Connection type | α-1, 4-glycosidic bond | β-1, 4-glycosidic bond | β-1, 4-glycosidic bond | 1, 4-glycosidic bond | - | - | - |
| Techniques | Radical polymerization; Click reaction; Nucleophilic addition/substitution | Photo- polymerization; Thermal polymerization | Chemical crosslinking; Free-radical polymerization; Grafting; Freeze-thaw | Enzymatically crosslinking; Chemical crosslinking | Photo-induced radical polymerization | Radiation-induced | Freeze-thaw |
| Kinds of drug delivery | Hydrophobic drug | Small molecules; peptides; proteins | Small molecules; peptides; proteins | Traditional low-molecular-weight drugs and macromolecules | Small molecules; proteins | Small molecules; peptides; proteins | Small molecules; peptides; proteins |
| Clinic trial | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Ref. | [27,28,29] | [30,31] | [32,33] | [34,35] | [36] | [37,38] | [39] |
| Types | Matrix | Preparation Methods | Characteristic | Ref. |
|---|---|---|---|---|
| Physically cross-linked cyclodextrin-containing hydrogels | Chitosan | Casting method | Bilayer hydrogels | [48] |
| Chitosan | Freeze-thaw cycling method | pH sensitivity | [49] | |
| Chitosan | Freezing method | Thermosensitive; Shortly gelation time (3 min or less) | [50] | |
| Chitosan/Poly(Vinyl Alcohol) | Dry at room temperature in vacuo | pH-specific release behavior | [51] | |
| Gellan gum | Gelation at room temperature | Biocompatible material | [52] | |
| Hydroxypropyl methylcellulose | Dispersion method | Benefit for skin | [53] | |
| Nanocellulose | 30 min with autoclaving (121 ℃, 103 kPa) | Sustained release | [54] | |
| poly (vinyl alcohol) | Freezing drying method | Long-term release | [55] | |
| Soy soluble polysaccharide | Reduced pressure and stored in a desiccator | 3D-nanocomposites, superabsorbent, malleable, bioadhesive | [56] | |
| Xanthan | Freezing drying method | Long-term release | [57] | |
| Chemically cross-linked cyclodextrin-containing hydrogels | 4-arm-Polyethylene glycol-Succinimidyl Glutarate | Nucleophilic substitution-based method | Improved the therapeutic effect | [58] |
| Agarose gel | Nucleophilic substitution-based method | Low gelling temperature for controlled drug delivery | [59] | |
| Carboxymethyl cellulose | Free radical polymerization crosslinking-based method | pH-responsive behaviour | [60] | |
| Carboxymethyl cellulose | Nucleophilic substitution-based method | Biocompatible, capable of controlling the release for a long duration | [61] | |
| Chitosan | Nucleophilic substitution-based method | Local antibiotic release | [62] | |
| Nanocellulose | Nucleophilic substitution-based method | Cell compatibility, non-cytotoxicity | [63] | |
| Polyvinyl alcohol | Nucleophilic substitution-based method | Good strength, elasticity, WVP, and swelling ability | [64] | |
| Poly(N-isopropylacrylamide) | Free radical polymerization crosslinking-based method | Thermoresponsive | [65] | |
| Poly(2-hydroxyethyl methacrylate) | Nucleophilic substitution-based method | Sustained drug delivery | [66] | |
| Sodium hyaluronan | Nucleophilic substitution-based method | Controlled release | [67] |
| Property | Physical Cross-Linked Hydrogel | Chemical Cross-Linked Hydrogel |
|---|---|---|
| Size of guest molecules | Small molecules (lipophilic) | Small molecules (lipophilic) |
| Drug loading strategies | Encapsulation | Encapsulation |
| Drug release speed | Can be controlled | Can be controlled |
| Drug release possible mechanisms | External stimulus; competition of external molecules | External stimulus; competition of external molecule |
| Duration times | Hours to days | Days to months |
| Drug delivery characteristic | High drug loading effectivity; low chance of drug deactivation | High drug loading effectivity; low chance of drug deactivation |
| Potential application | Drug delivery systems, injectable, wound dressings | Transdermal drug delivery, injectable, implantable, oral/ophthalmic drug carrier |
| Advantages | Non-toxic; cross-linking is reversible | Strong mechanical strength; the pore size can be adjusted; the variety of synthesis methods; difficult to degrade |
| Disadvantages | Low mechanical strength; difficult to adjust the pore size | Potentially toxic; no cross-linking is reversible |
| No. | Drug | Formation Materials | Hydrogel State | Type of Cells | Summary | Potential Application | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Berberine hydrochloride | β-CD; Bacterial cellulose; | Nano- particle | S. aureus; P. aeruginosa; E. coli | The ultra-fine network of bacterial cellulose resulted in different release characteristics of berberine hydrochloride. The drug-loaded hydrogel had a good antibacterial effect as revealed by in vitro experiments. | Oral administration medicine | [54] |
| 2 | Chlorhexidine | β-CD; NaCl; NaHCO3; CaCl2 | Contact lenses | E. coli | β-CD in eye drops significantly enhanced the delivery of chlorhexidine into the cornea. | Ocular delivery | [81] |
| 3 | Coumestrol | Hydroxypropyl-β-CD; methylcellulose | Not mentioned | Animals | Hydrogel has high efficacy in wound healing when compared to Dersani, with 50% wound healing achieved within a shorter period compared to this positive control. | Wound dressing materials | [53] |
| 4 | Curcumin | Hydroxypropyl-β-CD; silver nanoparticles; bacterial cellulose | Film | P. aeruginosa; S. aureus; C. aureus; Panc 1, U251, MSTO | The nano-silver particles loaded into the bacterial cellulose hydrogel showed high cytocompatibility and therapeutic effects against three common wound infection pathogens. | Wound dressing materials | [82] |
| 5 | Curcumin | β-CD; Polyvinyl alcohol | Film | Glioblastoma cell line C6; melanoma cell line B16F10; astrocyte cells | The hydrogel controlled the release of curcumin (48 h, 85% release). The polymer membrane had higher cytotoxicity than curcumin. The drug-loaded hydrogel showed prolonged cytotoxic effects (up to 96 h) at a lower concentration (50 μg/mL). | Local drug delivery system to treat cancer | [83] |
| 6 | Curcumin | 2-hydroxypropyl-β-CD; sodium alginate; chitosan | Film | E. coli; S. aureus; NCTC clone 929 cells; NHDF cells | High concentration of crosslinking agent concentration improved the mechanical properties of the hydrogel and decreased the hygroscopicity, water swelling, and weight loss. In addition, hydrogel showed a slow-release effect (t > 50 h). Curcumin-loaded double-layer hydrogel effectively treated E. coli and S. aureus. The double-layer hydrogel was not toxic to NCTC clone 929 cells and normal human dermal fibroblasts. | Wound dressing materials | [48] |
| 7 | Gallic acid | Hydroxypropyl-β-CD; bacterial cellulose; poly (vinyl alcohol) | Not mentioned | Not mentioned | The swelling properties during encapsulation were inferior. The release profile of the complex was slower compared with gallic acid. | Pharmaceutical and cosmetic products | [55] |
| 8 | Honey bee propolis extract | β-CD; κ-Carrageenan | Not mentioned | S. aureus; P. aeruginosa; Aspergillus Flavus; Candida albicans | Higher active compound concentration ensures sustained in vitro release. | Wound dressing | [80] |
| 9 | Levofloxacin; methotrexate | Hydroxypropyl-β-CD; xanthan gum | Film | E. coli; S. aureus | The hydrogel loaded with the methotrexate showed a well-controlled release profile (t > 600 min). The hydrogel loaded with levofloxacin had a good antibacterial effect. | Drug delivery system | [57] |
| 10 | Red thyme oil | γ-CD; polyvinyl alcohol; chitosan; clinoptilolite | Film | L929 cells | Hydrogels with clinoptilolite contained characteristics such as compressed structure, improved mechanical properties, decreased swelling values, and reduced release rate of the drug. In addition, prepared hydrogels were low-toxic based on MTT assay. | Drug delivery systems and wound dressings | [84] |
| 11 | Thyme oil | Methyl-β-CD; hydroxypropyl-β-CD; γ-CD; chitosan; polyvinyl alcohol | Film | E. coli; S. aureus | The water vapor transmission rate of the hydrogel was appropriate for application in wound dressing. The swelling degree of hydrogel loaded with thyme oil varied with the pH. The hydrogels containing γ-CD had good antibacterial activity. | Wound dressings | [49,85] |
| No. | Drug | Formation Materials | Hydrogel State | Types of Cell | Summary | Potential Application | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 5-Fluorouracil | β-CD; N-vinylcaprolactam; N, N′-methylene bisacrylamide | Nanogel | Human colon cancer cell lines (HCT 116); MRC-5 normal cells | The hydrogel had the best drug loading (659.7 mg/g) after controlling the feeding ratio. The drug release curve showed that the hydrogel could continue to release drugs for up to 30 h; especially in the intestinal juice with pH = 7.4, the 5-fluorouracil drug molecules contained therein were not completely released; and the maximum release rate was 68%. | Implantable hydrogels | [93] |
| 2 | Coumarin | β-CD; alginate; calcium homopoly-L-guluronate | Supramolecular hydrogel | RAW 264.7 cells; T. cruzi cells | The lowest release of substituted amidocoumarins from the hydrogels occurred at pH = 1.2 whereas the maximum release (34%) was observed at pH 8.0. | Biomedicine | [94] |
| 3 | Curcumin | β-CD; epiclon | Nanosponge | Non-tumorigenic human breast; invasive mouse breast cell lines (4T1) | The high degree of cross-linking led to the formation of mesoporous having high specific surface area and high loading capacity. Nanosponge showed no toxicity against MCF 10A and 4T1 cells as normal and cancerous cells, respectively. | Cancer therapy | [95] |
| 4 | Curcumin | Carboxymethyl-β-CD; gelatin; methacrylic anhydride | Microneedle arrays | B16F10 melanoma cell | The inclusion complex of curcumin maintained 90% of the initial concentration. Besides, the hydrogel could enhance the drug loading and adjust release. In vivo study showed that hydrogel had good biocompatibility and degradability. | Transdermal drug delivery | [96] |
| 5 | Dexamethasone | β-CD; low-acyl gellan gum; EDC | Injectable hydrogel | NIH/3T3 mouse embryo fibroblast | After drug loading, the gel-forming temperature of the modified hydrogel was reduced and the mechanical properties are improved. Hydrogel had a high affinity and release rate for drugs. In vivo studies had shown that the drug-loaded hydrogel improved the anti-inflammatory response. | Tissue engineering and regenerative medicine | [52] |
| 6 | Dexamethasone | β-CD; sodium hyaluronate | Delivery hydrogel | 3T3 cells | The novel hydrogels significantly improved the therapeutic effect of dexamethasone in burn wound healing. | Wound healing | [58] |
| 7 | Dexibuprofen | β-CD; acrylic acid; methylene bisacrylamide | Nanosponges | Not mentioned | The solubility of ibuprofen in the hydrogel was increased 6.3 times. In vitro release experiments demonstrated that the drug release rate of β-CD nanosponges reached 89% within 30 min under the condition of pH = 6.8. | Oral administration of lipophilic drugs | [97] |
| 8 | Diclofenac sodium | β-CD; sodium hyaluronan; EDC; | Contact lens materials | S. aureus; 3T3 fibroblasts | The hydrogel not only reduced the adsorption of tearing proteins due to electrostatic mutual repulsion but also improved encapsulation capacity and sustainable release of diclofenac (t > 72 h). In vitro cell viability analysis displayed that all hydrogels were non-toxic to 3T3 mouse fibroblasts. | Ophthalmic diseases | [66] |
| 9 | Doxorubicin | β-CD; 2-ethyl-2-oxazoline; aminopropyltriethoxy silane; FeCl2.4H2O; FeCl3.6H2O | Magnetic nanohydrogel | MCF7 cells | The magnetic nanohydrogel had a good drug loading rate (74%) and encapsulation rate (81%). Under acidic conditions (pH = 5.3), adding a small amount of GSH (10 mM) increased the release value (89.21%). The magnetic nanohydrogel had good cell compatibility even at high concentrations (10 mg/mL). | Implantable hydrogels | [98] |
| 10 | Doxorubicin | β-CD; agarose | Injectable hydrogel | Human embryonic kidney 239 cells; HeLa cells | The hydrogel was able to easily and uniformly load a drug at 30 °C. The drug-loaded hydrogel maintained the drug’s anti-cancer activity. In addition, the hydrogels did not exhibit toxicity toward the HEK-293 and HeLa cells. | Injectable hydrogel | [59] |
| 11 | Doxorubicin | β-CD; hyaluronic acid; bis(4-nitrophenyl) carbonate | Injectable hydrogel | Human colorectal cancer cells HCT-116 | Rheological tests showed that this hydrogel could be easily prepared and used on a schedule compatible with normal operating room procedures. In vitro experiments showed that the unique physical and chemical properties of the hydrogel ensured the sustained release of anticancer drugs (t > 32 d) and prevented the growth of colorectal cancer micelles under 3D culture conditions. | Device for localized chemotherapy of solid tumors | [99] |
| 12 | Doxorubicin; curcumin | β-CD; multiwalled carbon nanotubes; maleic anhydride; folic acid; hexamethylene diisocyanate | Nanocarrier | Not mentioned | This injectable hydrogel exhibited pH/thermo response and exerted a deleterious effect on the tumor. A sustained release of the two drugs was observed over a period of 30 h. The release rate of doxorubicin reached 90% under tumor microenvironmental conditions, and the release rate of curcumin reached 85% under high temperature and physiological pH conditions. | Injectable nanocarriers | [100] |
| 13 | Doxorubicin | β-CD; tetronic; adamantane | Injectable shear-thinning hydrogels | HeLa cell | The hydrogels showed shear-thinning behaviors, rapid recovery properties, pH-responsive properties, and long-term release of the hydrophobic drug. | Embolic material | [101] |
| 14 | Insulin | Carboxymethyl β-CD; carboxymethyl chitosan | Microparticles | Caco-2 cells | The insulin was loaded into the hydrogel, and the results of the drug release experiment found that the insulin was successfully retained in the stomach environment and slowly released after passing through the intestine. In vitro studies had shown that the hydrogel particles exhibited non-cytotoxicity and were mainly transported in the Caco-2 cell monolayer through paracellular pathways. | Oral drug delivery | [102] |
| 15 | Vitamin E | β-CD; soy soluble polysaccharides; galacturonic acid | Core-shell bionanomaterials hydrogel | Not mentioned | The hydrogel exhibited significant swelling adsorption and sustained release (t > 230 h) for the release of vitamin E in vitro. The encapsulation efficiency and drug loadings were 79.10% and 16.04%, respectively. In addition, after oral administration of the vitamin E-loaded hydrogel in rats, the vitamin E level in the plasma continued to increase within 12 h. | Oral drug carrier | [56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Tian, B.; Liu, Y.; Wan, J.-B. Cyclodextrin-Containing Hydrogels: A Review of Preparation Method, Drug Delivery, and Degradation Behavior. Int. J. Mol. Sci. 2021, 22, 13516. https://doi.org/10.3390/ijms222413516
Liu J, Tian B, Liu Y, Wan J-B. Cyclodextrin-Containing Hydrogels: A Review of Preparation Method, Drug Delivery, and Degradation Behavior. International Journal of Molecular Sciences. 2021; 22(24):13516. https://doi.org/10.3390/ijms222413516
Chicago/Turabian StyleLiu, Jiayue, Bingren Tian, Yumei Liu, and Jian-Bo Wan. 2021. "Cyclodextrin-Containing Hydrogels: A Review of Preparation Method, Drug Delivery, and Degradation Behavior" International Journal of Molecular Sciences 22, no. 24: 13516. https://doi.org/10.3390/ijms222413516
APA StyleLiu, J., Tian, B., Liu, Y., & Wan, J.-B. (2021). Cyclodextrin-Containing Hydrogels: A Review of Preparation Method, Drug Delivery, and Degradation Behavior. International Journal of Molecular Sciences, 22(24), 13516. https://doi.org/10.3390/ijms222413516