The Zinc-Sensing Receptor GPR39 in Physiology and as a Pharmacological Target
Abstract
1. Introduction
2. GPR39 Structure and Signaling
3. Ligands of GPR39
3.1. Endogenous Ligands of GPR39: Zn2+
3.2. Synthetic Ligands of GPR39
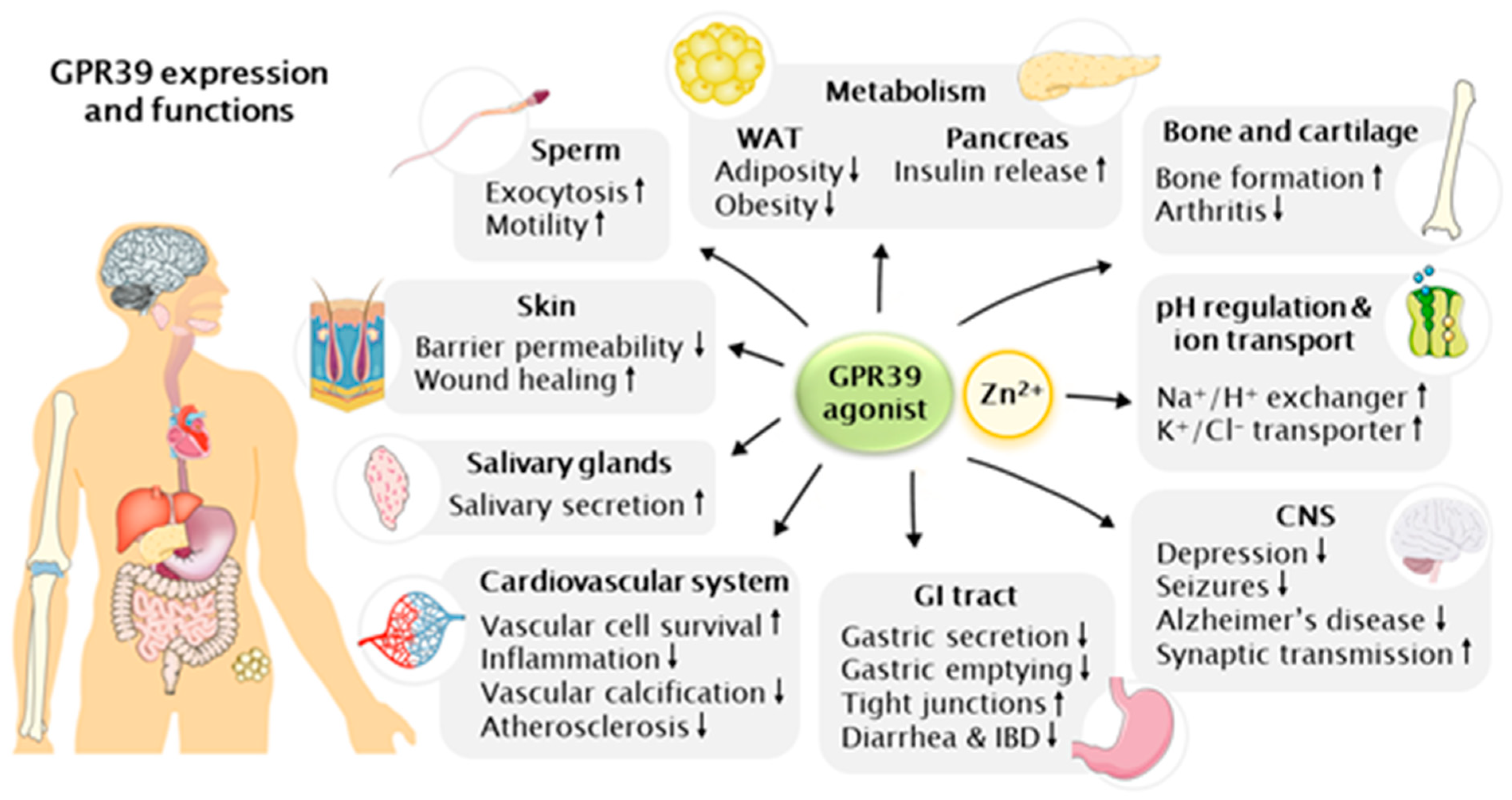
4. Physiological Functions of GPR39
4.1. pH Regulation and Ion Transport
4.2. Neuronal Functions
4.3. Skin and Wound Healing
4.4. Metabolic Diseases
4.5. Gastrointestinal Tract
4.6. Bone and Cartilage
4.7. Cardiovascular Functions
4.8. Other Recent Advances in Unraveling the Physiological Functions of GPR39
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Schwartz, T.W.; Frimurer, T.M.; Holst, B.; Rosenkilde, M.M.; Elling, C.E. Molecular mechanism of 7TM receptor activation—A global toggle switch model. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 481–519. [Google Scholar] [CrossRef]
- McKee, K.K.; Tan, C.P.; Palyha, O.C.; Liu, J.; Feighner, S.D.; Hreniuk, D.L.; Smith, R.G.; Howard, A.D.; der Ploeg, L.H. Cloning and characterization of two human G protein-coupled receptor genes (GPR38 and GPR39) related to the growth hormone secretagogue and neurotensin receptors. Genomics 1997, 46, 426–434. [Google Scholar] [CrossRef]
- Kaiya, H.; Kangawa, K.; Miyazato, M. Molecular evolution of GPCRs: Ghrelin/ghrelin receptors. J. Mol. Endocrinol. 2013, 52, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, E.; Landuyt, B.; Arckens, L.; Schoofs, L.; Luyten, W. Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem. Biophys. Res. Commun. 2006, 351, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Hershfinkel, M.; Moran, A.; Grossman, N.; Sekler, I. A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc. Natl. Acad. Sci. USA 2001, 98, 11749–11754. [Google Scholar] [CrossRef]
- Holst, B.; Egerod, K.L.; Schild, E.; Vickers, S.P.; Cheetham, S.; Gerlach, L.-O.; Storjohann, L.; Stidsen, C.E.; Jones, R.; Beck-Sickinger, A.G.; et al. GPR39 signaling is stimulated by zinc ions but not by obestatin. Endocrinology 2007, 148, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Miyazaki, T.; Munechika, K.; Yamashita, M.; Ikeda, Y.; Kamizono, A. Isolation of Zn2+ as an endogenous agonist of GPR39 from fetal bovine serum. J. Recept. Signal Transduct. Res. 2007, 27, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Holst, B.; Holliday, N.D.; Bach, A.; Elling, C.E.; Cox, H.M.; Schwartz, T.W. Common structural basis for constitutive activity of the ghrelin receptor family. J. Biol. Chem. 2004, 279, 53806–53817. [Google Scholar] [CrossRef] [PubMed]
- Chasapis, C.T.; Ntoupa, P.S.A.; Spiliopoulou, C.A.; Stefanidou, M.E. Recent aspects of the effects of zinc on human health. Arch. Toxicol. 2020, 94, 1443–1460. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc biochemistry: From a single zinc enzyme to a key element of life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar]
- Prasad, A.S. Discovery of human zinc deficiency: Its impact on human health and disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Levaot, N.; Hershfinkel, M. How cellular Zn2+ signaling drives physiological functions. Cell Calcium 2018, 75, 53–63. [Google Scholar] [CrossRef]
- Sharir, H.; Zinger, A.; Nevo, A.; Sekler, I.; Hershfinkel, M. Zinc released from injured cells is acting via the Zn2+-sensing receptor, ZnR, to trigger signaling leading to epithelial repair. J. Biol. Chem. 2010, 285, 26097–26106. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.J.; Gee, K.R.; Kennedy, R.T. Imaging of Zn2+ release from pancreatic β-cells at the level of single exocytotic events. Anal. Chem. 2003, 75, 3468–3475. [Google Scholar] [CrossRef]
- Paoletti, P.; Vergnano, A.M.; Barbour, B.; Casado, M. Zinc at glutamatergic synapses. Neuroscience 2009, 158, 126–136. [Google Scholar] [CrossRef]
- Egerod, K.L.; Holst, B.; Petersen, P.S.; Hansen, J.B.; Mulder, J.; Hökfelt, T.; Schwartz, T.W. GPR39 splice variants versus antisense gene LYPD1: Expression and regulation in gastrointestinal tract, endocrine pancreas, liver, and white adipose tissue. Mol. Endocrinol. 2007, 21, 1685–1698. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, S.; Ishida, J. GPR39-1b, the 5-transmembrane isoform of GPR39 interacts with neurotensin receptor NTSR1 and modifies its function. J. Recept. Signal Transduct. Res. 2014, 34, 307–312. [Google Scholar] [CrossRef]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.Y. Regulation, Signaling, and Physiological Functions of G-Proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, S.; Sahin, M.; Pantlen, A.; Saxena, A.; Toutzaris, D.; Pina, A.-L.; Geerts, A.; Golz, S.; Methner, A. The constitutively active orphan G-protein-coupled receptor GPR39 protects from cell death by increasing secretion of pigment epithelium-derived growth factor. J. Biol. Chem. 2008, 283, 7074–7081. [Google Scholar] [CrossRef]
- Cohen, L.; Sekler, I.; Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colonocytes and thereby tight junction formation in the colon. Cell Death Dis. 2014, 5, e1307. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Tang, S.; Zhang, W.; Gao, W.; Chen, Y. GPR39 activates proliferation and differentiation of porcine intramuscular preadipocytes through targeting the PI3K/AKT cell signaling pathway. J. Recept. Signal Transduct. Res. 2016, 36, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.V.; Ren, P.-G.; Avsian-Kretchmer, O.; Luo, C.-W.; Rauch, R.; Klein, C.; Hsueh, A.J.W. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 2005, 310, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Sharir, H.; Hershfinkel, M. The extracellular zinc-sensing receptor mediates intercellular communication by inducing ATP release. Biochem. Biophys. Res. Commun. 2005, 332, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.; Azriel-Tamir, H.; Arotsker, N.; Sekler, I.; Hershfinkel, M. Zinc sensing receptor signaling, mediated by GPR39, reduces butyrate-induced cell death in HT29 colonocytes via upregulation of clusterin. PLoS ONE 2012, 7, e35482. [Google Scholar] [CrossRef] [PubMed]
- Azriel-Tamir, H.; Sharir, H.; Schwartz, B.; Herskfinkel, M. Extracellular zinc triggers ERK-dependent activation of Na+/H+ exchange in colonocytes mediated by the zinc-sensing receptor. J. Biol. Chem. 2004, 279, 51804–51816. [Google Scholar] [CrossRef]
- Asraf, H.; Salomon, S.; Nevo, A.; Sekler, I.; Mayer, D.; Hershfinkel, M. The ZnR/GPR39 interacts with the CaSR to enhance signaling in prostate and salivary epithelia. J. Cell. Physiol. 2014, 229, 868–877. [Google Scholar] [CrossRef]
- Cho, D.; Mier, J.W.; Atkins, M.B. PI3K/Akt/mTOR pathway: A growth and proliferation pathway. In Renal Cell Carcinoma: Molecular Targets and Clinical Applications; Humana Press: Totowa, NJ, USA, 2009; pp. 267–285. ISBN 9781588297372. [Google Scholar]
- Lu, Z.; Xu, S. ERK1/2 MAP kinases in cell survival and apoptosis. IUBMB Life 2006, 58, 621–631. [Google Scholar] [CrossRef]
- Jing, W.; Sun, W.; Zhang, N.; Zhao, C.; Yan, X. The protective effects of the GPR39 agonist TC-G 1008 against TNF-α-induced inflammation in human fibroblast-like synoviocytes (FLSs). Eur. J. Pharmacol. 2019, 865, 172663. [Google Scholar] [CrossRef]
- Shan, W.; Qi, J.; Li, C.; Nie, X. Agonism of GPR39 displays protective effects against advanced glycation end-product (AGE)-induced degradation of extracellular matrix in human SW1353 cells. Arch. Biochem. Biophys. 2019, 677, 108164. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, M.; Xie, Y.; Jiang, Y.; Liu, M.; Yu, S.; Wang, B.; Liu, Q. Activation of GPR39 with the agonist TC-G 1008 ameliorates ox-LDL-induced attachment of monocytes to endothelial cells. Eur. J. Pharmacol. 2019, 858, 172451. [Google Scholar] [CrossRef]
- Holst, B.; Cygankiewicz, A.; Jensen, T.H.; Ankersen, M.; Schwartz, T.W. High Constitutive Signaling of the Ghrelin Receptor—Identification of a Potent Inverse Agonist. Mol. Endocrinol. 2003, 17, 2201–2210. [Google Scholar] [CrossRef]
- Zhang, L.; Song, J.; Zang, Z.; Tang, H.; Li, W.; Lai, S.; Deng, C. Adaptive evolution of GPR39 in diverse directions in vertebrates. Gen. Comp. Endocrinol. 2020, 299, 113610. [Google Scholar] [CrossRef]
- Shimizu, Y.; Koyama, R.; Kawamoto, T. Rho kinase-dependent desensitization of GPR39; a unique mechanism of GPCR downregulation. Biochem. Pharmacol. 2017, 140, 105–114. [Google Scholar] [CrossRef]
- Dubi, N.; Gheber, L.; Fishman, D.; Sekler, I.; Hershfinkel, M. Extracellular zinc and zinc-citrate, acting through a putative zinc-sensing receptor, regulate growth and survival of prostate cancer cells. Carcinogenesis 2008, 29, 1692–1700. [Google Scholar] [CrossRef]
- Rajagopal, S.; Shenoy, S.K. GPCR desensitization: Acute and prolonged phases. Cell. Signal. 2018, 41, 9–16. [Google Scholar] [CrossRef]
- Holliday, N.D.; Holst, B.; Rodionova, E.A.; Schwartz, T.W.; Cox, H.M. Importance of constitutive activity and arrestin-independent mechanisms for intracellular trafficking of the ghrelin receptor. Mol. Endocrinol. 2007, 21, 3100–3112. [Google Scholar] [CrossRef]
- González-Maeso, J. GPCR oligomers in pharmacology and signaling. Mol. Brain 2011, 4, 20. [Google Scholar] [CrossRef] [PubMed]
- Tena-Campos, M.; Ramon, E.; Borroto-Escuela, D.O.; Fuxe, K.; Garriga, P. The zinc binding receptor GPR39 interacts with 5-HT1A and GalR1 to form dynamic heteroreceptor complexes with signaling diversity. Biochim. Biophys. Acta 2015, 1852, 2585–2592. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Wind, N.; McClenaghan, C.; Verkuyl, J.M.; Watson, R.P.; Nash, M.S. GPR39 is coupled to TMEM16A in intestinal fibroblast-like cells. PLoS ONE 2012, 7, e47686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kovacs, Z.; Schacht, T.; Herrmann, A.-K.; Albrecht, P.; Lefkimmiatis, K.; Methner, A. Protein kinase inhibitor β enhances the constitutive activity of G-protein-coupled zinc receptor GPR39. Biochem. J. 2014, 462, 125–132. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Zhang, X.; Chen, D. Zac1/GPR39 phosphorylating CaMK-II contributes to the distinct roles of Pax3 and Pax7 in myogenic progression. Biochim. Biophys. Acta. Mol. Basis Dis. 2018, 1864, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gordillo-Martinez, F.; Jiang, L.; He, P.; Hong, W.; Wei, X.; Staines, K.A.; Macrae, V.E.; Zhang, C.; Yu, D.; et al. Zinc ameliorates human aortic valve calcification through GPR39 mediated ERK1/2 signaling pathway. Cardiovasc. Res. 2020. [Google Scholar] [CrossRef]
- Holst, B.; Egerod, K.L.; Jin, C.; Petersen, P.S.; Østergaard, M.V.; Hald, J.; Sprinkel, A.M.E.; Størling, J.; Mandrup-Poulsen, T.; Holst, J.J.; et al. G protein-coupled receptor 39 deficiency is associated with pancreatic islet dysfunction. Endocrinology 2009, 150, 2577–2585. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, M.; Schmidt, F.N.; Guterman-Ram, G.; Khayyeri, H.; Hiram-Bab, S.; Orenbuch, A.; Katchkovsky, S.; Aflalo, A.; Isaksson, H.; Busse, B.; et al. Perturbed bone composition and integrity with disorganized osteoblast function in zinc receptor/Gpr39-deficient mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 2507–2518. [Google Scholar] [CrossRef]
- Chorin, E.; Vinograd, O.; Fleidervish, I.; Gilad, D.; Herrmann, S.; Sekler, I.; Aizenman, E.; Hershfinkel, M. Upregulation of KCC2 activity by zinc-mediated neurotransmission via the mZnR/GPR39 receptor. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 12916–12926. [Google Scholar] [CrossRef]
- Nishida, K.; Hasegawa, A.; Yamasaki, S.; Uchida, R.; Ohashi, W.; Kurashima, Y.; Kunisawa, J.; Kimura, S.; Iwanaga, T.; Watarai, H.; et al. Mast cells play role in wound healing through the ZnT2/GPR39/IL-6 axis. Sci. Rep. 2019, 9, 10842. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Su, Y.; Zheng, Y.; Fu, B.; Tang, L.; Qin, Y.-X. Zinc regulates vascular endothelial cell activity through zinc-sensing receptor ZnR/GPR39. Am. J. Physiol. Cell Physiol. 2018, 314, C404–C414. [Google Scholar] [CrossRef]
- Voelkl, J.; Tuffaha, R.; Luong, T.T.D.; Zickler, D.; Masyout, J.; Feger, M.; Verheyen, N.; Blaschke, F.; Kuro-o, M.; Tomaschitz, A.; et al. Zinc Inhibits Phosphate-Induced Vascular Calcification through TNFAIP3-Mediated Suppression of NF-κB. J. Am. Soc. Nephrol. JASN 2018, 29, 1636–1648. [Google Scholar] [CrossRef]
- Giblin, L.J.; Chang, C.J.; Bentley, A.F.; Frederickson, C.; Lippard, S.J.; Frederickson, C.J. Zinc-secreting Paneth cells studied by ZP fluorescence. J. Histochem. Cytochem. 2006, 54, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Akita, M.; Sato, M.; Tomita, H. Localization of zinc in the rat submandibular gland and the effect of its deficiency on salivary secretion. Ann. Otol. Rhinol. Laryngol. 1999, 108, 300–308. [Google Scholar] [CrossRef]
- Perez-Rosello, T.; Anderson, C.T.; Schopfer, F.J.; Zhao, Y.; Gilad, D.; Salvatore, S.R.; Freeman, B.A.; Hershfinkel, M.; Aizenman, E.; Tzounopoulos, T. Synaptic Zn2+ inhibits neurotransmitter release by promoting endocannabinoid synthesis. J. Neurosci. Off. J. Soc. Neurosci. 2013, 33, 9259–9272. [Google Scholar] [CrossRef]
- Storjohann, L.; Holst, B.; Schwartz, T.W. Molecular mechanism of Zn2+ agonism in the extracellular domain of GPR39. FEBS Lett. 2008, 582, 2583–2588. [Google Scholar] [CrossRef]
- Storjohann, L.; Holst, B.; Schwartz, T.W. A second disulfide bridge from the N-terminal domain to extracellular loop 2 dampens receptor activity in GPR39. Biochemistry 2008, 47, 9198–9207. [Google Scholar] [CrossRef]
- Cohen, L.; Asraf, H.; Sekler, I.; Hershfinkel, M. Extracellular pH regulates zinc signaling via an Asp residue of the zinc-sensing receptor (ZnR/GPR39). J. Biol. Chem. 2012, 287, 33339–33350. [Google Scholar] [CrossRef] [PubMed]
- Ganay, T.; Asraf, H.; Aizenman, E.; Bogdanovic, M.; Sekler, I.; Hershfinkel, M. Regulation of neuronal pH by the metabotropic Zn(2+)-sensing Gq-coupled receptor, mZnR/GPR39. J. Neurochem. 2015, 135, 897–907. [Google Scholar] [CrossRef]
- Sakon, J.; Irwin, D.; Wilson, D.B.; Andrew Karplus, P. Structure and mechanism of endo/exocellulase E4 from Thermomonospora fusca. Nat. Struct. Biol. 1997, 4, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Boehm, M.; Hepworth, D.; Loria, P.M.; Norquay, L.D.; Filipski, K.J.; Chin, J.E.; Cameron, K.O.; Brenner, M.; Bonnette, P.; Cabral, S.; et al. Chemical Probe Identification Platform for Orphan GPCRs Using Focused Compound Screening: GPR39 as a Case Example. ACS Med. Chem. Lett. 2013, 4, 1079–1084. [Google Scholar] [CrossRef]
- Peukert, S.; Hughes, R.; Nunez, J.; He, G.; Yan, Z.; Jain, R.; Llamas, L.; Luchansky, S.; Carlson, A.; Liang, G.; et al. Discovery of 2-Pyridylpyrimidines as the First Orally Bioavailable GPR39 Agonists. ACS Med. Chem. Lett. 2014, 5, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Carlson, V.C.C.; Ford, M.M.; Carlson, T.L.; Lomniczi, A.; Grant, K.A.; Ferguson, B.; Cervera-Juanes, R.P. Modulation of Gpr39, a G-protein coupled receptor associated with alcohol use in non-human primates, curbs ethanol intake in mice. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2019, 44, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Muneoka, S.; Goto, M.; Nishimura, T.; Enomoto, K.; Kadoshima-Yamaoka, K.; Tomimori, Y. G Protein-Coupled Receptor 39 Agonist Improves Concanavalin A-Induced Hepatitis in Mice. Biol. Pharm. Bull. 2019, 42, 1415–1418. [Google Scholar] [CrossRef]
- Chai, X.; Zhang, W.; Chang, B.; Feng, X.; Song, J.; Li, L.; Yu, C.; Zhao, J.; Si, H. GPR39 agonist TC-G 1008 promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3569–3576. [Google Scholar] [CrossRef]
- Satianrapapong, W.; Pongkorpsakol, P.; Muanprasat, C. A G-protein coupled receptor 39 agonist stimulates proliferation of keratinocytes via an ERK-dependent pathway. Biomed. Pharmacother. 2020, 127, 110160. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Huang, X.-P.; Kroeze, W.K.; Roth, B.L. Discovery and Characterization of Novel GPR39 Agonists Allosterically Modulated by Zinc. Mol. Pharmacol. 2016, 90, 726–737. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Nishitsuji, H.; Sugiyama, M.; Nishida, N.; Mizokami, M.; Shimotohno, K. Orchestration of Intracellular Circuits by G Protein-Coupled Receptor 39 for Hepatitis B Virus Proliferation. Int. J. Mol. Sci. 2020, 21, 661. [Google Scholar] [CrossRef]
- Mo, F.; Tang, Y.; Du, P.; Shen, Z.; Yang, J.; Cai, M.; Zhang, Y.; Li, H.; Shen, H. GPR39 protects against corticosterone-induced neuronal injury in hippocampal cells through the CREB-BDNF signaling pathway. J. Affect. Disord. 2020, 272, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Pongkorpsakol, P.; Buasakdi, C.; Chantivas, T.; Chatsudthipong, V.; Muanprasat, C. An agonist of a zinc-sensing receptor GPR39 enhances tight junction assembly in intestinal epithelial cells via an AMPK-dependent mechanism. Eur. J. Pharmacol. 2019, 842, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Starowicz, G.; Jarosz, M.; Frąckiewicz, E.; Grzechnik, N.; Ostachowicz, B.; Nowak, G.; Mlyniec, K. Long-lasting antidepressant-like activity of the GPR39 zinc receptor agonist TC-G 1008. J. Affect. Disord. 2019, 245, 325–334. [Google Scholar] [CrossRef]
- Młyniec, K.; Starowicz, G.; Gaweł, M.; Frąckiewicz, E.; Nowak, G. Potential antidepressant-like properties of the TC G-1008, a GPR39 (zinc receptor) agonist. J. Affect. Disord. 2016, 201, 179–184. [Google Scholar] [CrossRef]
- Muneoka, S.; Goto, M.; Kadoshima-Yamaoka, K.; Kamei, R.; Terakawa, M.; Tomimori, Y. G protein-coupled receptor 39 plays an anti-inflammatory role by enhancing IL-10 production from macrophages under inflammatory conditions. Eur. J. Pharmacol. 2018, 834, 240–245. [Google Scholar] [CrossRef]
- Lu, H.; Wang, D.; Li, H.; Zhong, J.; Lin, Y.; Xu, X.; Wang, B. GPR39 agonist TC-G 1008 ameliorates IL-1β-induced chondrocyte senescence. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2612–2617. [Google Scholar] [CrossRef]
- Bassilana, F.; Carlson, A.; DaSilva, J.A.; Grosshans, B.; Vidal, S.; Beck, V.; Wilmeringwetter, B.; Llamas, L.A.; Showalter, T.B.; Rigollier, P.; et al. Target identification for a Hedgehog pathway inhibitor reveals the receptor GPR39. Nat. Chem. Biol. 2014, 10, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Fjellström, O.; Larsson, N.; Yasuda, S.-I.; Tsuchida, T.; Oguma, T.; Marley, A.; Wennberg-Huldt, C.; Hovdal, D.; Fukuda, H.; Yoneyama, Y.; et al. Novel Zn2+ Modulated GPR39 Receptor Agonists Do Not Drive Acute Insulin Secretion in Rodents. PLoS ONE 2015, 10, e0145849. [Google Scholar] [CrossRef] [PubMed]
- Frimurer, T.M.; Mende, F.; Graae, A.-S.; Engelstoft, M.S.; Egerod, K.L.; Nygaard, R.; Gerlach, L.-O.; Hansen, J.B.; Schwartz, T.W.; Holst, B. Model-Based Discovery of Synthetic Agonists for the Zn2+-Sensing G-Protein-Coupled Receptor 39 (GPR39) Reveals Novel Biological Functions. J. Med. Chem. 2017, 60, 886–898. [Google Scholar] [CrossRef] [PubMed]
- Grunddal, K.V.; Diep, T.A.; Petersen, N.; Tough, I.R.; Skov, L.J.; Liu, L.; Buijink, J.A.; Mende, F.; Jin, C.; Jepsen, S.L.; et al. Selective release of gastrointestinal hormones induced by an orally active GPR39 agonist. Mol. Metab. 2021, 101207. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, M.J.; Salgado, A.; Piñeiro, R.; Rodiño, B.K.; Otero, M.F.; Grigorian, L.; Gallego, R.; Diéguez, C.; Gualillo, O.; González-Juanatey, J.R.; et al. Lack of effect of the ghrelin gene-derived peptide obestatin on cardiomyocyte viability and metabolism. J. Endocrinol. Investig. 2007, 30, 470–476. [Google Scholar] [CrossRef]
- Yamamoto, I.; Kimura, N.; Arai, T.; Tanaka, M. cDNA cloning and mRNA expression of bovine GPR39. J. Vet. Med. Sci. 2009, 71, 641–644. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, I.; Numao, M.; Sakaguchi, Y.; Tsushima, N.; Tanaka, M. Molecular characterization of sequence and expression of chicken GPR39. Gen. Comp. Endocrinol. 2007, 151, 128–134. [Google Scholar] [CrossRef]
- Yamamoto, I.; Sakaguchi, Y.; Numao, M.; Tsukada, A.; Tsushima, N.; Tanaka, M. Primary structure and tissue distribution of GPR39 messenger ribonucleic acid in Japanese quail, Coturnix japonica. Poult. Sci. 2007, 86, 2472–2476. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Huang, X.; Liu, X.; Jiao, B.; Meng, Z.; Zhu, P.; Li, S.; Lin, H.; Cheng, C.H.K. Two alternatively spliced GPR39 transcripts in seabream: Molecular cloning, genomic organization, and regulation of gene expression by metabolic signals. J. Endocrinol. 2008, 199, 457–470. [Google Scholar] [CrossRef]
- Jackson, V.R.; Nothacker, H.-P.; Civelli, O. GPR39 receptor expression in the mouse brain. Neuroreport 2006, 17, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Metsuyanim, S.; Harari-Steinberg, O.; Buzhor, E.; Omer, D.; Pode-Shakked, N.; Ben-Hur, H.; Halperin, R.; Schneider, D.; Dekel, B. Expression of stem cell markers in the human fetal kidney. PLoS ONE 2009, 4, e6709. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Rotellar, F.; Silva, C.; Gil, M.J.; Rodríguez, A.; Cienfuegos, J.A.; Salvador, J.; Frühbeck, G. The obestatin receptor (GPR39) is expressed in human adipose tissue and is down-regulated in obesity-associated type 2 diabetes mellitus. Clin. Endocrinol. 2007, 66, 598–601. [Google Scholar] [CrossRef]
- Fontenot, E.; DeVente, J.E.; Seidel, E.R. Obestatin and ghrelin in obese and in pregnant women. Peptides 2007, 28, 1937–1944. [Google Scholar] [CrossRef]
- Aoi, W.; Marunaka, Y. Importance of pH Homeostasis in Metabolic Health and Diseases: Crucial Role of Membrane Proton Transport. BioMed Res. Int. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Takahashi, K.I.; Copenhagen, D.R. Modulation of neuronal function by intracellular pH. Neurosci. Res. 1996, 24, 109–116. [Google Scholar] [CrossRef]
- Blachier, F.; de Sá Resende, A.; da Silva Fogaça Leite, G.; Vasques da Costa, A.; Lancha Junior, A.H. Colon epithelial cells luminal environment and physiopathological consequences: Impact of nutrition and exercise. Nutrire 2018, 43, 1–9. [Google Scholar] [CrossRef]
- Hachem, J.P.; Behne, M.; Aronchik, I.; Demerjian, M.; Feingold, K.R.; Elias, P.M.; Mauro, T.M. Extracellular pH controls NHE1 expression in epidermis and keratinocytes: Implications for barrier repair. J. Investig. Dermatol. 2005, 125, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Ardura, J.A.; Friedman, P.A. Regulation of G protein-coupled receptor function by Na+/H+ exchange regulatory factors. Pharmacol. Rev. 2011, 63, 882–900. [Google Scholar] [CrossRef]
- Gillen, C.M.; Brill, S.; Payne, J.A.; Forbush, B. Molecular cloning and functional expression of the K-Cl cotransporter from rabbit, rat, and human: A new member of the cation-chloride cotransporter family. J. Biol. Chem. 1996, 271, 16237–16244. [Google Scholar] [CrossRef]
- Rivera, C.; Voipio, J.; Payne, J.A.; Ruusuvuori, E.; Lahtinen, H.; Lamsa, K.; Pirvola, U.; Saarma, M.; Kaila, K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 1999, 397, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Payne, J.A.; Stevenson, T.J.; Donaldson, L.F. Molecular characterization of a putative K-Cl cotransporter in rat brain: A neuronal-specific isoform. J. Biol. Chem. 1996, 271, 16245–16252. [Google Scholar] [CrossRef]
- Sunuwar, L.; Asraf, H.; Donowitz, M.; Sekler, I.; Hershfinkel, M. The Zn2+-sensing receptor, ZnR/GPR39, upregulates colonocytic Cl- absorption, via basolateral KCC1, and reduces fluid loss. Biochim. Biophys. Acta. Mol. Basis Dis. 2017, 1863, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Mero, M.; Asraf, H.; Sekler, I.; Taylor, K.M.; Hershfinkel, M. ZnR/GPR39 upregulation of K+/Cl−-cotransporter 3 in tamoxifen resistant breast cancer cells. Cell Calcium 2019, 81, 12–20. [Google Scholar] [CrossRef]
- Chakraborty, M.; Asraf, H.; Sekler, I.; Hershfinkel, M. ZnR/GPR39 controls cell migration by orchestrating recruitment of KCC3 into protrusions, re-organization of actin and activation of MMP. Cell Calcium 2021, 94, 102330. [Google Scholar] [CrossRef] [PubMed]
- McAllister, B.B.; Dyck, R.H. Zinc transporter 3 (ZnT3) and vesicular zinc in central nervous system function. Neurosci. Biobehav. Rev. 2017, 80, 329–350. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.B.; Wenzel, H.J.; Kafer, K.E.; Schwartzkroin, P.A.; Palmiter, R.D. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. USA 1999, 96, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Ketterman, J.K.; Li, Y.V. Presynaptic evidence for zinc release at the mossy fiber synapse of rat hippocampus. J. Neurosci. Res. 2008, 86, 422–434. [Google Scholar] [CrossRef]
- Qian, J.; Noebels, J.L. Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J. Physiol. 2005, 566, 747–758. [Google Scholar] [CrossRef]
- Besser, L.; Chorin, E.; Sekler, I.; Silverman, W.F.; Atkin, S.; Russell, J.T.; Hershfinkel, M. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 2890–2901. [Google Scholar] [CrossRef]
- Adlard, P.A.; Parncutt, J.M.; Finkelstein, D.I.; Bush, A.I. Cognitive loss in zinc transporter-3 knock-out mice: A phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J. Neurosci. 2010, 30, 1631–1636. [Google Scholar] [CrossRef]
- Suh, S.W.; Won, S.J.; Hamby, A.M.; Yoo, B.H.; Fan, Y.; Sheline, C.T.; Tamano, H.; Takeda, A.; Liu, J. Decreased brain zinc availability reduces hippocampal neurogenesis in mice and rats. J. Cereb. Blood Flow Metab. 2009, 29, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Cole, T.B.; Robbins, C.A.; Wenzel, H.J.; Schwartzkroin, P.A.; Palmiter, R.D. Seizures and neuronal damage in mice lacking vesicular zinc. Epilepsy Res. 2000, 39, 153–169. [Google Scholar] [CrossRef]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, magnesium, selenium and depression: A review of the evidence, potential mechanisms and implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Młyniec, K.; Doboszewska, U.; Szewczyk, B.; Sowa-Kućma, M.; Misztak, P.; Piekoszewski, W.; Trela, F.; Ostachowicz, B.; Nowak, G. The involvement of the GPR39-Zn(2+)-sensing receptor in the pathophysiology of depression. Studies in rodent models and suicide victims. Neuropharmacology 2014, 79, 290–297. [Google Scholar] [CrossRef]
- Młyniec, K.; Nowak, G. GPR39 up-regulation after selective antidepressants. Neurochem. Int. 2013, 62, 936–939. [Google Scholar] [CrossRef]
- Młyniec, K.; Gaweł, M.; Librowski, T.; Reczyński, W.; Bystrowska, B.; Holst, B. Investigation of the GPR39 zinc receptor following inhibition of monoaminergic neurotransmission and potentialization of glutamatergic neurotransmission. Brain Res. Bull. 2015, 115, 23–29. [Google Scholar] [CrossRef]
- Młyniec, K.; Nowak, G. Up-regulation of the GPR39 Zn2+-sensing receptor and CREB/BDNF/TrkB pathway after chronic but not acute antidepressant treatment in the frontal cortex of zinc-deficient mice. Pharmacol. Rep. 2015, 67, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Młyniec, K.; Budziszewska, B.; Holst, B.; Ostachowicz, B.; Nowak, G. GPR39 (zinc receptor) knockout mice exhibit depression-like behavior and CREB/BDNF down-regulation in the hippocampus. Int. J. Neuropsychopharmacol. 2014, 18. [Google Scholar] [CrossRef]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef]
- Omar, N.N.; Tash, R.F. Fluoxetine coupled with zinc in a chronic mild stress model of depression: Providing a reservoir for optimum zinc signaling and neuronal remodeling. Pharmacol. Biochem. Behav. 2017, 160, 30–38. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Narvaez, M.; Marcellino, D.; Parrado, C.; Narvaez, J.A.; Tarakanov, A.O.; Agnati, L.F.; Díaz-Cabiale, Z.; Fuxe, K. Galanin receptor-1 modulates 5-hydroxtryptamine-1A signaling via heterodimerization. Biochem. Biophys. Res. Commun. 2010, 393, 767–772. [Google Scholar] [CrossRef]
- Jia, W.; Song, Y.; Yang, L.; Kong, J.; Boczek, T.; He, Z.; Wang, Y.; Zhang, X.; Hu, H.; Shao, D.; et al. The changes of serum zinc, copper, and selenium levels in epileptic patients: A systematic review and meta-analysis. Expert Rev. Clin. Pharmacol. 2020, 13, 1047–1058. [Google Scholar] [CrossRef] [PubMed]
- Doboszewska, U.; Młyniec, K.; Wlaź, A.; Poleszak, E.; Nowak, G.; Wlaź, P. Zinc signaling and epilepsy. Pharmacol. Ther. 2019, 193, 156–177. [Google Scholar] [CrossRef] [PubMed]
- Heydarian, F.; Nakhaei, A.A.; Majd, H.M.; Bakhtiari, E. Zinc deficiency and febrile seizure: A systematic review and meta-analysis. Turk. J. Pediatr. 2020, 62, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Arul, J.; Kommu, P.P.K.; Kasinathan, A.; Ray, L.; Krishnan, L. Zinc Status and Febrile Seizures: Results from a Cross-sectional Study. J. Neurosci. Rural Pract. 2020, 11, 597–600. [Google Scholar] [CrossRef]
- Woo, N.S.; Lu, J.; England, R.; McClellan, R.; Dufour, S.; Mount, D.B.; Deutch, A.Y.; Lovinger, D.M.; Delpire, E. Hyperexcitability and epilepsy associated with disruption of the mouse neuronal-specific K-Cl cotransporter gene. Hippocampus 2002, 12, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lovinger, D.; Delpire, E. Cortical neurons lacking KCC2 expression show impaired regulation of intracellular chloride. J. Neurophysiol. 2005, 93, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Gilad, D.; Shorer, S.; Ketzef, M.; Friedman, A.; Sekler, I.; Aizenman, E.; Hershfinkel, M. Homeostatic regulation of KCC2 activity by the zinc receptor mZnR/GPR39 during seizures. Neurobiol. Dis. 2015, 81, 4–13. [Google Scholar] [CrossRef]
- Chen, N.-N.; Zhao, D.-J.; Sun, Y.-X.; Wang, D.-D.; Ni, H. Long-Term Effects of Zinc Deficiency and Zinc Supplementation on Developmental Seizure-Induced Brain Damage and the Underlying GPR39/ZnT-3 and MBP Expression in the Hippocampus. Front. Neurosci. 2019, 13, 920. [Google Scholar] [CrossRef]
- Sunuwar, L.; Gilad, D.; Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, in health and disease. Front. Biosci. 2017, 22, 1469–1492. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, G.; Liu, L.; Lang, M. Zinc transporters in Alzheimer’s disease. Mol. Brain 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Huang, X.; Atwood, C.S.; Moir, R.D.; Hartshorn, M.A.; Vonsattel, J.P.; Tanzi, R.E.; Bush, A.I. Zinc-induced Alzheimer’s Aβ1-40 aggregation is mediated by conformational factors. J. Biol. Chem. 1997, 272, 26464–26470. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Tamano, H.; Tempaku, M.; Sasaki, M.; Uematsu, C.; Sato, S.; Kanazawa, H.; Datki, Z.L.; Adlard, P.A.; Bush, A.I. Extracellular Zn2+ is essential for amyloid β1-42-induced cognitive decline in the normal brain and its rescue. J. Neurosci. 2017, 37, 7253–7262. [Google Scholar] [CrossRef]
- Abramovitch-Dahan, C.; Asraf, H.; Bogdanovic, M.; Sekler, I.; Bush, A.I.; Hershfinkel, M. Amyloid β attenuates metabotropic zinc sensing receptor, mZnR/GPR39, dependent Ca2+, ERK1/2 and Clusterin signaling in neurons. J. Neurochem. 2016, 139, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Loef, M.; Schrauzer, G.N.; Walach, H. Selenium and alzheimer’s disease: A systematic review. J. Alzheimer’s Dis. 2011, 26, 81–104. [Google Scholar] [CrossRef] [PubMed]
- Farbood, Y.; Sarkaki, A.; Mahdavinia, M.; Ghadiri, A.; Teimoori, A.; Seif, F.; Dehghani, M.A.; Navabi, S.P. Protective Effects of Co-administration of Zinc and Selenium Against Streptozotocin-Induced Alzheimer’s Disease: Behavioral, Mitochondrial Oxidative Stress, and GPR39 Expression Alterations in Rats. Neurotox. Res. 2020, 38, 398–407. [Google Scholar] [CrossRef]
- Kogan, S.; Sood, A.; Garnick, M.S. Zinc and Wound Healing: A Review of Zinc Physiology and Clinical Applications. Wounds Compend. Clin. Res. Pract. 2017, 29, 102–106. [Google Scholar]
- Bin, B.H.; Hojyo, S.; Seo, J.; Hara, T.; Takagishi, T.; Mishima, K.; Fukada, T. The role of the slc39a family of zinc transporters in zinc homeostasis in skin. Nutrients 2018, 10, 219. [Google Scholar] [CrossRef]
- Fukunaka, A.; Fujitani, Y. Role of zinc homeostasis in the pathogenesis of diabetes and obesity. Int. J. Mol. Sci. 2018, 19, 476. [Google Scholar] [CrossRef]
- Tremblay, F.; Perreault, M.; Klaman, L.D.; Tobin, J.F.; Smith, E.; Gimeno, R.E. Normal food intake and body weight in mice lacking the G protein-coupled receptor GPR39. Endocrinology 2007, 148, 501–506. [Google Scholar] [CrossRef]
- Moechars, D.; Depoortere, I.; Moreaux, B.; de Smet, B.; Goris, I.; Hoskens, L.; Daneels, G.; Kass, S.; Ver Donck, L.; Peeters, T.; et al. Altered gastrointestinal and metabolic function in the GPR39-obestatin receptor-knockout mouse. Gastroenterology 2006, 131, 1131–1141. [Google Scholar] [CrossRef]
- Petersen, P.S.; Jin, C.; Madsen, A.N.; Rasmussen, M.; Kuhre, R.; Egerod, K.L.; Nielsen, L.B.; Schwartz, T.W.; Holst, B. Deficiency of the GPR39 receptor is associated with obesity and altered adipocyte metabolism. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 3803–3814. [Google Scholar] [CrossRef]
- Pound, L.D.; Sarkar, S.A.; Benninger, R.K.P.; Wang, Y.; Suwanichkul, A.; Shadoan, M.K.; Printz, R.L.; Oeser, J.K.; Lee, C.E.; Piston, D.W.; et al. Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem. J. 2009, 421, 371–376. [Google Scholar] [CrossRef]
- Nicolson, T.J.; Bellomo, E.A.; Wijesekara, N.; Loder, M.K.; Baldwin, J.M.; Gyulkhandanyan, A.V.; Koshkin, V.; Tarasov, A.I.; Carzaniga, R.; Kronenberger, K.; et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 2009, 58, 2070–2083. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Richard, A.-M.T.; Will, S.; Syed, J.; Stedman, N.; Perreault, M.; Gimeno, R.E. Disruption of G protein-coupled receptor 39 impairs insulin secretion in vivo. Endocrinology 2009, 150, 2586–2595. [Google Scholar] [CrossRef] [PubMed]
- Verhulst, P.J.; Lintermans, A.; Janssen, S.; Loeckx, D.; Himmelreich, U.; Buyse, J.; Tack, J.; Depoortere, I. GPR39, a receptor of the ghrelin receptor family, plays a role in the regulation of glucose homeostasis in a mouse model of early onset diet-induced obesity. J. Neuroendocrinol. 2011, 23, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Egerod, K.L.; Jin, C.; Petersen, P.S.; Wierup, N.; Sundler, F.; Holst, B.; Schwartz, T.W. β-Cell Specific Overexpression of GPR39 Protects against Streptozotocin-Induced Hyperglycemia. Int. J. Endocrinol. 2011, 2011, 401258. [Google Scholar] [CrossRef]
- Moran, B.M.; Abdel-Wahab, Y.H.A.; Vasu, S.; Flatt, P.R.; McKillop, A.M. GPR39 receptors and actions of trace metals on pancreatic beta cell function and glucose homoeostasis. Acta Diabetol. 2016, 53, 279–293. [Google Scholar] [CrossRef]
- Moran, B.M.; Miskelly, M.G.; Abdel-Wahab, Y.H.A.; Flatt, P.R.; McKillop, A.M. Zinc-induced activation of GPR39 regulates glucose homeostasis through glucose-dependent insulinotropic polypeptide secretion from enteroendocrine K-cells. Biol. Chem. 2019. [Google Scholar] [CrossRef]
- Katayama, K. Zinc and protein metabolism in chronic liver diseases. Nutr. Res. 2020, 74, 1–9. [Google Scholar] [CrossRef]
- Mohommad, M.K.; Zhou, Z.; Cave, M.; Barve, A.; McClain, C.J. Zinc and liver disease. Nutr. Clin. Pract. 2012, 27, 8–20. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Chen, T.; Yao, B.; Wang, Y.; Li, Q.; Yang, W.; Liu, Z. microRNA-1914, which is regulated by lncRNA DUXAP10, inhibits cell proliferation by targeting the GPR39-mediated PI3K/AKT/mTOR pathway in HCC. J. Cell. Mol. Med. 2019, 23, 8292–8304. [Google Scholar] [CrossRef]
- Engevik, A.C.; Kaji, I.; Goldenring, J.R. The physiology of the gastric parietal cell. Physiol. Rev. 2020, 100, 573–602. [Google Scholar] [CrossRef]
- Vaghari-Tabari, M.; Jafari-Gharabaghlou, D.; Sadeghsoltani, F.; Hassanpour, P.; Qujeq, D.; Rashtchizadeh, N.; Ghorbanihaghjo, A. Zinc and Selenium in Inflammatory Bowel Disease: Trace Elements with Key Roles? Biol. Trace Elem. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Sellin, J.H.; Barrett, K.E. Pathophysiology, Evaluation, and Management of Chronic Watery Diarrhea. Gastroenterology 2017, 152, 515–532.e2. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Jayaratne, R.; Barrett, K.E. The Role of Ion Transporters in the Pathophysiology of Infectious Diarrhea. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Sunuwar, L.; Medini, M.; Cohen, L.; Sekler, I.; Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, triggers metabotropic calcium signalling in colonocytes and regulates occludin recovery in experimental colitis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef]
- Landy, J.; Ronde, E.; English, N.; Clark, S.K.; Hart, A.L.; Knight, S.C.; Ciclitira, P.J.; Al-Hassi, H.O. Tight junctions in inflammatory bowel diseases and inflammatory bowel disease associated colorectal cancer. World J. Gastroenterol. 2016, 22, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Skrovanek, S. Zinc and gastrointestinal disease. World J. Gastrointest. Pathophysiol. 2014, 5, 496. [Google Scholar] [CrossRef]
- Huang, T.; Yan, G.; Guan, M. Zinc homeostasis in bone: Zinc transporters and bone diseases. Int. J. Mol. Sci. 2020, 21, 1236. [Google Scholar] [CrossRef]
- Zhivotovsky, B.; Orrenius, S. Cell cycle and cell death in disease: Past, present and future. J. Intern. Med. 2010, 268, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. Secretory function of the salivary gland in patients with taste disorders or xerostomia: Correlation with zinc deficiency. Acta Oto-Laryngol. 2002, 134–141. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Perez-Clausell, J.; Danscher, G. Zinc-containing 7S-NGF complex. Evidence from zinc histochemistry for localization in salivary secretory granules. J. Histochem. Cytochem. 1987, 35, 579–583. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Jo, Y.; Lee, Y.-H.; Park, K.; Park, H.-K.; Choi, S.-Y. Zn2+ stimulates salivary secretions via metabotropic zinc receptor ZnR/GPR39 in human salivary gland cells. Sci. Rep. 2019, 9, 17648. [Google Scholar] [CrossRef]
- Ambudkar, I.S. Calcium signalling in salivary gland physiology and dysfunction. J. Physiol. 2016, 594, 2813–2824. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Cho, G.; Yuan, Z.; Inoue, N.; Nakae, Y. Aquaporin-5 water channel in lipid rafts of rat parotid glands. Biochim. Biophys. Acta-Biomembr. 2006, 1758, 1053–1060. [Google Scholar] [CrossRef]
- Allouche-Fitoussi, D.; Bakhshi, D.; Breitbart, H. Signaling pathways involved in human sperm hyperactivated motility stimulated by Zn2+. Mol. Reprod. Dev. 2019, 86, 502–515. [Google Scholar] [CrossRef]
- Michailov, Y.; Ickowicz, D.; Breitbart, H. Zn2+-stimulation of sperm capacitation and of the acrosome reaction is mediated by EGFR activation. Dev. Biol. 2014, 396, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Allouche-Fitoussi, D.; Breitbart, H. The Role of Zinc in Male Fertility. Int. J. Mol. Sci. 2020, 21, 7796. [Google Scholar] [CrossRef] [PubMed]
- Bafaro, E.; Liu, Y.; Xu, Y.; Dempski, R.E. The emerging role of zinc transporters in cellular homeostasis and cancer. Signal Transduct. Target. Ther. 2017, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Choi, S.; Ouadid-Ahidouch, H.; Yang, J.M.; Beattie, J.H.; Korichneva, I. Zinc transporters and dysregulated channels in cancers. Front. Biosci. -Landmark 2017, 22, 623–643. [Google Scholar] [CrossRef]
- Xie, F.; Liu, H.; Zhu, Y.-H.; Qin, Y.-R.; Dai, Y.; Zeng, T.; Chen, L.; Nie, C.; Tang, H.; Li, Y.; et al. Overexpression of GPR39 contributes to malignant development of human esophageal squamous cell carcinoma. BMC Cancer 2011, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, T.; Wu, Y.; Xu, H.; Xie, C.; Dong, Y.; Zhong, L.; Wang, Z.; Zhao, H.; Zhou, Y.; et al. GPR39 Overexpression in OSCC Promotes YAP-Sustained Malignant Progression. J. Dent. Res. 2020, 99, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Bixenshpaner, H.; Asraf, H.; Chakraborty, M.; Elkabets, M.; Sekler, I.; Taylor, K.M.; Hershfinkel, M. Enhanced ZnR/GPR39 Activity in Breast Cancer, an Alternative Trigger of Signaling Leading to Cell Growth. Sci. Rep. 2018, 8, 8119. [Google Scholar] [CrossRef] [PubMed]
- Dershem, R.; Metpally, R.P.R.; Jeffreys, K.; Krishnamurthy, S.; Smelser, D.T.; Hershfinkel, M.; Center, R.G.; Carey, D.J.; Robishaw, J.D.; Breitwieser, G.E. Rare-variant pathogenicity triage and inclusion of synonymous variants improves analysis of disease associations of orphan G protein-coupled receptors. J. Biol. Chem. 2019, 294, 18109–18121. [Google Scholar] [CrossRef] [PubMed]
- Selvanayagam, T.; Walker, S.; Gazzellone, M.J.; Kellam, B.; Cytrynbaum, C.; Stavropoulos, D.J.; Li, P.; Birken, C.S.; Hamilton, J.; Weksberg, R.; et al. Genome-wide copy number variation analysis identifies novel candidate loci associated with pediatric obesity. Eur. J. Hum. Genet. EJHG 2018, 26, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Green, B.D.; Grieve, D.J. Biochemical properties and biological actions of obestatin and its relevence in type 2 diabetes. Peptides 2018, 100, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.-Z.; Shen, M.; Qudirat, H.; Shi, J.-B.; Su, T.; Song, J.-W.; Wang, Z.-K.; Zhao, X.-X.; Jing, Q.; Zheng, X.; et al. Obestatin ameliorates water retention in chronic heart failure by downregulating renal aquaporin 2 through GPR39, V2R and PPARG signaling. Life Sci. 2019, 231, 116493. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Temprano, A.; Relova, J.L.; Camiña, J.P.; Pazos, Y. Concurrent Akt, ERK1/2 and AMPK Activation by Obestatin Inhibits Apoptotic Signaling Cascades on Nutrient-Deprived PC12 Cells. Cell. Mol. Neurobiol. 2021. [Google Scholar] [CrossRef]
- Santos-Zas, I.; Negroni, E.; Mamchaoui, K.; Mosteiro, C.S.; Gallego, R.; Butler-Browne, G.S.; Pazos, Y.; Mouly, V.; Camiña, J.P. Obestatin Increases the Regenerative Capacity of Human Myoblasts Transplanted Intramuscularly in an Immunodeficient Mouse Model. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 2345–2359. [Google Scholar] [CrossRef] [PubMed]
- Cid-Díaz, T.; Santos-Zas, I.; González-Sánchez, J.; Gurriarán-Rodríguez, U.; Mosteiro, C.S.; Casabiell, X.; García-Caballero, T.; Mouly, V.; Pazos, Y.; Camiña, J.P. Obestatin controls the ubiquitin-proteasome and autophagy-lysosome systems in glucocorticoid-induced muscle cell atrophy. J. Cachexia. Sarcopenia Muscle 2017, 8, 974–990. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laitakari, A.; Liu, L.; Frimurer, T.M.; Holst, B. The Zinc-Sensing Receptor GPR39 in Physiology and as a Pharmacological Target. Int. J. Mol. Sci. 2021, 22, 3872. https://doi.org/10.3390/ijms22083872
Laitakari A, Liu L, Frimurer TM, Holst B. The Zinc-Sensing Receptor GPR39 in Physiology and as a Pharmacological Target. International Journal of Molecular Sciences. 2021; 22(8):3872. https://doi.org/10.3390/ijms22083872
Chicago/Turabian StyleLaitakari, Anna, Lingzhi Liu, Thomas M. Frimurer, and Birgitte Holst. 2021. "The Zinc-Sensing Receptor GPR39 in Physiology and as a Pharmacological Target" International Journal of Molecular Sciences 22, no. 8: 3872. https://doi.org/10.3390/ijms22083872
APA StyleLaitakari, A., Liu, L., Frimurer, T. M., & Holst, B. (2021). The Zinc-Sensing Receptor GPR39 in Physiology and as a Pharmacological Target. International Journal of Molecular Sciences, 22(8), 3872. https://doi.org/10.3390/ijms22083872




