Abstract
The available tooth whitening products in the market contain high concentrations of hydrogen peroxide (H2O2) as an active ingredient. Therefore, in order to curb the high H2O2 concentration and instability of liquid H2O2, this study evaluated the efficacy and cytotoxicity of the bleaching gel composed of 10% calcium peroxide (CaO2) and visible-light-activating nitrogen-doped titanium dioxide (N-TiO2) with methyl cellulose as a thickener. Extracted bovine teeth were discolored using coffee and black tea stain solution and were divided into two groups (n = 6). Bleaching was performed thrice on each tooth specimen in both the groups, with one minute of visible light irradiation during each bleaching time. The CIELAB L*a*b* values were measured pre- and post-bleaching. The N-TiO2 calcinated at 350 °C demonstrated a shift towards the visible light region by narrowing the band gap energy from 3.23 eV to 2.85 eV. The brightness (ΔL) and color difference (ΔE) increased as bleaching progressed each time in both the groups. ANOVA results showed that the number of bleaching significantly affected ΔE (p < 0.05). The formulated bleaching gel exhibits good biocompatibility and non-toxicity upon exposure to 3T3 cells. Our findings showed that CaO2-based bleaching gel at neutral pH could be a stable, safe, and effective substitute for tooth whitening products currently available in the market.
1. Introduction
The desire to flaunt a beautiful smile has turned into an aesthetic necessity today. Hence, tooth whitening has become rampant in esthetic dentistry [1]. The demand for that near-perfect smile has triggered the production of several whitening products and the success of each such product is dependent on the type of tooth discoloration [2]. Tooth discoloration can be classified into two main categories: external staining, which is mainly due to poor oral hygiene, smoking, chlorhexidine in mouthwashes, pigmented food, dental caries, or beverages; whereas internal staining is attributed to factors like age, antibiotics, or excessive fluoride content [3,4].
Hydrogen peroxide is the most common active ingredient in dental bleaching products, due to its ability to release free radicals [5]. These free radicals trigger the oxidation and splitting of pigmented organic molecules into smaller molecules. In turn, these smaller molecules reflect more light, making the tooth appear brighter and whiter, achieving a successful bleaching [6,7,8]. For vital tooth bleaching, both in-office and home bleaching techniques are widely used [9]. Office bleaching products usually contain a high concentration of H2O2 (35–40%) to generate high levels of free radicals for a short treatment time in one appointment, while home bleaching products contain 6–10% H2O2 and take more than one month to achieve desired results [10,11]. The higher H2O2 concentration or longer exposure time in contact with bleaching gel is effective in whitening. However, side effects might be generated. The most common complications include tooth sensitivity and gingival irritation [9]. With the increasing concentration of H2O2, adverse side effects, such as dental hypersensitivity, soft tissue irritation, and cytotoxicity, of dental bleach may occur. Moreover, 0.1 to 6.0% hydrogen peroxide or equivalent for hydrogen peroxide releasing from dental bleaching products is reported safe as per the European Scientific Committee on Consumer Products (SCCP) [12]. However, dental bleaching gel consisting of varying concentrations of H2O2 showed toxicity in different cell lines, such as fibroblasts and dental pulp cells (DPCs), in previous reports [13,14,15,16]. Therefore, a safer yet efficient bleaching product is long overdue.
Calcium peroxide (CaO2) can be a potential substitute of liquid H2O2 in dental bleaching products because of its characteristics, such as a more effective source of H2O2, and relatively stable nature than liquid H2O2. CaO2 is considered to be the safest form of solid peroxy compounds. Upon dissolution in water, CaO2 is capable of reacting in the medium for a longer time and releases H2O2 in a controlled manner. The maximum H2O2 released per gram of CaO2 is 0.47 g, which is considered safe in dentistry [17,18]. According to previous studies, CaO2 is capable of releasing H2O2 and O2 independently. Moreover, the releasing rates can be controlled by various factors, such as pH and temperature; that is, an increase in pH leads to a decrease in the release of H2O2 and increases the O2 yield, while the increased temperature improves the release of O2 [19].
In the past, there have been studies showing the use of high-energy ultraviolet (UV)-light-assisted catalysts to reduce the concentration of hydrogen peroxide in dental bleaching products [20]. However, UV-light imposed greater risks on the eyes and skin of the patients as well as that of the dentists [21]. To address the challenges imposed by the harmful UV light, a visible light-activating photocatalyst titanium dioxide was recently introduced. The feasibility of TiO2 can be attributed to its nontoxicity, low price, and photostability [22]. Titanium dioxide (TiO2), in its crude form, is a well-known photocatalytic material activated by UV light but can be modified to achieve photo response at visible light. Doping TiO2 with nitrogen turns out to be the most effective way to reduce the band gap, and transition its photo response from UV light to visible light [23,24,25].
Given this background, the focus of this study was to develop a safe and effective dental bleaching product for clinical use. This product comprises calcium peroxide and visible light-activating nitrogen-doped TiO2 (N-TiO2) as a photocatalyst to increase the rate of bleaching. The cytotoxicity and efficacy of the fabricated dental bleach were examined in vitro by co-culturing with 3T3 cells, while the effectiveness of the prepared dental bleach was further evaluated using stained bovine teeth model in vitro.
2. Results
2.1. X-ray Diffraction (XRD) Analysis
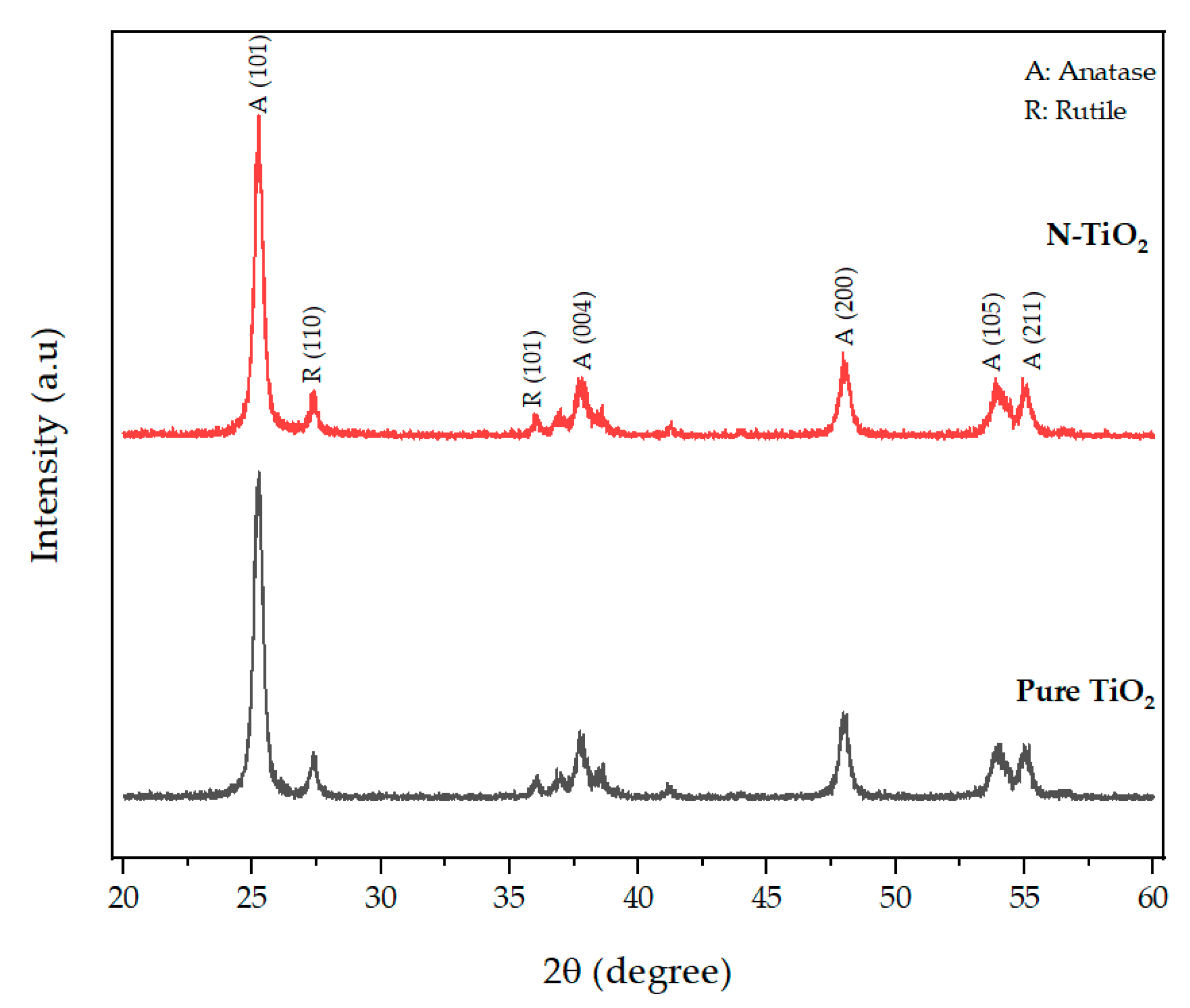
XRD analysis was used to characterize the crystal phase structure of the prepared photocatalytic TiO2. The XRD pattern of N-TiO2, along with the pattern of pure TiO2 used as a reference, is presented in Figure 1. The crystal phase composition of pure TiO2 and N-TiO2 contained a mixture of major anatase phase and minor rutile phase. The XRD profile of N-TiO2 calcined at 350 °C exhibited anatase peaks at 25.25°, 37.68°, 47.94°, 53.87°, and 55.01° and were in good accordance with the (101), (004), (200), (105), and (211) peak position of anatase TiO2 (JCPDS 86-1157) and rutile peaks at 27.4° and 36.03°, which were in conformity with the (110) and (101) peak position of rutile TiO2 (JCPDS 21-1276).
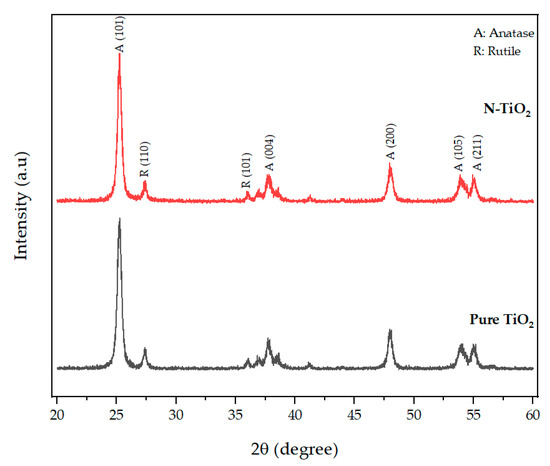
Figure 1.
XRD patterns of TiO2 and nitrogen-doped TiO2.
2.2. X-ray Photoelectron Spectroscopy (XPS) Analysis
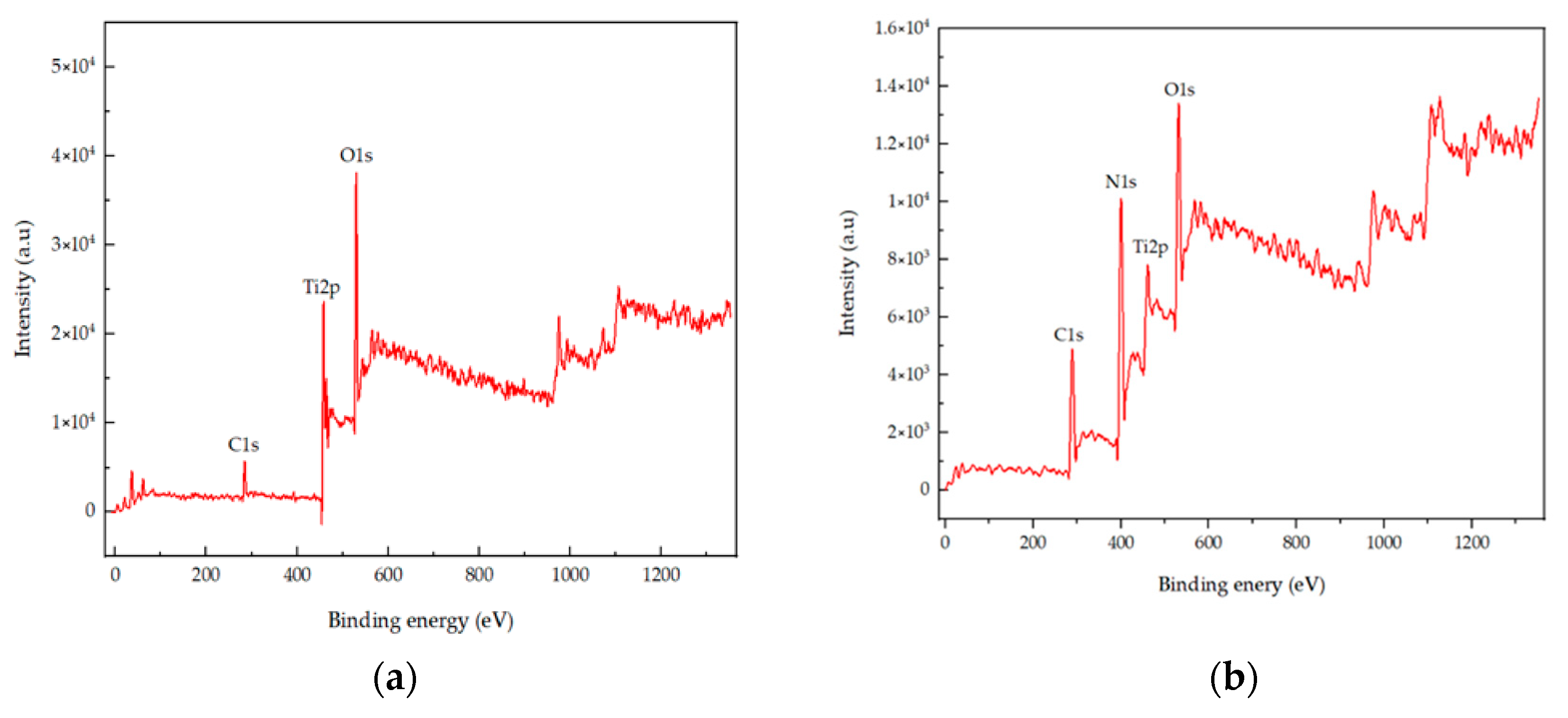
XPS was performed in order to characterize the chemical state and surface composition of the prepared photocatalytic TiO2. The spectrum of pure TiO2 and N-TiO2 is presented in Figure 2a,b, respectively. TiO2 predominantly contained Ti, O, and C elements, while TiO2 upon heat treatment indicated the presence of Ti, O, N, and C elements. Amongst these elements in N-TiO2, the C1s peak was located at 288.6 eV, and it represented the contaminated residual precursor, which was not completely removed during heat treatment. Moreover, accidental carbon during the process may cause the presence of the C element. The N1s peak was found to be at 400.6 eV, while Ti 2p doublets were located at 464.4 and 458.4 eV corresponding to Ti 2p3/2 and Ti 2p1/2, which were approximately in accordance with the actual values. Subsequently, the O1s peak could be fitted into two peaks located at binding energies of 529.8 and 532.05 eV.
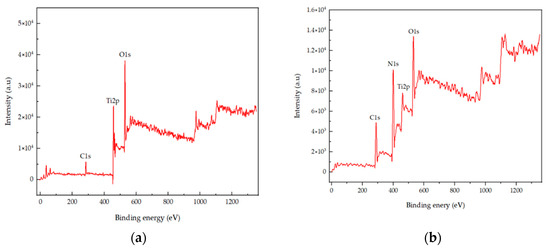
Figure 2.
(a) XPS survey spectra of TiO2 and (b) XPS survey spectra of N-TiO2.
2.3. Morphology Analysis
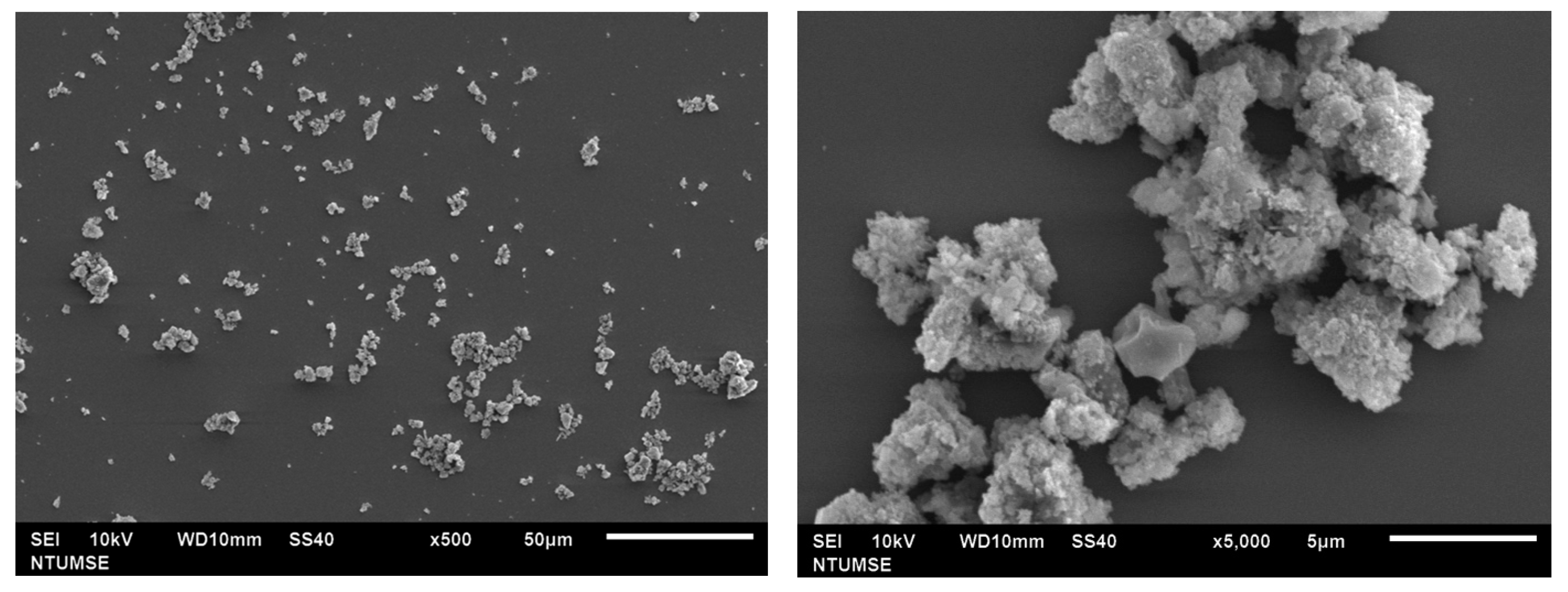
The morphology of doped TiO2 was observed using SEM and is shown in Figure 3. According to the results, N-TiO2 calcinated at 350 °C showed agglomerated clusters with spherical morphology. At high temperatures, particles tend to aggregate due to the particle growth process.
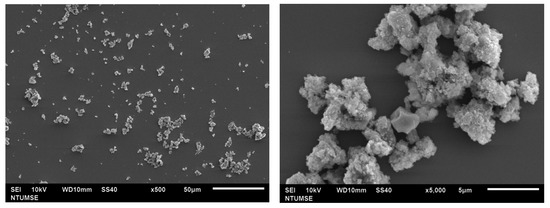
Figure 3.
SEM micrograph of N-TiO2.
2.4. UV-Vis Absorption Spectra
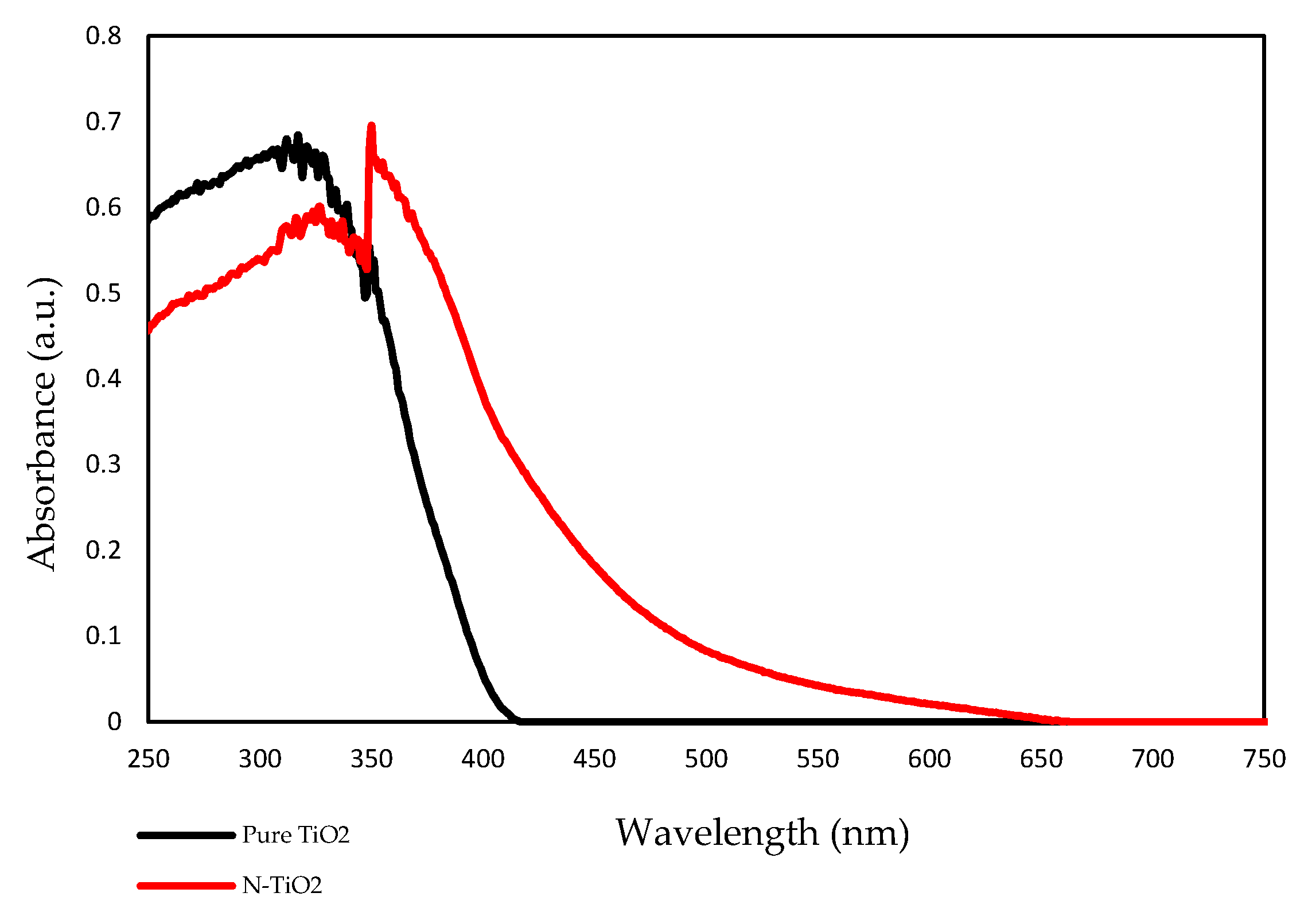
The optical property of the prepared TiO2 photocatalyst was measured using UV-vis spectroscopy. Figure 4 shows the UV-Vis absorption spectra of pure TiO2 (undoped) and N-TiO2. According to the results, pure TiO2 had an absorption edge around 390 nm, while the absorption edge of N-TiO2 was found to be around 450 nm. This shift toward the visible light in the absorption spectra of N-TiO2 was due to the incorporation of nitrogen into the TiO2 lattice, eventually leading to band gap narrowing.
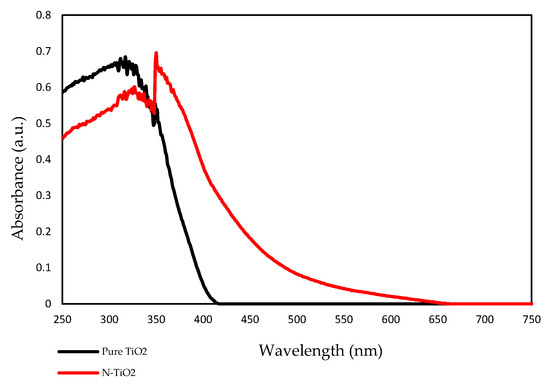
Figure 4.
UV-Vis absorption spectrum of pure TiO2 and N-TiO2.
2.5. Color Analysis of Tooth Bleaching
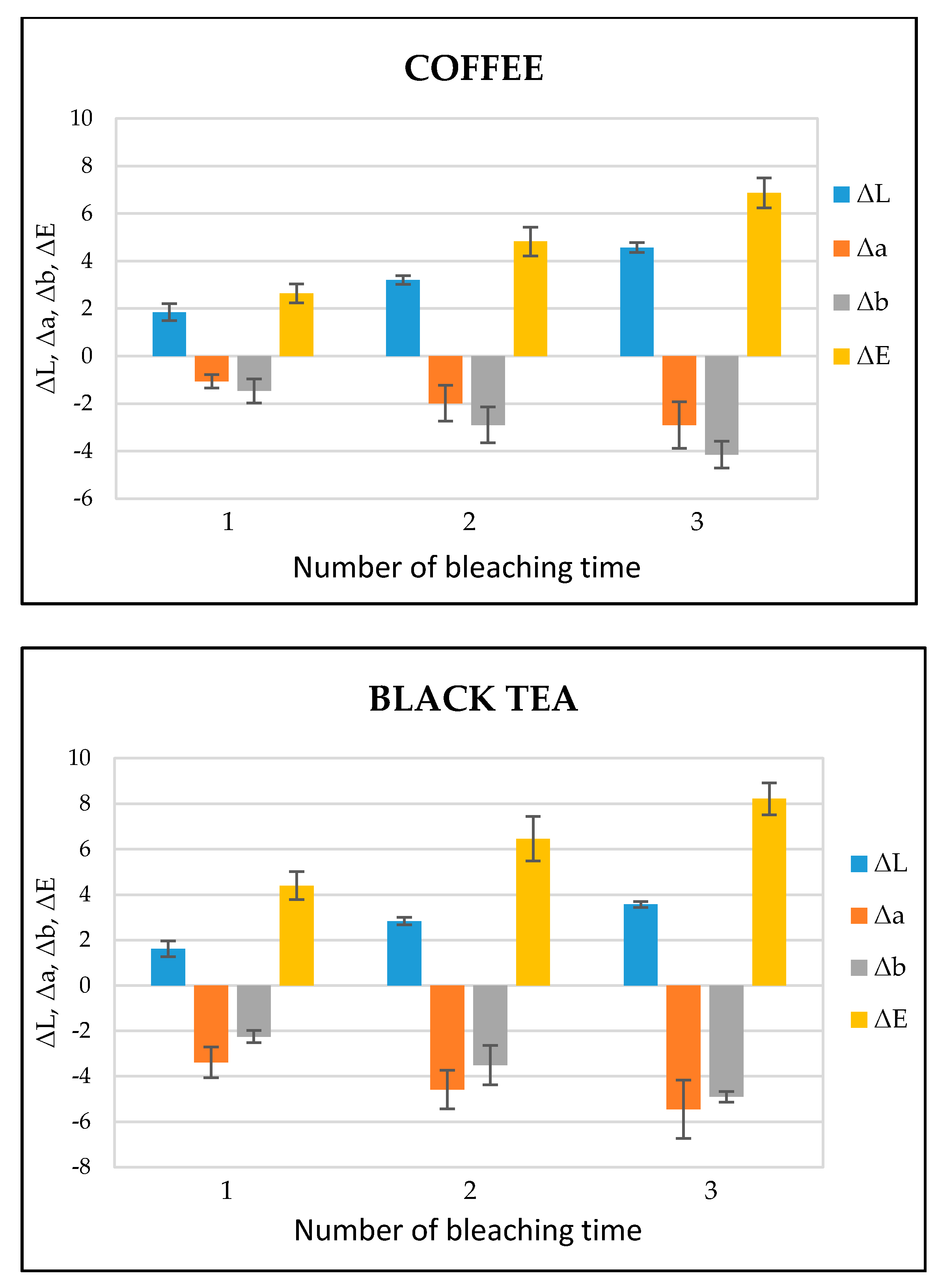
Figure 5 shows the ΔL, Δa, Δb, and ΔE values of each bleaching time in the coffee- and black tea-stained bovine teeth groups, respectively. ΔL, Δa, and Δb stand for the difference of L, a, and b between baseline and each bleaching time while the color difference (ΔE) was calculated according to the formula ΔE = [(ΔL)2 + (Δa)2 + (Δb)2]1/2. After the bleaching treatment, the values of ΔL and ΔE gradually increased in both the groups, while the values of Δa and Δb exhibited a decrease. According to the statistical analysis, ΔE in both the groups showed significant differences (p < 0.05) between bleaching times (Table 1). The photographs of a representative image of tooth bleaching from black tea and coffee stained groups at the baseline and 1, 2, 3 times of bleaching is shown in Figures S1 and S2 respectively.
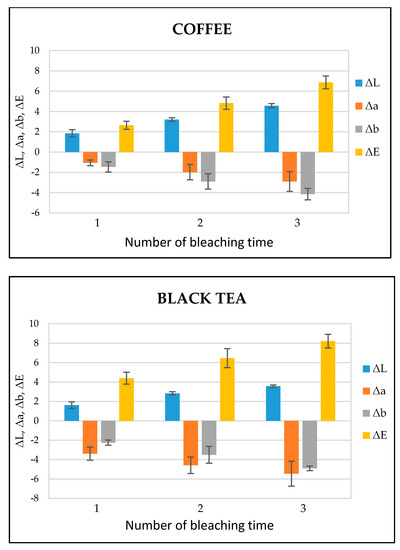
Figure 5.
ΔL, Δa, Δb, and ΔE values of coffee- and black tea-stained bovine tooth samples treated with dental bleaching gel (n = 6).

Table 1.
Mean ΔE and standard deviation.
2.6. Biological Assays
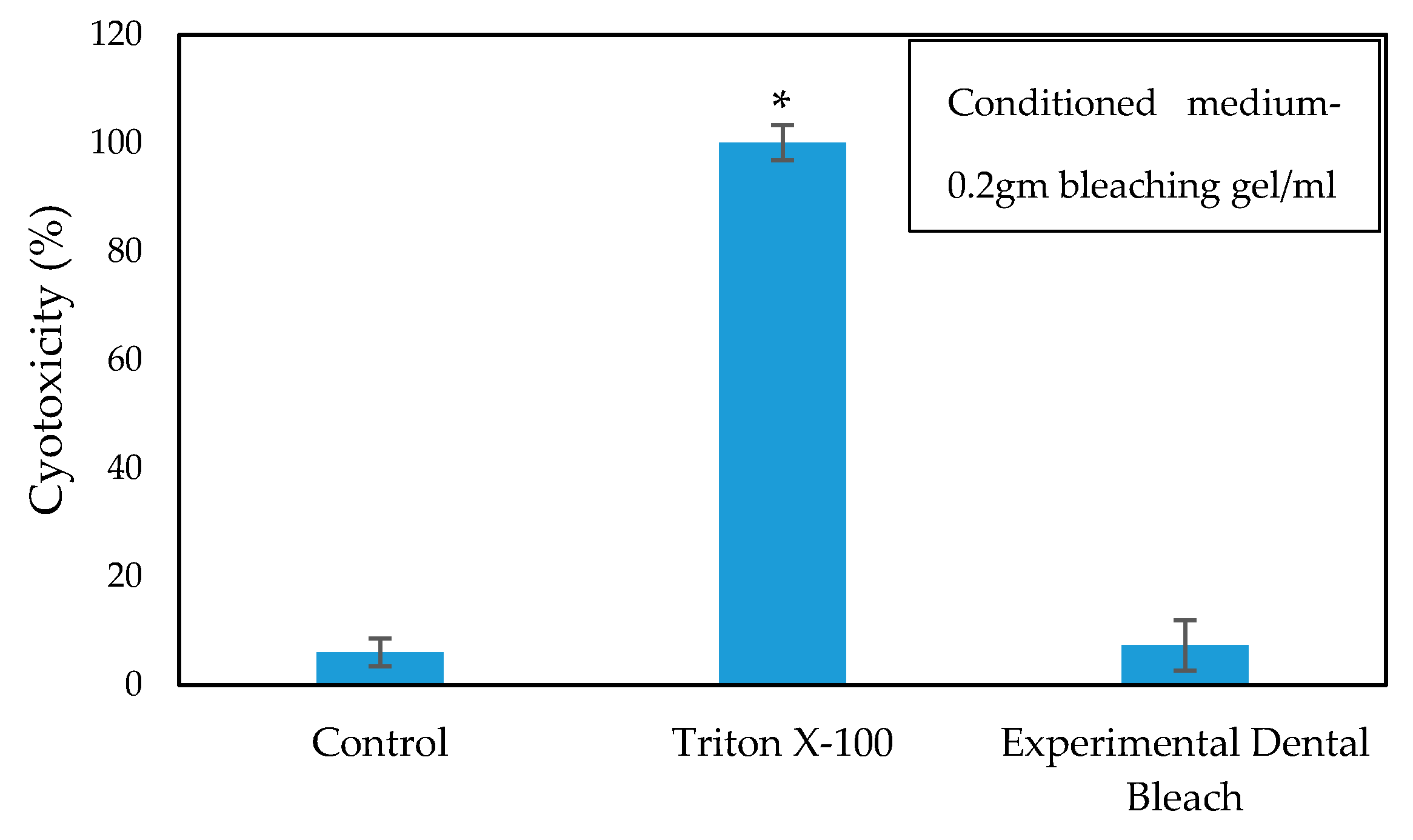
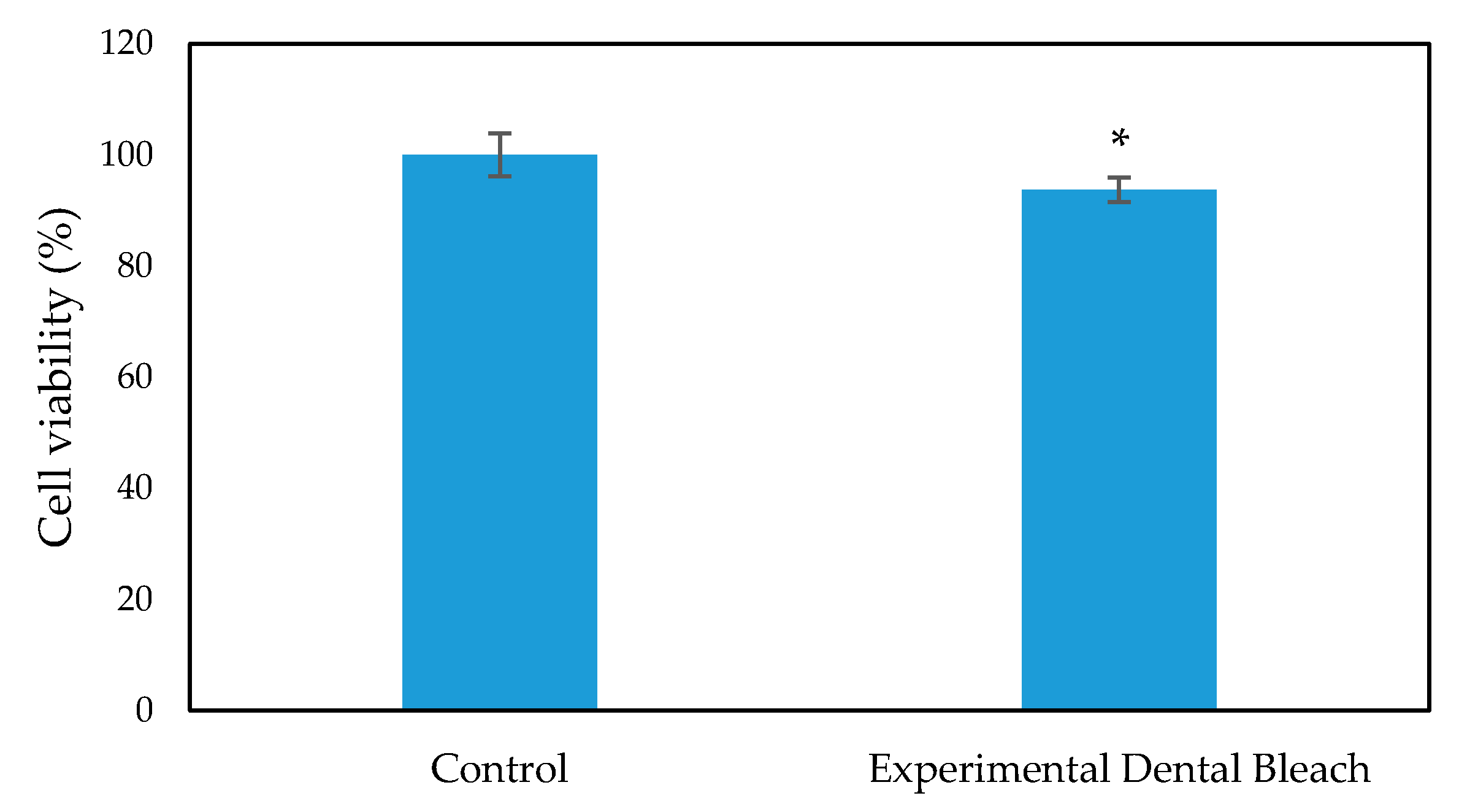
The cytotoxicity and biocompatibility of the bleaching gel were determined via LDH and WST-1 assay, respectively, and the results are presented in Figure 6 and Figure 7, respectively. WST-1 is a colorimetric cell proliferation assay used to measure the biocompatibility while the LDH assay determines the cellular cytotoxicity. 3T3 cells were used in this study. Conditioned medium containing 0.2 g of bleaching gel (10% CaO2 + 1% N-TiO2 + 1.5% methylcellulose) per mL was prepared and used in this study. The WST-1 result showed a cell viability of 93.7% at 24 h while the LDH assay showed very low cytotoxicity of 7.3% when exposed to dental bleach-conditioned medium for 24 h. No significant toxicity was observed in 3T3 cells when cultured with the experimental bleaching gel.
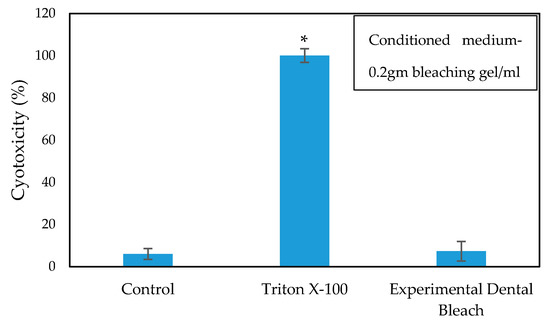
Figure 6.
Cytotoxicity of the cells cultured in dental bleach-conditioned medium (n = 6), * p < 0.05 compared to the control group by student t-test.
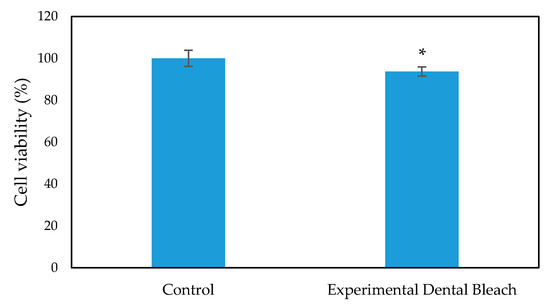
Figure 7.
WST-1 assay for 3T3 cell viability evaluation on exposure to dental bleach-conditioned medium (n = 6), * p < 0.05 compared to the control group by student t-test.
3. Discussion
The study focused on the bleaching efficiency and cytotoxicity of the formulated dental bleaching gel using a discolored bovine tooth model. In view of the existing literature, there have been several in vitro models appointed for evaluating the efficacy of the dental bleaching agent, the important ones being the human or bovine tooth model, either cut or whole [26,27,28]. In this current study, bovine teeth were used as an in vitro model because of its similarity with human teeth in terms of physical and chemical properties, such as hardness, permeability, and density of the dentin tubule. Moreover, the collection of extracted human teeth is difficult for experiments with large sample sizes, and the consistency in the results may be affected by various factors, such as shade, age, thickness of enamel, and mineralization of the extracted human tooth surface [29].
In this study, discoloration of the bovine teeth was achieved by tea and coffee staining solutions, respectively, due to their cost effectiveness, easy preparation, and regular use by the majority of the population [30,31]. Shade change of the teeth after bleaching can be evaluated using several methods, such as visual comparison of the tooth surface with the standard tooth shade card or parametric analysis using a chroma meter. In this study, a chroma meter was used to evaluate the tooth shade as it can provide quantitative analysis in color matching, so that statistical analysis can be easily achieved and is more accurate than the visual analysis using a tooth shade guide.
The main and effective attraction of in-office dental bleaching products is the use of H2O2 as an active ingredient, but due to the diverse side effects of using high-concentration H2O2 as mentioned before, many researchers have now directed their research towards finding alternatives to control high-concentration H2O2 to provide effective bleaching without harm to the dental tissues. Therefore, CaO2, used as a bleaching agent in this study, is expected to be a potential alternative as compared to the other commercial agents with high concentrations of H2O2 (20–40%). H2O2 released from CaO2 can be controlled with the alterations in temperature and pH. Therefore, the release rate of H2O2 was controlled by adjusting the pH of the bleaching gel to 7. Moreover, the neutral pH of the dental bleaching products has been reported to be safe on enamel because it does not alter the surface roughness of the tooth even after several applications [10]. CaO2 is also capable of reacting for a longer duration in the medium, making its functionality even more efficient.
In previous studies, it was demonstrated that the efficiency of H2O2-based bleaching products was increased with the addition of visible light-activating TiO2 when exposed to visible light [32,33]. Therefore, in this study, N-TiO2 was used as a photocatalyst to increase the efficiency of CaO2-based bleaching gel. The nitrogen doping in the TiO2 lattice results in the formation of a new electronic state above the valance band, which leads to the shift in the absorption spectrum of N-TiO2 from UV light to the visible light region as analyzed by the UV-vis spectrophotometer (Figure 4). The formation of a new electronic band led to a decrease in the band gap energy value and was calculated by the Tauc plot method. The band energy value for pure TiO2 was found to be 3.2 eV while on the other hand, the nitrogen-incorporated TiO2 lattice exhibited a decrease in the band gap value from 3.23 eV to 2.85 eV. Moreover, to investigate the effect of nitrogen doping on the crystal phase of TiO2, XRD was performed. The phase composition of N-TiO2 contains major intensity of the anatase phase and minor rutile phase, similar to that of pure TiO2 (Figure 1). In view of the existing literature, the anatase phase is a better photocatalyst than the rutile phase [34]. Therefore, in the present study, it was important to retain the intensity of the anatase phase in N-TiO2 in order to fabricate a strong photocatalyst to make a stronger dental bleach. Additionally, the results of XPS analysis (Figure 2b) confirmed the existence of N in the TiO2 lattice along with the elements Ti, O, and C. The observed N1s peak with binding energy at 400.1 eV is assigned to N-O-Ti linkage, i.e., nitrogen bonded to oxygen sites (interstitial doping). The high nitrogen content results in an effective visible light-activating photocatalyst even though there is slight particle aggregation at high temperatures due to the particle growth process.
The bleaching efficiency of the experimental bleaching gel was determined by comparing the color values at each bleaching time. The important indicator for the bleaching evaluation is the determination of the L value; the higher the L value, the brighter or whiter the teeth appear. In the present study, the value of L increased with each bleaching time in both the groups, indicating the brightness or whiteness was upgraded with each consecutive bleaching. On the other hand, the total color difference, i.e., ΔE, gradually increased during each bleaching treatment. ΔE is categorized into six ranks: 0.5 or less ΔE value, wherein macroscopically no color difference was seen; between 0.5 and 1.5, a small difference was observed with effort to the naked eye; between 1.5 and 3.0, a slight difference was clearly noticed; 3.0–6.0 values, when a substantial difference was seen; between 6.0 and 12.0, a marked difference was observed; and 12.0 or higher, when a different color line was noticed [35]. In this study, ΔE after the last post-treatment was in the range of 6.0–12.0, representing a significant difference (Figure 5).
Successful bleaching treatment brightens the teeth effectively without compromising safety. Therefore, a cytotoxicity test was conducted in order to evaluate the safety and biologic properties of the bleaching gel. We found that the bleaching components were non-toxic to 3T3 cells (Figure 6 and Figure 7). No significant difference was observed between the experimental group and control group. Therefore, the biocompatibility of the experimental bleaching gel was good when tested on 3T3 cell lines and should be safe in clinical use. Future study will focus on the quantification of released H2O2 from CaO2 and a more delicate mechanism under different conditions.
4. Materials and Methods
4.1. Preparation of N-TiO2
An organic nitrogen source urea was used for nitrogen doping. Briefly, 3M urea solution was prepared, and titanium (IV) oxide (21-nm primary particle size, Sigma-Aldrich, St. Louis, MI, USA) was dispersed in it at a ratio of 0.1 g/mL. The mixture was stirred for 24 h and later dried in the oven at 80 °C, followed by calcination in a furnace at 350 °C for 2 h.
4.2. Characterization
The phase identification of pure TiO2 and N-TiO2 was carried out using an X-ray diffractometer (XRD) (TTRAX 3, Rigaku, Tokyo, Japan). The surface composition and electron binding energy of TiO2 and N-TiO2 was measured using an X-ray photoelectron spectroscopy (XPS) (Theta probe, Thermo Scientific, Waltham, MA, USA). The morphology was observed using a scanning electron microscope (SEM) (JSM6510, JEOL, Tokyo, Japan). The UV-Vis spectroscopy (CARY 300nc, Agilent, Santa Clara, CA, USA) was used to record the absorption spectra of the samples.
4.3. Preparation of Stained Teeth
Twelve freshly extracted bovine incisors purchased from a local meat market in Taipei, Taiwan were used in this study. After extraction, the teeth were dipped in hot boiling water for 20 s, and the soft tissues were removed using a scalpel. The enamel surface was polished with ascending grit silicon carbide papers starting from #100 up to #1000 under running water, removing 300 µm from the enamel surface to create a smooth and flat surface. The teeth were then stored in water at 4 °C.
Black tea and coffee stain solutions were prepared. Briefly, two black tea bags (Earl Grey Twinings) each weighing 2 g were immersed in 100 mL of boiling water for 5 min to prepare a black tea stain solution while the coffee stain solution was prepared by adding 4 g of ground coffee powder (Nescafe) in 100 mL of boiling water. The teeth were then divided into two groups (n = 6) and immersed into the respective above-mentioned staining solutions for a week in the incubator at 37 °C. The solutions were renewed after every 3 days and stirred once a day to avoid sedimentation.
4.4. Color Analysis
After the teeth were immersed in the staining solutions for a week, they were rinsed under tap water to remove excess coffee and tea from the surface, respectively, followed by drying using kimwipes. Prior to bleaching, the CIELAB values of the stained enamel surface were recorded as a baseline value using a dental chroma meter (VITA EasyShade Compact, Vident, Model # DEASYCBU, Yorba Linda, CA, USA).
4.5. Tooth Bleaching
The experimental bleaching gel was composed of 10% calcium peroxide, 1% N-TiO2,, and 1.5% methylcellulose as a thickener. The pH of the gel was adjusted to 7.0 before the application onto the test tooth surface.
A thin layer of the experimental bleaching gel was applied on the test tooth surface with a brush and irradiated for 1 min with a light emitting diode (LED) light curing device (LITEX 696, Dentamerica Asia Inc., Taipei, Taiwan). Following which, the bleaching agent was left for 5 min on the tooth surface, and the bleaching gel was then rinsed with fresh water and dried. A VITA EasyShade Compact chroma meter was used to measure the L, a, and b values of the bleached teeth after rinsing and drying of teeth, where L* represents the lightness of the sample from black (0) to white (100), a* represents the green-red coordinate, and b* represents the blue-yellow coordinate. The bleaching and color measurements were repeated thrice per tooth. The difference between baseline and each bleaching time for L, a, and b values were denoted as ΔL, Δa, and Δb, respectively. The color difference (ΔE) was calculated according to the equation below:
ΔE = [(ΔL)2 + (Δa)2 + (Δb)2]1/2
4.6. Biological Assays
4.6.1. Cell Culture
The 3T3 cell line (mouse embryonic fibroblasts) (Bioresource Collection and Research Center, Taiwan) was used as a cell source in this study. Dulbecco’s modified Eagle’s medium (DMEM) (Sigma, USA) supplemented with 10% fetal calf serum (FBS) (Gibco, Gaithersburg, MD, USA) and 1% antibody (Gibco, USA) was used for the cell culture. The cells were incubated at 37 °C in a 5% CO2-containing atmosphere.
4.6.2. Cell Viability
Cells were seeded in 96-well plates at a cell density of 1 × 104 cells/well and incubated for 24 h at 37 °C. After 24h, the used DMEM was aspirated, washed with PBS, and then cultured with the conditioned medium for 24 h at 37 °C. The conditioned medium was obtained according to the ISO standard 10993-12:2012. Briefly, 5 mL of the conditioned medium was prepared (0.2 g bleaching gel per 1 mL of DMEM culture medium incubated for 30 min at room temperature). The conditioned medium was filtered through a sterile filter and subsequently used for cell culture.
Cell viability was evaluated using the WST-1 assay kit (Takara, Japan) according to the manufacturer’s instruction. The conditioned medium was replaced with WST-1 working solution for 2 h, and the absorbance of formazan, a colored dye produced by viable cells using WST-1, was measured at 450 nm by a microplate reader (Spectramax plus 384 microplate reader, Molecular Devices, CA, USA). Untreated cells, cultured with DMEM medium, were used as controls.
4.6.3. Cell Cytotoxicity
The cytotoxicity of the prepared dental bleach was quantified using the LDH assay kit (Takara, Japan) according to the manufacturer’s instructions. The presence of LDH in the medium is an indicator of cellular toxicity. The conditioned medium after incubation with cells for 24 h was transferred and mixed with LDH working solution in a 1:1 ratio. The absorbance of LDH was measured at 450 nm, after incubating for 30 min in the dark using a microplate reader (Spectramax plus 384 microplate reader, Molecular Devices, CA, USA). The cells cultured with DMEM medium were used as controls, while the cells treated with medium containing 0.1% Triton X-100 were used as the positive control.
4.7. Statistical Analysis
After the calculation of ΔE data, it was subjected to statistical analysis and analysis of variance test (ANOVA) was performed, following which post hoc tukey’s test was used for comparison between the groups. A probability (p) value of 0.05 was considered statistically significant. The statistical difference between the control and experimental group in the WST-1 and LDH assay was evaluated by student’s t-test using GraphPad (Prism for Mac, GraphPaD software, San Diego, CA, USA).
5. Conclusions
In this work, nitrogen-doped titanium dioxide was successfully prepared and characterized. The synthesized N-TiO2 calcinated at 350 °C contained majorly the photocatalytic anatase phase and the absorbed nitrogen in the TiO2 lattice caused the band gap narrowing, resulting in visible light-activating N-TiO2. The dental bleaching gel comprising 10% CaO2 as an active ingredient and N-TiO2 as a photocatalyst demonstrated an efficient bleaching effect with a gradual increase in ΔL and ΔE on a coffee- and black tea-stained bovine tooth model in vitro, while the cell viability and cytotoxicity data provided an affirmation of the safety of bleaching gel. The overall findings of this study suggest the CaO2-based bleaching gel with N-TiO2 not only results in effective bleaching but can also decrease the potential side effects that are usually caused by a high-concentrated hydrogen peroxide-based dental bleaching procedure.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms22073759/s1, Figure S1. Representative image of tooth bleaching in black tea stained group; Figure S2. Representative image of tooth bleaching in coffee stained group.
Author Contributions
Conceptualization, F.-H.L.; Methodology, M.T. and F.-H.L.; Validation, C.-P.L.; Formal Analysis, M.T.; Investigation, M.T., Y.-N.C. and C.-P.L.; Writing, M.T.; Supervision, F.-H.L. and C.-P.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the members of the School of Dentistry and College of Medicine, National Taiwan University for their assistance and technical advice.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barry, T.; Bailey, C.; Ashcraft-Olmscheid, D.; Vandewalle, K. Effect of a new bleaching gel on tooth whitening. Oper. Dent. 2017, 42, 559–566. [Google Scholar] [CrossRef]
- Kurzmann, C.; Verheyen, J.; Coto, M.; Kumar, R.V.; Divitini, G.; Shokoohi-Tabrizi, H.A.; Verheyen, P.; De Moor, R.J.G.; Moritz, A.; Agis, H. In vitro evaluation of experimental light activated gels for tooth bleaching. Photochem. Photobiol. Sci. 2019, 18, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.M. Tooth whitening: What we now know. J. Évid. Based Dent. Pract. 2014, 14, 70–76. [Google Scholar] [CrossRef]
- Manuel, S.T.; Abhishek, P.; Kundabala, M. Etiology of tooth discoloration—A review. Etiol. Tooth Discoloration Rev. 2010, 18. [Google Scholar] [CrossRef]
- Bizhang, M.; Domin, J.; Danesh, G.; Zimmer, S. Effectiveness of a new non-hydrogen peroxide bleaching agent after single use—A double-blind placebo-controlled short-term study. J. Appl. Oral Sci. 2017, 25, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.R.; Wertz, P.W. Review of the mechanism of tooth whitening. J. Esthet. Restor. Dent. 2015, 27, 240–257. [Google Scholar] [CrossRef]
- Kihn, P.W. Vital tooth whitening. Dent. Clin. N. Am. 2007, 51, 319–331. [Google Scholar] [CrossRef]
- Alqahtani, M.Q. Tooth-bleaching procedures and their controversial effects: A literature review. Saudi Dent. J. 2014, 26, 33–46. [Google Scholar] [CrossRef]
- Abdullah, A.O.; Muhammed, F.K.; Zheng, B.; Liu, Y. An overview of extrinsic tooth bleaching and its impact on oral restorative materials. World J. Dent. 2017, 8, 503–510. [Google Scholar] [CrossRef]
- Marson, F.; Gonçalves, R.; Silva, C.; Cintra, L.; Pascotto, R.; Dos Santos, P.H.; Briso, A.; Silva, C.; Cintra, L. Penetration of hydrogen peroxide and degradation rate of different bleaching products. Oper. Dent. 2015, 40, 72–79. [Google Scholar] [CrossRef]
- Sundfeld, R.; Neto, D.; Machado, L.; De Oliveira, F.; De Alexandre, R.; Palo, R.; Sundefeld, M.L.M.; Rh, S.; Ds, N.; Ls, M.; et al. Dental bleaching with a 10% hydrogen peroxide product: A six-month clinical observation. Indian J. Dent. Res. 2014, 25, 4. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Scientific Committee on Consumer Products SCCP Opinion on Hydrogen Peroxide in Tooth Whitening Products. 2005. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjtn5yoh9rvAhWa_7sIHc_OB50QFjAAegQIAxAD&url=http%3A%2F%2Fec.europa.eu%2Fhealth%2Fph_risk%2Fcommittees%2F04_sccp%2Fdocs%2Fsccp_cons_01_en.pdf&usg=AOvVaw3CMBtpf_qa6iSdvIB9c_xd (accessed on 31 March 2021).
- Lilaj, B.; Dauti, R.; Agis, H.; Schmid-Schwap, M.; Franz, A.; Kanz, F.; Moritz, A.; Schedle, A.; Cvikl, B. Comparison of bleaching products with up to 6% and with more than 6% hydrogen peroxide: Whitening efficacy using BI and WID and side effects—An in vitro study. Front. Physiol. 2019, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.; Pallesen, U. Tooth bleaching—A critical review of the biological aspects. Crit. Rev. Oral Biol. Med. 2003, 14, 292–304. [Google Scholar] [CrossRef]
- Duque, C.C.D.O.; Soares, D.G.; Basso, F.G.; Hebling, J.; Costa, C.A.D.S. Bleaching effectiveness, hydrogen peroxide diffusion, and cytotoxicity of a chemically activated bleaching gel. Clin. Oral Investig. 2013, 18. [Google Scholar] [CrossRef]
- Dantas, C.M.G.; Vivan, C.L.; Ferreira, L.S.; De Freitas, P.M.; Marques, M.M. In vitro effect of low intensity laser on the cytotoxicity produced by substances released by bleaching gel. Braz. Oral Res. 2010, 24, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Northup, A.; Cassidy, D. Calcium peroxide (CaO2) for use in modified Fenton chemistry. J. Hazard. Mater. 2008, 152, 1164–1170. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, X.; Xue, Y. Application of calcium peroxide in water and soil treatment: A review. J. Hazard. Mater. 2017, 337, 163–177. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Li, T.; Chen, Z.; Wang, Y.; Qin, C. Properties of calcium peroxide for release of hydrogen peroxide and oxygen: A kinetics study. Chem. Eng. J. 2016, 303, 450–457. [Google Scholar] [CrossRef]
- Ziemba, S.L.; Felix, H.; Macdonald, J.; Ward, M. Clinical evaluation of a novel dental whitening lamp and light-catalyzed peroxide gel. J. Clin. Dent. 2005, 16, 123–127. [Google Scholar]
- Bruzell, E.M.; Johnsen, B.; Aalerud, T.N.; Dahl, J.E.; Christensen, T. In vitro efficacy and risk for adverse effects of light-assisted tooth bleaching. Photochem. Photobiol. Sci. 2009, 8, 377–385. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Weng, C.-H.; Hsu, H.-J.; Lin, Y.-H.; Shiesh, C.-C. The synergistic effect of nitrogen dopant and calcination temperature on the visible-light-induced photoactivity of N-doped TiO2. Int. J. Photoenergy 2013, 2013, 268723. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.A.; Khan, M.M.; Ansari, M.O.; Cho, M.H. Nitrogen-doped titanium dioxide (N-doped TiO2) for visible light photocatalysis. New J. Chem. 2016, 40, 3000–3009. [Google Scholar] [CrossRef]
- Nosaka, Y.; Matsushita, M.; Nishino, J.; Nosaka, A.Y. Nitrogen-doped titanium dioxide photocatalysts for visible response prepared by using organic compounds. Sci. Technol. Adv. Mater. 2005, 6, 143–148. [Google Scholar] [CrossRef]
- Chainarong, S.; Sikong, L.; Pavasupree, S.; Niyomwas, S. Synthesis and characterization of nitrogen-doped TiO2 nanomaterials for photocatalytic activities under visible light. Energy Procedia 2011, 9, 418–427. [Google Scholar] [CrossRef]
- Joiner, A.; Philpotts, C.J.; Alonso, C.; Ashcroft, A.T.; Sygrove, N.J. A novel optical approach to achieving tooth whitening. J. Dent. 2008, 36, 8–14. [Google Scholar] [CrossRef]
- Yui, K.C.K.; Rodrigues, J.R.; Mancini, M.N.G.; Balducci, I.; Gonçalves, S.E.P. Ex vivo evaluation of the effectiveness of bleaching agents on the shade alteration of blood-stained teeth. Int. Endod. J. 2008, 41, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Kishi, A.; Otsuki, M.; Sadr, A.; Ikeda, M.; Tagami, J. Effect of light units on tooth bleaching with visible-light activating titanium dioxide photocatalyst. Dent. Mater. J. 2011, 30, 723–729. [Google Scholar] [CrossRef]
- Tano, E.; Otsuki, M.; Kato, J.; Sadr, A.; Ikeda, M.; Tagami, J. Effects of 405 nm diode laser on titanium oxide bleaching activation. Photomed. Laser Surg. 2012, 30, 648–654. [Google Scholar] [CrossRef]
- Sulieman, M.; Addy, M.; Rees, J. Development and evaluation of a method in vitro to study the effectiveness of tooth bleaching. J. Dent. 2003, 31, 415–422. [Google Scholar] [CrossRef]
- Penha, K.-V.-D.F.; Sousa, A.-C.-S.; Oliveira, C.-A.; De Andrade, R.-S.-B.; Vasconcelos, D.-F.-P. A swift, easy and cheap protocol to evaluate the tooth bleaching in vitro. J. Clin. Exp. Dent. 2018, 10, e579–e584. [Google Scholar] [CrossRef]
- Suyama, Y.; Otsuki, M.; Ogisu, S.; Kishikawa, R.; Tagami, J.; Ikeda, M.; Kurata, H.; Cho, T. Effects of light sources and visible light-activated titanium dioxide photocatalyst on bleaching. Dent. Mater. J. 2009, 28, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Suemori, T.; Kato, J.; Nakazawa, T.; Akashi, G.; Igarashi, A.; Hirai, Y.; Kumagai, Y.; Kurata, H. Effects of light irradiation on bleaching by a 3.5% hydrogen peroxide solution containing titanium dioxide. Laser Phys. Lett. 2008, 5, 379–383. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Kato, J.; Nakazawa, T.; Hirai, Y. Bleaching effect of a 405-nm diode laser irradiation used with titanium dioxide and 3.5% hydrogen peroxide. Laser Phys. 2007, 17, 1166–1170. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).