Lipid Metabolism in Bovine Oocytes and Early Embryos under In Vivo, In Vitro, and Stress Conditions
Abstract
1. Introduction
2. Cumulus–Oocyte-Complex Lipid Metabolism
3. Oocytes
4. Follicular Fluid
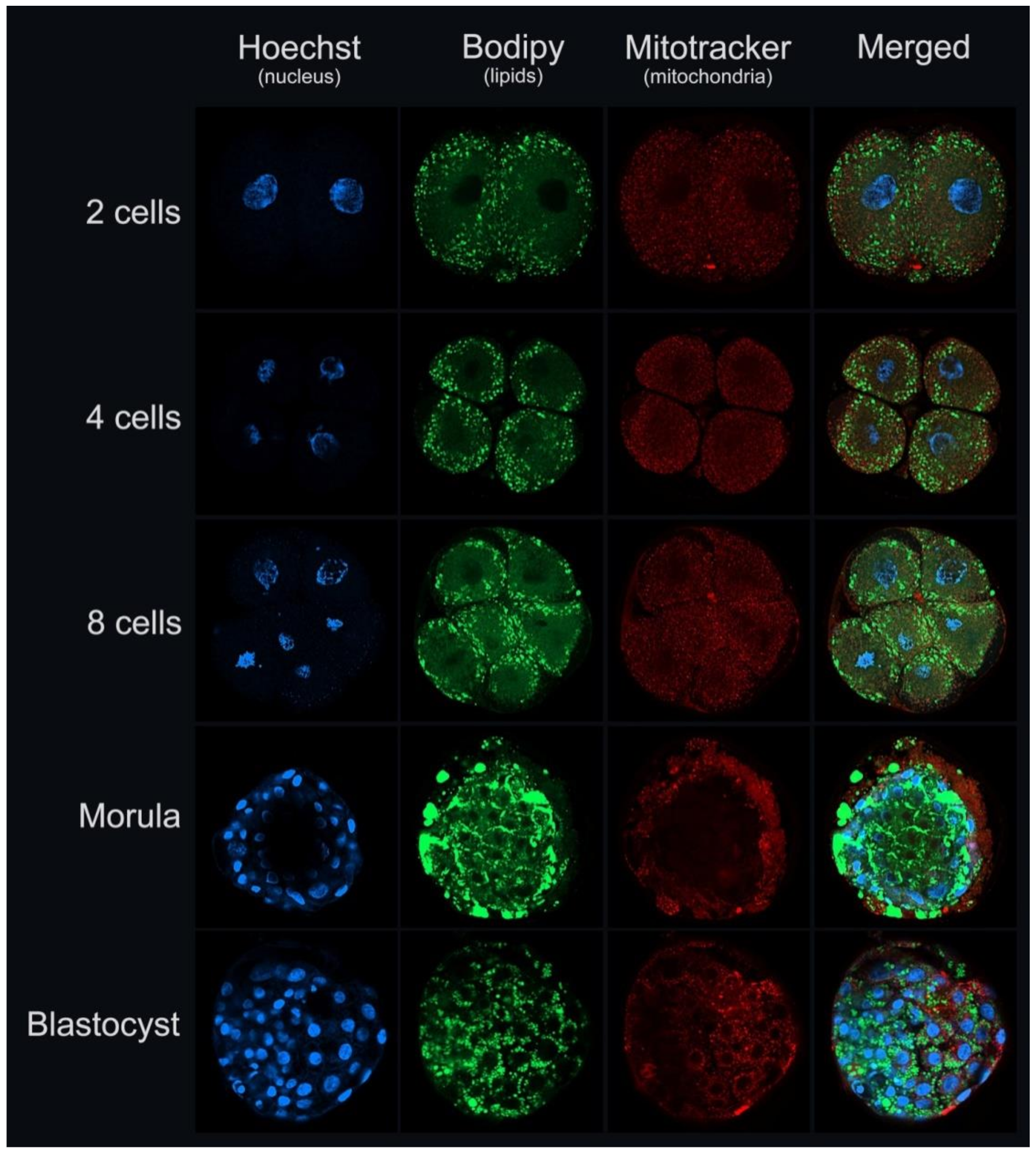
5. Embryos
6. Tubal Fluids
7. Heat Stress
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IVEP | in vitro embryo production |
| FF | follicular fluid |
| FFA | Free fatty acids |
| HDL | high-density lipoprotein |
| NEB | negative energy balance |
| COC | cumulus–oocyte complex |
| CC | cumulus cells |
| ATP | adenosine triphosphate |
| FAO | fatty acid oxidation |
| CPT | carnitine palmitoyltransferase |
| GC | granulosa cell |
| TZP | transzonal projections |
| FABP3 | fatty acid-binding protein 3 |
| LD | lipid droplet |
| TAG | triacylglycerol |
| FSK | forskolin |
| GV | germinal vesicle |
| PAT | Perilipin Adipophilin Tail-interacting |
| ER | endoplasmic reticulum |
| ROS | reactive oxygen species |
| FBS | fetal bovine serum |
| IVD | in vivo derived |
| IVP | in vitro-produced |
| BSA | bovine serum albumin |
| PC | phosphatidylcholines |
| CLA | conjugated linoleic acid |
| NEFA | non-esterified fatty acids |
| ICSI | intracytoplasmic sperm injection |
| SNT | somatic nuclear transfer |
References
- Hansen, P.J. Reproductive physiology of the heat-stressed dairy cow: Implications for fertility and assisted reproduction. Anim. Reprod. 2019, 16, 497–507. [Google Scholar] [CrossRef]
- Summers, M.C.; Biggers, J.D. Chemically defined media and the culture of mammalian preimplantation embryos: Historical perspective and current issues. Hum. Reprod. Updat. 2003, 9, 557–582. [Google Scholar] [CrossRef] [PubMed]
- Jewgenow, K.; Braun, B.C.; Dehnhard, M.; Zahmel, J.; Goeritz, F. Research on reproduction is essential for captive breeding of endangered carnivore species. Reprod. Domest. Anim. 2016, 52, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Paris, M.C.J.; Mastromonaco, G.F.; Paris, D.B.B.P.; Krisher, R.L. A perspective on the role of emerging technologies for the propagation of companion animals, non-domestic and endangered species. Reprod. Fertil. Dev. 2007, 19, iii–iv. [Google Scholar] [CrossRef]
- Ealy, A.D.; Wooldridge, L.K.; McCoski, S.R. Board Invited Review: Post-transfer consequences of in vitro-produced embryos in cattle. J. Anim. Sci. 2019, 97, 2555–2568. [Google Scholar] [CrossRef]
- Young, L.E.; Sinclair, K.D.; Wilmut, I. Large offspring syndrome in cattle and sheep. Rev. Reprod. 1998, 3, 155–163. [Google Scholar] [CrossRef]
- Sudano, M.J.; Paschoal, D.M.; Rascado, T.D.S.; Magalhães, L.C.O.; Crocomo, L.F.; De Lima-Neto, J.F.; Landim-Alvarenga, F.D.C. Lipid content and apoptosis of in vitro-produced bovine embryos as determinants of susceptibility to vitrification. Theriogenology 2011, 75, 1211–1220. [Google Scholar] [CrossRef]
- Dias, L.R.O.; Leme, L.O.; Sprícigo, J.F.W.; Pivato, I.; Dode, M.A.N. Effect of delipidant agents during in vitro culture on the development, lipid content, gene expression and cryotolerance of bovine embryos. Reprod. Domest. Anim. 2019, 55, 11–20. [Google Scholar] [CrossRef]
- Cetica, P.; Pintos, L.; Dalvit, G.; Beconi, M. Activity of key enzymes involved in glucose and triglyceride catabolism during bovine oocyte maturation in vitro. Reproduction 2002, 124, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Lazo, L.; Brisard, D.; Elis, S.; Maillard, V.; Uzbekov, R.; Labas, V.; Desmarchais, A.; Papillier, P.; Monget, P.; Uzbekova, S. Fatty Acid Synthesis and Oxidation in Cumulus Cells Support Oocyte Maturation in Bovine. Mol. Endocrinol. 2014, 28, 1502–1521. [Google Scholar] [CrossRef]
- Ferguson, E.M.; Leese, H.J. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol. Reprod. Dev. 2006, 73, 1195–1201. [Google Scholar] [CrossRef]
- Sturmey, R.G.; Leese, H.J. Energy metabolism in pig oocytes and early embryos. Reproduction 2003, 126, 197–204. [Google Scholar] [CrossRef]
- Dunning, K.R.; Cashman, K.; Russell, D.L.; Thompson, J.G.; Norman, R.J.; Robker, R.L. Beta-Oxidation Is Essential for Mouse Oocyte Developmental Competence and Early Embryo Development1. Biol. Reprod. 2010, 83, 909–918. [Google Scholar] [CrossRef]
- Bonnefont, J.-P. Carnitine palmitoyltransferases 1 and 2: Biochemical, molecular and medical aspects. Mol. Asp. Med. 2004, 25, 495–520. [Google Scholar] [CrossRef]
- Vireque, A.A.; Tata, A.; Belaz, K.R.A.; Grázia, J.G.V.; Santos, F.N.; Arnold, D.R.; Basso, A.C.; Eberlin, M.N.; Silva-De-Sá, M.F.; Ferriani, R.A.; et al. MALDI mass spectrometry reveals that cumulus cells modulate the lipid profile ofin vitro-matured bovine oocytes. Syst. Biol. Reprod. Med. 2017, 63, 86–99. [Google Scholar] [CrossRef]
- Collado-Fernandez, E.; Picton, H.M.; Dumollard, R. Metabolism throughout follicle and oocyte development in mammals. Int. J. Dev. Biol. 2012, 56, 799–808. [Google Scholar] [CrossRef]
- Del Collado, M.; Da Silveira, J.C.; Oliveira, M.L.F.; Alves, B.M.S.M.; Simas, R.C.; Godoy, A.T.; Coelho, D.S.J.; Marques, A.B.M.D.S.; Carriero, M.M.; Nogueira, E.M.; et al. In vitro maturation impacts cumulus–oocyte complex metabolism and stress in cattle. Reproduction 2017, 154, 881–893. [Google Scholar] [CrossRef]
- Bradley, J.; Swann, K. Mitochondria and lipid metabolism in mammalian oocytes and early embryos. Int. J. Dev. Biol. 2019, 63, 93–103. [Google Scholar] [CrossRef]
- Santos, R.R.; Schoevers, E.J.; Roelen, B.A.J. Usefulness of bovine and porcine IVM/IVF models for reproductive toxicology. Reprod. Biol. Endocrinol. 2014, 12, 117. [Google Scholar] [CrossRef]
- Nagano, M. Acquisition of developmental competence and in vitro growth culture of bovine oocytes. J. Reprod. Dev. 2019, 65, 195–201. [Google Scholar] [CrossRef]
- Babayev, E.; Seli, E. Oocyte mitochondrial function and reproduction. Curr. Opin. Obstet. Gynecol. 2015, 27, 175–181. [Google Scholar] [CrossRef]
- Trimarchi, J.R.; Liu, L.; Porterfield, D.M.; Smith, P.J.; Keefe, D.L. Oxidative Phosphorylation-Dependent and -Independent Oxygen Consumption by Individual Preimplantation Mouse Embryos. Biol. Reprod. 2000, 62, 1866–1874. [Google Scholar] [CrossRef]
- Cummins, J. The role of mitochondria in the establishment of oocyte functional competence. Eur. J. Obstet. Gynecol. Reprod. Biol. 2004, 115, S23–S29. [Google Scholar] [CrossRef] [PubMed]
- Tarazona, A.; Rodriguez, J.; Restrepo, L.; Olivera-Angel, M. Mitochondrial Activity, Distribution and Segregation in Bovine Oocytes and in Embryos Produced in Vitro. Reprod. Domest. Anim. 2006, 41, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Tamassia, M.; Nuttinck, F.; May-Panloup, P.; Reynier, P.; Heyman, Y.; Charpigny, G.; Stojkovic, M.; Hiendleder, S.; Renard, J.-P.; Chastant-Maillard, S. In Vitro Embryo Production Efficiency in Cattle and Its Association with Oocyte Adenosine Triphosphate Content, Quantity of Mitochondrial DNA, and Mitochondrial DNA Haplogroup. Biol. Reprod. 2004, 71, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Fair, T. Follicular oocyte growth and acquisition of developmental competence. Anim. Reprod. Sci. 2003, 78, 203–216. [Google Scholar] [CrossRef]
- Stojkovic, M.; Machado, S.A.; Stojkovic, P.; Zakhartchenko, V.; Hutzler, P.; Gonçalves, P.B.; Wolf, E. Mitochondrial Distribution and Adenosine Triphosphate Content of Bovine Oocytes Before and After In Vitro Maturation: Correlation with Morphological Criteria and Developmental Capacity After In Vitro Fertilization and Culture1. Biol. Reprod. 2001, 64, 904–909. [Google Scholar] [CrossRef]
- Jeseta, M.; Knitlova, D.C.; Hanzalova, K.; Hulinska, P.; Hanulakova, S.; Milakovic, I.; Nemcova, L.; Kanka, J.; Machatkova, M. Mitochondrial Patterns in Bovine Oocytes with Different Meiotic Competence Related to Their in vitro Maturation. Reprod. Domest. Anim. 2014, 49, 469–475. [Google Scholar] [CrossRef]
- Kątska-Książkiewicz, L.; Alm, H.; Torner, H.; Heleil, B.; Tuchscherer, A.; Ryńska, B. Mitochondrial aggregation patterns and activity in in vitro cultured bovine oocytes recovered from early antral ovarian follicles. Theriogenology 2011, 75, 662–670. [Google Scholar] [CrossRef]
- Torner, H.; Brüssow, K.-P.; Alm, H.; Ratky, J.; Pöhland, R.; Tuchscherer, A.; Kanitz, W. Mitochondrial aggregation patterns and activity in porcine oocytes and apoptosis in surrounding cumulus cells depends on the stage of pre-ovulatory maturation. Theriogenology 2004, 61, 1675–1689. [Google Scholar] [CrossRef]
- Torner, H.; Alm, H.; Kanitz, W.; Goellnitz, K.; Becker, F.; Poehland, R.; Bruessow, K.-P.; Tuchscherer, A. Effect of Initial Cumulus Morphology on Meiotic Dynamic and Status of Mitochondria in Horse Oocytes during IVM. Reprod. Domest. Anim. 2007, 42, 176–183. [Google Scholar] [CrossRef]
- Hyttel, P.; Fair, T.; Callesen, H.; Greve, T. Oocyte growth, capacitation and final maturation in cattle. Theriogenology 1997, 47, 23–32. [Google Scholar] [CrossRef]
- Bickel, P.E.; Tansey, J.T.; Welte, M.A. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2009, 1791, 419–440. [Google Scholar] [CrossRef] [PubMed]
- Wolins, N.E.; Quaynor, B.K.; Skinner, J.R.; Schoenfish, M.J.; Tzekov, A.; Bickel, P.E. S3-12, Adipophilin, and TIP47 Package Lipid in Adipocytes. J. Biol. Chem. 2005, 280, 19146–19155. [Google Scholar] [CrossRef]
- Prates, E.G.; Nunes, J.T.; Pereira, R.M. A Role of Lipid Metabolism during Cumulus-Oocyte Complex Maturation: Impact of Lipid Modulators to Improve Embryo Production. Mediat. Inflamm. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Brusentsev, E.Y.; Mokrousova, V.I.; Igonina, T.N.; Rozhkova, I.N.; Amstislavsky, S.Y. Role of Lipid Droplets in the Development of Oocytes and Preimplantation Embryos in Mammals. Russ. J. Dev. Biol. 2019, 50, 230–237. [Google Scholar] [CrossRef]
- Valm, A.M.; Cohen, S.; Legant, W.R.; Melunis, J.; Hershberg, J.M.U.; Wait, E.; Cohen, A.R.; Davidson, M.W.; Betzig, E.; Lippincott-Schwartz, W.R.L.E.B.J. Applying systems-level spectral imaging and analysis to reveal the organelle interactome. Nat. Cell Biol. 2017, 546, 162–167. [Google Scholar] [CrossRef]
- Cardoso, C.J.T.; Melo-Sterza, F.A.; Drawert, B.; Poehland, R. Lipid accumulation and mitochondrial activity during in vitro maturation of bovine oocytes. Reprod. Domest. Anim. 2020, 55, 7. [Google Scholar] [CrossRef]
- Sastre, D.; Da Costa, N.N.; De Sá, A.L.A.; Conceição, S.D.B.; Chiaratti, M.R.; Adona, P.R.; Guemra, S.; Meirelles, F.V.; Santos, S.D.S.D.; Sena, L.; et al. Expression of PLIN2 and PLIN3 during oocyte maturation and early embryo development in cattle. Theriogenology 2014, 81, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Welte, M.A.; Gould, A.P. Lipid droplet functions beyond energy storage. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 1260–1272. [Google Scholar] [CrossRef]
- Ordoñez-Leon, E.; Merchant, H.; Medrano, A.; Kjelland, M.; Romo, S. Lipid Droplet Analysis Using In Vitro Bovine Oocytes and Embryos. Reprod. Domest. Anim. 2014, 49, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Zolini, A.M.; Carrascal-Triana, E.; De King, A.R.; Hansen, P.J.; Torres, C.A.A.; Block, J. Effect of addition of l-carnitine to media for oocyte maturation and embryo culture on development and cryotolerance of bovine embryos produced in vitro. Theriogenology 2019, 133, 135–143. [Google Scholar] [CrossRef]
- Razza, E.M.; Sudano, M.J.; Fontes, P.K.; Franchi, F.F.; Belaz, K.R.A.; Santos, P.H.; Castilho, A.C.S.; Rocha, D.F.O.; Eberlin, M.N.; Machado, M.F.; et al. Treatment with cyclic adenosine monophosphate modulators prior to in vitro maturation alters the lipid composition and transcript profile of bovine cumulus–oocyte complexes and blastocysts. Reprod. Fertil. Dev. 2018, 30, 1314. [Google Scholar] [CrossRef]
- Hashimoto, S.; Yamanaka, M.; Yamochi, T.; Iwata, H.; Kawahara-Miki, R.; Inoue, M.; Morimoto, Y. Mitochondrial function in immature bovine oocytes is improved by an increase of cellular cyclic AMP. Sci. Rep. 2019, 9, 5167. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.A.; Braga, D.P.D.A.F.; Borges, E.; Camargo, M.; Cordeiro, F.B.; Pilau, E.J.; Gozzo, F.C.; Fraietta, R.; Turco, E.G.L. Understanding mechanisms of oocyte development by follicular fluid lipidomics. J. Assist. Reprod. Genet. 2019, 36, 1003–1011. [Google Scholar] [CrossRef]
- Moore, S.G.; O’Gorman, A.; Brennan, L.; Fair, T.; Butler, S.T. Follicular fluid and serum metabolites in Holstein cows are predictive of genetic merit for fertility. Reprod. Fertil. Dev. 2017, 29, 658–669. [Google Scholar] [CrossRef] [PubMed]
- O’Gorman, A.; Wallace, M.; Cottell, E.; Gibney, M.J.; McAuliffe, F.M.; Wingfield, M.; Brennan, L. Metabolic profiling of human follicular fluid identifies potential biomarkers of oocyte developmental competence. Reproduction 2013, 146, 389–395. [Google Scholar] [CrossRef]
- Leroy, J.L.M.R.; Vanholder, T.; Mateusen, B.; Christophe, A.; Opsomer, G.; De Kruif, A.; Genicot, G.; Van Soom, A. Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction 2005, 130, 485–495. [Google Scholar] [CrossRef]
- Azhar, S.; Tsai, L.; Medicherla, S.; Chandrasekher, Y.; Giudice, L.; Reaven, E. Human Granulosa Cells Use High Density Lipoprotein Cholesterol for Steroidogenesis. J. Clin. Endocrinol. Metab. 1998, 83, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Von Otte, S.; Paletta, J.R.J.; Becker, S.; König, S.; Fobker, M.; Greb, R.R.; Kiesel, L.; Assmann, G.; Diedrich, K.; Nofer, J.-R. Follicular Fluid High Density Lipoprotein-associated Sphingosine 1-Phosphate Is a Novel Mediator of Ovarian Angiogenesis. J. Biol. Chem. 2006, 281, 5398–5405. [Google Scholar] [CrossRef]
- Aardema, H.; Lolicato, F.; Van De Lest, C.H.; Brouwers, J.F.; Vaandrager, A.B.; Van Tol, H.T.; Roelen, B.A.; Vos, P.L.; Helms, J.B.; Gadella, B.M. Bovine Cumulus Cells Protect Maturing Oocytes from Increased Fatty Acid Levels by Massive Intracellular Lipid Storage. Biol. Reprod. 2013, 88, 164. [Google Scholar] [CrossRef]
- Lolicato, F.; Brouwers, J.F.; Van De Lest, C.H.; Wubbolts, R.; Aardema, H.; Priore, P.; Roelen, B.A.; Helms, J.B.; Gadella, B.M. The Cumulus Cell Layer Protects the Bovine Maturing Oocyte Against Fatty Acid-Induced Lipotoxicity. Biol. Reprod. 2015, 92, 1–16. [Google Scholar] [CrossRef]
- Marei, W.F.; De Bie, J.; Mohey-Elsaeed, O.; Wydooghe, E.; Bols, P.E.; Leroy, J.L. Alpha-linolenic acid protects the developmental capacity of bovine cumulus–oocyte complexes matured under lipotoxic conditions in vitro. Biol. Reprod. 2017, 96, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Marei, W.F.A.; Bosch, L.V.D.; Pintelon, I.; Mohey-Elsaeed, O.; Bols, P.E.J.; Leroy, J.L.M.R. Mitochondria-targeted therapy rescues development and quality of embryos derived from oocytes matured under oxidative stress conditions: A bovine in vitro model. Hum. Reprod. 2019, 34, 1984–1998. [Google Scholar] [CrossRef] [PubMed]
- Warzych, E.; Pawlak, P.; Pszczola, M.; Cieslak, A.; Madeja, Z.E.; Lechniak, D. Interactions of bovine oocytes with follicular elements with respect to lipid metabolism. Anim. Sci. J. 2017, 88, 1491–1497. [Google Scholar] [CrossRef] [PubMed]
- Matoba, S.; Bender, K.; Fahey, A.G.; Mamo, S.; Brennan, L.; Lonergan, P.; Fair, T. Predictive value of bovine follicular components as markers of oocyte developmental potential. Reprod. Fertil. Dev. 2014, 26, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Belaz, K.R.A.; Tata, A.; França, M.R.; Da Silva, M.I.S.; Vendramini, P.H.; Fernandes, A.M.A.; D’Alexandri, F.L.; Eberlin, M.N.; Binelli, M. Phospholipid Profile and Distribution in the Receptive Oviduct and Uterus During Early Diestrus in Cattle. Biol. Reprod. 2016, 95, 127. [Google Scholar] [CrossRef]
- Sudano, M.J.; Santos, V.G.; Tata, A.; Ferreira, C.R.; Paschoal, D.M.; Machado, R.; Buratini, J.; Eberlin, M.N.; Landim-Alvarenga, F.D. Phosphatidylcholine and Sphingomyelin Profiles Vary in Bos taurus indicus and Bos taurus taurus In Vitro- and In Vivo-Produced Blastocysts. Biol. Reprod. 2012, 87, 130. [Google Scholar] [CrossRef] [PubMed]
- Annes, K.; Sudano, M.J.; Belaz, K.R.A.; Tata, A.; Santos, V.G.; Junior, A.M.D.F.; Dos Santos, É.C.; Eberlin, M.N.; Milazzotto, M.P. Lipid characterization of in vitro-produced bovine embryos with distinct kinetics of development. Zygote 2019, 27, 413–422. [Google Scholar] [CrossRef]
- Leese, H.J.; Guerif, F.; Allgar, V.; Brison, D.R.; Lundin, K.; Sturmey, R.G. Biological optimization, the Goldilocks principle, and how much islagomin the preimplantation embryo. Mol. Reprod. Dev. 2016, 83, 748–754. [Google Scholar] [CrossRef]
- Thompson, J. In vitro culture and embryo metabolism of cattle and sheep embryos—A decade of achievement. Anim. Reprod. Sci. 2000, 60–61, 263–275. [Google Scholar] [CrossRef]
- Leese, H.J. Metabolism of the preimplantation embryo: 40 years on. Reproduction 2012, 143, 417–427. [Google Scholar] [CrossRef] [PubMed]
- García-Herreros, M.; Simintiras, C.A.; Lonergan, P. Temporally differential protein expression of glycolytic and glycogenic enzymes during in vitro preimplantation bovine embryo development. Reprod. Fertil. Dev. 2018, 30, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.G.; Sturmey, R.G. Parallels between embryo and cancer cell metabolism. Biochem. Soc. Trans. 2013, 41, 664–669. [Google Scholar] [CrossRef]
- Sudano, M.J.; Rascado, T.D.; Tata, A.; Belaz, K.R.; Santos, V.G.; Valente, R.S.; Mesquita, F.S.; Ferreira, C.R.; Araújo, J.P.; Eberlin, M.N.; et al. Lipidome signatures in early bovine embryo development. Theriogenology 2016, 86, 472–484.e1. [Google Scholar] [CrossRef] [PubMed]
- Abe, H.; Yamashita, S.; Satoh, T.; Hoshi, H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Dev. 2001, 61, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Del Collado, M.; Saraiva, N.Z.; Lopes, F.L.; Gaspar, R.C.; Padilha, L.C.; Costa, R.R.; Rossi, G.F.; Vantini, R.; Garcia, J.M. Influence of bovine serum albumin and fetal bovine serum supplementation during in vitro maturation on lipid and mitochondrial behaviour in oocytes and lipid accumulation in bovine embryos. Reprod. Fertil. Dev. 2016, 28, 1721–1732. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.-H.; Park, B.-Y.; Kong, R.; Son, M.-J.; Park, C.-S.; Shin, N.-H.; Cheon, H.-Y.; Yang, Y.-R.; Lee, J.-W.; Jin, J.-I.; et al. Effect of Serum and Serum Free Media on the Developmental Competence of OPU Derived Bovine IVP Embryo. J. Anim. Reprod. Biotechnol. 2019, 34, 305–310. [Google Scholar] [CrossRef][Green Version]
- Jeong, W.; Cho, S.; Lee, H.; Deb, G.; Lee, Y.; Kwon, T.; Kong, I. Effect of cytoplasmic lipid content on in vitro developmental efficiency of bovine IVP embryos. Theriogenology 2009, 72, 584–589. [Google Scholar] [CrossRef]
- Rizos, D.; Gutiérrez-Adán, A.; Pérez-Garnelo, S.; De La Fuente, J.; Boland, M.; Lonergan, P. Bovine embryo culture in the presence or absence of serum: Implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol. Reprod. 2003, 68, 236–243. [Google Scholar] [CrossRef]
- Hosoe, M.; Inaba, Y.; Hashiyada, Y.; Imai, K.; Kajitani, K.; Hasegawa, Y.; Irie, M.; Teramoto, H.; Takahashi, T.; Niimura, S. Effect of supplemented sericin on the development, cell number, cryosurvival and number of lipid droplets in cultured bovine embryos. Anim. Sci. J. 2016, 88, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Held-Hoelker, E.; Klein, S.; Rings, F.; Salilew-Wondim, D.; Saeed-Zidane, M.; Neuhoff, C.; Tesfaye, D.; Schellander, K.; Hoelker, M. Cryosurvival of in vitro produced bovine embryos supplemented with l -Carnitine and concurrent reduction of fatty acids. Theriogenology 2017, 96, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Keicho, K.; Takahashi, H.; Ogawa, H.; Schultz, R.M.; Okano, A.; Schulte, R. Effect of oxidative stress on development and DNA damage in in-vitro cultured bovine embryos by comet assay. Theriogenology 2000, 54, 137–145. [Google Scholar] [CrossRef]
- Guerin, P.; El Mouatassim, S.; Ménézo, Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum. Reprod. Updat. 2001, 7, 175–189. [Google Scholar] [CrossRef]
- Cagnone, G.L.; Sirard, M.-A. Transcriptomic signature to oxidative stress exposure at the time of embryonic genome activation in bovine blastocysts. Mol. Reprod. Dev. 2013, 80, 297–314. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-B.; Choi, S.-A.; Sim, B.-W.; Kim, J.-S.; Mun, S.-E.; Jeong, P.-S.; Yang, H.-J.; Lee, Y.; Park, Y.-H.; Song, B.-S.; et al. Developmental Competence of Bovine Early Embryos Depends on the Coupled Response Between Oxidative and Endoplasmic Reticulum Stress. Biol. Reprod. 2014, 90, 104. [Google Scholar] [CrossRef]
- Leite, R.F.; Annes, K.; Ispada, J.; De Lima, C.B.; Dos Santos, É.C.; Fontes, P.K.; Nogueira, M.F.G.; Milazzotto, M.P. Oxidative Stress Alters the Profile of Transcription Factors Related to Early Development on In Vitro Produced Embryos. Oxidative Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Takahashi, T.; Inaba, Y.; Somfai, T.; Kaneda, M.; Geshi, M.; Nagai, T.; Manabe, N. Supplementation of culture medium with L-carnitine improves development and cryotolerance of bovine embryos produced in vitro. Reprod. Fertil. Dev. 2013, 25, 589–599. [Google Scholar] [CrossRef]
- Ghanem, N.; Ha, A.-N.; Fakruzzaman, M.; Bang, J.-I.; Lee, S.-C.; Kong, I.-K. Differential expression of selected candidate genes in bovine embryos produced in vitro and cultured with chemicals modulating lipid metabolism. Theriogenology 2014, 82, 238–250. [Google Scholar] [CrossRef]
- Pereira, D.M.; Cardoso, C.J.T.; Da Silva, W.A.L.; Souza-Cáceres, M.B.; Santos, M.; Pöhland, R.; Couto, A.M.; Moslaves, I.S.B.; Kadri, M.C.T.; Sterza, F.D.A.M. Production of in vitro bovine embryos supplemented with l-carnitine in different oxygen tensions and the relation to nitric oxide. Zygote 2020, 28, 1–6. [Google Scholar] [CrossRef]
- Shahzad, Q.; Pu, L.; Wadood, A.A.; Waqas, M.; Xie, L.; Pareek, C.S.; Xu, H.; Liang, X.; Lu, Y. Proteomics Analysis Reveals that Warburg Effect along with Modification in Lipid Metabolism Improves In Vitro Embryo Development under Low Oxygen. Int. J. Mol. Sci. 2020, 21, 1996. [Google Scholar] [CrossRef]
- Lanzarini, F.; Pereira, F.; Camargo, J.; Oliveira, A.; Belaz, K.; Melendez-Perez, J.; Eberlin, M.; Brum, M.; Mesquita, F.; Sudano, M. ELOVL5 Participates in Embryonic Lipid Determination of Cellular Membranes and Cytoplasmic Droplets. Int. J. Mol. Sci. 2021, 22, 1311. [Google Scholar] [CrossRef] [PubMed]
- Valente, R.S.; De Almeida, T.G.; Alves, M.F.; De Camargo, J.; Basso, A.C.; Belaz, K.R.A.; Eberlin, M.N.; Landim-Alvarenga, F.D.C.; Fontes, P.K.; Nogueira, M.F.G.; et al. Modulation of long-chain Acyl-CoA synthetase on the development, lipid deposit and cryosurvival of in vitro produced bovine embryos. PLoS ONE 2019, 14, e0220731. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.S.; De Barros, B.A.F.; Monteiro, C.A.S.; Rosa, P.M.S.; Leal, G.R.; Serapião, R.V.; Camargo, L.S.A. Individual assessment of bovine embryo development using a homemade chamber reveals kinetic patterns of success and failure to reach blastocyst stage. Syst. Biol. Reprod. Med. 2019, 65, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.J.T.; Drawert, B.; Poehland, R.; Melo-Sterza, F.A. Lipid content and mitochondrial activity of bovine embryos with different developmental kinetics. XXXIV Reunião Anual da Sociedade Brasileira de Tecnologia de Embriões, Animal Reproduction. Belo Horizonte: Brazilian College of Animal Reproduction. Anim. Reprod. 2020, 17, 18. [Google Scholar]
- Lamy, J.; Gatien, J.; Dubuisson, F.; Nadal-Desbarats, L.; Salvetti, P.; Mermillod, P.; Saint-Dizier, M. Metabolomic profiling of bovine oviductal fluid across the oestrous cycle using proton nuclear magnetic resonance spectroscopy. Reprod. Fertil. Dev. 2018, 30, 1021. [Google Scholar] [CrossRef]
- Banliat, C.; Tomas, D.; Teixeira-Gomes, A.-P.; Uzbekova, S.; Guyonnet, B.; Labas, V.; Saint-Dizier, M. Stage-dependent changes in oviductal phospholipid profiles throughout the estrous cycle in cattle. Theriogenology 2019, 135, 65–72. [Google Scholar] [CrossRef]
- Jordaens, L.; Van Hoeck, V.; De Bie, J.; Berth, M.; Marei, W.F.; Desmet, K.L.; Bols, P.E.; Leroy, J.L. Non-esterified fatty acids in early luteal bovine oviduct fluid mirror plasma concentrations: An ex vivo approach. Reprod. Biol. 2017, 17, 281–284. [Google Scholar] [CrossRef]
- Banliat, C.; Le Bourhis, D.; Bernardi, O.; Tomas, D.; Labas, V.; Salvetti, P.; Guyonnet, B.; Mermillod, P.; Saint-Dizier, M. Oviduct Fluid Extracellular Vesicles Change the Phospholipid Composition of Bovine Embryos Developed In Vitro. Int. J. Mol. Sci. 2020, 21, 5326. [Google Scholar] [CrossRef]
- Nakamura, K.; Sheps, S.; Arck, P.C. Stress and reproductive failure: Past notions, present insights and future directions. J. Assist. Reprod. Genet. 2008, 25, 47–62. [Google Scholar] [CrossRef]
- Hansen, P. Physiological and cellular adaptations of zebu cattle to thermal stress. Anim. Reprod. Sci. 2004, 82–83, 349–360. [Google Scholar] [CrossRef]
- Souza-Cácares, M.; Fialho, A.; Silva, W.; Cardoso, C.; Pöhland, R.; Martins, M.; Melo-Sterza, F. Oocyte quality and heat shock proteins in oocytes from bovine breeds adapted to the tropics under different conditions of environmental thermal stress. Theriogenology 2019, 130, 103–110. [Google Scholar] [CrossRef]
- Da Silva, W.A.L.; Poehland, R.; De Oliveira, C.C.; Ferreira, M.G.C.R.; De Almeida, R.G.; Cáceres, M.B.S.; Macedo, G.G.; Silva, E.V.D.C.E.; Alves, F.V.; Nogueira, E.; et al. Shading effect on physiological parameters and in vitro embryo production of tropical adapted Nellore heifers in integrated crop-livestock-forest systems. Trop. Anim. Health Prod. 2020, 52, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Pöhland, R.; Souza-Cácares, M.B.; Datta, T.K.; Vanselow, J.; Martins, M.I.M.; Da Silva, W.A.L.; Cardoso, C.J.T.; Melo-Sterza, F.D.A. Influence of long-term thermal stress on the in vitro maturation on embryo development and Heat Shock Protein abundance in zebu cattle. Anim. Reprod. 2020, 17, 20190085. [Google Scholar] [CrossRef] [PubMed]
- Shehab-El-Deen, M.; Leroy, J.; Fadel, M.; Saleh, S.; Maes, D.; Van Soom, A. Biochemical changes in the follicular fluid of the dominant follicle of high producing dairy cows exposed to heat stress early post-partum. Anim. Reprod. Sci. 2010, 117, 189–200. [Google Scholar] [CrossRef]
- Hooper, L.M.; Payton, R.R.; Rispoli, L.A.; Saxton, A.M.; Edwards, J.L. Impact of heat stress on germinal vesicle breakdown and lipolytic changes during in vitro maturation of bovine oocytes. J. Reprod. Dev. 2015, 61, 459–464. [Google Scholar] [CrossRef]

| Lipids | Cell/Fluid | Association with Higher Fertility | Reference |
|---|---|---|---|
| Higher concentrations of the n-3 PUFA linolenic acid | Follicular Fluid | Oocyte competence—potential to develop to the blastocyst stage in vitro | [56] (bovine) |
| Higher concentrations of Phosphatidic acid (PA; 745.5563 m/z) | Follicular Fluid | Pregnancy probability | [45] (woman) |
| Higher concentrations of Triacylglycerol (TAG; 773.6153 m/z) | Follicular Fluid | Pregnancy probability | [45] (woman) |
| Higher concentrations of Phosphatidylglycerol (PG; 749.5693 m/z). | Follicular Fluid | Pregnancy probability | [45] (woman) |
| Lower concentrations of Glucosylceramide (GluCer) (796.6948 m/z) | Follicular Fluid | Pregnancy probability | [45] (woman) |
| Higher concentrations of Arachidonic acid (C20:4n6) | Follicular Fluid | Oocyte competence—potential of human oocyte to cleave. | [47] (woman) |
| Higher concentrations of Stearic acid (C18:0) | Follicular Fluid | Oocyte competence—potential of human oocyte to cleave. | [47] (woman) |
| Lower concentrations of palmitic acid (C 16:00) | Follicular Fluid | Oocyte competence—potential of bovine oocyte to develop to the blastocyst stage in vitro and of human oocyte to cleave. | [56] (bovine); [47] (woman) |
| Lower concentrations of total saturated fatty acids | Follicular Fluid | Oocyte competence—potential of bovine oocyte to develop to the blastocyst stage in vitro and of human oocyte to cleave. | [56] (bovine), [47] (woman) |
| Lower n-6:n-3 PUFA ratio | Follicular Fluid | Oocyte competence—potential of human oocyte to cleave. | [47] (woman) |
| Lower concentrations of Arachidic acid (C20:0) | Follicular Fluid | Oocyte competence—potential of human oocyte to cleave. | [47] (woman) |
| Lower concentration of Phosphatidylcholines (PC 36:4; 38:7; 38:5; 40:7; 40:6) | Uterus Fluid D4 | Present in cows with bigger pre-ovulatory follicle and bigger corpus luteum | [57] (bovine) |
| Higher concentrations of Phosphatidylcholines (PC 32:0; 32:1; 34:4) | Uterus Fluid D4 | Present in cows with bigger pre-ovulatory follicle and bigger corpus luteum | [57] (bovine) |
| Higher concentrations Ceramides (CER 42:1) | Uterus Fluid D4 | Present in cows with bigger pre-ovulatory follicle and bigger corpus luteum | [57] (bovine) |
| Higher concentration of Phosphatidylcholines (PC 32:1; 35:2) | Uterus Fluid D7 | Present in cows with bigger pre-ovulatory follicle and bigger corpus luteum | [57] (bovine) |
| Higher concentrations of Sphingomyelins (PC 34:2; 34:1) | Uterus Fluid D7 | Present in cows with bigger pre-ovulatory follicle and bigger corpus luteum | [57] (bovine) |
| Higher concentration of Phosphatidylcholines—PC 34:2 | Blastocyst | Potential for survival to cryopreservation | [58] (bovine) |
| Lower concentration of Phosphatidylcholines—PC 32:0 | Blastocyst | Potential for survival to cryopreservation | [58] (bovine) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Andrade Melo-Sterza, F.; Poehland, R. Lipid Metabolism in Bovine Oocytes and Early Embryos under In Vivo, In Vitro, and Stress Conditions. Int. J. Mol. Sci. 2021, 22, 3421. https://doi.org/10.3390/ijms22073421
de Andrade Melo-Sterza F, Poehland R. Lipid Metabolism in Bovine Oocytes and Early Embryos under In Vivo, In Vitro, and Stress Conditions. International Journal of Molecular Sciences. 2021; 22(7):3421. https://doi.org/10.3390/ijms22073421
Chicago/Turabian Stylede Andrade Melo-Sterza, Fabiana, and Ralf Poehland. 2021. "Lipid Metabolism in Bovine Oocytes and Early Embryos under In Vivo, In Vitro, and Stress Conditions" International Journal of Molecular Sciences 22, no. 7: 3421. https://doi.org/10.3390/ijms22073421
APA Stylede Andrade Melo-Sterza, F., & Poehland, R. (2021). Lipid Metabolism in Bovine Oocytes and Early Embryos under In Vivo, In Vitro, and Stress Conditions. International Journal of Molecular Sciences, 22(7), 3421. https://doi.org/10.3390/ijms22073421






