Ascorbic Acid Supplementation Improves Skeletal Muscle Growth in Pacu (Piaractus mesopotamicus) Juveniles: In Vivo and In Vitro Studies
Abstract
1. Introduction
2. Results
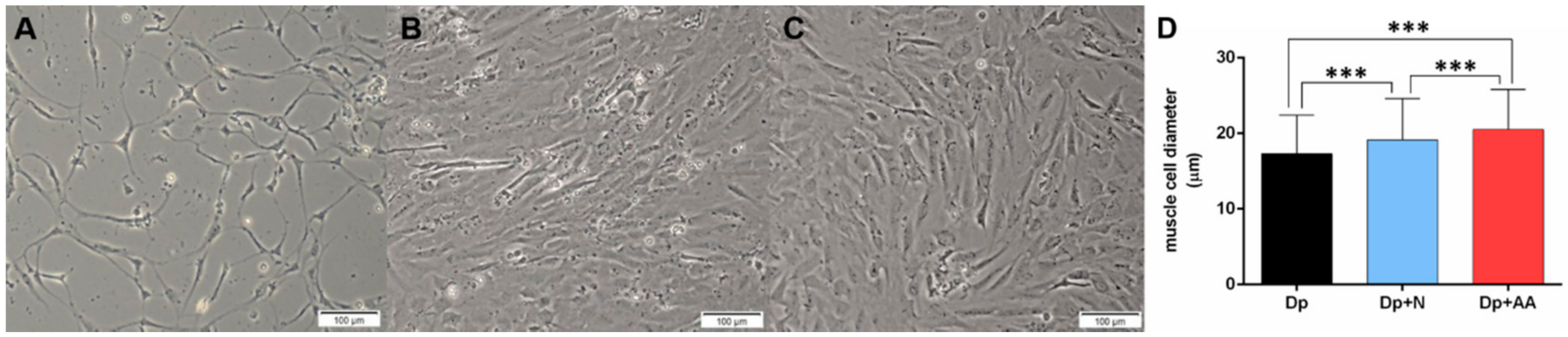
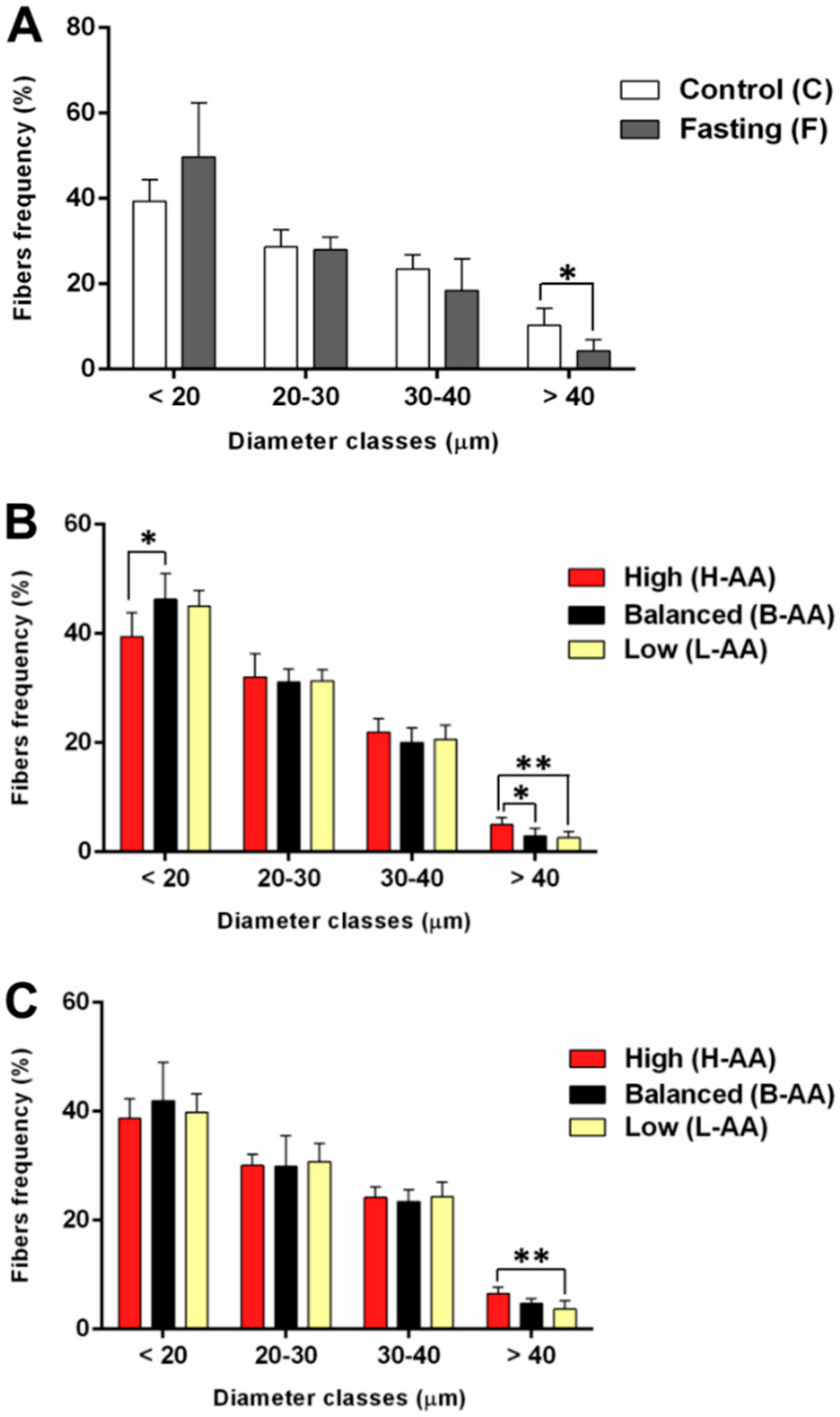
2.1. Fasting Followed by AA Supplementation Promotes Morphological Alterations in Skeletal Muscle In Vitro and In Vivo
2.2. AA Supplementation Promotes Changes in Antioxidant Enzyme Activity In Vitro and In Vivo
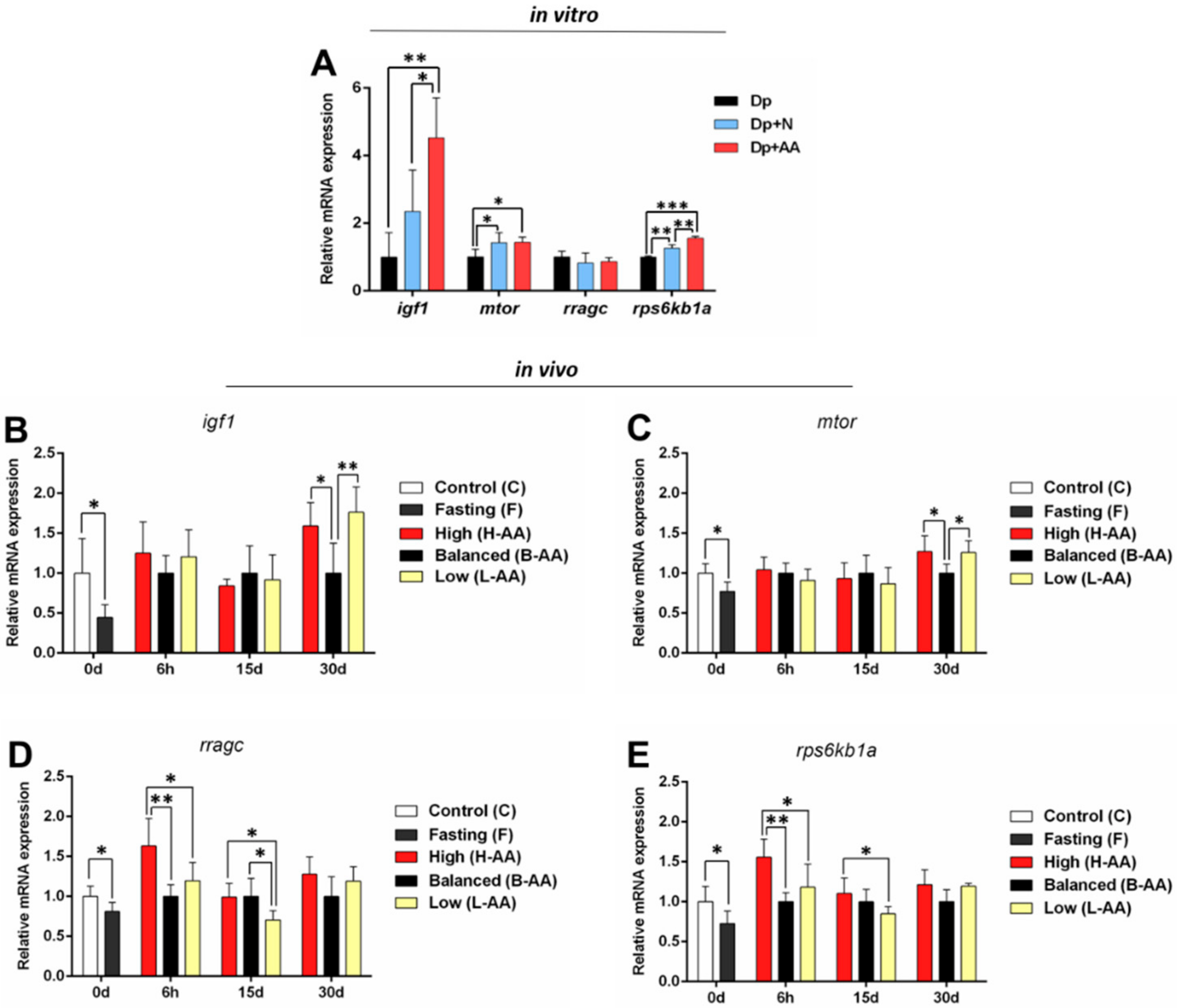
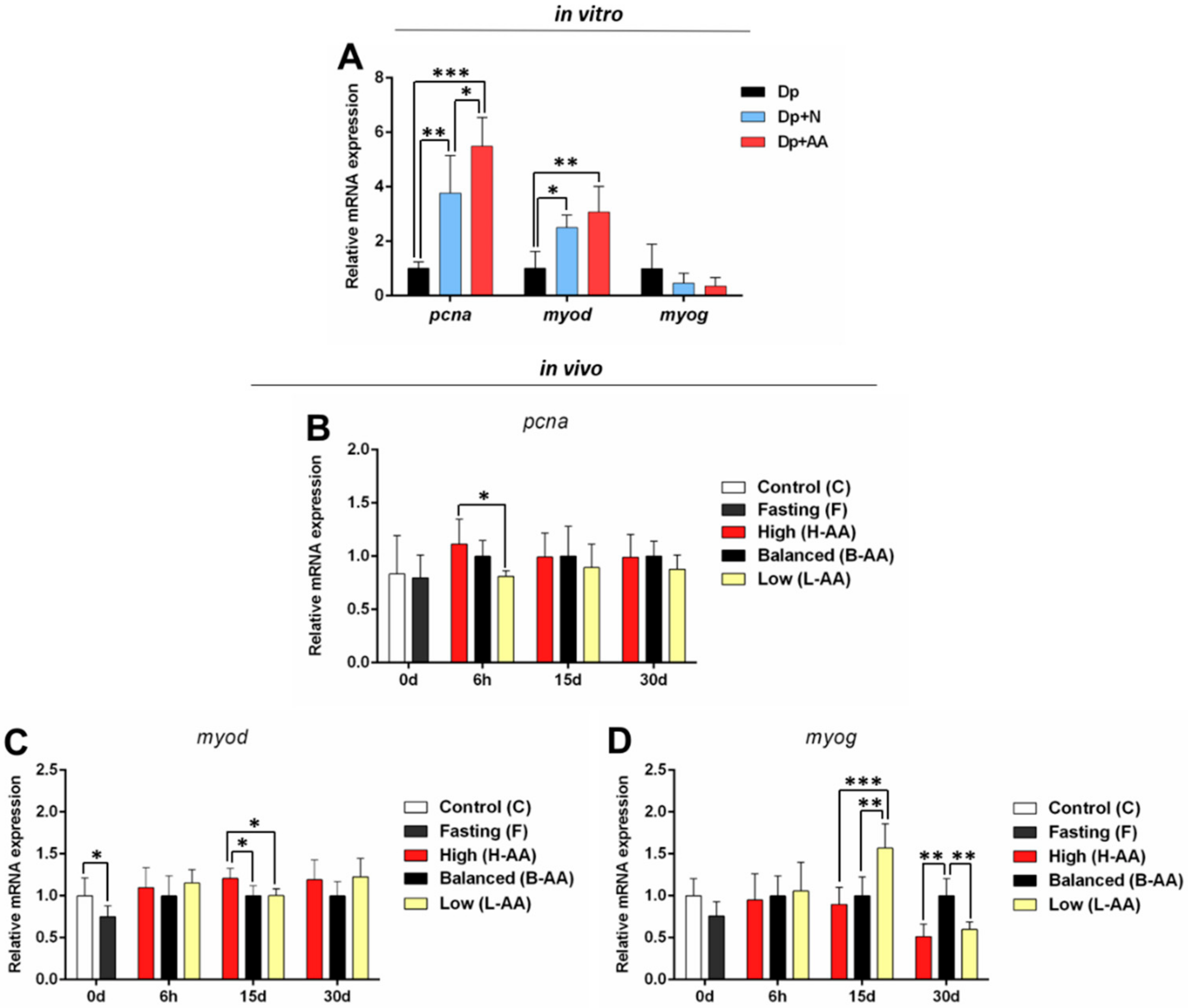
2.3. AA Supplementation Affects Genes Expression In Vitro and In Vivo
2.3.1. Expression of Anabolic Genes Increases with AA Supplementation
2.3.2. Catabolic Gene Expression Is Modulated Throughout the Experimental Protocols
2.3.3. Myogenic Genes May Contribute to Muscle Recovery in Response to AA Supplementation
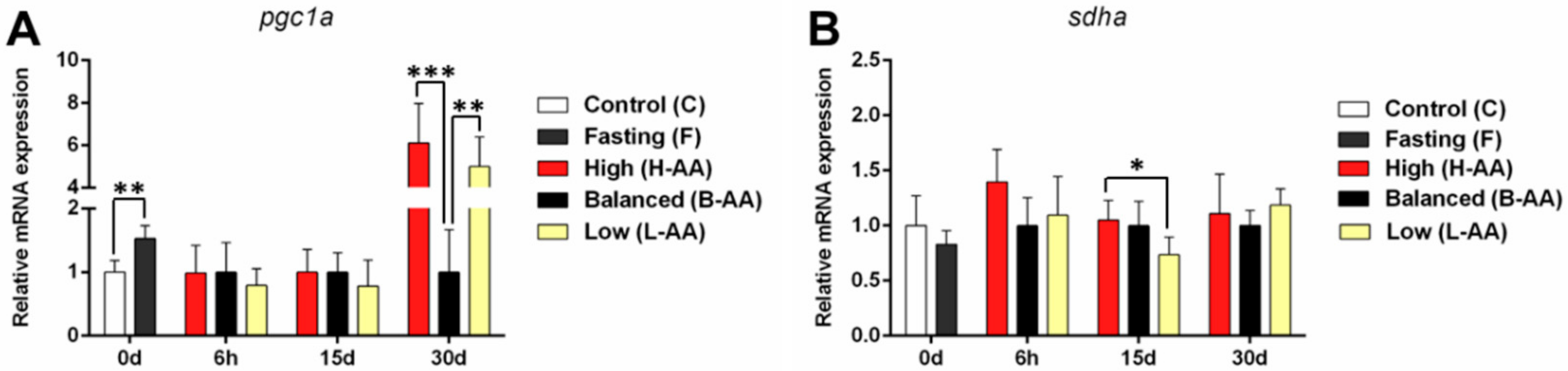
2.3.4. Changes in the Expression of Mitochondrial Genes Contribute to Muscle Maintenance during Fasting and Recovery during Refeeding
3. Discussion
4. Materials and Methods
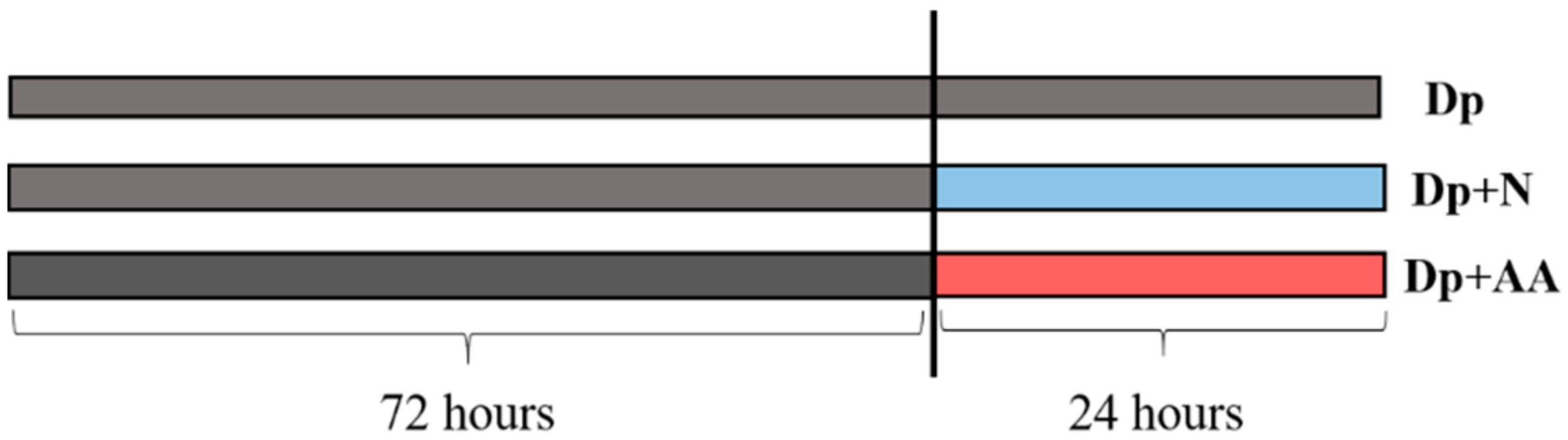
4.1. Experimental Conditions
4.2. Primary Muscle Cell Culture
4.3. Morphological Analyzes
4.4. Gene Expression Analyzes
4.5. Antioxidant Enzymes Activity
4.6. Statistical Analyzes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bone, Q. Locomotor muscle. In Fish Physiology; Academic Press: San Diego, CA, USA, 1978; Volume 7, pp. 361–424. ISBN 0123504074. [Google Scholar]
- Sänger, A.M.; Stoiber, W. Muscle fiber diversity and plasticity. In Muscle Development and Growth; Johnston, I.A., Ed.; Academic Press: San Diego, CA, USA, 2001; pp. 187–250. [Google Scholar]
- Vélez, E.J.; Lutfi, E.; Azizi, S.; Perelló, M.; Salmerón, C.; Riera-Codina, M.; Ibarz, A.; Fernández-Borràs, J.; Blasco, J.; Capilla, E.; et al. Understanding fish muscle growth regulation to optimize aquaculture production. Aquaculture 2017, 467, 28–40. [Google Scholar] [CrossRef]
- Keenan, S.R.; Currie, P.D. The Developmental Phases of Zebrafish Myogenesis. J. Dev. Biol. 2019, 7, 12. [Google Scholar] [CrossRef]
- Navarro, I.; Gutiérrez, J. Fasting and starvation. In Biochemistry and Molecular Biology of Fishes; Hochachka, P.W., Mommsen, T.P., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1995; Volume 4, pp. 393–434. ISBN 9780444820822. [Google Scholar]
- Johnston, I.A. Environment and plasticity of myogenesis in teleost fish. J. Exp. Biol. 2006, 209, 2249–2264. [Google Scholar] [CrossRef]
- Johnston, I.A.; Bower, N.I.; Macqueen, D.J. Growth and the regulation of myotomal muscle mass in teleost fish. J. Exp. Biol. 2011, 214, 1617–1628. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.N.; Einarsdottir, I.E.; Paredes, R.; Hidalgo, C.; Valdes, J.A.; Björnsson, B.T.; Molina, A. The TORC1/P70S6K and TORC1/4EBP1 signaling pathways have a stronger contribution on skeletal muscle growth than MAPK/ERK in an early vertebrate: Differential involvement of the IGF system and atrogenes. Gen. Comp. Endocrinol. 2015, 210, 96–106. [Google Scholar] [CrossRef]
- Rescan, P.Y.; Montfort, J.; Rallière, C.; Le Cam, A.; Esquerré, D.; Hugot, K. Dynamic gene expression in fish muscle during recovery growth induced by a fasting-refeeding schedule. BMC Genom. 2007, 8, 438. [Google Scholar] [CrossRef] [PubMed]
- Rescan, P.Y.; Cam, A.; Rallière, C.; Montfort, J. Global gene expression in muscle from fasted/refed trout reveals up-regulation of genes promoting myofibre hypertrophy but not myofibre production. BMC Genom. 2017, 18, 447. [Google Scholar] [CrossRef]
- Paula, T.G.; Zanella, B.T.T.; Fantinatti, B.E.d.A.; Moraes, L.N.d.; Duran, B.O.d.S.; Oliveira, C.B.d.; Salomão, R.A.S.; Silva, R.N.d.; Padovani, C.R.; dos Santos, V.B.; et al. Food restriction increase the expression of mTORC1 complex genes in the skeletal muscle of juvenile pacu (Piaractus mesopotamicus). PLoS ONE 2017, 12, e0177679. [Google Scholar] [CrossRef]
- Fuentes, E.N.; Safian, D.; Einarsdottir, I.E.; Valdes, J.A.; Elorza, A.A.; Molina, A.; Bjornsson, B.T. Nutritional status modulates plasma leptin, AMPK and TOR activation, and mitochondrial biogenesis: Implications for cell metabolism and growth in skeletal muscle of the fine flounder. Gen. Comp. Endocrinol. 2013, 186, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tan, Q.; Kong, F.; Yu, H.; Zhu, Y.; Yao, J.; Abouel Azm, F.R. Fish growth in response to different feeding regimes and the related molecular mechanism on the changes in skeletal muscle growth in grass carp (Ctenopharyngodon idellus). Aquaculture 2019, 512. [Google Scholar] [CrossRef]
- Valente, M.P.; Bower, N.I.; Johnston, I.A.; Valente, L.M.P.; Bower, N.I.; Johnston, I.A. Postprandial expression of growth-related genes in Atlantic salmon (Salmo salar L.) juveniles fasted for 1 week and fed a single meal to satiation. Br. J. Nutr. 2012, 108, 2148–2157. [Google Scholar] [CrossRef]
- Nebo, C.; Portella, M.C.; Carani, F.R.; de Almeida, F.L.A.; Padovani, C.R.; Carvalho, R.F.; Dal-Pai-Silva, M. Short periods of fasting followed by refeeding change the expression of muscle growth-related genes in juvenile Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2013, 164, 268–274. [Google Scholar] [CrossRef]
- Zhu, K.; Chen, L.; Zhao, J.; Wang, H.; Wang, W.; Li, Z.; Wang, H. Molecular characterization and expression patterns of myogenin in compensatory growth of Megalobrama amblycephala. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 170, 10–17. [Google Scholar] [CrossRef]
- Ali, M.; Nicieza, A.; Wootton, R.J. Compensatory growth in fishes: A response to growth depression. Fish Fish. 2003, 4, 147–190. [Google Scholar] [CrossRef]
- Seiliez, I.; Gabillard, J.-C.; Skiba-Cassy, S.; Garcia-Serrana, D.; Gutierrez, J.; Kaushik, S.; Panserat, S.; Tesseraud, S. An in vivo and in vitro assessment of TOR signaling cascade in rainbow trout (Oncorhynchus mykiss). AJP Regul. Integr. Comp. Physiol. 2008, 295, R329–R335. [Google Scholar] [CrossRef]
- Montserrat, N.; Gabillard, J.C.; Capilla, E.; Navarro, M.I.; Gutiérrez, J. Role of insulin, insulin-like growth factors, and muscle regulatory factors in the compensatory growth of the trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 2007, 150, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Mareco, E.A.; Garcia de la Serrana, D.; Johnston, I.A.; Dal-Pai-Silva, M. Characterization of the transcriptome of fast and slow muscle myotomal fibres in the pacu (Piaractus mesopotamicus). BMC Genom. 2015, 16, 182. [Google Scholar] [CrossRef]
- Kuniyoshi, M.L.G.; Silva-Gomes, R.N.; Vieira, J.C.S.; Hessel, M.C.; Mareco, E.A.; Dos Santos, V.B.; Carvalho, R.F.; Padilha, P.D.M.; Dal-Pai-Silva, M. Proteomic analysis of the fast-twitch muscle of pacu (Piaractus mesopotamicus)after prolonged fasting and compensatory growth. Comp. Biochem. Physiol. Part D Genom. Proteom. 2019, 30, 321–332. [Google Scholar] [CrossRef]
- Cleveland, B.M.; Weber, G.M.; Blemings, K.P.; Silverstein, J.T.; Bm, C.; Gm, W.; Kp, B.; Jt, S. Insulin-like growth factor-I and genetic effects on indexes of protein degradation in response to feed deprivation in rainbow trout (Oncorhynchus mykiss). Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, 1332–1342. [Google Scholar] [CrossRef]
- Fuentes, E.N.; Björnsson, B.T.; Valdés, J.A.; Einarsdottir, I.E.; Lorca, B.; Alvarez, M.; Molina, A. IGF-I/PI3K/Akt and IGF-I/MAPK/ERK pathways in vivo in skeletal muscle are regulated by nutrition and contribute to somatic growth in the fine flounder. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Bower, N.I.; Johnston, I.A. Transcriptional regulation of the IGF signaling pathway by amino acids and insulin-like growth factors during myogenesis in Atlantic salmon. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, B.M.; Weber, G.M. Effects of insulin-like growth factor-I, insulin, and leucine on protein turnover and ubiquitin ligase expression in rainbow trout primary myocytes. Am. J. Physiol. Integr. Comp. Physiol. 2010, 298, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Vélez, E.J.; Lutfi, E.; Jiménez-Amilburu, V.; Riera-Codina, M.; Capilla, E.; Navarro, I.; Gutiérrez, J. IGF-I and amino acids effects through TOR signaling on proliferation and differentiation of gilthead sea bream cultured myocytes. Gen. Comp. Endocrinol. 2014, 205, 296–304. [Google Scholar] [CrossRef]
- Rodríguez-Vargas, J.M.; Ruiz-Magaña, M.J.; Ruiz-Ruiz, C.; Majuelos-Melguizo, J.; Peralta-Leal, A.; Rodríguez, M.I.; Muñoz-Gámez, J.A.; de Almodóvar, M.R.; Siles, E.; Rivas, A.L.; et al. ROS-induced DNA damage and PARP-1 are required for optimal induction of starvation-induced autophagy. Cell Res. 2012, 22, 1181–1198. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. WAO J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F.; MccCung, J.P. Vitamin C. In The Vitamins Fundamental Aspects in Nutrition and Health; Combs, G.F., MccCung, J.P., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 267–295. ISBN 9780128029657. [Google Scholar]
- Takisawa, S.; Funakoshi, T.; Yatsu, T.; Nagata, K.; Aigaki, T.; Machida, S.; Ishigami, A. Vitamin C deficiency causes muscle atrophy and a deterioration in physical performance. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Ryan, M.J.; Dudash, H.J.; Docherty, M.; Geronilla, K.B.; Baker, B.A.; Haff, G.G.; Cutlip, R.G.; Alway, S.E. Vitamin E and C supplementation reduces oxidative stress, improves antioxidant enzymes and positive muscle work in chronically loaded muscles of aged rats. Exp. Gerontol. 2010, 45, 882–895. [Google Scholar] [CrossRef]
- Shima, A.; Pham, J.; Blanco, E.; Barton, E.R.; Sweeney, H.L.; Matsuda, R. IGF-I and vitamin C promote myogenic differentiation of mouse and human skeletal muscle cells at low temperatures. Exp. Cell Res. 2011, 317, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, K.; Ito, A.; Sato, M.; Kanno, S.; Kawabe, Y.; Kamihira, M. Effects of heat stimulation and l-ascorbic acid 2-phosphate supplementation on myogenic differentiation of artificial skeletal muscle tissue constructs. J. Tissue Eng. Regen. Med. 2015, 11, 1322–1331. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Regional Review on Status and Trends in Aquaculture Development in Latin America and the Caribbean 2015; FAO: Rome, Italy, 2017; Volume 1, ISBN 9789250068688. [Google Scholar]
- IBGE Produção da Aquicultura. Available online: https://sidra.ibge.gov.br/tabela/3940 (accessed on 1 October 2019).
- Urbinati, E.C.; Gonçalves, F.D. Pacu (Piaractus mesopotamicus). In Espécies Nativas Para Piscicultura No Brasil; Baldisseroto, B., Gomes, L.C., Eds.; UFSM Santa Maria: Santa Maria, Brazil, 2005; pp. 225–255. [Google Scholar]
- Volkoff, H.; Esatevan Sabioni, R.; Coutinho, L.L.; Cyrino, J.E.P. Appetite regulating factors in pacu (Piaractus mesopotamicus): Tissue distribution and effects of food quantity and quality on gene expression. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 203, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Dal Pai, V.; Dal Pai-Silva, M.; Carvalho, E.D.; Fujihara, C.Y.; Gregorio, E.A.; Curi, P.R. Morphological, Histochemical and Morphometric Study of the Myotomal Muscle Tissue of the Pacu (Piaractus mesopotamicus Holmberg 1887: Serrasalminae, Characidae, Teleostei). Anat. Histol. Embryol. J. Vet. Med. Ser. C 2000, 29, 283–289. [Google Scholar] [CrossRef]
- Fracalossi, D.M.; Allen, M.E.; Yuyama, L.K.; Oftedal, O.T. Ascorbic acid biosynthesis in Amazonian fishes. Aquaculture 2001, 192, 321–332. [Google Scholar] [CrossRef]
- Darias, M.J.; Mazurais, D.; Koumoundouros, G.; Cahu, C.L.; Zambonino-Infante, J.L. Overview of vitamin D and C requirements in fish and their influence on the skeletal system. Aquaculture 2011, 315, 49–60. [Google Scholar] [CrossRef]
- Cahu, C.; Infante, J.Z.; Takeuchi, T. Nutritional components affecting skeletal development in fish larvae. Aquaculture 2003, 227, 245–258. [Google Scholar] [CrossRef]
- Guha, B.; Khuda-Bukhsh, A.R. Efficacy of vitamin-C (L-ascorbic acid) in reducing genotoxicity in fish (Oreochromis mossambicus) induced by ethyl methane sulphonate. Chemosphere 2002, 47, 49–56. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, J.C. Effects of sub-chronic exposure to lead (Pb) and ascorbic acid in juvenile rockfish: Antioxidant responses, MT gene expression, and neurotransmitters. Chemosphere 2017, 171, 520–527. [Google Scholar] [CrossRef]
- Kim, J.H.; Kang, J.C. Changes in hematological parameters, plasma cortisol, and acetylcholinesterase of juvenile rockfish, Sebastes schlegelii supplemented with the dietary ascorbic acid. Aquac. Rep. 2016, 4, 80–85. [Google Scholar] [CrossRef][Green Version]
- Duran, B.O.S.; Góes, G.A.; Zanella, B.T.T.; Freire, P.P.; Valente, J.S.; Salomão, R.A.S.; Fernandes, A.; Mareco, E.A.; Carvalho, R.F.; Dal-Pai-Silva, M. Ascorbic acid stimulates the in vitro myoblast proliferation and migration of pacu (Piaractus mesopotamicus). Sci. Rep. 2019, 9, 2229. [Google Scholar] [CrossRef] [PubMed]
- Vélez, E.J.; Lutfi, E.; Azizi, S.; Montserrat, N.; Riera-Codina, M.; Capilla, E.; Navarro, I.; Gutiérrez, J. Contribution of in vitro myocytes studies to understanding fish muscle physiology. Comp. Biochem. Physiol. Part. B Biochem. Mol. Biol. 2015, 199, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Codina, M.; Martínez, M.L.; Navarro, I.; Gutiérrez, J. Metabolic and mitogenic effects of IGF-I and insulin on muscle cells of rainbow trout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.; Ammendrup-Johnsen, I.; Codina, M.; Navarro, I.; Gutiérrez, J. IGF-I and insulin receptor signal transduction in trout muscle cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Seiliez, I.; Taty, G.C.T.; Bugeon, J.; Dias, K.; Sabin, N.; Gabillard, J. Myostatin induces atrophy of trout myotubes through inhibiting the TORC1 signaling and promoting Ubiquitin–Proteasome and Autophagy-Lysosome degradative pathways. Gen. Comp. Endocrinol. 2013, 186, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Amilburu, V.; Salmerón, C.; Codina, M.; Navarro, I.; Capilla, E.; Gutiérrez, J. Insulin-like growth factors effects on the expression of myogenic regulatory factors in gilthead sea bream muscle cells. Gen. Comp. Endocrinol. 2013, 188, 151–158. [Google Scholar] [CrossRef]
- Duran, B.O.S.; Fernandez, G.J.; Mareco, E.A.; Moraes, L.N.; Salomão, R.A.S.; Gutierrez De Paula, T.; Santos, V.B.; Carvalho, R.F.; Dal-Pai-Silva, M. Differential microRNA expression in fast- and slow-twitch skeletal muscle of Piaractus mesopotamicus during growth. PLoS ONE 2015, 10, e0141967. [Google Scholar] [CrossRef]
- Duran, B.O.S.; Dal-Pai-Silva, M.; de la Serrana, D.G. Rainbow trout slow myoblast cell culture as a model to study slow skeletal muscle, and the characterization of mir-133 and mir-499 families as a case study. J. Exp. Biol. 2020, 223. [Google Scholar] [CrossRef] [PubMed]
- Freshney, R.I. Advantages of Tissue Culture. In Culture of Animal Cells; John Wiley & Sons, Inc.: New Jersey, NY, USA, 2010; pp. 1–11. ISBN 9780470528129. [Google Scholar]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef] [PubMed]
- Bonaldo, P.; Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef]
- Watabe, S. Myogenic regulatory factors and muscle differentiation during ontogeny in fish. J. Fish. Biol. 1999, 55, 1–18. [Google Scholar] [CrossRef]
- Watabe, S. Myogenic Regulatory factors. In Muscle Development and Growth; Johnston, I.A., Ed.; Academic Press: San Diego, CA, USA, 2001; pp. 19–36. [Google Scholar]
- Turrens, J.F. Superoxide Production by the Mitochondrial Respiratory Chain. Biosci. Rep. 1997, 17, 3–8. [Google Scholar] [CrossRef]
- Puigserver, P.; Spiegelman, B.M. Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α): Transcriptional coactivator and metabolic regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef]
- De la Serrana, D.G.; Johnston, I.A. Expression of Heat Shock Protein (Hsp90) Paralogues Is Regulated by Amino Acids in Skeletal Muscle of Atlantic Salmon. PLoS ONE 2013, 8, e74295. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Shimozuru, M.; Tsubota, T. Skeletal muscles of hibernating black bears show minimal atrophy and phenotype shifting despite prolonged physical inactivity and starvation. PLoS ONE 2019, 14, e0215489. [Google Scholar] [CrossRef] [PubMed]
- Cotton, C.J. Skeletal muscle mass and composition during mammalian hibernation. J. Exp. Biol. 2016, 219, 226–234. [Google Scholar] [CrossRef]
- Wu, P.; Li, Y.; Cheng, J.; Chen, L.; Zeng, M.; Wu, Y.; Wang, J.; Zhang, J.; Chu, W. Transcriptome Analysis and Postprandial Expression of Amino Acid Transporter Genes in the Fast Muscles and Gut of Chinese Perch (Siniperca chuatsi). PLoS ONE 2016, 11, e0159533. [Google Scholar] [CrossRef]
- Doupé, D.P.; Perrimon, N. Visualizing and Manipulating Temporal Signaling Dynamics with Fluorescence-Based Tools. Sci. Signal. 2014, 7, 1–16. [Google Scholar] [CrossRef]
- Huang, K.; Fingar, D.C. Growing knowledge of the mTOR signaling network. Semin. Cell Dev. Biol. 2014, 36, 79–90. [Google Scholar] [CrossRef]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-peled, L.; Sabatini, D.M. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef]
- Glass, D.J. Skeletal muscle hypertrophy and atrophy signaling pathways. Int. J. Biochem. Cell Biol. 2005, 37, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Kraut, J. Do Enzymes Work? Science 1988, 242, 533–540. [Google Scholar] [CrossRef]
- Chance, B. The kinetics of the enzyme-substrate compound of peroxidase. J. Biol. Chem 1943, 151, 553–577. [Google Scholar] [CrossRef]
- Hidalgo, M.C.; Morales, A.E.; Arizcun, M.; Abellán, E.; Cardenete, G. Regional asymmetry of metabolic and antioxidant profile in the sciaenid fish shi drum (Umbrina cirrosa) white muscle. Response to starvation and refeeding. Redox Biol. 2017, 11, 682–687. [Google Scholar] [CrossRef] [PubMed]
- García de la Serrana, D.; Codina, M.; Capilla, E.; Jiménez-Amilburu, V.; Navarro, I.; Du, S.J.; Johnston, I.A.; Gutiérrez, J. Characterisation and expression of myogenesis regulatory factors during in vitro myoblast development and in vivo fasting in the gilthead sea bream (Sparus aurata). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2014, 167, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Koumans, J.T.M.; Akster, H.A. Myogenic Cells in Development and Growth of Fish. Science 1995, 110, 3–20. [Google Scholar] [CrossRef]
- Mommsen, T.P. Paradigms of growth in fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001, 129, 207–219. [Google Scholar] [CrossRef]
- Villasante, A.; Powell, M.S.; Moutou, K.; Murdoch, G.K.; Overturf, K.; Wacyk, J.; Hardy, R.W. Effects of anthocyanidins on myogenic differentiation and antioxidant defense in primary myogenic cells isolated from rainbow trout (Oncorhynchus mykiss). Aquaculture 2016, 454, 81–89. [Google Scholar] [CrossRef]
- Baehr, L.M.; Tunzi, M.; Bodine, S.C. Muscle hypertrophy is associated with increases in proteasome activity that is independent of MuRF1 and MAFbx expression. Front. Physiol. 2014, 5, 1–8. [Google Scholar] [CrossRef]
- Abimorad, E.G.; Carneiro, D.J. Digestibility and performance of pacu (Piaractus mesopotamicus) juveniles–Fed diets containing different protein, lipid and carbohydrate levels. Aquac. Nutr. 2007, 13, 1–9. [Google Scholar] [CrossRef]
- Jobling, M.; Meløy, O.H.; dos Santos, J.; Christiansen, B. The compensatory growth response of the Atlantic cod: Effects of nutritional history. Aquac. Int. 1994, 2, 75–90. [Google Scholar] [CrossRef]
- Garcia, F.; Pilarski, F.; Onaka, E.M.; de Moraes, F.R. Performance and hematology of pacu fed diets supplemented with vitamins C and/or E. Sci. Agric. 2011, 68, 314–319. [Google Scholar] [CrossRef]
- Sevgili, H.; Hoşsu, B.; Emre, Y.; Kanyilmaz, M. Compensatory growth after various levels of dietary protein restriction in rainbow trout, Oncorhynchus mykiss. Aquaculture 2012, 344–349, 126–134. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Belo, M.A.d.A.; de Moraes, J.R.E.; Soares, V.E.; Martins, M.L.; Brum, C.D.; de Moraes, F.R. Vitamin C and endogenous cortisol in foreign-body inflammatory response in pacus. Pesqui. Agropecuária Bras. 2012, 47, 1015–1021. [Google Scholar] [CrossRef][Green Version]
- Martins, M.L. Evaluation of the addition of ascorbic acid to the ration of cultivated Piaractus mesopotamicus (Characidae) on the infrapopulation of Anacanthorus penilabiatus (Monogenea). Braz. J. Med. Biol. Res. 1998, 31, 655–658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garcia, F.; Pilarski, F.; Onaka, E.M.; de Moraes, F.R.; Martins, M.L. Hematology of Piaractus mesopotamicus fed diets supplemented with vitamins C and E, challenged by Aeromonas hydrophila. Aquaculture 2007, 271, 39–46. [Google Scholar] [CrossRef]
- Bower, N.I.; Johnston, I.A. Selection of reference genes for expression studies with fish myogenic cell cultures. BMC Mol. Biol. 2009, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Rocha, E.; Gomes, E.F.; Silva, M.W.; Oliveira, M.H.; Monteiro, R.A.F.; Fauconneau, B. Growth dynamics of white and red muscle fibres in fast- and slow-growing strains of rainbow trout. J. Fish. Biol. 1999, 55, 675–691. [Google Scholar] [CrossRef]
- de Almeida, F.L.A.; Carvalho, R.F.; Pinhal, D.; Padovani, C.R.; Martins, C.; Dal Pai-Silva, M. Differential expression of myogenic regulatory factor MyoD in pacu skeletal muscle (Piaractus mesopotamicus Holmberg 1887: Serrasalminae, Characidae, Teleostei) during juvenile and adult growth phases. Micron 2008, 39, 1306–1311. [Google Scholar] [CrossRef]
- Johnston, I.A. Quantitative studies of muscle breakdown during starvation in the marine flat fish Pleuronectes platessa. Cell Tissue Res. 1981, 214, 369–386. [Google Scholar] [CrossRef]
- Ohno, Y.; Oyama, A.; Kaneko, H.; Egawa, T.; Yokoyama, S.; Sugiura, T.; Ohira, Y.; Yoshioka, T.; Goto, K. Lactate increases myotube diameter via activation of MEK/ERK pathway in C2C12 cells. Acta Physiol. 2018, 223. [Google Scholar] [CrossRef] [PubMed]
- Williamson, D.L.; Butler, D.C.; Alway, S.E. AMPK inhibits myoblast differentiation through a PGC-1α-dependent mechanism. Am. J. Physiol. Endocrinol. Metab. 2009, 297, 304–314. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Sinha, A.K. Colorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Flohé, L.; Günzler, W.A. Assays of Glutathione Peroxidase. In Methods in Enzymology; Packer, L., Ed.; Academic Press: San Diego, CA, USA, 1984; Volume 105, pp. 112–121. ISBN 9780121820053. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanella, B.T.T.; Magiore, I.C.; Duran, B.O.S.; Pereira, G.G.; Vicente, I.S.T.; Carvalho, P.L.P.F.; Salomão, R.A.S.; Mareco, E.A.; Carvalho, R.F.; Paula, T.G.d.; et al. Ascorbic Acid Supplementation Improves Skeletal Muscle Growth in Pacu (Piaractus mesopotamicus) Juveniles: In Vivo and In Vitro Studies. Int. J. Mol. Sci. 2021, 22, 2995. https://doi.org/10.3390/ijms22062995
Zanella BTT, Magiore IC, Duran BOS, Pereira GG, Vicente IST, Carvalho PLPF, Salomão RAS, Mareco EA, Carvalho RF, Paula TGd, et al. Ascorbic Acid Supplementation Improves Skeletal Muscle Growth in Pacu (Piaractus mesopotamicus) Juveniles: In Vivo and In Vitro Studies. International Journal of Molecular Sciences. 2021; 22(6):2995. https://doi.org/10.3390/ijms22062995
Chicago/Turabian StyleZanella, Bruna Tereza Thomazini, Isabele Cristina Magiore, Bruno Oliveira Silva Duran, Guilherme Gutierrez Pereira, Igor Simões Tiagua Vicente, Pedro Luiz Pucci Figueiredo Carvalho, Rondinelle Artur Simões Salomão, Edson Assunção Mareco, Robson Francisco Carvalho, Tassiana Gutierrez de Paula, and et al. 2021. "Ascorbic Acid Supplementation Improves Skeletal Muscle Growth in Pacu (Piaractus mesopotamicus) Juveniles: In Vivo and In Vitro Studies" International Journal of Molecular Sciences 22, no. 6: 2995. https://doi.org/10.3390/ijms22062995
APA StyleZanella, B. T. T., Magiore, I. C., Duran, B. O. S., Pereira, G. G., Vicente, I. S. T., Carvalho, P. L. P. F., Salomão, R. A. S., Mareco, E. A., Carvalho, R. F., Paula, T. G. d., Barros, M. M., & Dal-Pai-Silva, M. (2021). Ascorbic Acid Supplementation Improves Skeletal Muscle Growth in Pacu (Piaractus mesopotamicus) Juveniles: In Vivo and In Vitro Studies. International Journal of Molecular Sciences, 22(6), 2995. https://doi.org/10.3390/ijms22062995








