Exercise–Linked Irisin: Consequences on Mental and Cardiovascular Health in Type 2 Diabetes
Abstract
1. Introduction
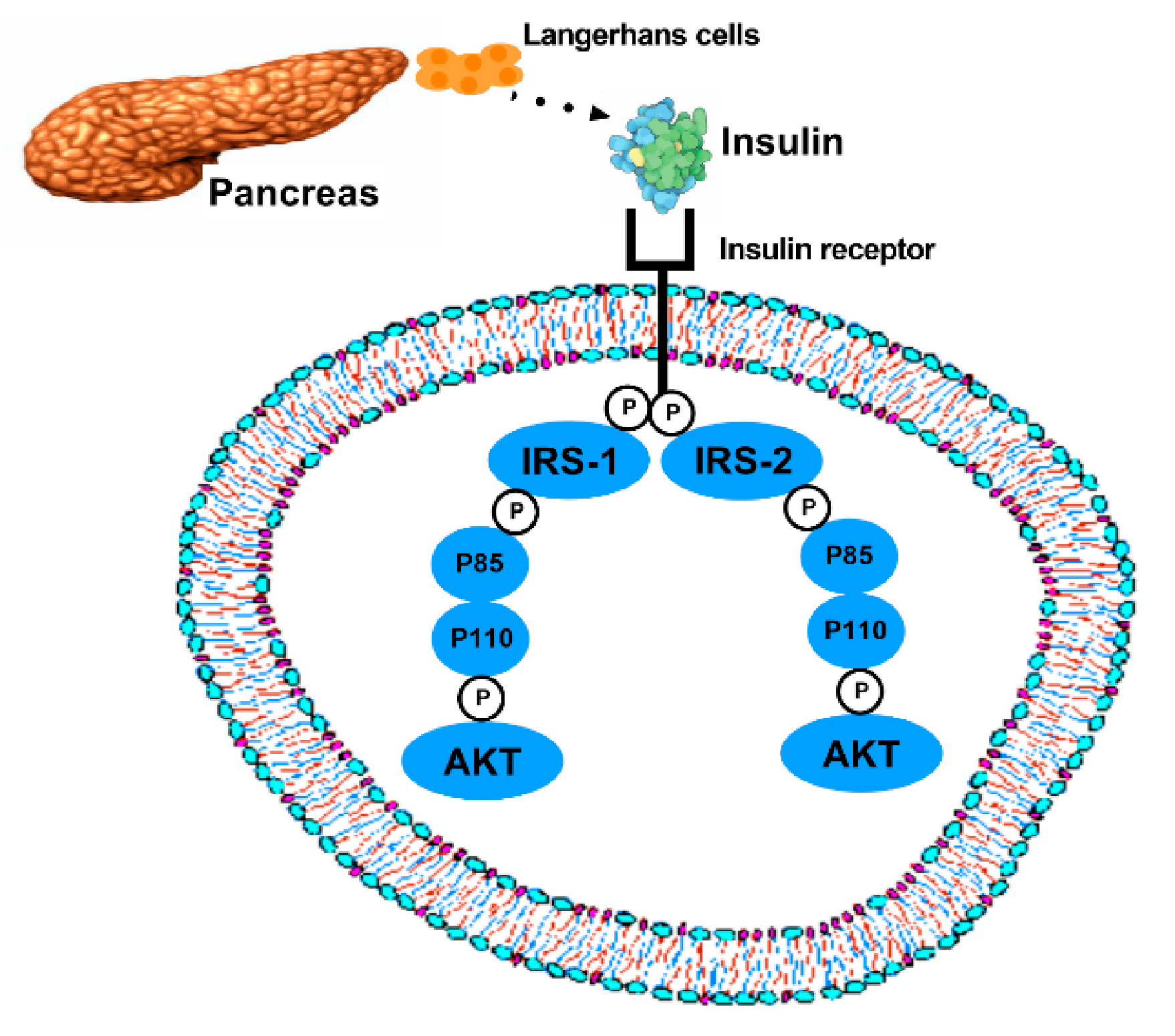
2. Molecular Mechanisms of T2DM
The Role of Adipocytokines in T2DM
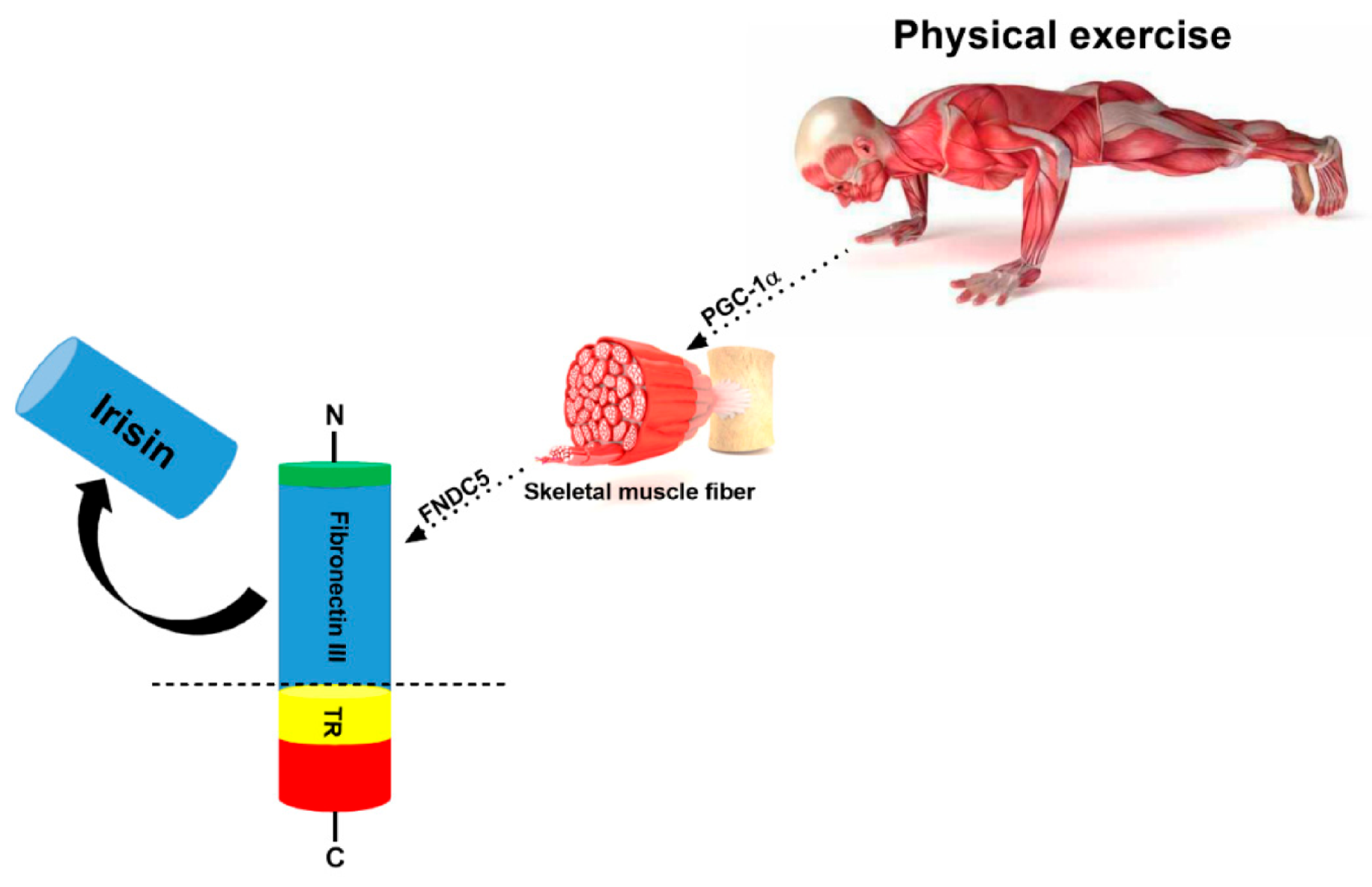
3. Irisin
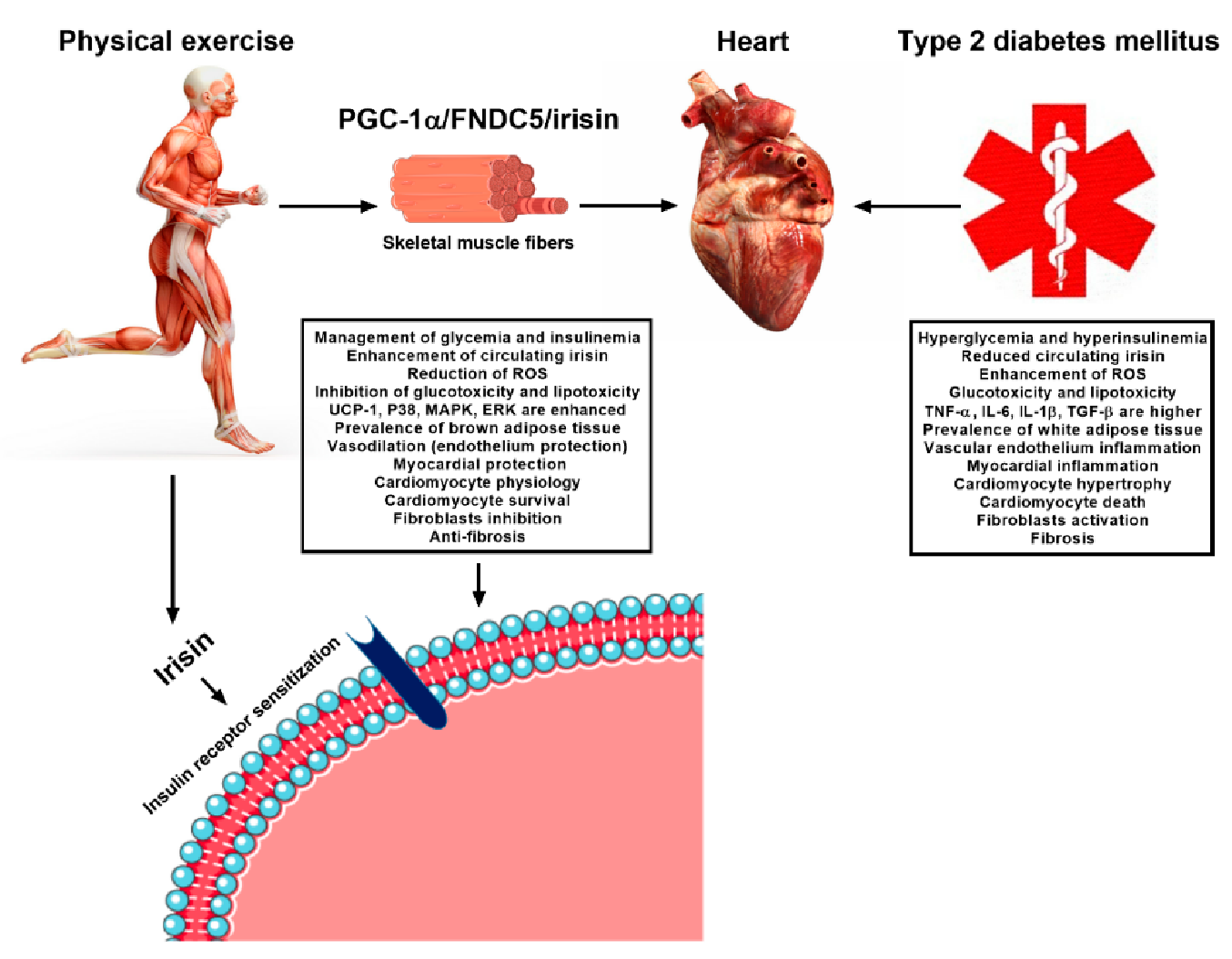
4. Exercise-Linked Irisin: Consequences on Cardiovascular Health in T2DM
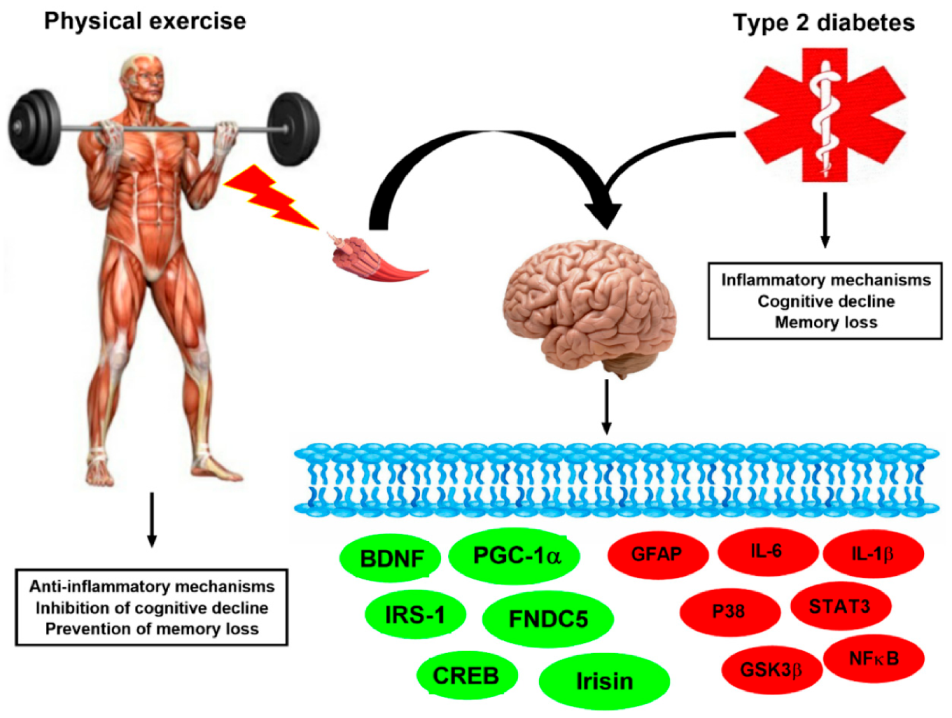
5. Exercise-Linked Irisin: Consequences on Mental Health in T2DM
5.1. Exercise-Linked Irisin: Consequences on Cognitive Function and Memory in T2DM
5.2. Exercise-Linked irisin: Consequences on Depression and Anxiety in T2DM
6. Exercise in T2DM: Types, Variables and Outcomes
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Michos, E.D.; McEvoy, J.W.; Miedema, M.D.; Himmelfarb, C.D.; et al. 2019 ACC / AHA Guideline on the Primary Prevention of Cardiovascular Disease. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef]
- De Sousa, R.A.L.; Harmer, A.R.; Freitas, D.A.; Mendonça, V.A.; Lacerda, A.C.R.; Leite, H.R. An update on potential links between type 2 diabetes mellitus and Alzheimer’s disease. Mol. Biol. Rep. 2020. [Google Scholar] [CrossRef]
- Saltiel, A.R.; Kahn, C.R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. Recommendations for Managing Type 2 Diabetes in Primary Care; International Diabetes Federation: Brussel, Belgium, 2017; ISBN 9782930229850. [Google Scholar]
- Kelly, A.S.; Bergenstal, R.M.; Gonzalez-Campoy, J.M.; Katz, H.; Bank, A.J. Effects of exenatide vs. metformin on endothelial function in obese patients with pre-diabetes: A randomized trial. Cardiovasc. Diabetol. 2012, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Roriz-Filho, J.S.; Sá-Roriz, T.M.; Rosset, I.; Camozzato, A.L.; Santos, A.C.; Chaves, M.L.F.; Moriguti, J.C.; Roriz-Cruz, M. (Pre)diabetes, brain aging, and cognition. Biochim. Biophys. Acta Mol. Basis Dis. 2009, 1792, 432–443. [Google Scholar] [CrossRef]
- Bordier, L.; Doucet, J.; Boudet, J.; Bauduceau, B. Update on cognitive decline and dementia in elderly patients with diabetes. Diabetes Metab. 2014, 40, 331–337. [Google Scholar] [CrossRef]
- Sousa, R.A.L. de Brief report of the effects of the aerobic, resistance, and high-intensity interval training in type 2 diabetes mellitus individuals Diabetes mellitus. Int. J. Diabetes Dev. Ctries. 2018, 38, 138–145. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Sirtuins and Type 2 Diabetes: Role in Inflammation, Oxidative Stress, and Mitochondrial Function. Front. Endocrinol. 2019, 10, 1–12. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Colley, R.C.; Saunders, T.J.; Healy, G.N.; Owen, N. Physiological and health implications of a sedentary lifestyle. Appl. Physiol. Nutr. Metab. 2010, 35, 725–740. [Google Scholar] [CrossRef]
- Tompkins, C.L.; Soros, A.; Sothern, M.S.; Vargas, A. Effects of Physical Activity on Diabetes Management and Lowering Risk For Type 2 Diabetes. Am. J. Health Educ. 2009, 40, 286–290. [Google Scholar] [CrossRef]
- De Sousa, R.A.L.; Hagenbeck, K.F.; Arsa, G.; Pardono, E. Moderate / high resistance exercise is better to reduce blood glucose and blood pressure in middle-aged diabetic subjects. Rev. Bras. Educ. Física Esporte 2020, 34, 165–175. [Google Scholar] [CrossRef]
- Ranasinghe, C.; Hills, A.P.; Constantine, G.R.; Finlayson, G.; Katulanda, P.; King, N.A. Study protocol: A randomised controlled trial of supervised resistance training versus aerobic training in Sri Lankan adults with type 2 diabetes mellitus: SL-DART study. BMC Public Health 2018, 18, 176. [Google Scholar] [CrossRef] [PubMed]
- Zisser, H.; Sueyoshi, M.; Krigstein, K.; Szigiato, A.; Riddell, M.C. Advances in exercise, physical activity and diabetes mellitus. Int. J. Clin. Pract. 2012, 66, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Feter, N.; Spanevello, R.M.; Soares, M.S.P.; Spohr, L.; Pedra, N.S.; Bona, N.P.; Freitas, M.P.; Gonzales, N.G.; Ito, L.G.M.S.; Stefanello, F.M.; et al. How does physical activity and different models of exercise training affect oxidative parameters and memory? Physiol. Behav. 2019, 201, 42–52. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, R.A.L.; Caria, A.C.I.; De Jesus Silva, F.M.; Magalhães, C.O.D.; Freitas, D.A.; Lacerda, A.C.R.; Mendonça, V.A.; Cassilhas, R.C.; Leite, H.R. High-intensity resistance training induces changes in cognitive function, but not in locomotor activity or anxious behavior in rats induced to type 2 diabetes. Physiol. Behav. 2020, 223, 1–7. [Google Scholar] [CrossRef]
- Benedini, S.; Dozio, E.; Invernizzi, P.L.; Vianello, E.; Banfi, G.; Terruzzi, I.; Luzi, L.; Romanelli, M.M.C. Irisin: A Potential Link between Physical Exercise and Metabolism—An Observational Study in Differently Trained Subjects, from Elite Athletes to Sedentary People. J. Diabetes Res. 2017, 2017. [Google Scholar] [CrossRef]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszevski, A.; et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165–175. [Google Scholar] [CrossRef]
- Czech, M.P.; Corvera, S. Signaling Mechanisms That Regulate Glucose Transport. J. Biol. Chem. 1999, 274, 1865–1868. [Google Scholar] [CrossRef]
- Camandola, S.; Mattson, M. Brain metabolism in health, aging, and neurodegeneration. EMBO J. 2017, 1–19. [Google Scholar] [CrossRef]
- Friedman, J.E.; Sherman, W.M.; Reed, M.J.; Elton, C.W.; Dohm, G.L. Exercise training increases glucose transporter protein GLUT-4 in skeletal muscle of obese Zucker (fa/fa) rats. FEBS Lett. 1990, 268, 13–16. [Google Scholar] [CrossRef]
- Hardin, D.S.; Dominguez, H.; Timothy, W. Muscle Group-Specific Regulation of Glut 4 Glucose Transporters in Control, Diabetic, and Insulin Treated Diabetic Rats. Metabolism 1993, 42, 1310–1315. [Google Scholar] [CrossRef]
- Egawa, T.; Tsuda, S.; Ma, X.; Hamada, T.; Hayashi, T. Caffeine modulates phosphorylation of insulin receptor substrate-1 and impairs insulin signal transduction in rat skeletal muscle. J. Appl. Physiol. 2011, 111, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.A.L. de Gestational diabetes is associated to the development of brain insulin resistance in the offspring. Int. J. Diabetes Dev. Ctries. 2018, 39, 408–416. [Google Scholar] [CrossRef]
- Yi, S.S. Effects of exercise on brain functions in diabetic animal models. World J. Diabetes 2015, 6, 583–597. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.S.S.; Fernandes, C.S.; Vieira, M.N.N.; De-Felice, F.G. Insulin resistance in Alzheimer’s disease. Front. Neurosci. 2018, 12, 1–11. [Google Scholar] [CrossRef]
- Folch, J.; Ettcheto, M.; Busquets, O.; Sánchez-López, E.; Castro-Torres, R.D.; Verdaguer, E.; Manzine, P.R.; Poor, S.R.; García, M.L.; Olloquequi, J.; et al. The implication of the brain insulin receptor in late onset Alzheimer’s disease dementia. Pharmaceuticals 2018, 11, 11. [Google Scholar] [CrossRef]
- De Sousa, R.A.L.; Rodrigues, C.M.; Mendes, B.F.; Improta-caria, A.C.; Peixoto, M.F.D.; Cassilhas, R.C. Physical exercise protocols in animal models of Alzheimer’ s disease: A systematic review. Metab. Brain Dis. 2020, 1–11. [Google Scholar] [CrossRef]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 809–824. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, X.; Chen, W. Role of oxidative stress in Alzheimer’s disease (Review). Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Poblete-Aro, C.; Russell-Guzmán, J.; Parra, P.; Soto-Muñoz, M.; Villegas-González, B.; Cofré-Bola-Dos, C.; Herrera-Valenzuela, T. Exercise and oxidative stress in type 2 diabetes mellitus. Rev. Med. Chil. 2018, 146, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications review-Article. Cell Death Dis. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Sau, C.; Lai, W.; Cichon, J.; Li, W. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Maximus, P.S.; Al Achkar, Z.; Hamid, P.F.; Hasnain, S.S.; Peralta, C.A. Adipocytokines: Are they the Theory of Everything? Cytokine 2020, 133, 155144. [Google Scholar] [CrossRef]
- Banerjee, A.; Singh, J. Remodeling adipose tissue inflammasome for type 2 diabetes mellitus treatment: Current perspective and translational strategies. Bioeng. Transl. Med. 2020, 5, 1–19. [Google Scholar] [CrossRef]
- Kim, J.A.; Choi, K.M. Newly Discovered Adipokines: Pathophysiological Link Between Obesity and Cardiometabolic Disorders. Front. Physiol. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Wędrychowicz, A.; Zajac, A.; Pilecki, M.; Koscielniak, B.; Tomasik, P.J. Peptides from adipose tissue in mental disorders. World J. Psychiatry 2014, 4, 103. [Google Scholar] [CrossRef]
- Weber-Hamann, B.; Kratzsch, J.; Kopf, D.; Lederbogen, F.; Gilles, M.; Heuser, I.; Deuschle, M. Resistin and adiponectin in major depression: The association with free cortisol and effects of antidepressant treatment. J. Psychiatr. Res. 2007, 41, 344–350. [Google Scholar] [CrossRef]
- Elizondo-Montemayor, L.; Gonzalez-Gil, A.M.; Tamez-Rivera, O.; Toledo-Salinas, C.; Peschard-Franco, M.; Rodríguez-Gutiérrez, N.A.; Silva-Platas, C.; Garcia-Rivas, G. Association between irisin, hs-CRP, and metabolic status in children and adolescents with type 2 diabetes mellitus. Mediat. Inflamm. 2019, 2019. [Google Scholar] [CrossRef]
- Jorge, M.L.M.P.; De Oliveira, V.N.; Resende, N.M.; Paraiso, L.F.; Calixto, A.; Diniz, A.L.D.; Resende, E.S.; Ropelle, E.R.; Carvalheira, J.B.; Espindola, F.S.; et al. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism 2011, 60, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Rana, K.S.; Pararasa, C.; Afzal, I.; Nagel, D.A.; Hill, E.J.; Bailey, C.J.; Griffiths, H.R.; Kyrou, I.; Randeva, H.S.; Bellary, S.; et al. Plasma irisin is elevated in type 2 diabetes and is associated with increased E-selectin levels. Cardiovasc. Diabetol. 2017, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Corleo, D.; Buscemi, C.; Giordano, C. Does iris(in) bring bad news or good news? Eat. Weight Disord. 2018, 23, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1a dependent myokine that derives browning of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Chinnam, N.; Ohashi, T.; Shah, R.S.; Erickson, H.P. The structure of Irisin reveals a novel intersubunit β-sheet fibronectin type III (FNIII) dimer: Implications for receptor activation. J. Biol. Chem. 2013, 288, 33738–33744. [Google Scholar] [CrossRef] [PubMed]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A Cold-Inducible Coactivator of Nuclear Receptors Linked to Adaptive Thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Lu, J.; Xiang, G.; Liu, M.; Mei, W.; Xiang, L.; Dong, J. Irisin protects against endothelial injury and ameliorates atherosclerosis in apolipoprotein E-Null diabetic mice. Atherosclerosis 2015, 243, 438–448. [Google Scholar] [CrossRef]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivo and in vitro studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef]
- Amri, J.; Parastesh, M.; Sadegh, M.; Latifi, S.A.; Alaee, M. High-intensity interval training improved fasting blood glucose and lipid profiles in type 2 diabetic rats more than endurance training; Possible involvement of irisin and betatrophin. Physiol. Int. 2019, 106, 213–224. [Google Scholar] [CrossRef]
- Liu, S.X.; Zheng, F.; Xie, K.L.; Xie, M.R.; Jiang, L.J.; Cai, Y. Exercise Reduces Insulin Resistance in Type 2 Diabetes Mellitus via Mediating the lncRNA MALAT1/MicroRNA-382-3p/Resistin Axis. Mol. Ther. Nucleic Acids 2019, 18, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Boudina, S.; Abel, E.D. Diabetic cardiomyopathy revisited. Circulation 2007, 115, 3213–3223. [Google Scholar] [CrossRef] [PubMed]
- Frati, G.; Schirone, L.; Chimenti, I.; Yee, D.; Biondi-Zoccai, G.; Volpe, M.; Sciarretta, S. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc. Res. 2017, 113, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.; Wainwright, S.R.; Galea, L.A.M. Sex hormones and adult hippocampal neurogenesis: Regulation, implications, and potential mechanisms. Front. Neuroendocr. 2016, 41, 129–152. [Google Scholar] [CrossRef] [PubMed]
- Chengji, W.; Xianjin, F. Exercise protects against diabetic cardiomyopathy by the inhibition of the endoplasmic reticulum stress pathway in rats. J. Cell. Physiol. 2019, 234, 1682–1688. [Google Scholar] [CrossRef]
- Gimenes, C.; Gimenes, R.; Rosa, C.M.; Xavier, N.P.; Campos, D.H.S.; Fernandes, A.A.H.; Cezar, M.D.M.; Guirado, G.N.; Cicogna, A.C.; Takamoto, A.H.R.; et al. Low Intensity Physical Exercise Attenuates Cardiac Remodeling and Myocardial Oxidative Stress and Dysfunction in Diabetic Rats. J. Diabetes Res. 2015, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.D.; Dunstan, D.W.; Robinson, C.; Vulikh, E.; Zimmet, P.Z.; Shaw, J.E. Improved endothelial function following a 14-month resistance exercise training program in adults with type 2 diabetes. Diabetes Res. Clin. Pract. 2008, 79, 405–411. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Perrea, D.; Iliadis, F.; Angelopoulou, N.; Liapis, C.; Alevizos, M. Exercise reduces resistin and inflammatory cytokines in patients with type 2 diabetes. Diabetes Care 2007, 30, 719–721. [Google Scholar] [CrossRef]
- Wang, H.; Bei, Y.; Lu, Y.; Sun, W.; Liu, Q.; Wang, Y.; Cao, Y.; Chen, P.; Xiao, J.; Kong, X. Exercise prevents cardiac injury and improves mitochondrial biogenesis in advanced diabetic cardiomyopathy with PGC-1α and Akt activation. Cell. Physiol. Biochem. 2015, 35, 2159–2168. [Google Scholar] [CrossRef]
- Caria, A.C.I.; Nonaka, C.K.V.; Pereira, C.S.; Soares, M.B.P.; Macambira, S.G.; de Souza, B.S.F. Exercise Training-Induced Changes in MicroRNAs: Beneficial Regulatory Effects in Hypertension, Type 2 Diabetes, and Obesity. Int. J. Mol. Sci. 2018, 19, 3608. [Google Scholar] [CrossRef]
- Gizaw, M.; Anandakumar, P.; Debela, T. A Review on the Role of Irisin in Insulin Resistance and Type 2 Diabetes Mellitus. J. Pharmacopunct. 2017, 20, 235–242. [Google Scholar]
- Baker, L.D.; Frank, L.L.; Foster-Schubert, K.; Green, P.S.; Wilkinson, C.W.; McTiernan, A.; Plymate, S.R.; Fishel, M.A.; Watson, G.S.; Cholerton, B.A.; et al. Effects of aerobic exercise on mild cognitive impairment: A controlled trial. Arch. Neurol. 2010, 67, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Shima, T.; Matsui, T.; Jesmin, S.; Okamoto, M.; Soya, M.; Inoue, K.; Liu, Y.F.; Torres-Aleman, I.; McEwen, B.S.; Soya, H. Moderate exercise ameliorates dysregulated hippocampal glycometabolism and memory function in a rat model of type 2 diabetes. Diabetologia 2017, 60, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Cassilhas, R.C.; Antunes, H.K.M.; Tufik, S.; de Mello, M.T. Mood, Anxiety, and Serum IGF-1 in Elderly Men Given 24 Weeks of High Resistance Exercise. Percept. Mot. Skills 2010, 110, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Lee, J.; Cho, H.; Park, S.; Kim, T. Physical exercise ameliorates mood disorder-like behavior on high fat diet- induced obesity in mice. Psychiatry Res. 2017, 250, 71–77. [Google Scholar] [CrossRef]
- Botta, A.; Laher, I.; Beam, J.; DeCoffe, D.; Brown, K.; Halder, S.; Devlin, A.; Gibson, D.L.; Ghosh, S. Short Term Exercise Induces PGC-1α, Ameliorates Inflammation and Increases Mitochondrial Membrane Proteins but Fails to Increase Respiratory Enzymes in Aging Diabetic Hearts. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Wang, K.; Li, H.; Wang, H.; Wang, J.H.; Song, F.; Sun, Y. Irisin exerts neuroprotective effects on cultured neurons by regulating astrocytes. Mediat. Inflamm. 2018, 1–7. [Google Scholar] [CrossRef]
- Bourdel-Marchasson, I.; Lapre, E.; Laksir, H.; Puget, E. Insulin resistance, diabetes and cognitive function: Consequences for preventative strategies. Diabetes Metab. 2010, 36, 173–181. [Google Scholar] [CrossRef]
- Espeland, M.A.; Lipska, K.; Miller, M.E.; Rushing, J.; Cohen, R.A.; Verghese, J.; McDermott, M.M.; King, A.C.; Strotmeyer, E.S.; Blair, S.N.; et al. Effects of Physical Activity Intervention on Physical and Cognitive Function in Sedentary Adults With and Without Diabetes. J. Gerontol. Ser. A 2017, 72, 861–866. [Google Scholar] [CrossRef]
- Eakin, K.A.; Saleem, M.; Herrmann, N.; Cogo-Moreira, H.; Mielke, M.M.; Oh, P.I.; Haughey, N.J.; Venkata, S.L.V.; Lanctôt, K.L.; Swardfager, W. Plasma Sphingolipids Mediate a Relationship between Type 2 Diabetes and Memory Outcomes in Patients with Coronary Artery Disease Undertaking Exercise. J. Alzheimer’s Dis. 2019, 69, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Shellington, E.M.; Reichert, S.M.; Heath, M.; Gill, D.P.; Shigematsu, R.; Petrella, R.J. Results From a Feasibility Study of Square-Stepping Exercise in Older Adults With Type 2 Diabetes and Self-Reported Cognitive Complaints to Improve Global Cognitive Functioning. Can. J. Diabetes 2018, 42, 603–612.e1. [Google Scholar] [CrossRef] [PubMed]
- Olson, E.A.; Mullen, S.P.; Raine, L.B.; Kramer, A.F.; Hillman, C.H.; McAuley, E. Integrated social- and neuro-cognitive model of physical activity behavior in older aduts with metabolic disease. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Lin, H.; Yuan, Y.; Tian, S.; Han, J.; Huang, R.; Guo, D.; Wang, J.; An, K.; Wang, S. In Addition to Poor Glycemic Control, a High Level of Irisin in the Plasma Portends Early Cognitive Deficits Clinically in Chinese Patients With Type 2 Diabetes Mellitus. Front. Endocrinol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Song, F.; Xu, K.; Liu, Z.; Han, S.; Li, F.; Sun, Y. Irisin attenuates neuroinflammation and prevents the memory and cognitive deterioration in streptozotocin-induced diabetic mice. Mediat. Inflamm. 2019, 1–8. [Google Scholar] [CrossRef]
- Wang, S.; Pan, J. Irisin ameliorates depressive-like behaviors in rats by regulating energy metabolism. Biochem. Biophys. Res. Commun. 2016, 474, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Zhang, C.; Wang, H.; Yang, M.; Guo, Y.; Li, G.; Zhang, H.; Wang, C.; Chen, D.; Geng, C.; et al. Alterations of irisin, adropin, preptin and BDNF concentrations in coronary heart disease patients comorbid with depression. Ann. Transl. Med. 2019, 7, 298. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Zhang, J.; Yu, K.; Song, F. Irisin ameliorates the postoperative depressive-like behavior by reducing the surface expression of epidermal growth factor receptor in mice. Neurochem. Int. 2020, 135. [Google Scholar] [CrossRef]
- Knapen, J.; Vancampfort, D.; Moriën, Y.; Marchal, Y. Exercise therapy improves both mental and physical health in patients with major depression. Disabil. Rehabil. 2015, 37, 1490–1495. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, X.; Li, H.; Ji, L. Depression-like behaviors in mice subjected to co-treatment of high-fat diet and corticosterone are ameliorated by AICAR and exercise. J. Affect. Disord. 2014, 156, 171–177. [Google Scholar] [CrossRef]
- Uysal, N.; Yuksel, O.; Kizildag, S.; Yuce, Z.; Gumus, H.; Karakilic, A.; Guvendi, G.; Koc, B.; Kandis, S.; Ates, M. Regular aerobic exercise correlates with reduced anxiety and incresed levels of irisin in brain and white adipose tissue. Neurosci. Lett. 2018, 676, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.; Lindblad, U.; Bennet, L. Physical inactivity is strongly associated with anxiety and depression in Iraqi immigrants to Sweden: A cross-sectional study. BMC Public Health 2014, 14, 1–8. [Google Scholar] [CrossRef][Green Version]
- Dinel, A.-L.; André, C.; Aubert, A.; Ferreira, G.; Layé, S.; Castanon, N. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS ONE 2011, 6, e24325. [Google Scholar] [CrossRef] [PubMed]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Liegro, I. Di Physical activity and brain health. Genes 2020, 10, 1–40. [Google Scholar] [CrossRef]
- Cassilhas, R.C.; Tufik, S.; De Mello, M.T. Physical exercise, neuroplasticity, spatial learning and memory. Cell. Mol. Life Sci. 2016, 73, 975–983. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sport. 2015, 25, 1–72. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Bhaskaran, S.; Van Remmen, H. Muscle fiber type diversification during exercise and regeneration. Free Radic. Biol. Med. 2016, 98, 56–67. [Google Scholar] [CrossRef] [PubMed]
- World Health Assembly Resolution WHA57.17. Physical Activity and Older Adults; World Health Organization: Geneva, Switzerland, 22 May 2004; pp. 1–21. [Google Scholar]
- Haskell, W.L.; Lee, I.-M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A. Physical activity and public health: Updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1081–1093. [Google Scholar] [CrossRef]
- Care, D. ADA Standards of medical care in diabetes—2020. Diabetes Care 2020, 43, S1–S224. [Google Scholar] [CrossRef]
- Franz, M.J.; Boucher, J.L.; Rutten-Ramos, S.; VanWormer, J.J. Lifestyle Weight-Loss Intervention Outcomes in Overweight and Obese Adults with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. J. Acad. Nutr. Diet. 2015, 115, 1447–1463. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90 056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Yoo, J.H.; Kang, S.H.; Woo, J.H.; Shin, K.O.; Kim, K.B.; Cho, S.Y.; Roh, H.T.; Il Kim, Y. The Effects of 12 Weeks Regular Aerobic Exercise on Brain-derived Neurotrophic Factor and Inflammatory Factors in Juvenile Obesity and Type 2 Diabetes Mellitus. J. Physiol. Ther. Sci. 2014, 26, 2014. [Google Scholar] [CrossRef] [PubMed]
- Da Marques, N.S.F.; de Abreu, L.C.; dos Santos, B.V.; Neto, C.F.R.; da Silva, J.R.C.; de Braga, K.K.S.; da Uchôa, K.S.; Moraes, L.M.S.; de Ferreira, L.C.P.; Ribeiro, N.G.; et al. Cardiorespiratory parameters and glycated hemoglobin of patients with type 2 diabetes after a rehabilitation program. Medicine 2018, 97, e9321. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.P.; Moes, K.A.; Shiu, M.Y.; Hutchison, M.G.; Churchill, N.; Thomas, S.G.; Rhind, S.G. High-Intensity Interval Training Is Associated With Alterations in Blood Biomarkers Related to Brain Injury. Front. Physiol. 2018, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.; Rocha-vieira, E.; de Sousa, R.A.L.; Alvarenga, B.; Rocha-gomes, A.; Garcia, B.; Cassilhas, R.C.; Mendonça, V.A.; Camargos, A.C.R.; Lacerda, A.C.; et al. High-intensity interval training improves cerebellar antioxidant capacity without affecting cognitive functions in rats. Behav. Brain Res. 2019, 376, 1–7. [Google Scholar] [CrossRef]
- Gibala, M.J.; McGee, S.L. Metabolic Adaptations to Short-term High-Intensity Interval Training: A Little Pain For a Lot of Gain? Exerc. Sport Sci. Rev. 2008, 36, 58–63. [Google Scholar] [CrossRef]
- Little, J.P.; Safdar, A.; Wilkin, G.P.; Tarnopolsky, M.A.; Gibala, M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J. Physiol. 2010, 588, 1011–1022. [Google Scholar] [CrossRef]
- Mendes, R.; Sousa, N.; Themudo-Barata, J.L.; Reis, V.M. High-intensity interval training versus moderate-intensity continuous training in middle-aged and older patients with type 2 diabetes: A randomized controlled crossover trial of the acute effects of treadmill walking on glycemic control. Int. J. Environ. Res. Public Health 2019, 16, 4163. [Google Scholar] [CrossRef]
- Roden, M. Exercise in type 2 diabetes: To resist or to endure? Diabetologia 2012, 55, 1235–1239. [Google Scholar] [CrossRef][Green Version]
- VanDijk, J.; Manders, R.; Tummers, K.; Bonomi, A.; Stehouwer, C.; Hartgens, F.; VanLoon, L. Both resistance- and endurance-type exercise reduce the prevalence of hyperglycaemia in individuals with impaired glucose tolerance and in insulin-treated and non-insulin-treated type 2 diabetic patients. Diabetologia 2012, 55, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Afzalpour, M.E.; Chadorneshin, H.T.; Foadoddini, M.; Eivari, H.A. Comparing interval and continuous exercise training regimens on neurotrophic factors in rat brain. Physiol. Behav. 2015, 147, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Jamurtas, A.Z.; Fatouros, I.G.; Deli, C.K.; Georgakouli, K.; Poulios, A.; Draganidis, D.; Papanikolaou, K.; Tsimeas, P.; Chatzinikolaou, A.; Avloniti, A.; et al. The effects of acute low-volume HIIT and aerobic exercise on leukocyte count and redox status. J. Sport. Sci. Med. 2018, 17, 501–508. [Google Scholar]
- Holloway, T.M.; Bloemberg, D.; Da Silva, M.L.; Simpson, J.A.; Quadrilatero, J.; Spriet, L.L. High intensity interval and endurance training have opposing effects on markers of heart failure and cardiac remodeling in hypertensive rats. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef] [PubMed][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, R.A.L.D.; Improta-Caria, A.C.; Souza, B.S.d.F. Exercise–Linked Irisin: Consequences on Mental and Cardiovascular Health in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 2199. https://doi.org/10.3390/ijms22042199
Sousa RALD, Improta-Caria AC, Souza BSdF. Exercise–Linked Irisin: Consequences on Mental and Cardiovascular Health in Type 2 Diabetes. International Journal of Molecular Sciences. 2021; 22(4):2199. https://doi.org/10.3390/ijms22042199
Chicago/Turabian StyleSousa, Ricardo Augusto Leoni De, Alex Cleber Improta-Caria, and Bruno Solano de Freitas Souza. 2021. "Exercise–Linked Irisin: Consequences on Mental and Cardiovascular Health in Type 2 Diabetes" International Journal of Molecular Sciences 22, no. 4: 2199. https://doi.org/10.3390/ijms22042199
APA StyleSousa, R. A. L. D., Improta-Caria, A. C., & Souza, B. S. d. F. (2021). Exercise–Linked Irisin: Consequences on Mental and Cardiovascular Health in Type 2 Diabetes. International Journal of Molecular Sciences, 22(4), 2199. https://doi.org/10.3390/ijms22042199





